Abstract
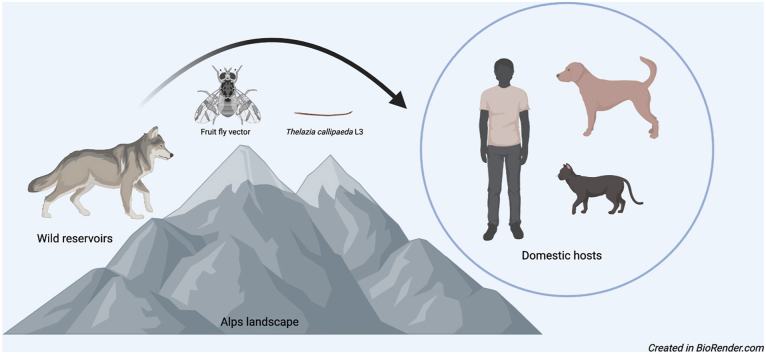
Thelazia callipaeda is a zoonotic parasite causing ocular disease in domestic dogs, cats, several wild carnivores, hares, and humans. This nematode is widely distributed in Europe, where it is transmitted by the drosophilid fly Phortica variegata. Since the first report of infection in grey wolves (Canis lupus) from southern Italy, other cases of thelaziosis have been recorded in this animal species throughout Europe, raising questions about their role in spreading T. callipaeda. Indeed, for their wandering behavior through long distances and living in woody areas where the vectors thrive, wolves may act as reservoirs and spreaders of thelaziosis. In this study we reviewed the literature about wolves acting as reservoirs of T. callipaeda in Europe. In addition, we report the first detection of T. callipaeda eyeworms in grey wolves in the Italian Alps, discussing its possible implications in the epidemiology of thelaziosis in the Alpine landscape. Animals (n = 3) included in this study were originated from the Italian Alps, one juvenile male wolf was found dead, and the other two were seven-year-old males translocated from Piedmont region to a Zoological Garden, in Tuscany. All animals were infected with eyeworms, which were morphologically and molecularly identified as T. callipaeda. Data herein presented confirm those available in the literature about the circulation of a unique cox1 haplotype in Europe. In addition, the report of T. callipaeda in wolves from the Alps suggests an ecological continuity of habitats which are suitable for the distribution of T. callipaeda from the southern to northern Italy through the Apennine backbone. Retrospectively, it could also explain the spreading of the oriental eyeworm infection in Europe over the last 20 years with many wild carnivores, such as foxes and possibly wolves, playing a pivotal role as reservoirs of the infection for dogs, cats and humans.
Keywords: Alps, Eyeworm, Thelazia callipaeda, Wildlife, Wolf, Zoonosis
Graphical abstract
Highlights
-
•
Wolves may play important role in the transmission cycle of Thelazia callipaeda.
-
•
There is a need of passive-surveillance programs of this zoonotic nematode in wild carnivores.
-
•
This is the first report of Thelazia callipaeda in wolves from the Italian Alps.
1. Introduction
Among the zoonotic parasites affecting wildlife and humans, Thelazia callipaeda is widely distributed in Europe, representing a health concern for domestic dogs and cats, several wild carnivores (e.g., foxes, wolves, bears), hares, and humans (Dorchies et al., 2007; Otranto et al., 2009; Miró et al., 2011; Baneth et al., 2016; Papadopoulos et al., 2021). This nematode is known as eyeworm since its late larval stages (i.e., L3, L4 and L5) and adults develop in the conjunctival sac of the above-mentioned vertebrate hosts (Otranto et al., 2004; Vale et al., 2020), after being transmitted by drosophilid flies of the Phortica genus (Otranto et al., 2005). To date, two Phortica spp. are recognized as vectors of T. callipaeda (i.e., Phortica variegata in Europe, and Phortica okadai in Asia) under natural and experimental conditions (Otranto et al., 2005; Jin et al., 2021). Recently, a third species, Phortica oldenbergi has shown to be a competent vector of this nematode, under experimental conditions (Bezerra-Santos et al., 2022 [submitted]).
Records of T. callipaeda in wildlife species in the European Alps are scanty, with an autochthonous case of thelaziosis reported in a dog from this landscape in Germany (Magnis et al., 2010). In addition, the infection was reported in foxes, dogs and cats in surrounding areas in Southern Switzerland, as well as in Phortica spp. vectors (Roggero et al., 2010; Malacrida et al., 2008) suggesting that a transmission cycle of T. callipaeda in the Alps may occur among domestic and wildlife carnivores. The wild fauna of this area includes several vertebrates such as foxes, wolves, red and roe deer, Northern chamois, Alpine ibex, wild boars, hares, rodents, lizards, amphibians, and birds (Spagnesi et al., 2017), including rare and protected taxa. The most iconic one, the grey wolf (Canis lupus), successfully recolonized the Western Alps in the last three decades, after more than 50 years of extirpation due to formerly legal persecution (Musto et al., 2021). Nowadays, the expansion of grey wolf population has also being observed in anthropized areas due to the saturation of undisturbed environments (Bassi et al., 2015; Meriggi et al., 2020; Musto et al., 2021), leading to human-wildlife contacts and increasing the risks of transmission of zoonotic parasites (Jenkins et al., 2015; Moroni et al., 2020). Here, we report the first detection of this eyeworm species in grey wolves in the Italian Alps, discussing its possible implications in the epidemiology of thelaziosis.
2. Material and methods
2.1. Case reports
The study was carried out in two locations in the Italian Western Alps. Wolf 1 was an illegally culled juvenile (5-month-old) from Lozzolo in the province of Vercelli, Piedmont, Italy (45°38′N 8°19′E). Eyeworms in active motion were observed during the forensic necropsy of the fresh wolf cadaver carried out at the Department of Veterinary Sciences of the University of Turin (Italy) in the frame of the Life WolfAlps EU programme (https://www.lifewolfalps.eu/). Nematodes were collected and placed in 70% alcohol for posterior analysis. Wolves 2 and 3 were 7 years old siblings translocated from “La Torbiera” wildlife park in Agrate Conturbia in the province of Novara (45°40′35″N 8°33′08″E) to the “Zoological Gardens” of Pistoia in Tuscany (Italy). Animals arrived with ocular discharge, and 20 days later one of them presented blepharospasm, corneal redness, and palpebral wounds (i.e., paracentral corneal ulcer with perforation and prolapse of the iris probably caused by scratching). Ocular thelaziosis was suspected and both animals sharing the same enclosure were examined under general anesthesia by a veterinarian. Several adult worms were manually collected and stored in 70% alcohol. Both animals were treated with ivermectin (Noromectin® 1%, Vetoquinol, 0.2 mg/kg, once subcutaneous), and the one with palpebral injuries also received an antibiotic therapy with injectable cefovecin sodium (Convenia®, Zoetis Italia s. r.l., 8 mg/kg, once subcutaneous).
2.2. Morphological and molecular analysis
Adult nematodes were firstly identified according to morphological keys (Otranto et al., 2003, 2004). Molecular analysis was performed to confirm the morphological identification. For this, genomic DNA was extracted from individual worms using Dneasy Blood & Tissue Kit (Qiagen, Hilden, Germany). Thereafter PCR analysis was performed using the primers NTF (5′-TGATTGGTGGTTTTGGTAA-3′) and NTR (5′-ATAAGTACGAGTATCAATATC-3′) that amplify a portion (689bp) of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene. Amplicons were purified and sequenced in both directions using the Big Dye Terminator v.3.1 chemistry in a 3130 Genetic analyzer (Applied Biosystems, California, USA) in an automated sequencer (ABI-PRISM 377). Sequences were analyzed using MEGA7 software and compared with sequences available in GenBank through the BLAST search tool.
3. Results
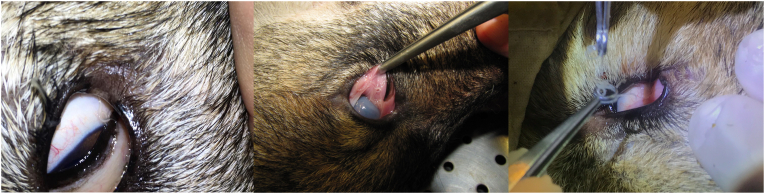
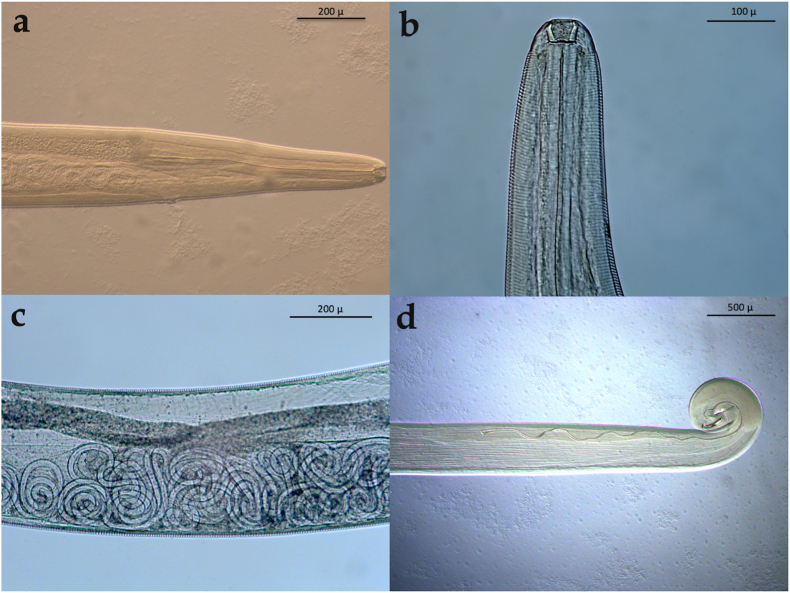
Nematodes were detected in both eyes of all three animals (n = 41 in wolf 1; n = 8 in wolf 2; n = 7 in wolf 3; Fig. 1). Specimens were morphologically and morphometrically identified as T. callipaeda (Table 1). The specimens were characterized by striated cuticle throughout the body. Females were 13.5 mm (SD ± 1.24) long and 0.42 mm (SD ± 0.03) wide, with the vulva located anteriorly to the oesophagus-intestinal junction (Fig. 2a and b). All females observed were gravid with presence of L1 in the uterus and close to the vaginal opening (Fig. 2c). Males were 10.8 mm (SD ± 1.47) long and 0.39 mm (SD ± 0.007) wide, presenting a short spicule on the right side, and a long spicule on the left side (Fig. 2d). Molecular analysis and sequencing of a portion of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene confirmed the species identification. Sequences presented 100% nucleotide identity with T. callipaeda haplotype 1 available in GenBank (AM042549.1; OK662943.1). Obtained nucleotide sequences were submitted to GenBank under the accession number: OM462655.
Fig. 1.
Presence of Thelazia callipaeda in the conjunctival sacs of wolves (Canis lupus) from the Italian Alps.
Table 1.
Morphometry of 5 specimens (2 male and 3 female) of Thelazia callipaeda from naturally infected Canis lupus.
| Representative samples | S1♂ | S2♂ | S3♀ | S4♀ | S5♀ |
|---|---|---|---|---|---|
| Body length (mm) | 11.84 | 9.76 | 12.61 | 12.90 | 14.88 |
| Body width (μ) | 375 | 388 | 390 | 420 | 447 |
| Buccal capsule length (μ) | 28 | 27 | 28 | 30 | 31 |
| Buccal capsule width (μ) | 25 | 24 | 26 | 27 | 27 |
| Nerve-ring from anterior extremity (μ) | 300 | 260 | 341 | 310 | 351 |
| Oesophagus length (mm) | 680 | 630 | 710 | 720 | 728 |
| Vulva from anterior extremity (μ) | – | – | 602 | 654 | 671 |
| Left spicule length (mm) | 1.980 | 1.620 | – | – | – |
| Right spicule length (μ) | 171 | 154 | – | – | |
| Tail length (μ) | 65 | 61 | 69 | 71 | 74 |
| Eggs (μ) | – | – | 60 × 41 | 58 × 41 | 59 × 44 |
| Larvae L1 length (μ) -range | – | – | 376–420 | 391–400 | 390–421 |
Fig. 2.
Thelazia callipaeda collected from the eyes of wolves. a) anterior end of a female; b) anterior end of a female highlighting the striated cuticle throughout the body; c) presence of L1 in the uterus; d) posterior end of a male highlighting the short and long spicules.
4. Discussion
This study reports the first cases of ocular thelaziosis by T. callipaeda in grey wolves from peripheral zones of the Italian Alps. Since the first report of T. callipaeda in wolves in Italy (Otranto et al., 2007), other records on this host species have been published in different European countries such as Romania (Mihalca et al., 2016), Serbia (Bojan et al., 2019), Spain (Nájera et al., 2020), and Greece (Papadopoulos et al., 2021), demonstrating that this ecologically plastic canid is involved in the transmission cycle of T. callipaeda in diverse habitat types in Europe.
In general, wild canids are particularly susceptible to infection by T. callipaeda eyeworm and may harbor heavy infections by this parasite (Vale et al., 2020). For example, red foxes (Vulpes vulpes), have been reported with infection intensity of up to 192 T. callipaeda specimens (Ionică et al., 2018). The infection in carnivore hosts may vary from asymptomatic to clinical signs characterized by conjunctivitis, mucus, corneal ulcers, red eyes, blepharospasm, corneal oedema, and mucopurulent discharge (Sargo et al., 2014; Papadopoulos et al., 2021). In addition, the presence of adult worms in the eyes causes discomfort and may expose the animals to opportunistic bacterial infections, which may have more severe consequences (Rolbiecki et al., 2021).
Wolves are characterized by ample home-ranges, a dispersal behavior of subadult individuals over long distances and an ecological preference for woody areas where the Thelazia vectors also thrive. The association of these characteristics make the wolf a candidate for acting as a sylvatic reservoir and spreader of thelaziosis (Hodžić et al., 2014; Liang et al., 2019). Indeed, several studies have demonstrated the direct and indirect contact of these wild canids with humans and companion animals (Bassi et al., 2021), which may contribute to the exchange of zoonotic pathogens among wildlife, people and domestic hosts (Otranto et al., 2015; Jenkins et al., 2015; Moroni et al., 2020; Bezerra-Santos et al., 2021a, Bezerra-Santos et al., 2021b). For example, a recent study on Dirofilaria immitis, demonstrated the role of these wild canids as competent hosts of this zoonotic nematode in a complex multi-environmental scenario involving urban and domestic interface (Moroni et al., 2020). This interaction in endemic areas for ocular thelaziosis may be an important route of transmission of T. callipaeda to humans and domestic hosts. Data herein reported highlight the need for passive-surveillance programs of this zoonotic nematode in wild carnivores, eventually leading to reinforcement of awareness raising initiatives on actual ocular thelaziosis distribution amongst companion animal practitioners.
In the Italian Alps, wolves thrived until the early 1900's when they were gradually extinguished; however, this wild carnivore became legally protected in Italy in the early 1970's, resulting in strengthening of the survived peninsular population and the successive recolonization of the alpine bioregion since the late Eighties, with an increase to a minimum of 300 individuals by winter 2016/2017 (Marucco et al., 2012; Pilgrim et al., 2018). Under the above circumstances, the first record of T. callipaeda infecting grey wolves from the Italian Alps is of veterinary and public health interest, as this wildlife species, along with other carnivores (e.g., foxes, wild cats), may be acting not only as regional wildlife sentinel but also in the maintenance and spreading of the oriental eyeworm, increasing the risk of infection to humans and companion animals inhabiting the Alps.
It is reasonable to assume that T. callipaeda infection risk in colonizing wolves recently started when these wild carnivores established themselves in low altitude peripheral zones of the Alps following the saturation of remote wilderness areas at higher altitude and consequently harsher climate. Modelling studies showed that optimum ecological conditions for T. callipaeda vectors occurrence are found mainly in hilly areas at a mean altitude of 804.8 m above sea level (Marino et al., 2020). Furthermore, it is known that besides overwintering as adult in the conjunctival sac of competent hosts, T. callipaeda is also capable to overwinter in Phortica spp. vectors before the transmission to susceptible vertebrate hosts, in the next spring (Otranto et al., 2006; Pombi et al., 2020). Nevertheless, further studies are needed to understand if low mountain-dwelling wolves are able to play a role as additional maintenance host of this zoonotic nematode, aside dogs and red foxes.
5. Conclusions
The records of T. callipaeda in wolves from the Alps suggest an ecological continuity of habitats which are compatible with the occurrence of this zoonotic nematode from the southern to northern Italy through the Apennine backbone. Retrospectively, it could also explain the spreading of these oriental eyeworm infection in Europe over the last 20 years with many wild carnivores such as red foxes and possibly wolves, playing a pivotal role as reservoirs of the infection for dogs, cats and humans.
Author's contribution
Marcos Antônio Bezerra-Santos: Formal analysis; Investigation; Methodology; Writing - original draft; Writing - review & editing. Barbara Moroni: Investigation; Methodology; Writing - review & editing. Jairo Alfonso Mendonza-Roldan: Writing - review & editing. Stefania Perrucci: Investigation; Methodology; Writing - review & editing. Paolo Cavicchio: Methodology; Writing - review & editing. Rossana Cordon - Methodology; Writing - review & editing. Caterina Cianfanelli: Methodology; Writing - review & editing. Riccardo Paolo Lia: Methodology; Writing - review & editing. Luca Rossi: Investigation; Methodology; Writing - review & editing. Domenico Otranto: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors thank Giada Annoscia (University of Bari) for the support on laboratory work.
References
- Baneth G., Thamsborg S.M., Otranto D., Guillot J., Blaga R., Deplazes P., Solano-Gallego L. Major parasitic zoonoses associated with dogs and cats in Europe. J. Comp. Pathol. 2016;155(1 Suppl. 1):S54–S74. doi: 10.1016/j.jcpa.2015.10.179. [DOI] [PubMed] [Google Scholar]
- Bassi E., Willis S.G., Passilongo D., Mattioli L., Apollonio M. Predicting the spatial distribution of wolf (Canis lupus) breeding areas in a mountainous region of Central Italy. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi E., Pervan I., Ugarković D., Kavčić K., Maksan M.T., Krofel M., Šprem N. Attacks on hunting dogs: the case of wolf–dog interactions in Croatia. Eur. J. Wildl. Res. 2021;67:1–9. doi: 10.1007/s10344-020-01451-5. [DOI] [Google Scholar]
- Bezerra-Santos M.A., Bernardini I., Lia R.P., Mendoza-Roldan J.A., Beugnet F., Pombi M., Otranto D. Submitted paper; 2022. Phortica oldenbergi (Diptera: Drosophilidae): a new vector of the zoonoticThelazia callipaeda eyeworm. [DOI] [PubMed] [Google Scholar]
- Bezerra-Santos M.A., Mendoza-Roldan J.A., Thompson R.C.A., Dantas-Torres F., Otranto D. Legal versus illegal wildlife trade: zoonotic disease risks. Trends Parasitol. 2021;37:360–361. doi: 10.1016/j.pt.2021.02.003. [DOI] [PubMed] [Google Scholar]
- Bezerra-Santos Marcos Antonio, Mendoza-Roldan Jairo Alfonso, Thompson R.C.A., Dantas-Torres Filipe, Otranto Domenico. Illegal wildlife trade: A gateway to zoonotic infectious diseases. Trends Parasitol. 2021;37:181–184. doi: 10.1016/j.pt.2020.12.005. [DOI] [PubMed] [Google Scholar]
- Bojan G., Vanja B.S., Aleksandra P., Milica K., Neda B., Duško Ć. First report of eyeworm infection by Thelazia callipaeda in gray wolf (Canis lupus) from Serbia. Parasitol. Res. 2019;118:3549–3553. doi: 10.1007/s00436-019-06519-z. [DOI] [PubMed] [Google Scholar]
- Dorchies P., Chaudieu G., Siméon L.A., Cazalot G., Cantacessi C., Otranto D. First reports of autochthonous eyeworm infection by Thelazia callipaeda (Spirurida, Thelaziidae) in dogs and cat from France. Vet. Parasitol. 2007;149(3–4):294–297. doi: 10.1016/j.vetpar.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Hodžić A., Latrofa M.S., Annoscia G., Alić A., Beck R., Lia R.P., Dantas-Torres F., Otranto D. The spread of zoonotic Thelazia callipaeda in the Balkan area. Parasites Vectors. 2014;7:1–6. doi: 10.1186/1756-3305-7-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionică A.M., Deak G., Matei I.A., D'Amico G., Cotuţiu V.D., Gherman C.M., Mihalca A.D. Thelazia callipaeda, an endemic parasite of red foxes (Vulpes vulpes) in Western Romania. J. Wildl. Dis. 2018;54:829–833. doi: 10.7589/2017-10-251. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Simon A., Bachand N., Stephen C. Wildlife parasites in a one health world. Trends Parasitol. 2015;31:174–180. doi: 10.1016/j.pt.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Liu Z., Wei J., Wen Y., He N., Tang L., Lin D., Lin J. A first report of Thelazia callipaeda infection in Phortica okadai and wildlife in national nature reserves in China. Parasites Vectors. 2021;14:13. doi: 10.1186/s13071-020-04509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., He J., Wang S., Yang L., Chen F. Improved cluster collaboration algorithm based on wolf pack behavior. Cluster Comput. 2019;22:6181–6196. doi: 10.1007/s10586-018-1891-y. [DOI] [Google Scholar]
- Magnis J., Naucke T.J., Mathis A., Deplazes P., Schnyder M. Local transmission of the eye worm Thelazia callipaeda in southern Germany. Parasitol. Res. 2010;106:715–717. doi: 10.1007/s00436-009-1678-4. [DOI] [PubMed] [Google Scholar]
- Malacrida F., Hegglin D., Bacciarini L., Otranto D., Nägeli F., Nägeli C., Bernasconi C., Scheu U., Balli A., Marenco M., Togni L. Emergence of canine ocular thelaziosis caused by Thelazia callipaeda in southern Switzerland. Vet. Parasitol. 2008;157:321–327. doi: 10.1016/j.vetpar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Marino V., Gálvez R., Montoya A., Mascuñán C., Hernández M., Barrera J.P., Domínguez I., Zenker C., Checa R., Sarquis J., Miró G. Spain as a dispersion model for Thelazia callipaeda eyeworm in dogs in Europe. Prev. Vet. Med. 2020;175:104883. doi: 10.1016/j.prevetmed.2020.104883. [DOI] [PubMed] [Google Scholar]
- Marucco F., Avanzinelli E., Boitani L. Non-invasive integrated sampling design to monitor the wolf population in Piemonte, Italian Alps. Hystrix. 2012;23:5–13. doi: 10.4404/hystrix-23.1-4584. [DOI] [Google Scholar]
- Meriggi A., Torretta E., Dondina O. In: Problematic Wildlife II. Angelici F., Rossi L., editors. Springer; Cham: 2020. Recent changes in wolf habitat occupancy and feeding habits in Italy: implications for conservation and reducing conflict with humans. [DOI] [Google Scholar]
- Mihalca A.D., Ionică A.M., D'Amico G., Daskalaki A.A., Deak G., Matei I.A., Șimonca V., Iordache D., Modrý D., Gherman C.M. Thelazia callipaeda in wild carnivores from Romania: new host and geographical records. Parasites Vectors. 2016;9:350. doi: 10.1186/s13071-016-1628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miró G., Montoya A., Hernández L., Dado D., Vázquez M.V., Benito M., Villagrasa M., Brianti E., Otranto D. Thelazia callipaeda: infection in dogs: a new parasite for Spain. Parasites Vectors. 2011;4:148. doi: 10.1186/1756-3305-4-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni B., Rossi L., Meneguz P.G., Orusa R., Zoppi S., Robetto S., Marucco F., Tizzani P. Dirofilaria immitis in wolves recolonizing northern Italy: are wolves competent hosts? Parasites Vectors. 2020;13:1–7. doi: 10.1186/s13071-020-04353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musto C., Cerri J., Galaverni M., Caniglia R., Fabbri E., Apollonio M., Mucci N., Bonilauri P., Maioli G., Fontana M.C., Gelmini L. Men and wolves: anthropogenic causes are an important driver of wolf mortality in human-dominated landscapes in Italy. Glob. Ecol. Conserv. 2021;32 doi: 10.1016/j.gecco.2021.e01892. [DOI] [Google Scholar]
- Nájera F., de Lucas-Veguillas J., Vela Á., López-Fernández M., Martínez-Martínez P., Mata-Huete M., Cáceres-Urones J., Annoscia G., Otranto D., Calero-Bernal R. First report of Thelazia callipaeda in a free-ranging Iberian wolf (Canis lupus signatus) from Spain. Parasitol. Res. 2020;119:2347–2350. doi: 10.1007/s00436-020-06735-y. [DOI] [PubMed] [Google Scholar]
- Otranto D., Lia R.P., Traversa D., Giannetto S. Thelazia callipaeda (Spirurida,Thelaziidae) of carnivores and humans: morphological study by light and scanning electron microscopy. Parassitologia. 2003;45:125–133. [PubMed] [Google Scholar]
- Otranto D., Lia R.P., Buono V., Traversa D., Giangaspero A. Biology of Thelazia callipaeda (Spirurida, Thelaziidae) eyeworms in naturally infected definitive hosts. Parasitology. 2004;129:627–633. doi: 10.1017/s0031182004006018. [DOI] [PubMed] [Google Scholar]
- Otranto D., Lia R.P., Cantacessi C., Testini G., Troccoli A., Shen J.L., Wang Z.X. Nematode biology and larval development of Thelazia callipaeda (Spirurida, Thelaziidae) in the drosophilid intermediate host in Europe and China. Parasitology. 2005;131:847–855. doi: 10.1017/S0031182005008395. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Testini G., Lia R.P. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: evidence for pathogen transmission by a male arthropod vector. Int. J. Parasitol. 2006;36:1167–1173. doi: 10.1016/j.ijpara.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Mallia E., Lia R.P. First report of Thelazia callipaeda (Spirurida, Thelaziidae) in wolves in Italy. J. Wildl. Dis. 2007;43:508–511. doi: 10.7589/0090-3558-43.3.508. [DOI] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Mallia E., Di Geronimo P.M., Brianti E., Testini G., Traversa D., Lia R.P. Thelazia callipaeda (Spirurida, Thelaziidae) in wild animals: report of new host species and ecological implications. Vet. Parasitol. 2009;166:262–267. doi: 10.1016/j.vetpar.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Dantas-Torres F., Brianti E., Pfeffer M., Genchi C., Guberti V., Capelli G., Deplazes P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: helminths and arthropods. Vet. Parasitol. 2015;213(1–2):24–37. doi: 10.1016/j.vetpar.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Papadopoulos E., Komnenou A., Karamanlidis A.A., Bezerra-Santos M.A., Otranto D. Zoonotic Thelazia callipaeda eyeworm in brown bears (Ursus arctos): a new host record in Europe. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14414. [DOI] [PubMed] [Google Scholar]
- Pilgrim K., Engkjer C., Schwartz M. In: La popolazione di lupo sulle Alpi Italiane 2014-2018. Marucco F., Avanzinelli E., Bassano B., Bionda R., Bisi F., Calderola S., Chioso C., Fattori U., et al., editors. Life Wolfalps; 2018. Genetic analysis summary and preliminary population genetic evaluation of wolves (Canis lupus) in the Italian Alps in 2014–2018.http://www.lifewolfalps.eu/wp-content/uploads/2014/05/Report_Alpi.pdf Relazione tecnica, Progetto Life 12 Nat/IT/00080 Wolfalps - Azione A4 e D1. 2018. [Google Scholar]
- Pombi M., Marino V., Jaenike J., Graham-Brown J., Bernardini I., Lia R.P., Beugnet F., Miro G., Otranto D. Temperature is a common climatic descriptor of lachryphagous activity period in Phortica variegata (Diptera: drosophilidae) from multiple geographical locations. Parasites Vectors. 2020;13:1–9. doi: 10.1186/s13071-020-3955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggero C., Schaffner F., Bächli G., Mathis A., Schnyder M. Survey of Phortica drosophilid flies within and outside of a recently identified transmission area of the eye worm Thelazia callipaeda in Switzerland. Vet. Parasitol. 2010;171:58–67. doi: 10.1016/j.vetpar.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Rolbiecki L., Izdebska J.N., Franke M., Iliszko L., Fryderyk S. The vector-borne zoonotic nematode Thelazia callipaeda in the eastern part of Europe, with a clinical case report in a dog in Poland. Pathogens. 2021;10:55. doi: 10.3390/pathogens10010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargo R., Loureiro F., Catarino A.L., Valente J., Silva F., Cardoso L., Otranto D., Maia C. First report of Thelazia callipaeda in red foxes (Vulpes vulpes) from Portugal. J. Zoo Wildl. Med. 2014;45:458–460. doi: 10.1638/2013-0294R.1. [DOI] [PubMed] [Google Scholar]
- Spagnesi M., Catalano U., Riva L., Zambotti L. vol. 9. 2017. http://www.uomoenatura.it/project/vertebrati-terrestri-della-pianura-padana/ (Vertebrati terrestri delle Alpi italiane. Quaderni di Educazione Naturalistica). [Google Scholar]
- Vale B., Lopes A.P., da Conceição Fontes M., Silvestre M., Cardoso L., Coelho A.C. Systematic review on infection and disease caused by Thelazia callipaeda in Europe: 2001-2020. Revue systématique de l’infection et de la maladie provoquées par Thelazia callipaeda en Europe (2001–2020) Parasite. 2020;27:52. doi: 10.1051/parasite/2020048. [DOI] [PMC free article] [PubMed] [Google Scholar]