Abstract
The objective of this study was to investigate oral health-related quality of life (OHRQoL) in times of the COVID-19 pandemic and to examine a possible association to psychosocial factors like psychological stress and symptoms of depression and anxiety disorders. Secondary research questions were whether people changed oral hygiene regimens during the COVID-19 pandemic and to what extent dental symptoms existed and developed compared to pre-pandemic. For this cross-sectional study a survey has been conceptualized to determine OHRQoL, stress, depression and anxiety and their specific confounders in a German cohort. Validated questionnaires as OHIP-G14, PHQ-Stress and PHQ-4 have been implemented. Altogether 1178 participants completed the survey between May and August 2020. The overall OHIP-G14 sum score of 4.8 ± 7.5 indicated good OHRQoL. 21% of the participants (n = 248) reported toothache, 23% (n = 270) mucosal problems, 31% (n = 356) hypersensitivity of the teeth and 27% (n = 305) myofacial pain. The PHQ-Stress score (4.5 ± 3.5) demonstrated a mild severity of stress. Depression and anxiety level has been mild to moderate (PHQ-4 score: 2.4 ± 2.6). 38% of the participants stated subjectively greater emotional burden compared to pre-pandemic. Statistically significant differences exist for OHRQoL, stress, anxiety and depression levels between participants with greater, equal or less emotional burden compared to pre-pandemic. COVID-19 history and aggravated levels of depression, anxiety, and stress seem to associate with lower OHRQoL. Psychosocial consequences during pandemic times and their association to oral health should be further investigated.
Subject terms: Epidemiology, Dental public health, Public health, Quality of life, Psychology and behaviour, Oral manifestations, Anxiety, Depression
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory disease caused by the virus SARS-CoV-2. It was declared a pandemic by the World Health Organization in March 2020. The course of the disease is diverse and varies. In addition to asymptomatic infections, mild to moderate courses were observed, as well as severe progressions with pneumonia up to lung failure and death1. In order to contain the rapidly increasing number of infections and to prevent an overload of the health system, public life in Germany was severely restricted from March 2020. Hence, people were asked to stay at home and adhere to contact restrictions. Numerous shops and services had to close temporarily. In the meantime, outpatient dental care had also been reduced to a minimum. For children, classroom-teaching in schools and child care in day-care centers were temporarily cancelled. This led to a change of working conditions (home office, furlough, terminations, etc.) and social situations (e.g. family, friends)2,3. Due to decreasing incidences, public life was gradually allowed to resume again as of May 2020, but with continuing substantial restrictions. Effects of the COVID-19 pandemic and its countermeasures on the perception of psychological stress and mental health or on the intensification of already existing mental conditions were shown4,5.
The effect of psychological stress and mental conditions such as depression and anxiety disorders on the course of oral conditions as e.g. periodontitis or functional conditions and vice versa has not been conclusively established, but several studies show an association6. On the one hand, stress can influence the immune response and is generally associated with inflammation. On the other hand, health-related behavior as preventive oral hygiene can be negatively influenced by psychosocial factors, which in return can lead to increased infection or may aggravate existing inflammation6–12.
Oral health or disease in general, can be assessed objectively and clinically based, while neglecting the impact on patient’s quality of life13,14. A more patient-reported but subjective outcome is the concept of oral health-related quality of life (OHRQoL). Consequently, this measure cannot reveal the clinical oral status, but the individual’s perception of oral health and its impact on life13. It has to be interpreted as a multidimensional concept involving biopsychosocial aspects related to oral health13. It can be influenced by cultural context and age13. Instruments as the most widely used Oral Health Impact Profile (OHIP) capture the patient-perceived impact and make OHRQoL measurable13,14.
The objective of this study was to examine how adults in Germany rate their OHRQoL in times of the COVID-19 pandemic and whether an association to perceived psychosocial stress and symptoms of depressive disorders and generalized anxiety exist. Secondary research questions were whether people changed oral hygiene regimens during the COVID-19 pandemic and to what extent oral symptoms as toothache, mucosal pain, dental hypersensitivity or myofacial pain existed and developed compared to pre-pandemic.
Methods
Study design
This questionnaire-based cross-sectional study was approved by the ethics committee of the Medical Faculty of Heidelberg (# S-303/2020) and is in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The trial was registered at the US National Institute of Health (ClinicalTrials.gov, # NCT04381273). This observational study is being reported using the STROBE statement for cross-sectional studies15.
Survey development
For this study, a digital questionnaire consisting of validated items was conceptualized to determine oral health and psychosocial factors and queries experiences in times of the COVID-19 pandemic. Hence, the questionnaire is conducted of sub-questionnaires and free questions (total: 27 questions). To query oral-health related and also sociodemographic data, the seven-item questionnaire from the German Society for Periodontology (DG PARO) “the periodontitis risk score” was integrated. The age range was slightly modified in order to include all ages from 18 years onwards16. The score ranges from 0 points indicating lowest periodontitis risk up to 20 points indicating very high periodontitis risk. A question was also added asking whether toothache, mucosal pain, dental hypersensitivity or myofacial pain were perceived symptoms and whether they were felt the same, stronger or weaker compared to before the pandemic. OHRQoL was assessed by the German version of the 14-item Oral Health Impact Profile (OHIP-G14). It consists of 14 questions which can be grouped into seven distinct sub-domains: (1) functional limitation (items 1 and 2), (2) handicap (items 3 and 10), (3) psychological disability (items 4 and 11), (4) psychological discomfort (items 5 and 14), (5) physical disability (items 6 and 12), (6) physical pain (items 7 and 13), and (7) social disability (items 8 and 9). The answers are recorded on a Likert scale with values ranging from 0 to 4 coded as 0 “never”, 1 “hardly ever”, 2 “occasionally”, 3 “fairly often”, or 4 “very often”. The OHIP-G14 sum score can range from 0 to 56 with a higher score indicating a poorer OHRQoL17. To determine psychosocial stress during the COVID-19 pandemic, the validated ten-item PHQ-Stress module (Patient Health Questionnaire) was chosen. The psychosocial impairment caused by the queried factors can be assessed from 0 “not impaired”, over 1 “slightly impaired” to 2 “severely impaired”. Score values of 0–4 can be viewed as minimally pronounced psychosocial stress factors. Total values of 5–9 represent mild, 10–14 moderate and 15–20 severe stress manifestation18. As a validated screening instrument for depressive disorders and generalized anxiety, the four-item PHQ-4 was included. The frequency of complaints inquired can be evaluated on a scale from 0 “not at all” to 3 “almost every day”. Total values can range from 0–12. A PHQ-4 score of 0–2 designates none-to-minimal, 3–5 mild, 6–8 moderate and 9–12 severe level of depression and anxiety18,19. Questions were added on the subjective assessment of the extent of pandemic-related restrictions in life (none/little/strong), as well as the extent to which the pandemic had an influence on work and family/social life (none/little/strong). An additional question was implemented on subjectively perceived emotional burden during the COVID-19 pandemic compared to before the pandemic (greater/equal/less) to also match these self-assessments in relation to the more objectified scores of the psychosocial sub-questionnaires as the PHQ-Stress and PHQ-4. In order to take known confounders for oral health and psychosocial factors into account, questions about the oral hygiene behavior, systemic diseases, body weight and height (to calculate the body mass index (BMI)) as well as the aforementioned demographic and socioeconomic questions regarding age, sex, smoking educational and employment status were added.
Recruitment & investigation
The questionnaire was created using a “free access” for scientific research at “umfrageonline.com” (enuvo GmbH, Zurich, Switzerland) and was available online for twelve weeks from May 16th, 2020 to August 8th, 2020. Answering the questionnaire took about 5–10 min. Because the survey was anonymous and voluntary, and the purpose of the study was described at the beginning of the questionnaire, no additional informed consent was required, as approved by the ethics committee of the Medical Faculty of Heidelberg (# S-303/2020). The digital link was initially distributed to patients at the Clinic of Conservative Dentistry at Heidelberg University Hospital and published on a flyer. Passing on the link e.g. through social media was anticipated with the intention of reaching as many people of all ages as possible across Germany. Additionally, a printed version of the questionnaire was made available for people without internet access, reaching especially local respondents. Additional samples were distributed by some study participants. In order to fit the inclusion criteria participants had to be over 18 years of age, have a place of residence in Germany and give consent to participate in the study.
Sample size & statistical analysis
Due to the nature of the survey, no formal sample size calculation was performed. Nevertheless, a power calculation using a Kruskal–Wallis one-way ANOVA in a study population of 1097 (365.7 per group) with mean OHIP-G14 sum score of 7.74, 3.05 and 3.80 for the three levels of emotional burden (greater/equal/less compared to pre-pandemic), respectively, yields a power of 100% using a type-I-error of 5%. Power calculation was performed by the software PASS v 16.0.3.
Demographics and general data were described using descriptive measures. Continuous variables were described using the number of non-missing values, mean and standard deviation. For binary or categorical variables absolute and relative frequencies were provided.
To investigate OHRQoL (primary objective), data from the OHIP-G14 was collected and analyzed using Kruskal–Wallis tests between participants with a greater, equal or lower emotional burden compared to pre-pandemic for each of the seven sub-domains and the sum score of the OHIP-G14. Non-parametric tests were chosen because of the skewed allocation ratio and the not normally distributed data. All missing values were excluded for statistical testing.
The influence of psychosocial factors and confounders on OHRQoL was examined using a linear regression model with stepwise (bidirectional elimination) variable selection based on Akaike information criterion. As dependent variable, the OHIP-G14 sum score and as independent variables, the following were used (variables marked with a * were considered clinically relevant and hence were enforced to be included in the final model and only the variables not marked with a * were used in the variable selection process): “PHQ-Stress sum score”*, “PHQ-4 sum score”*, “sex” (male/female)*, “age group” (18–39/40–59/60–100)*, “systemic diseases” (yes/no), “COVID-19 history” (yes/no), “employment status” (employed/self-employed/unemployed/student/in training/retired), “restrictions due to the COVID-19-pandemic” (strong/little/none), “BMI”, “periodontitis-history” (yes/no), “educational status” (≤ 10 years/ > 10 years of school), “interdental cleaning” (yes/no), “dentist visit frequency” (2/1/ < 1 per year), “tooth brushing frequency” (≤ 1/ ≥ 2 per day) and “emotional burden due to the COVID-19-pandemic compared to pre-pandemic” (greater/equal/less).
The secondary objective whether people changed oral hygiene regimens during the COVID-19 pandemic was analyzed using descriptive measures as described above and additionally Chi-squared tests between patients with greater, equal and less emotional burden compared to pre-pandemic.
Furthermore, if one item of a sub-questionnaire was missing, the whole sub-questionnaire was defined missing for this participant and excluded for statistical testing. In addition, due to the exploratory character of the survey, p-values can only be interpreted descriptively, so that no formal adjustment was made for multiple testing. P-values smaller than 0.05 were considered to be statistically significant. Statistical analyses were conducted using the statistic software R (version 4.0.2, R Core Team, Auckland, New Zealand) and were carried out at the Institute for Medical Biometry at Heidelberg University Hospital.
Results
Study population
A total of 1178 participants (426 men (36%), 747 women (64%)) took part in the survey, n = 795 (67%) digitally and n = 383 (33%) analogously. All age groups were represented, but not evenly distributed. The participants included residents of all 16 federal states, but with local differences in distribution. In particular, there were many participants from Baden-Württemberg (n = 488, 44%) and Hesse (n = 271, 25%). In Table 1, further demographic information is listed and additionally divided into study participants with a greater (n = 429), equal (n = 594) or lower (n = 134) emotional burden compared to pre-pandemic.
Table 1.
Demographic and general data of the study cohort.
| Variables | Total (n = 1178) | Greater emotional burden compared to pre-pandemic (n = 429) | Equal emotional burden compared to pre-pandemic (n = 594) | Less emotional burden compared to pre-pandemic (n = 134) | Emotional burden compared to pre-pandemic data missing (n = 21) |
|---|---|---|---|---|---|
| Age group | |||||
| 18–29 years | 215 (18%) | 83 (19%) | 99 (17%) | 30 (22%) | 3 (17%) |
| 30–39 years | 192 (16%) | 83 (19%) | 86 (15%) | 19 (14%) | 4 (22%) |
| 40–49 years | 129 (11%) | 57 (13%) | 57 (10%) | 14 (10%) | 1 (6%) |
| 50–59 years | 236 (20%) | 109 (25%) | 109 (18%) | 18 (13%) | 0 (0%) |
| 60–69 years | 194 (17%) | 64 (15%) | 106 (18%) | 21 (16%) | 3 (17%) |
| 70–79 years | 134 (11%) | 18 (4%) | 94 (16%) | 21 (16%) | 1 (6%) |
| 80–89 years | 67 (6%) | 13 (3%) | 39 (7%) | 10 (7%) | 5 (28%) |
| 90–99 years | 3 (0%) | 0 (0%) | 2 (0%) | 1 (1%) | 0 (0%) |
| ≥ 100 years | 2 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | 1 (6%) |
| Missing | 6 | 1 | 2 | 0 | 3 |
| Sex | |||||
| Male | 426 (36%) | 106 (25%) | 251 (42%) | 58 (44%) | 11 (58%) |
| Female | 747 (64%) | 322 (75%) | 342 (58%) | 75 (56%) | 8 (42%) |
| Missing | 5 | 1 | 1 | 1 | 2 |
| Smoking | |||||
| Never smoker | 623 (53%) | 221 (52%) | 322 (54%) | 66 (50%) | 14 (74%) |
| Former smoker | 363 (31%) | 130 (30%) | 190 (32%) | 39 (29%) | 4 (21%) |
| Active smoker, < 10 cigarettes per day | 84 (7%) | 38 (9%) | 35 (6%) | 11 (8%) | 0 (0%) |
| Active smoker, ≥ 10 cigarettes per day | 104 (9%) | 40 (9%) | 46 (8%) | 17 (13%) | 1 (5%) |
| Missing | 4 | 0 | 1 | 1 | 2 |
| Body mass index (BMI) | |||||
| n (missing) | 1132 (46; 4%) | 416 (13; 3%) | 577 (17; 3%) | 128 (6; 4%) | 11 (10; 48%) |
| BMI ± SD | 25.0 ± 4.8 | 25.0 ± 5.3 | 25.0 ± 4.5 | 25.0 ± 4.6 | 25.0 ± 3.3 |
| Educational status | |||||
| ≤ 10 years of school | 352 (30%) | 118 (28%) | 183 (31%) | 40 (31%) | 11 (61%) |
| > 10 years of school | 817 (70%) | 311 (72%) | 408 (69%) | 91 (69%) | 7 (39%) |
| Missing | 9 | 0 | 3 | 3 | 3 |
| Employment status | |||||
| Employed | 538 (47%) | 228 (54%) | 261 (44%) | 47 (36%) | 2 (18%) |
| Self-employed | 138 (12%) | 68 (16%) | 55 (9%) | 15 (11%) | 0 (0%) |
| Unemployed | 33 (3%) | 11 (3%) | 15 (3%) | 7 (5%) | 0 (0%) |
| Student/In Training | 126 (11%) | 50 (12%) | 58 (10%) | 18 (14%) | 0 (0%) |
| Retired | 320 (28%) | 66 (16%) | 200 (34%) | 45 (34%) | 9 (82%) |
| Missing | 23 | 6 | 5 | 2 | 10 |
| Systemic diseases | |||||
| Lung disease (COPD, Asthma, etc.) | 93 (8%) | 46 (11%) | 37 (6%) | 10 (7%) | 0 (0%) |
| Cardiovascular disease | 161 (14%) | 54 (13%) | 87 (15%) | 17 (13%) | 3 (1%) |
| Diabetes mellitus | 63 (5%) | 19 (44%) | 29 (5%) | 12 (9%) | 3 (1%) |
| Other systemic diseases | 215 (18%) | 92 (21%) | 96 (16%) | 26 (19%) | 1 (0%) |
| No systemic diseases | 698 (59%) | 243 (57%) | 373 (63%) | 78 (58%) | 4 (2%) |
| Federal state | |||||
| Baden-Württemberg | 488 (44%) | 153 (37%) | 263 (47%) | 66 (54%) | 6 (60%) |
| Bavaria | 55 (5%) | 33 (8%) | 17 (3%) | 5 (4%) | 0 (0%) |
| Berlin | 16 (1%) | 8 (2%) | 7 (1%) | 1 (1%) | 0 (0%) |
| Brandenburg | 3 (0%) | 2 (0%) | 1 (0%) | 0 (0%) | 0 (0%) |
| Bremen | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hamburg | 4 (0%) | 2 (0%) | 2 (0%) | 0 (0%) | 0 (0%) |
| Hesse | 271 (25%) | 84 (20%) | 155 (28%) | 29 (24%) | 3 (30%) |
| Mecklenburg-Vorpommern | 2 (0%) | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) |
| Lower Saxony | 41 (4%) | 26 (6%) | 14 (2%) | 1 (1%) | 0 (0%) |
| North Rhine-Westphalia | 105 (10%) | 49 (12%) | 47 (8%) | 9 (7%) | 0 (0%) |
| Rhineland-Palatinate | 83 (8%) | 33 (8%) | 41 (7%) | 8 (7%) | 1 (10%) |
| Saarland | 3 (0%) | 3 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Saxony | 10 (1%) | 6 (1%) | 4 (1%) | 0 (0%) | 0 (0%) |
| Saxony-Anhalt | 5 (0%) | 3 (1%) | 2 (0%) | 0 (0%) | 0 (0%) |
| Schleswig–Holstein | 2 (0%) | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) |
| Thuringia | 16 (1%) | 8 (2%) | 5 (1%) | 3 (2%) | 0 (0%) |
| Missing | 73 | 16 | 34 | 12 | 11 |
| COVID-19 anamnesis | |||||
| No COVID-19 history | 1136 (98%) | 418 (98%) | 583 (98%) | 130 (99%) | 5 (100%) |
| Confirmed COVID-19 history without hospitalization | 14 (1%) | 4 (1%) | 9 (2%) | 1 (1%) | 0 (0%) |
| Confirmed COVID-19 history and hospitalization | 5 (0%) | 5 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 23 | 2 | 2 | 3 | 16 |
| Pandemic-related restrictions in life | |||||
| Strong restrictions | 295 (26%) | 192 (45%) | 83 (14%) | 18 (14%) | 2 (18%) |
| Little restrictions | 710 (62%) | 218 (51%) | 401 (68%) | 84 (65%) | 7 (64%) |
| No restrictions | 146 (13%) | 14 (3%) | 102 (17%) | 28 (22%) | 2 (18%) |
| Missing | 27 | 5 | 8 | 4 | 10 |
| Pandemic-related influence on work | |||||
| Strong influence | 431 (41%) | 238 (59%) | 153 (29%) | 40 (34%) | 0 (0%) |
| Little influence | 319 (30%) | 105 (26%) | 179 (34%) | 34 (29%) | 1 (17%) |
| No influence | 308 (29%) | 59 (15%) | 200 (38%) | 44 (37%) | 5 (83%) |
| Missing | 120 | 27 | 62 | 16 | 15 |
| Pandemic-related influence on family/social life | |||||
| Strong influence | 519 (46%) | 291 (69%) | 185 (32%) | 42 (33%) | 1 (12%) |
| Little influence | 494 (43%) | 122 (29%) | 306 (53%) | 60 (47%) | 6 (75%) |
| No influence | 125 (11%) | 8 (2%) | 91 (16%) | 25 (20%) | 1 (12%) |
| Missing | 40 | 8 | 12 | 7 | 13 |
n number of participants, COVID-19 coronavirus disease 2019, SD standard deviation.
Of all respondents, 295 (26%) stated that their daily life was severely restricted by the COVID-19 pandemic, 710 (62%) stated that they were only slightly restricted and 146 (13%) evaluated no restrictions. The COVID-19 pandemic exerted a strong influence on everyday work for n = 431 (41%), a minor influence for n = 319 (30%) and no influence for n = 308 (29%). With regard to family and social environment, n = 519 (46%) felt a strong influence, n = 494 (43%) a slight and n = 125 (11%) no influence.
Oral health-related parameters
The first seven questions of the questionnaire included validated questions of the DG PARO periodontitis risk score. Overall (n = 1149), a mean value of 6.9 ± 4.0 was given. 421 participants with subjectively greater emotional burden compared to pre-pandemic reported a periodontitis risk score of 6.2 ± 3.6 compared to 587 participants with equal emotional burden and a score of 7.4 ± 4.1 and 124 with less emotional burden and a score of 7.1 ± 4.3, respectively (p < 0.001KW). Perception of periodontitis and dentist visit frequency are shown in Table 2. With regard to oral hygiene measures, 899 participants (77%) reported that they brushed their teeth at least twice per day, 257 (22%) once per day and 15 (1%) less than once per day. There was no statistically significant difference between the three groups on this topic (p = 0.270chi2), in contrast to interdental cleaning (p = 0.032chi2). Overall, n = 512 (44%) used interdental brushes, n = 317 (27%) used floss, n = 82 (7%) other aids and n = 259 (22%) did not use any aids for interdental care. In total, 1083 (93%) of those surveyed had not changed toothbrushing frequency during the pandemic. Participants with greater emotional burden stated more frequently that they had noticed worsening of toothache (6% vs. 3% vs. 3%, p = 0.006chi2), mucosal pain (7% vs. 3% vs. 4%, p = 0.029chi2), hypersensitivity (9% vs. 3% vs. 4%, p < 0.001chi2) and myofacial pain (13% vs. 3% vs. 2%, p < 0.001chi2) during the pandemic compared to those with equal or less emotional burden compared to pre-pandemic.
Table 2.
Oral health-related parameters.
| Variables | Total (n = 1178) | Greater emotional burden compared to pre-pandemic (n = 429) | Equal emotional burden compared to pre-pandemic (n = 594) | Less emotional burden compared to pre-pandemic (n = 134) | Emotional burden compared to pre-pandemic data missing (n = 21) | p-Value |
|---|---|---|---|---|---|---|
| Bleeding of the gums | ||||||
| No | 787 (67%) | 271 (63%) | 410 (69%) | 92 (70%) | 14 (74%) | |
| Sometimes | 357 (30%) | 143 (33%) | 171 (29%) | 38 (29%) | 5 (26%) | |
| Often | 27 (2%) | 14 (3%) | 11 (2%) | 2 (2%) | 0 (0%) | |
| Missing | 7 | 1 | 2 | 2 | 2 | |
| Tooth mobility | ||||||
| No | 1052 (91%) | 382 (91%) | 539 (91%) | 115 (88%) | 16 (84%) | |
| Yes | 110 (9%) | 40 (9%) | 52 (9%) | 15 (12%) | 3 (16%) | |
| Missing | 16 | 7 | 3 | 4 | 2 | |
| Periodontitis risk score | < 0.001KW | |||||
| n | 1149 | 421 | 587 | 124 | 17 | |
| Missing | 29 (2%) | 8 (2%) | 7 (1%) | 10 (7%) | 4 (19%) | |
| Mean | 6.9 | 6.2 | 7.4 | 7.1 | 8.2 | |
| SD | 4.0 | 3.6 | 4.1 | 4.3 | 5.0 | |
| Median | 7 | 6 | 8 | 8 | 9 | |
| Q1–Q3 | 3–10 | 3–9 | 4–11 | 3–11 | 4–12 | |
| Min–Max | 0–17 | 0–16 | 0–17 | 0–15 | 2–16 | |
| Periodontitis anamnesis | ||||||
| Yes, treated | 361 (31%) | 109 (26%) | 199 (34%) | 49 (37%) | 4 (21%) | |
| Yes, not treated | 26 (2%) | 9 (2%) | 14 (2%) | 3 (2%) | 0 (0%) | |
| No | 659 (56%) | 256 (61%) | 324 (55%) | 66 (50%) | 10 (53%) | |
| Unknown | 122 (10%) | 48 (11%) | 55 (9%) | 14 (11%) | 5 (26%) | |
| Missing | 10 | 4 | 2 | 2 | 2 | |
| Dental status | ||||||
| I have natural teeth | 1031 (88%) | 386 (90%) | 516 (87%) | 116 (87%) | 13 (62%) | |
| I am edentulous | 11 (1%) | 3 (1%) | 6 (1%) | 1 (1%) | 1 (5%) | |
| I have implants | 264 (22%) | 92 (21%) | 140 (24%) | 27 (20%) | 5 (24%) | |
| I wear dentures | 113 (10%) | 32 (7%) | 61 (10%) | 17 (13%) | 3 (14%) | |
| Dentist visit frequency | 0.671chi2 | |||||
| 2/year | 658 (56%) | 252 (59%) | 322 (54%) | 77 (58%) | 7 (39%) | |
| 1/year | 380 (32%) | 128 (30%) | 201 (34%) | 42 (32%) | 9 (50%) | |
| < 1/year | 133 (11%) | 48 (11%) | 69 (12%) | 14 (11%) | 2 (11%) | |
| Missing | 7 | 1 | 2 | 1 | 3 | |
| Tooth brushing frequency | 0.270chi2 | |||||
| ≥ 2/day | 899 (77%) | 333 (78%) | 456 (77%) | 96 (72%) | 14 (78%) | |
| 1/day | 257 (22%) | 92 (21%) | 128 (22%) | 33 (25%) | 4 (22%) | |
| < 1/day | 15 (1%) | 3 (1%) | 8 (1%) | 4 (3%) | 0 (0%) | |
| Missing | 7 | 1 | 2 | 1 | 3 | |
| Interdental cleaning | 0.032chi2 | |||||
| Yes, with interdental brushes | 512 (44%) | 165 (39%) | 282 (48%) | 56 (42%) | 9 (50%) | |
| Yes, with dental floss | 317 (27%) | 136 (32%) | 143 (24%) | 34 (26%) | 4 (22%) | |
| Yes, with other tools | 82 (7%) | 26 (6%) | 41 (7%) | 14 (11%) | 1 (6%) | |
| No | 259 (22%) | 101 (24%) | 126 (21%) | 28 (21%) | 4 (22%) | |
| Missing | 8 | 1 | 2 | 2 | 3 | |
| Oral hygiene frequency during COVID-19 pandemic compared to before | 0.001chi2 | |||||
| Less often | 34 (3%) | 23 (5%) | 7 (1%) | 3 (2%) | 1 (6%) | |
| Unchanged | 1083 (93%) | 384 (90%) | 562 (95%) | 121 (91%) | 16 (94%) | |
| More often | 53 (5%) | 20 (5%) | 24 (4%) | 9 (7%) | 0 (0%) | |
| Missing | 8 | 2 | 1 | 1 | 4 | |
| Toothache | 0.006chi2 | |||||
| Worse than pre-pandemic | 46 (4%) | 25 (6%) | 17 (3%) | 4 (3%) | 0 (0%) | |
| Equivalent to pre-pandemic | 200 (17%) | 90 (21%) | 93 (16%) | 17 (13%) | 0 (0%) | |
| Better than pre-pandemic | 2 (0%) | 0 (0%) | 1 (0%) | 1 (1%) | 0 (0%) | |
| None | 904 (78%) | 308 (73%) | 479 (81%) | 111 (83%) | 6 (100%) | |
| Missing | 26 | 6 | 4 | 1 | 15 | |
| Mucosal symptoms | 0.029chi2 | |||||
| Worse than pre-pandemic | 55 (5%) | 30 (7%) | 20 (3%) | 5 (4%) | 0 (0%) | |
| Equivalent to pre-pandemic | 212 (18%) | 89 (21%) | 104 (18%) | 19 (14%) | 0 (0%) | |
| Better than pre-pandemic | 3 (0%) | 2 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | |
| None | 882 (77%) | 302 (71%) | 464 (79%) | 110 (82%) | 6 (100%) | |
| Missing | 26 | 6 | 5 | 0 | 15 | |
| Dental hypersensitivity | < 0.001chi2 | |||||
| Worse than pre-pandemic | 58 (5%) | 38 (9%) | 15 (3%) | 5 (4%) | 0 (0%) | |
| Equivalent to pre-pandemic | 291 (25%) | 120 (29%) | 148 (25%) | 23 (17%) | 0 (0%) | |
| Better than pre-pandemic | 7 (1%) | 2 (0%) | 4 (1%) | 1 (1%) | 0 (0%) | |
| None | 796 (69%) | 261 (62%) | 424 (72%) | 105 (78%) | 6 (100%) | |
| Missing | 26 | 8 | 3 | 0 | 15 | |
| Myofacial pain | < 0.001chi2 | |||||
| Worse than pre-pandemic | 75 (7%) | 55 (13%) | 17 (3%) | 3 (2%) | 0 (0%) | |
| Equivalent to pre-pandemic | 223 (19%) | 89 (21%) | 110 (19%) | 24 (18%) | 0 (0%) | |
| Better than pre-pandemic | 7 (1%) | 2 (0%) | 2 (0%) | 3 (2%) | 0 (0%) | |
| None | 846 (74%) | 276 (65%) | 460 (78%) | 104 (78%) | 6 (100%) | |
| Missing | 27 | 7 | 5 | 0 | 15 | |
Significant values are in bold.
All missing values were excluded for statistical testing.
n number of participants, COVID-19 coronavirus disease 2019, SD standard deviation, Q Quartile, Min Minimum, Max Maximum, MWU Mann–Whitney U test, chi2 chi-squared test, KW Kruskal–Wallis one-way ANOVA.
Psychosocial factors
Overall, the respondents gave a total sum score of 4.5 ± 3.5 for the validated questions of the PHQ-Stress module. Regarding depression and anxiety, the participants gave a total of 2.4 ± 2.6 points in the PHQ-4 sum score (Table 3). Concerning whether people were emotionally strained differently during the COVID-19 pandemic compared to before, 38% of all respondents (n = 429) stated a much greater or greater burden, while 12% (n = 134) stated less or much less burden. When dividing the study cohort in participants with greater, equal and less emotional burden compared to pre-pandemic, the PHQ-Stress sum score differed statistically significantly (p < 0.001KW) by total values of 6.6 ± 3.4, 3.2 ± 2.7 and 3.4 ± 3.1, respectively. Also, the PHQ-4 sum score differed in this group comparison statistically significantly (p < 0.001KW) by 4.0 ± 2.8, 1.4 ± 1.7 and 1.4 ± 1.8, respectively.
Table 3.
Psychosocial factors.
| Variables | Total (n = 1178) | Greater emotional burden compared to pre-pandemic (n = 429) | Equal emotional burden compared to pre-pandemic (n = 594) | Less emotional burden compared to pre-pandemic (n = 134) | Emotional burden compared to pre-pandemic data missing (n = 21) | p-Value |
|---|---|---|---|---|---|---|
| PHQ-stress | < 0.001KW | |||||
| n | 1043 | 395 | 530 | 115 | 3 | |
| Missing | 135 (11%) | 34 (8%) | 64 (11%) | 19 (14%) | 18 (86%) | |
| Mean | 4.5 | 6.6 | 3.2 | 3.4 | 2.7 | |
| SD | 3.5 | 3.4 | 2.7 | 3.1 | 3.8 | |
| Median | 4 | 6 | 3 | 3 | 1 | |
| Q1–Q3 | 2–7 | 4–9 | 1–5 | 1–4 | 0–7 | |
| Min–Max | 0–20 | 0–19 | 0–13 | 0–20 | 0–7 | |
| PHQ-4 | < 0.001KW | |||||
| n | 1141 | 420 | 584 | 129 | 8 | |
| Missing | 37 (3%) | 9 (2%) | 10 (2%) | 5 (4%) | 13 (62%) | |
| Mean | 2.4 | 4 | 1.4 | 1.4 | 1.8 | |
| SD | 2.6 | 2.8 | 1.7 | 1.8 | 2.5 | |
| Median | 2 | 4 | 1 | 1 | 0.5 | |
| Q1–Q3 | 0–4 | 2–5 | 0–2 | 0–2 | 0–3 | |
| Min–Max | 0–12 | 0–12 | 0–12 | 0–12 | 0–7 | |
| Emotional burden compared to pre-pandemic | ||||||
| Much greater | 64 (6%) | 64 (15%) | 0 (0%) | 0 (0%) | ||
| Greater | 365 (32%) | 365 (85%) | 0 (0%) | 0 (0%) | ||
| Equal | 594 (51%) | 0 (0%) | 594 (100%) | 0 (0%) | ||
| Less | 100 (9%) | 0 (0%) | 0 (0%) | 100 (75%) | ||
| Much less | 34 (3%) | 0 (0%) | 0 (0%) | 34 (25%) | ||
| Missing | 21 | 0 | 0 | 0 | 21 | |
Significant values are in bold.
All missing values were excluded for statistical testing.
n number of participants, COVID-19 coronavirus disease 2019, SD standard deviation, Q Quartile, Min Minimum, Max Maximum, KW Kruskal–Wallis one-way ANOVA.
OHRQoL
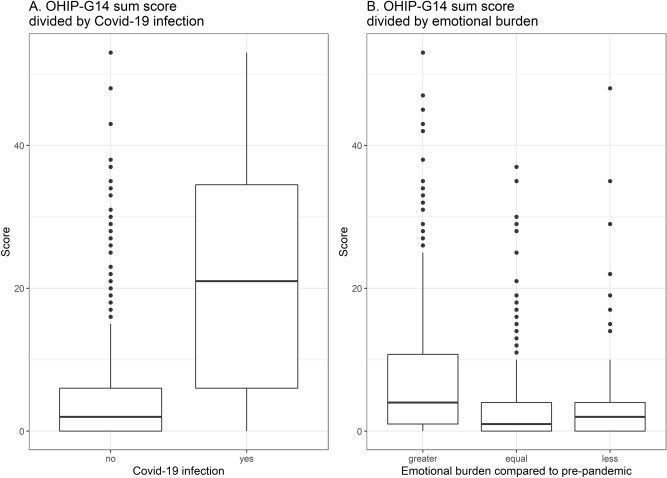
On average, 1103 study participants reached an OHIP-G14 sum score of 4.8 ± 7.5 (Table 4). When dividing the study cohort in participants with greater, equal and less emotional burden compared to pre-pandemic, the overall OHIP-G14 sum score was 7.7 ± 9.4, 3.1 ± 5.1 and 3.8 ± 7.1, respectively, with statistically significant difference (p < 0.001KW). These differences are present in all the seven OHIP-G14 sub-domains.
Table 4.
Oral health-related quality of life.
| Variables | Total (n = 1178) | Greater emotional burden compared to pre-pandemic (n = 429) | Equal emotional burden compared to pre-pandemic (n = 594) | Less emotional burden compared to pre-pandemic (n = 134) | Emotional burden compared to pre-pandemic data missing (n = 21) | p-Value |
|---|---|---|---|---|---|---|
| OHIP-G14—total sum score | < 0.001KW | |||||
| n | 1103 | 402 | 567 | 128 | 6 | |
| Missing | 75 (6%) | 27 (6%) | 27 (5%) | 6 (4%) | 15 (71%) | |
| Mean | 4.8 | 7.7 | 3.1 | 3.8 | 2 | |
| SD | 7.5 | 9.4 | 5.1 | 7.1 | 2.6 | |
| Median | 2 | 4 | 1 | 1.5 | 1.5 | |
| Q1–Q3 | 0–6 | 1–11 | 0–4 | 0–4 | 0–2 | |
| Min–Max | 0–53 | 0–53 | 0–37 | 0–48 | 0–7 | |
| OHIP-G14—sub-domain 1 (functional limitation) | 0.007KW | |||||
| n | 1145 | 417 | 589 | 132 | 7 | |
| Missing | 33 (3%) | 12 (3%) | 5 (1%) | 2 (1%) | 14 (67%) | |
| Mean | 0.28 | 0.37 | 0.22 | 0.27 | 0.14 | |
| SD | 0.91 | 1 | 0.79 | 1 | 0.38 | |
| Median | 0 | 0 | 0 | 0 | 0 | |
| Q1–Q3 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | |
| Min–Max | 0–8 | 0–8 | 0–6 | 0–8 | 0–1 | |
| OHIP-G14—sub-domain 2 (handicap) | < 0.001KW | |||||
| n | 1143 | 417 | 586 | 134 | 6 | |
| Missing | 35 (3%) | 12 (3%) | 8 (1%) | 0 (0%) | 15 (71%) | |
| Mean | 0.88 | 1.5 | 0.48 | 0.6 | 0.67 | |
| SD | 1.4 | 1.8 | 0.92 | 1.3 | 0.82 | |
| Median | 0 | 1 | 0 | 0 | 0.5 | |
| Q1–Q3 | 0–1 | 0–2 | 0–1 | 0–1 | 0–1 | |
| Min–Max | 0–8 | 0–8 | 0–8 | 0–8 | 0–2 | |
| OHIP-G14—sub-domain 3 (psychological disability) | < 0.001KW | |||||
| n | 1137 | 415 | 583 | 133 | 6 | |
| Missing | 41 (3%) | 14 (3%) | 11 (2%) | 1 (1%) | 15 (71%) | |
| Mean | 1 | 1.7 | 0.6 | 0.65 | 0.5 | |
| SD | 1.5 | 1.8 | 1 | 1.2 | 0.55 | |
| Median | 0 | 1 | 0 | 0 | 0.5 | |
| Q1–Q3 | 0–2 | 0–3 | 0–1 | 0–1 | 0–1 | |
| Min–Max | 0–8 | 0–8 | 0–6 | 0–8 | 0–1 | |
| OHIP-G14—sub-domain 4 (psychological discomfort) | < 0.001KW | |||||
| n | 1144 | 418 | 587 | 133 | 6 | |
| Missing | 34 (3%) | 11 (3%) | 7 (1%) | 1 (1%) | 15 (71%) | |
| Mean | 0.77 | 1.2 | 0.53 | 0.59 | 0.17 | |
| SD | 1.5 | 1.9 | 1.1 | 1.1 | 0.41 | |
| Median | 0 | 0 | 0 | 0 | 0 | |
| Q1–Q3 | 0–1 | 0–2 | 0–1 | 0–1 | 0–0 | |
| Min–Max | 0–8 | 0–8 | 0–6 | 0–6 | 0–1 | |
| OHIP-G14—sub-domain 5 (physical disability) | < 0.001KW | |||||
| n | 1146 | 420 | 588 | 132 | 6 | |
| Missing | 32 (3%) | 9 (2%) | 6 (1%) | 2 (1%) | 15 (71%) | |
| Mean | 0.48 | 0.79 | 0.27 | 0.47 | 0 | |
| SD | 1.2 | 1.5 | 0.81 | 1.2 | 0 | |
| Median | 0 | 0 | 0 | 0 | 0 | |
| Q1–Q3 | 0–0 | 0–1 | 0–0 | 0–0 | 0–0 | |
| Min–Max | 0–8 | 0–8 | 0–7 | 0–7 | 0–0 | |
| OHIP-G14—sub-domain 6 (physical pain) | < 0.001KW | |||||
| n | 1147 | 419 | 589 | 133 | 6 | |
| Missing | 31 (3%) | 10 (2%) | 5 (1%) | 1 (1%) | 15 (71%) | |
| Mean | 0.6 | 0.87 | 0.43 | 0.5 | 0 | |
| SD | 1.2 | 1.4 | 1 | 1.1 | 0 | |
| Median | 0 | 0 | 0 | 0 | 0 | |
| Q1–Q3 | 0–1 | 0–1 | 0–0 | 0–0 | 0–0 | |
| Min–Max | 0–8 | 0–8 | 0–7 | 0–6 | 0–0 | |
| OHIP-G14—sub-domain 7 (social disability) | < 0.001KW | |||||
| n | 1147 | 420 | 587 | 134 | 6 | |
| Missing | 31 (3%) | 9 (2%) | 7 (1%) | 0 (0%) | 15 (71%) | |
| Mean | 0.94 | 1.5 | 0.58 | 0.81 | 0.5 | |
| SD | 1.6 | 2 | 1.2 | 1.6 | 1.2 | |
| Median | 0 | 0 | 0 | 0 | 0 | |
| Q1–Q3 | 0–1 | 0–3 | 0–1 | 0–1 | 0–0 | |
| Min–Max | 0–8 | 0–8 | 0–8 | 0–8 | 0–3 |
Significant values are in bold.
All missing values were excluded for statistical testing.
n number of participants, COVID-19 coronavirus disease 2019, OHIP Oral Health Impact Profile, SD standard deviation, Q Quartile, Min Minimum, Max Maximum, KW Kruskal–Wallis one-way ANOVA.
Influencing factors on OHRQoL
The variable “periodontitis-history” was not included in the stepwise linear regression due to many missing values. The factors “BMI”, “educational status”, “interdental cleaning”, “dentist visit frequency”, “tooth brushing frequency” and “emotional burden due to the COVID-19-pandemic compared to pre-pandemic” cannot be found in the final output of the regression (Table 5) due to the elimination process of the performed variable selection (bidirectional elimination) based on Akaike information criterion.
Table 5.
Stepwise linear regression result for modeling OHIP-G14 sum score with fixed effects (n = 928).
| Variable | Estimate | Lower 95% CI | Upper 95% CI | p-value |
|---|---|---|---|---|
| Intercept | − 1.625 | − 2.614 | − 0.636 | 0.001 |
| PHQ-Stress sum score | 0.585 | 0.440 | 0.730 | < 0.001 |
| PHQ-4 sum score | 0.973 | 0.778 | 1.169 | < 0.001 |
| Sex: female | − 0.240 | − 1.035 | 0.555 | 0.554 |
| Age group: 40–59 years | 0.848 | − 0.059 | 1.755 | 0.067 |
| Age group: 60–100 years | 1.706 | 0.707 | 2.704 | < 0.001 |
| COVID-19 history: yes | 12.050 | 9.078 | 15.022 | < 0.001 |
| Systemic diseases: yes | 0.890 | 0.064 | 1.716 | 0.035 |
| Pandemic-related restrictions in life: strong | 0.287 | − 1.134 | 1.707 | 0.692 |
| Pandemic-related restrictions in life: little | − 0.617 | − 1.817 | 0.583 | 0.313 |
Significant values are in bold.
CI confidence interval, OHIP Oral Health Impact Profile, PHQ Patient Health Questionnaire, COVID-19 coronavirus disease 2019.
The variable with the strongest impact on a lower OHIP-G14 sum score is a confirmed COVID-19 history with an estimate of 12.05, meaning that participants with COVID-19 history have on average a 12.05 significantly increased OHIP-G14 sum score compared to participants without COVID-19 history. In addition, an increase of one unit of the PHQ-4 sum score associates with a statistically significant increase of 0.973 in the OHIP-G14 sum score. PHQ-Stress sum score is significantly relevant with an estimate of 0.585, as well as the age group of 60–100 years with an estimate of 1.706 compared to the age groups of 40–59 years (0.848) and 18–39 years (0). Participants with systemic diseases have an increase of 0.89 in the OHIP-G14 sum score compared to participants without systemic diseases. Sex and pandemic related restrictions in life were not statistically significantly relevant in the final model. Figure 1 depicts the differences of the OHIP-G14 sum scores stratified to participants with and without COVID-19 history as well as to participants with greater, equal or less emotional burden compared to pre-pandemic.
Figure 1.
OHIP-G14 sum score stratified to participants with and without COVID-19 history (A) and greater, equal or less emotional burden compared to pre-pandemic (B).
Discussion
In Germany, public and private life has been restricted by lockdown and contact restrictions since March 2020 due to the COVID-19 pandemic. To our knowledge, this is the first paper that uses validated questions to investigate associations of psychosocial factors on oral health and oral afflictions during the COVID-19 pandemic.
In contrast to a low OHIP-G14 sum score of 4.8 ± 7.5 in the overall cohort, suggesting a rather high OHRQoL17, the sum scores of participants with self-assessed greater (7.7 ± 9.4), equal (3.1 ± 5.1) and less (3.8 ± 7.1) emotional burden compared to pre-pandemic were statistically significantly different indicating an influential factor of psychosocial factors during the COVID-19 pandemic on OHRQoL. The differentiation of participants with subjectively rated higher, equal, or less emotional burden compared to pre-pandemic is reflected in statically significant group-differences in the more objectified scores of the PHQ-4 and PHQ-Stress sub-questionnaires.
The study design does not allow the detection of causal relationships, but the results might indicate an association between a stated COVID-19 history and increased anxiety and depression level as well as stress on OHRQoL. This can be seen in the linear regression result for modeling the OHIP-G14 score and the variables “PHQ-4” and “PHQ-Stress”. The significant differences in the PHQ-4 sum scores between emotionally greater burdened (4.0 ± 2.8) and equally (1.4 ± 1.7) or less (1.4 ± 1.8) burdened compared to pre-pandemic should be emphasized, since from a sum score of 3.0 onwards a moderate severity of depression and anxiety has to be assumed19. The results on stress exposure suggest a mild manifestation in participants with greater emotional burden compared to participants with equal or less emotional burden compared to pre-pandemic and a minimal stress exposure18. Explanations could be the altered living conditions for many people with uncertain employment states, financial worries or loneliness3,20,21. Brooks et al. showed negative psychological effects of quarantine measures during past pandemics. Stressors included longer quarantine duration, infection fear, frustration, boredom, inadequate supplies, inadequate information, financial loss and stigma. Long-lasting effects are assumed22. The COVID-19 pandemic has generally led to short term as well as long term psychosocial and mental health implications23. Peters et al. also concluded psychosocial effects of the COVID-19 pandemic from a national cohort study in Germany. In May 2020—at a similar point in time as in this observation—stress was also evaluated with the PHQ-Stress module, depression with PHQ-9 and anxiety with GAD-7. Due to the possibility of comparison with pre-pandemic results, a longitudinal assessment was possible and showed an increase in the PHQ-Stress score of 1.14 ± 0.02, an increase in PHQ-9 by 0.38 ± 0.02 and an increase of the GAD-9 by 0.36 ± 0.02.24 Samuel et al. presented similar results, as higher values of the Fear of COVID-19 scale suggest a reduced OHRQoL25.
Due to different incidence rates on regional and national levels as well as varying socio-political circumstances and reactions, the effects on social, professional and personal life may be diverse. Therefore, generalization of the present results, but also previous research on this topic, should only be transferred to similar circumstances and conditions. Nonetheless, Kishi et al. also demonstrated disaster-related effects on OHRQoL and mental health following the East Japan Earthquake and tsunami in 201126.
During the COVID-19 pandemic in Germany, dental care was partially limited and essential oral health care and inequities were a matter of debates. The results of the present study underline a continuing need for dental care. 21% of the respondents reported toothache, 23% mucosal problems, 31% hypersensitivity and 27% reported myofacial pain—the same applies to discussions on mental health care27–30.
One limitation of this study is that there are no clinical but rather questionnaire-based data for evaluation. However, for the most part, the questionnaire consists of validated items used in epidemiological studies, such as the OHIP-G14, the PHQ-Stress and the PHQ-4 along with the proposed DG PARO periodontitis risk score16–19. Furthermore, the known confounders for periodontitis and oral diseases, which can also influence OHRQoL, were integrated31.
In addition, a possible Hawthorne effect must be considered, since psychosocial consequences of the COVID-19 pandemic are generally discussed in social discourse32. Differences between the two testing formats—digital vs. paper-based questionnaire—have been shown, since the paper-based version was distributed especially to older adults with no access to the internet.
In the study cohort all age groups are represented, the genders and socio-economic status are adequately distributed and people from all federal states took part in this survey. However, due to the localization of the study center and the associated accessibility, many participants are from Baden-Württemberg and Hesse and possibly former patients of the Clinic of Conservative Dentistry at Heidelberg University. Therefore, this study cohort is not representative for the overall German population. This selection bias needs to be taken into consideration, when interpreting e.g. oral hygiene-related parameters. For example, interdental care is rated rather high which is reflected by 43.8% of the study participants using interdental brushes. In contrast, the broad-based and latest Fifth German Oral Health Study from 2013/2014 indicated a prevalence for the usage of interdental brushes of 16.5% for younger adults aged 35–44 years and a prevalence of 29% for younger seniors aged 65–74. Interestingly, in the preceding study of 2005, these values were 10.8% and 14.4%, respectively. This development could indicate that a further increase at an empirical level could be expected through enhanced education in preventive measures in recent years33. Thus, the results of this study could either due to the explicit patient collective or follow a positive trend in the usage of interdental brushes in general.
As shown in the regression analysis with variable selection, a COVID-19 history, the PHQ-4 sum score, the PHQ-Stress sum score, age and systemic diseases exerted an influence on OHRQoL. Consensually, the impact of sociodemographic factors on OHRQoL and periodontitis has been described34,35.
Conclusion
Within the limitations of this study, the perception of stress during the first wave of the COVID-19 pandemic in Germany was rated as mild and the severity of depression and anxiety level mild to moderate. A COVID-19 history as well as aggravated level of depression and anxiety and psychological stress were negatively associated with OHRQoL. The depicted oral afflictions underline the continuous need for dental treatment in pandemic times. As the COVID-19 pandemic and its implications progress and in context of future pandemics, psychosocial consequences and their association to oral health should be considered and further investigated.
Acknowledgements
The authors are grateful to all colleagues and supporters of this study who helped recruiting study participants. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors received no financial support for the research, authorship, and/or publication of this article. A.C. is supported by the Medical Faculty of Heidelberg with a Physician Scientist-scholarship.
Author contributions
A.C. contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M.M.S. and S.K.S. contributed to data acquisition and critically revised the manuscript; C.B. contributed to data analysis and critically revised the manuscript; T.K. contributed to conception, data interpretation and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Funding
Open Access funding enabled and organized by Projekt DEAL and supported by Deutsche Forschungsgemeinschaft (DFG) within the funding programme “Open Access Publikationskosten” as well as by Heidelberg University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazza C, et al. How personality relates to distress in parents during the Covid-19 lockdown: The mediating role of child's emotional and behavioral difficulties and the moderating effect of living with other people. Int. J. Environ. Res. Public Health. 2020 doi: 10.3390/ijerph17176236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benke C, Autenrieth LK, Asselmann E, Pané-Farré CA. Lockdown, quarantine measures, and social distancing: Associations with depression, anxiety and distress at the beginning of the COVID-19 pandemic among adults from Germany. Psychiatry Res. 2020;293:113462. doi: 10.1016/j.psychres.2020.113462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torales J, O'Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatry. 2020;66:317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 5.Stanton R, et al. Depression, anxiety and stress during COVID-19: Associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int. J. Environ. Res. Public Health. 2020 doi: 10.3390/ijerph17114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cademartori MG, Gastal MT, Nascimento GG, Demarco FF, Corrêa MB. Is depression associated with oral health outcomes in adults and elders? A systematic review and meta-analysis. Clin. Oral Invest. 2018;22:2685–2702. doi: 10.1007/s00784-018-2611-y. [DOI] [PubMed] [Google Scholar]
- 7.Coelho JMF, et al. Common mental disorder is associated with periodontitis. J. Periodontal. Res. 2020;55:221–228. doi: 10.1111/jre.12705. [DOI] [PubMed] [Google Scholar]
- 8.Decker A, Askar H, Tattan M, Taichman R, Wang HL. The assessment of stress, depression, and inflammation as a collective risk factor for periodontal diseases: A systematic review. Clin. Oral Invest. 2020;24:1–12. doi: 10.1007/s00784-019-03089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashioka S, et al. Implications of systemic inflammation and periodontitis for major depression. Front. Neurosci. 2018;12:483. doi: 10.3389/fnins.2018.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren KR, et al. Role of chronic stress and depression in periodontal diseases. Periodontol. 2014;2000(64):127–138. doi: 10.1111/prd.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nascimento GG, et al. Is there an association between depression and periodontitis? A birth cohort study. J. Clin. Periodontol. 2019;46:31–39. doi: 10.1111/jcpe.13039. [DOI] [PubMed] [Google Scholar]
- 12.Persson K, Olin E, Ostman M. Oral health problems and support as experienced by people with severe mental illness living in community-based subsidised housing—a qualitative study. Health Soc. Care Community. 2010;18:529–536. doi: 10.1111/j.1365-2524.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- 13.Campos LA, Peltomäki T, Marôco J, Campos J. Use of oral health impact profile-14 (OHIP-14) in different contexts. What is being measured? Int. J. Environ. Res. Public Health. 2021 doi: 10.3390/ijerph182413412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John MT. Foundations of oral health-related quality of life. J. Oral. Rehabil. 2020 doi: 10.1111/joor.13040. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet (London, England) 2007;370:1453–1457. doi: 10.1016/s0140-6736(07)61602-x. [DOI] [PubMed] [Google Scholar]
- 16.Kocher T, Holtfreter B, Dietrich T, Völzke H, Dannewitz B. The periodontitis risk score—via self-test to screening. Zahnarztl. Mitt. 2018;2:80–87. [Google Scholar]
- 17.John MT, et al. German short forms of the oral health impact profile. Community Dent. Oral Epidemiol. 2006;34:277–288. doi: 10.1111/j.1600-0528.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 18.Berth HB, Löwe RL, Spitzer S, Zipfel WH. PHQ-D. Gesundheitsfragebogen für Patienten. Z. Med. Psychol. 2003;12:90–93. [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra-brief screening scale for anxiety and depression: The PHQ-4. Psychosomatics. 2009;50:613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 20.Loades ME, et al. Rapid systematic review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59:1218–1239.e1213. doi: 10.1016/j.jaac.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks SK, et al. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet (Lond., Engl.) 2020;395:912–920. doi: 10.1016/s0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, et al. Impact of COVID-19 and lockdown on mental health of children and adolescents: A narrative review with recommendations. Psychiatry Res. 2020;293:113429. doi: 10.1016/j.psychres.2020.113429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters A, Rospleszcz S, Greiser KH, Dallavalle M, Berger K. The impact of the COVID-19 pandemic on self-reported health. Dtsch Arztebl Int. 2020;117:861–867. doi: 10.3238/arztebl.2020.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuel SR, et al. Impact of pain, psychological-distress, SARS-CoV2 fear on adults' OHRQOL during COVID-19 pandemic. Saudi J. Biol. Sci. 2021;28:492–494. doi: 10.1016/j.sjbs.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishi M, et al. Oral health-related quality of life and related factors among residents in a disaster area of the Great East Japan Earthquake and giant tsunami. Health Qual. Life Outcomes. 2015;13:143. doi: 10.1186/s12955-015-0339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha-Gomes G, et al. Impact of the coronavirus disease 2019 pandemic on periodontal practice: A questionnaire survey. J. Clin. Periodontol. 2021 doi: 10.1111/jcpe.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benzian H, Beltrán-Aguilar E, Mathur MR, Niederman R. Pandemic considerations on essential oral health care. J. Dent. Res. 2021;100:221–225. doi: 10.1177/0022034520979830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.León S, Giacaman RA. COVID-19 and inequities in oral health care for older people: An opportunity for emerging paradigms. JDR Clin. Trans. Res. 2020;5:290–292. doi: 10.1177/2380084420934742. [DOI] [PubMed] [Google Scholar]
- 30.Otu A, Charles CH, Yaya S. Mental health and psychosocial well-being during the COVID-19 pandemic: The invisible elephant in the room. Int. J. Ment. Health Syst. 2020;14:38. doi: 10.1186/s13033-020-00371-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtfreter B, et al. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: Proposed standards from the joint EU/USA periodontal epidemiology working group. J. Clin. Periodontol. 2015;42:407–412. doi: 10.1111/jcpe.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jordan AR, Micheelis W. Fünfte Deutsche Mundgesundheitsstudie-(DMS IV) Deutscher: Deutscher Zahnärzte Verlag DÄV Köln; 2016. [Google Scholar]
- 34.El Sayed N, et al. Twenty years later: Oral health-related quality of life and standard of treatment in patients with chronic periodontitis. J. Periodontol. 2019;90:323–330. doi: 10.1002/jper.18-0417. [DOI] [PubMed] [Google Scholar]
- 35.Bäumer A, et al. Oral health-related quality of life and standard of treatment in aggressive periodontitis patients more than 5 years after therapy. J. Clin. Periodontol. 2018;45:1347–1355. doi: 10.1111/jcpe.13011. [DOI] [PubMed] [Google Scholar]