Abstract
Bipolar disorder (BD) is a severe psychiatric disorder affecting approximately 1–3% of the population and characterized by a chronic and recurrent course of debilitating symptoms. An increasing focus has been directed to discover and explain the function of Blood-Brain Barrier (BBB) integrity and its association with a number of psychiatric disorders; however, there has been limited research in the role of BBB integrity in BD. Multiple pathways may play crucial roles in modulating BBB integrity in BD, such as inflammation, insulin resistance, and alterations of neuronal plasticity. In turn, BBB impairment is hypothesized to have a significant clinical impact in BD patients. Based on the high prevalence of medical and psychiatric comorbidities in BD and a growing body of evidence linking inflammatory and neuroinflammatory mechanisms to the disorder, recent studies have suggested that BBB dysfunction may play a key role in BD's pathophysiology. In this comprehensive narrative review, we aim to discuss studies investigating biological markers of BBB in patients with BD, mechanisms that modulate BBB integrity, their clinical implications on patients, and key targets for future development of novel therapies.
Keywords: Bipolar disorder, Blood-brain barrier, Inflammation, Claudin, Tight junction, Mania
Highlights
-
•
Bipolar disorder (BD) is associated with a blood-brain barrier (BBB) dysfunction.
-
•
Postmortem studies have reported altered levels of tight junction proteins in BD.
-
•
Extensive BBB dysfunction is associated with worse clinical outcomes in patients.
-
•
BBB dysfunction may relate to gut microbiome, substance abuse, insulin resistance.
-
•
Lithium and valproate have protective effects on BBB function and integrity.
1. Introduction
Bipolar disorder (BD) is a severe psychiatric disorder affecting approximately 1–3% of the population and characterized by a chronic course, recurrent pattern, and high clinical morbidity and mortality (Carvalho et al., 2020). Individuals with BD also present with high rates of co-occurring psychiatric and general medical conditions, which are known to increase disease burden and worsen prognosis. Notably, individuals with BD are more likely than the general population to be affected by many general medical conditions that have been linked to inflammation, resulting in decreased life expectancy (Kessing et al., 2021). In fact, increased inflammation, as evidenced by multiple reports of altered levels of inflammatory markers in blood of patients, has been described as a core pathophysiological finding in BD (Fries et al., 2019). These inflammatory changes, in association with oxidative stress and alterations in neurotrophic factors, characterize a process of systemic toxicity (Kapczinski et al., 2011) and may ultimately lead to alterations in the blood-brain barrier (BBB) structure and function. The BBB is the body's system involved in the selective uptake of oxygen, various ions, nutrients, and other chemicals to supply the central nervous system (CNS), while simultaneously protecting the brain from exogenous and endogenous toxins. The dysregulation of the normal homeostatic nature of the BBB has been linked to neuroinflammation in several pathogenic states. An increasing focus has been directed to discover and explain the function of BBB integrity and its association with inflammatory mechanisms in psychiatric disorders, though current understanding is still largely early in development.
Given the growing body of evidence suggesting an association between inflammatory and neuroinflammatory mechanisms and BD partnered with the high prevalence of medical and psychiatric comorbidities in BD, recent studies have suggested a potential key role for BBB dysfunction in the pathophysiology of BD. In this comprehensive narrative review, we sought to compile and discuss studies investigating markers of BBB in patients with BD and their clinical implications. We also explored mechanisms potentially underlying BBB disruption in BD, including changes in gut microbiome, substance abuse, physical exercise, and insulin resistance, and identified key targets for future development of novel therapies.
2. Blood-brain barrier (BBB)
The BBB is a highly specialized cellular interface between the blood and the CNS that regulates the entry of most blood-derived factors into the brain. Tight regulation of the brain environment is critical for adequate neural activity and is carried out by a dense network represented by a membrane formed by endothelial cells and brain microvessels. The central unit of the BBB is the neurovascular unit (NVU), which is a combination of various cell types working in coordinated effort to form the BBB and maintain homeostasis through regulation of tight junctions for proper ion balance and cerebral blood supply. Brain microvascular endothelial cells (BMECs) make up the transition zone between the blood supply and the CNS and are the foundational point of the NVU. The NVU also includes extracellular matrix components, pericytes, astrocytes, and microglia, with each component having a unique contribution (Fig. 1).
Fig. 1.
Neurovascular unit (NVU). The NVU is formed by the interaction of microvascular endothelial cells, a basal lamina, pericytes, astrocytes, microglia, neurons, and an extracellular matrix.
BMECs serve as a barrier and gatekeeper. These cells contain dense concentrations of mitochondria to aid in the production of ATP necessary to maintain BBB gradients and are held together by tight junctions, which allow only selective ions and molecules to pass. Pericytes, a type of mural cell, sit on the abluminal surface of the endothelium and incompletely cover the vasculature. Astrocytes, on the other hand, are a type of glial cell that ensheaths the vasculature and neuronal processes and are thought to communicate with BMECs through their long foot processes. Finally, the basement membrane is a key part of the NVU consisting of extracellular matrix proteins that are essential for protein communication.
Tight junctions regulate diffusion across the BBB through maintenance of close contact between cells, thereby preventing most unwanted infiltration or leakage into or out of the CNS. These paracellular barriers have membrane-bound transport proteins that allow specific substances through, and are tightly controlled by the NVU and its signaling proteins. Tight junction protein structures include but are not limited to claudins, occludins, and junctional adhesion molecules. Excessive up- or downregulation of such membrane-bound proteins has been linked to pathogenic processes. Of these membrane transport proteins, claudins, and particularly claudin-5 levels, have been consistently linked to psychiatric disorders (Greene et al., 2019).
Finally, another important line of defense is the blood-cerebrospinal fluid barrier formed by the choroid plexus, an epithelio-endothelial structure comprising a highly vascularized stroma with fenestrated capillaries and a continuous lining of epithelial cells joined by apical tight junctions (Solár et al., 2020). Recent evidence suggests that a closure of the brain choroid plexus may correlate with mental deficits (Carloni et al., 2021), which makes this an interesting structure in the context of BD.
Although under normal conditions, many mechanisms are in place to guarantee an intact BBB structure and function, multiple factors can cause disruptions of the BBB under pathological conditions. These disruptions may be led by increases in BBB permeability (by a reduction in expression or redistribution of tight junction proteins, for example), an impaired transporter function, insufficient clearance function, pericyte detachment, astrocyte loss, or disrupted basement membrane, among other mechanisms (Obermeier et al., 2013). Many of these mechanisms are activated or enhanced by a dysregulation of the immune response and glial activation, although some processes may be established in the absence of an inflammatory activation (for example, exogenous or endogenous damage to or defects in BMECs, including apoptosis, can lead to an inherent BBB disruption (Li et al., 2003)).
3. Evidence of BBB dysfunction in BD
As previously discussed, the BBB protects the brain from exogenous and endogenous toxins and releases waste products while simultaneously ensuring appropriate growth and functioning of the CNS through the uptake of ions, nutrients, and chemicals. As described below, the findings in BD suggest BBB dysfunction to be both structural (with altered levels of NVU proteins detected in the brain) and functional (with evidence of increased permeability as measured by molecular and neuroimaging methods), although the question as to whether these alterations and inherent to the disorder or a result of increased inflammation and other stressors remains open. A schematic summary of BBB alterations in BD is shown in Fig. 2.
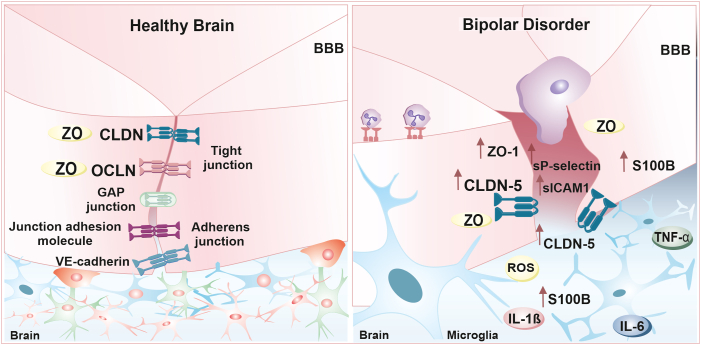
Fig. 2.
Proposed model for the blood-brain barrier (BBB) dysfunction in bipolar disorder (BD). The pathophysiology of BD is thought to exacerbate the host immune response by releasing proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6. This process may reduce the levels of tight junction proteins, such as claudin-5 (CLDN-5) and zona occludes (ZO)-1, in the BBB, thereby enhancing its permeability and leading to further neuroinflammatory processes. This is also reflected by increased levels of CLDN-5, ZO-1/TJP1, sP-selectin, soluble intercellular adhesion molecule 1 (sICAM1), endothelial injury biomarkers (such as urotensin-II and endocan), and S100 calcium-binding protein B (S100B) in the circulatory system of BD patients, as well as increased protein levels in the cerebrospinal fluid (CSF) and an increased CSF/albumin ratio.
BBB dysfunction can be explored in terms of tight junction protein disruption (e.g. claudins and membrane proteins), with initial evidence coming from animal studies not directly linked to BD. Animal models of major depressive disorder, for instance, have demonstrated reduced expression of claudin-5 (the most enriched tight junction protein) in the nucleus accumbens and subsequent depressive-like behaviors (Menard et al., 2017). In addition, suppression of claudin-5 in the hippocampus and medial prefrontal cortex of mice was associated with significant impairments in learning and memory, anxiety-like behavior, and sensorimotor gating (Greene et al., 2018).
In line with these non BD-specific preclinical findings, postmortem analyses in brains from BD patients revealed significant associations between several key components of the BBB, including claudin-5, claudin-12, and zonula occludens-1 (ZO-1, also known as tight junction protein 1 (TJP1)), and BD outcomes (Greene et al., 2020a). While no changes in claudin-5 protein levels were detected in the hippocampus or orbitofrontal cortex gray matter of BD patients compared to controls, earlier onset of BD was associated with reduced claudin-5 levels in the hippocampus white matter, gray matter, and orbitofrontal white matter (Greene et al., 2020a). Additionally, the protein levels of claudin-5, claudin-12, and ZO-1/TJP1 were negatively correlated with illness duration, suggesting a possible link between BD chronicity and increasing BBB permeability and dysfunction (Greene et al., 2020a). When assessing gene expression levels, claudin-5 (CLDN5) mRNA was found to be increased in the occipital cortex and cerebellum of BD patients, with no further differences in the expression of other tight junction proteins (Greene et al., 2020b). Furthermore, mRNA levels of TJP1 and CLDN12 in the occipital cortex and premotor frontal cortex, respectively, were also found to be negatively correlated with illness duration in BD patients (Greene et al., 2020b). Given that patients with BD often have progressively worsening symptoms and significant negative outcomes, particularly if unmedicated, tight junction dysregulation may be a promising drug target for future BD therapies. These different results may seem contradictory at first (for instance, with claudin-5 mRNA levels being increased in occipital cortex and cerebellum and their protein levels unchanged in the hippocampus and orbitofrontal cortex) and warrant replication in future postmortem studies, but they also suggest that BBB alterations may look differently in different brain regions. Moreover, it is possible that between-groups differences (or lack thereof) in this study were masked by the heterogeneity of the patients’ group (for instance, with different ages at onset and duration of illness).
Further evidence of BBB disruption in BD comes from studies investigating the BMEC function, which could represent a mechanism of inherent BBB deficit in the disorder. Indeed, vascular/endothelial dysfunction has been reported during acute mood symptoms and remission in BD patients (Schmitz et al., 2018), although this association was not found in all studies (Tong et al., 2018). Moreover, higher levels of endothelial injury biomarkers (urotensin-II and endocan) have also been reported in patients during both acute and remission phases (Oral et al., 2019), further emphasizing a potential role for endothelial dysfunction, and consequently BBB dysfunction in BD. Of note, preliminary in vitro evidence of BMEC dysfunction was not detected in a cellular model of BD, although this result may not be conclusive given the study's limited sample size (Pong et al., 2020).
Alterations of cerebrospinal fluid (CSF) and peripheral markers of neuroinflammation have also been used as potential biomarkers of BBB disruption within individuals with BD (Endres et al., 2016). Specifically, BD patients have been shown to present increased CSF protein levels (Endres et al., 2016) and increased serum and CSF albumin quotients (the reference standard for measuring BBB function). This increased CSF/serum albumin ratio in BD has been more recently confirmed by a meta-analysis, as well (Orlovska-Waast et al., 2019).
In terms of blood markers, an astrocytic protein known as S100 calcium-binding protein B (S100B) has repeatedly served as a peripherally-measured biomarker for BBB disruption in psychiatric disorders, with evidence that BBB dysfunction leads to the release of S100B into circulation (Marchi et al., 2003). Indeed, a meta-analysis reported significantly elevated S100B levels in patients during acute major depressive and manic episodes compared to healthy controls (Schroeter et al., 2008). Interestingly, Falcone and colleagues found that adolescents exposed to child abuse had significantly higher S100B levels in comparison to patients who had not experienced childhood trauma (Falcone, Lovell, Janigro, Anand), suggesting deleterious effects of early-life trauma on BBB integrity. Of note, BD patients are known to present with significantly higher rates of childhood trauma than the general population (Watson et al., 2014), suggesting it as a potential mechanism underlying BBB dysfunction. Other blood-based markers of BBB dysfunction found to be increased in BD include several cell adhesion molecules that facilitate leukocyte migration across epithelial cells of the BBB, such as the soluble intercellular adhesion molecule 1 (sICAM1) and sP-selectin. BD has also been associated with a dysregulation of matrix metalloproteinases (MMPs), which are proteolytic enzymes that contribute to BBB dysfunction by breaking down basal lamina proteins, tight junctions, and the BBB extracellular matrix. Specifically, blood MMP-7 and MMP-2 levels were found to be elevated and decreased in BD, respectively, while the levels of MMP-1 and tissue inhibitors of metalloproteinase 1 did not differ between patients and controls in a recent meta-analysis (Futtrup et al., 2020).
Proteins directly involved with the NVU have also been measured in peripheral tissues and explored as proxies of BBB integrity. Increased levels of claudin-5 and zonulin, a protein known to increase intestinal and BBB permeability by regulating tight junctions and other membrane protein interactions, have been found in peripheral blood of BD patients (Kılıç et al., 2020). Such increases have been hypothesized to reflect lower levels of these proteins in the BBB itself, although this has not been confirmed by the only postmortem evidence of claudin-5 levels in BD (Greene et al., 2020b) and requires further studies to be confirmed.
In addition, BD has been repeatedly associated with increased levels of inflammatory cytokines in peripheral blood, such as TNF-α, IL-6 and acute phase C-reactive protein (CRP)(Fries et al., 2019). CRP, particularly at high levels, has been shown to disrupt the BBB through a mechanism dependent on oxidative stress increase (Kuhlmann et al., 2009) and can ultimately increase permeability and facilitate CRP entry into the brain (Hsuchou et al., 2012). Accordingly, this inflammation in peripheral tissue parallels neuroinflammatory events within the CNS in BD (Fries et al., 2019; Giridharan et al., 2020), potentially contributing to BBB disruption. Importantly, the specific effects of CRP on BBB seem to be dependent on context and diagnosis. For instance, in clinically depressed populations, CRP has been associated with reductions in BBB permeability (Turkheimer et al., 2021). Of note, TNF-α has been hypothesized to contribute to chronic neurodegeneration seen in patients with BD through activation of a positive feedback loop. Specifically, by increasing BBB permeability, peripheral cytokine-recruiting monocytes enter the CNS and can cause up-regulation of NLRP3 inflammasome activity and modulation of glycogen synthase kinase-3β (GSK-3β) activity, which is known to regulate inflammation, mitochondrial metabolism, DNA repair, and apoptosis (Jones et al., 2021). The role of TNF-α in mediation of BBB function is further supported by in vitro models of BMECs derived from pluripotent stem cells of patients with BD and schizophrenia, which demonstrate decreased BBB integrity and increased permeability compared to controls. Samples with decreased BBB function also showed increased MMP-1 activity and decreased claudin-5 levels. Interestingly, claudin-5 levels and BBB function were improved with TNF-α and MMP-1 inhibition (Lizano et al., 2022).
Finally, BBB imaging with dynamic contrast-enhanced MRI (DCE-MRI) performed within a subgroup of BD patients demonstrated that extensive BBB dysfunction was associated with greater psychiatric morbidity in patients. Of note, this extensive BBB leakage was found to be diffuse (widespread) and not restricted to specific brain regions. Specifically, BD patients with extensive BBB dysfunction presented with an overall worse outcome and greater psychiatric morbidity, including higher rates of chronic illness, more frequent and/or severe affective episodes, and greater socio/occupational dysfunction (Kamintsky et al., 2020). Interestingly, BBB dysfunction was also highly associated with metabolic dysregulation, greater body mass index, insulin resistance, and elevated risk of cardiovascular diseases in patients (Kamintsky et al., 2020). This study also found no significant associations between BBB leakage and use of specific mood stabilizing treatments. Overall, this MRI study empirically reinforces a hypothesis suggested by the postmortem and peripheral studies, i.e., BBB dysfunction may not be present in all patients. Rather, BBB dysfunction may be specifically related to a subgroup of BD patients with a more severe presentation and significant metabolic dysregulation who may ultimately better benefit from drugs targeting the BBB or inflammatory mechanisms. Such a group-specific finding in the BBB may be accompanied by a similar specificity regarding the understudied blood-CSF barrier, as well, with recent evidence of enlarged choroid plexus associated with cognitive deficits in BD type I patients with psychosis symptoms (Lizano et al., 2019).
4. Potential mechanisms affecting the BBB in BD
4.1. Gut microbiome and brain dysfunction
BD has been previously associated with an altered gut microbiome composition (Sublette et al., 2021). Evidence for the gut microbiome's effects on the brain comes primarily from animal studies that assess brain structure and function pre- and post-microbial exposure and colonization in germ-free rodents. Rodents lacking gut microbes show a decreased expression of tight junction proteins, i.e., an increased permeability of the BBB (Braniste et al., 2014), and the permeability of the BBB decreases when fecal material from specific pathogen-free mice is transplanted to germ-free rodents (Braniste et al., 2014). Accordingly, it has been postulated that the gut microbiome may exert its influences on the brain and BBB via at least four different pathways: 1) circulatory system delivery of neuroactive metabolites and neurotransmitters directly produced in the gut; 2) the gut neuroendocrine system; 3) the immune system; and 4) the parasympathetic nervous system via mainly the vagus nerve (Berger et al., 2009).
One major function of the gut microbiome is transforming dietary components into different metabolites that may affect the BBB integrity and brain function, such as short chain fatty acids (SCFA). Specifically, SCFA may directly affect BBB integrity (MacFabe, 2012). One study showed that when butyrate, a type of SCFA, is introduced to germ-free mice either via colonization with a butyrate producer or oral administration of sodium butyrate, the permeability of the BBB decreases (Braniste et al., 2014). Of note, administration of sodium butyrate has been previously shown to reverse manic-like behavior and oxidative damage in a rodent model of mania (Valvassori et al., 2016).
Neuroendocrine cells may also play a role in brain and BBB function. Enteroendocrine cells exist at the intestinal epithelium, expressing a range of G protein-coupled receptors, transporters, and other receptors. When binding to such receptors occur, neuroendocrine cells often release peptide hormones such as neuropeptides cholecystokinin, peptide YY, and serotonin. Subsequently, these peptide hormones may enter the circulation and act on the brain either directly or indirectly through the innervation of the vagus nerve. All three of the neuropeptides have been associated with BD (Wilson et al., 2012; Haenisch et al., 2015; Mahmood and Silverstone, 2001).
Other than their actions on the neuroendocrine cells, gut microbiome metabolites can direct the development and normal functioning of the mucosa immune system. Gut metabolites utilize three main routes to affect immune cells and subsequently, the brain. The first of these routes is via the cytokine pathway. When the intestinal epithelial barrier is broken, bacteria and their metabolites can trigger an inflammatory immune response. This immune response may then propagate a chronic systemic inflammatory state characterized by elevated serum levels of TNF-α, IL-6, and IL-1β, which are hypothesized to disrupt the BBB integrity (Mark and Miller, 1999). This first route also leads to immune cell extravasation from the periphery into the brain, thereby leading to a cycle of brain inflammation and neurodegeneration (Perry, 2007). The second route by which gut metabolites can alter the secretion of cytokines is through local action on enteric neurons. Intestinal macrophages may alter enteric neural responses to inflammatory signals and lead to apoptosis of enteric neurons, ultimately affecting the gut's communication to the brain (Becker et al., 2018). Lastly, metabolite-activated immune cells may travel via the bloodstream to the BBB and release soluble factors to promote further recruitment of leukocytes and macrophages to the brain in order to decrease BBB permeability.
4.2. Substance abuse and its role in modulating BBB
Mood disorders and substance abuse often coexist with each other, and their comorbidity is generally associated with a worse course of disease (Małkiewicz et al., 2020). Substance use is increased in BD patients compared to the general population, with the current prevalence of comorbid substance use disorders in BD being as high as 61% in BD type I46. In addition, a meta-analysis revealed that increased substance abuse is correlated with a higher number of manic episodes in BD patients (Messer et al., 2017). Of note, there is evidence that drugs of abuse are associated with significant disrupting effects on the BBB, impacting BMECs and many aspects of the NVU (Sajja et al., 2016).
Chronic alcohol abuse, which is highly prevalent in BD (Grunze et al., 2021), is known to damage BBB. In mouse models, chronic ethanol exposure has been shown to reduce the expression of tight junction proteins, including occludin, ZO-1/TJP1, and claudin-5, and increase the permeability of the BBB for cytokines to enter and stimulate neuroinflammation (Muneer et al., 2011). Not only does alcohol induce the proinflammatory pathway, it also causes oxidative damage to the BBB through glutamate excitotoxicity (Somkuwar et al., 2016) and by inducing oxidative stress in the BMECs (Haorah et al., 2005).
It has been suggested that some psychostimulants may exacerbate psychopathology by increasing the permeability of the BBB and inducing inflammatory pathways (Małkiewicz et al., 2020). For instance, methamphetamine can cause BBB disruption by altering the levels of tight junction proteins, including claudin-5 and ZO-1/TJP1 (Mahajan et al., 2008a). In addition, several human studies have implicated the role of methamphetamine on oxidative stress and proinflammatory pathways. Specifically, the TNF-α pathway has been identified as playing an important role altering the BBB in methamphetamine users (Coelho-Santos et al., 2015). Similarly, cocaine, which has been described as one of the most frequently reported substances of abuse in BD (Chengappa et al., 2000), may damage the BBB through its negative effects on the tight junction complexes and by inducing a rise in the proinflammatory cytokines such as TNF-α, IL-6, and IL-8 (Chang et al., 2000).
Another substance that is potentially harmful to the integrity and function of the BBB is nicotine, a dependence to which is the most frequent substance use disorder in BD with a lifetime rate of 83% (Salloum and Brown, 2017). Like methamphetamine and cocaine, nicotine damages BBB through modulation of tight junction proteins and proinflammatory cytokines (Hawkins et al., 2004). Cigarette smoke extract, for instance, has been shown to induce an endothelial inflammatory response in BBB in vitro associated with an increase in oxidative stress (Kaisar et al., 2015). Finally, opioid use is increased among patients with BD and is also theorized to be a cause of BBB disruption and neuroinflammation (Cerullo and Strakowski, 2007). Several studies have shown that opioids damage the BBB through proinflammatory cytokines, endothelial expression of adhesion molecules, alterations of tight junction, and perivascular glial cell activation (Mahajan et al., 2008b). In summary, substances of abuse, whose use is known to be highly prevalent in BD populations, share the common theme of disrupting the BBB through altering its tight junctions and increasing the proinflammatory state.
4.3. Physical exercise protects BBB
One readily available therapy for combating neuroinflammation and BBB alteration is physical exercise. In diseases such as cerebral ischemia and multiple sclerosis, exercise has already been shown to serve as a protective factor against BBB dysfunction (Souza et al., 2017), with its beneficial effects being traced to its improvement in antioxidant defense mechanisms (Aguiar et al., 2008). A key aspect of BBB disruption is facilitated by angiotensin II (Ang II). Ang II has the capability to recruit inflammatory cells and promote vascular permeability, and it has been shown that this process can be attenuated by exercise (Małkiewicz et al., 2020). Previous studies have demonstrated that skeletal muscle releases anti-inflammatory cytokines IL-1ra and IL-6 to counteract the effects of proinflammatory cytokines during exercise (Petersen and Pedersen, 2005). Furthermore, exercise strengthens the antioxidant defense through the reduction of reactive oxygen species (McKee et al., 2014) and increases peripheral levels of circulating norepinephrine, which can shift the body into an anti-inflammatory state by suppressing inducible nitric oxide synthase, IL-1β, TNF-α, and intracellular adhesion molecule-1 (Feinstein et al., 1993). Finally, during exercise, more of the metabolite kynurenine is converted into kynurenic acid; unlike kynurenine, which is toxic and can cross the BBB, kynurenic acid is neuroprotective and does not cross the BBB (Fukui et al., 1991).
Several studies have shown that physical exercise may have the potential to reduce depressive episodes in BD (Lin and Liu, 2019). When exercise was examined in a retrospective cohort study, it was found that exercise reduced depressive and anxious symptoms (Ng et al., 2007). Another study examined exercise and healthy eating's contribution to BD depression symptoms; again, those patients who participated in the exercise and healthy eating group had a more significant improvement in depressive symptoms (Sylvia et al., 2019). Finally, physical exercise has also been shown to positively impact the gut microbiota (Monda et al., 2017) and thereby influence BBB properties, as discussed earlier. These beneficial depression-reducing effects of exercise may be a product of its anti-inflammatory properties. Patients with BD have been shown to present with significantly lower physical activity levels compared to controls and first-degree relatives (la Cour Karottki et al., 2020). This may be a source of increased vulnerability for BBB disruption and subsequently possibly increased BD symptom severity in patients who do not exercise regularly.
4.4. Insulin resistance may cause BBB dysfunction
Metabolic dysregulation is a common finding in BD patients, with insulin resistance present in more than half of all patients (Calkin et al., 2015). While no causal link has been shown between insulin resistance and increased BBB permeability yet, several animal studies have suggested that hyperglycemia can cause inflammation and damage to BBB (Ennis and Keep, 2007). Moreover, insulin resistance and BBB dysfunction share common pathways involved in the inflammatory states such as vascular endothelial growth factor and protein kinase C (Van Dyken and Lacoste, 2018). Insulin resistance leads to oxidative stress and chronic inflammation, which may play a role in diabetic encephalopathy (Bogush et al., 2017). Animal studies with metformin, an anti-diabetic drug, also support that BBB dysfunction can be slowed by reducing insulin resistance. Accordingly, a previous mice study showed that metformin can reduce systemic and CNS inflammation (Mudgal et al., 2019). Taken together, it is possible that insulin resistance may contribute to BBB disruption in BD.
5. The BBB and pharmacologic implications in BD
5.1. BBB & pharmacologic interactions
Alongside growing evidence indicating BBB dysfunction as part of the pathogenesis of BD, there has also been growing interest in how BBB dysfunction affects medication response and the effects of medications on the BBB itself. Notably, successful treatment of depressive and manic episodes has been linked to decreases in markers of BBB disruption (Tsai and Huang, 2017). This is particularly relevant not only because BBB dysfunction has been linked to BD, but also because the majority of medications used to treat BD need to cross and thereby interact with the BBB in a variety of ways. One significant way the BBB interacts with pharmacological agents is through P-glycoprotein (P-gp), a transmembrane protein expressed on BMECs that functions as a drug efflux pump helping to eliminate potentially dangerous substances. Interestingly, drugs utilized in BD are known to have varying effects on P-gp function (Husain et al., 2022). Notably, positron emission tomography studies in psychiatric patients have found increased P-gp function in the BBB (de Klerk et al., 2010). Moreover, polymorphisms within the ABCB1 gene, also known as the multi-drug resistance 1 (MDR1) gene, have been associated with BD (Turgut et al., 2009) and altered P-gp function and response to treatment (Zheng et al., 2021). Finally, given the association between mood disorders and inflammation, several studies have also investigated the utilization of adjunct agents with anti-inflammatory properties, suggesting them as promising therapeutic agents (Calkin et al., 2015).
5.2. Lithium
Lithium is considered a pharmacological standard in acute and chronic treatment of BD. Remarkably, the mechanisms by which lithium exhibits its effects are largely elusive. A well supported potential mechanism for lithium's effects on the BBB is through inhibition of GSK-3β. GSK-3β is thought to contribute to rearranging the subcellular distribution of tight junctions and inhibition of the Wnt/β-catenin pathway, which when activated serves to protect BBB integrity through increasing tight junction protein formation (Luo et al., 2021).
Animal studies have indicated that lithium likely has a protective effect on the BBB. Ischemic-stroke and reperfusion mouse models were found to have significantly reduced BBB breakdown in the ischemic hemisphere with lithium treatment (Ji et al., 2021). Moreover, lithium-treated mice maintained claudin-5 and ZO-1/TJP1 protein expression levels after implementation of the ischemia and reperfusion protocol (Ji et al., 2021). Of note, although stroke's pathophysiology may be distant from that of BD, these models may still help to inform molecular mechanisms associated with lithium's actions at the cellular and physiological levels. In fact, many of lithium's neuroprotective effects thought to underlie its mood stabilizing properties were initially identified in stroke models (Chuang et al., 2002).
Unsurprisingly, animal models of mood disorders have also demonstrated improvement in BBB disruption with lithium (Taler et al., 2021). Lithium-exposed rats were found to have significantly decreased hippocampal BBB permeability and trend toward decreased expression of hippocampal aquaporin-4 expression after chronic mild stress (Taler et al., 2021). In addition, lithium treatment was found to upregulate hippocampal claudin-5 and brain derived neurotrophic factor protein expression (Taler et al., 2021). In vitro studies of lithium compounds have demonstrated variable effects on P-gp expression depending on the dosage administered, where low doses have been associated with inhibitory effects and doses outside the therapeutic range have been shown to induce P-gp expression (Luo et al., 2021; Newman et al., 2017).
We were unable to find any studies that directly assessed BBB integrity patients with mood disorders treated with lithium. However, a recent review summarized current literature on “the role of brain barriers in the neurokinetics and pharmacodynamics of lithium” indicating that lithium may not only have an effect on the BBB, but patient BBB and blood-cerebrospinal fluid barrier integrity may also impact lithium response. More than half of patients with BD do not respond to lithium treatment, and lithium transport to and throughout the brain may play a role in patients' variable treatment responsivity (Luo et al., 2021). In light of this, further study of lithium's interaction with the BBB and BBB-associated proteins such as GSK-3β in patients may serve as fruitful areas for drug development and personalized medicine.
5.3. Anticonvulsants
Many anticonvulsants are also commonly used as first line mood stabilizers BD treatment. Like lithium, valproic acid has been shown to be neuroprotective in mice that suffered traumatic injuries. Treatment of intracerebral hemorrhage in mice with valproic acid was associated with better preserved BBB integrity than controls (Zhao et al., 2020). Valproic acid-treated mice have also been shown to have decreased levels of proinflammatory molecules and increased BMEC junction protein levels, such as claudin-5 and occludin (Zhao et al., 2020). Valproic acid has also been shown to decrease MMP-9 expression after subarachnoid hemorrhage in mice, which in turn, prevented degradation of occludin and claudin-5 (Ying et al., 2016). Not only does valproic acid seem to protect the BBB through increasing levels of BMEC junction proteins, but it also seems to affect the nutrient transporters (Mann Brukner et al., 2018). Specifically, it downregulates Glut1 mRNA expression, possibly indicating its role in nutrient transport across the BBB after injury (Mann Brukner et al., 2018). Moreover, combined sub-effective doses of valproic acid and lithium may reduce lesion volume and preserve BBB integrity in mice suffering from traumatic brain injury (Yu et al., 2013). Valproic acid has also been shown to interact with P-gp and may have inhibitory effects on P-gp expression (Nicolae et al., 2016).
5.4. Antipsychotics
Antipsychotics are also often used as first-line or adjunct therapies in BD and can have effects on BBB integrity. In particular, several studies have shown that quetiapine has neuroprotective effects when used in the setting of traumatic brain injury, where it helps preserve tight junction integrity and thereby results in decreased BBB hyperpermeability (Morra and Alao, 2020). One mechanism in which this was shown to occur is through binding to MMP-9, which is known to disrupt β-catenin and the functioning of tight and adherens junctions, such as ZO-1/TJP1 (Robinson et al., 2018). Of note, while low doses of most antipsychotics are associated with neuroprotection, high doses have been associated with cytotoxic effects on BBB endothelial cells, leading to apoptosis and BBB disruption (Elmorsy et al., 2014; Schmidt et al., 2010).
Quetiapine, along with other antipsychotics including risperidone and olanzapine, are also substrates for P-gp and thereby actively shunted out of the brain. This leads to decreased drug concentrations in the brain necessitating higher doses to reach therapeutic levels. In order to minimize this, efforts have been made to determine if inhibiting P-gp could improve drug delivery to the brain. Unsurprisingly, co-treatment with P-gp inhibitors has led to increased antipsychotic concentrations in the brain as well as longer lasting behavioral effects in animal studies. These investigations have been furthered through efforts to develop dimer compounds that can act as both an antipsychotic and P-gp inhibitor to minimize negative drug-drug interactions and improve drug delivery (Emmert et al., 2014).
Interestingly, not all antipsychotics interact with P-gp in the same way. Clozapine, for instance, has been shown to have inhibitory effects on P-gp, notably when combined with risperidone, allowing higher risperidone concentrations in the brain (Liu et al., 2021). Moreover, there is evidence that drug-drug interactions can modulate whether some drugs work as a P-gp inhibitor or activator (Bebawy and Chetty, 2008).
5.5. Antidepressants
Antidepressants are generally not considered to be highly effective treatments for BD. Nevertheless, antidepressants remain commonly used in the treatment of BD, particularly for depressive symptoms. In fact, antidepressant treatment leading to clinical improvement has been associated with decreased S100B levels, which are known to be elevated in BD and associated with BBB disruption (Schroeter et al., 2008).
In vitro and in vivo studies have shown that antidepressants interact with P-gp in varying ways (O'Brien et al., 2012). Furthermore, ABCB1 polymorphisms have been linked to depression severity and antidepressant treatment response, although with mixed results (Zheng et al., 2021). Nevertheless, it is clear that a relationship between P-gp function, BBB permeability, and some antidepressants exists and further elucidation could lead to targeted antidepressant selection for affected patients and/or targets for adjunct therapy, such as P-gp modulators to improve antidepressant response (Zheng et al., 2021; O'Brien et al., 2012). Sertraline has been shown to contribute to decreased BBB integrity (Ma et al., 2020), and co-administration of sertraline and risperidone has been shown to lead to significantly increased risperidone concentration in the brain (O'Brien et al., 2012).
6. Conclusions and future directions
As discussed in the previous sections, multiple pathways may play crucial roles in modulating BBB integrity in BD, such as inflammation, insulin resistance, and alteration of neuronal plasticity. As a consequence, a disruption of the BBB can have a significant clinical impact in BD patients. Certain molecules, such as claudin-5, zonulin, P-gp, and S100B, have been shown to modulate BBB integrity and can serve as useful biomarkers to detect BBB disruption and as targets for novel medications. In addition, although medications such as lithium, anticonvulsants, and antipsychotics are well established as clinical treatments of BD, their various specific effects on the BBB in the context of BD are promising areas of further research and can possibly be targeted specifically for future clinical trials. Of note, targeting the BBB may present its own nuances when developing and proposing novel medications for BD. Specifically, increasing BBB integrity, while correcting its initial disruption, may reduce its permeability to substances and drugs that need to reach the CNS (thereby limiting therapeutic options). This suggests that targeting BBB in BD will need a balanced goal of achieving its optimal, healthy integrity to simultaneously reduce influx of peripheral inflammatory mediators while still allowing the delivery of psychotropic medications and their metabolites into the brain.
Due to the limited number of studies investigating the role of the BBB in psychiatric disorders, particularly BD, we decided that a narrative review with a wider search criteria would be the most effective method to study the state of the field. Accordingly, an important limitation of this narrative review is the fact that we did not perform a systematic search for the manuscripts included, potentially leading to bias in the presentation of our results. We purposely included studies with both positive and negative studies; nevertheless, future studies with a systematic review and meta-analyses, especially once new original data emerges, will be warranted.
Although of promising clinical implications, the study of BBB dysfunction in BD is still in its infancy, and no study has been able to identify whether it may be a cause or a consequence of the disease. A recent MRI study suggests it to be a consequence of the disease (or, rather, a cause of neuroprogression)(Kamintsky et al., 2020), but it is possible that an innate BBB dysfunction (possibly (epi)genetically determined) may already be present in unaffected subjects at a high risk for the disorder. As discussed earlier, it is also evident that a BBB dysfunction may be characteristic of only a subgroup of patients with a worse disease presentation and prognosis. The relatively small number of studies on BBB integrity in BD samples also limits our ability to make definitive inferences about mechanisms underlying BBB in the disorder. This is particularly important given that many of the mechanisms reviewed in this study may have patterns and directions in BD that are different (and possibly even opposite) of what is seen in BD. For instance, a reduction in BBB permeability has been observed in subjects with inflamed gut (Carloni et al., 2021), which is counterintuitive to the BD mechanisms proposed here. Since many of the findings available for this review are from different backgrounds other than BD, it is possible that some of the mechanisms discussed are incorrectly being proposed for the disorder. Future studies with a focus on BD samples will be able to identify BD-specific markers of BBB functionality (if they exist).
Importantly, many of the findings of BBB dysfunction reported in BD samples, including the underlying mechanisms discussed in this study (e.g. gut microbiota dysbiosis, systemic inflammation, and metabolic alterations), are at least partially shared with other major psychiatric disorders, including major depressive disorder and schizophrenia. This suggests that BBB dysfunction may not be a BD-specific finding, although its specific drivers may differ significantly between diagnoses or even between subgroups of patients with specific characteristics.
Overall, understanding these clinical implications and their underlying molecular bases may provide targets for the development of novel therapeutics and personalized therapies focusing on BBB restoration in BD. These may include the use of stem cells, biomaterials, anti-inflammatory drugs, and the production of novel mood stabilizers directed towards the BBB and its integrity.
Role of funding source
This study was funded by the National Institute of Mental Health (NIMH, K01 MH121580 to GRF) and the American Foundation for Suicide Prevention (GRF). Translational Psychiatry Program (USA) is funded by the Department of Psychiatry and Behavioral Sciences, McGovern Medical School at UTHealth and Linda Gail Behavioral Health Research Fund. These funding sources had no role in study design, collection, analysis and interpretation of data, in writing of the report, or in the decision to submit this article for publication.
Declaration of competing interest
None.
References
- Aguiar A.S., Jr., et al. The effect of n-acetylcysteine and deferoxamine on exercise-induced oxidative damage in striatum and hippocampus of mice. Neurochem. Res. 2008;33:729–736. doi: 10.1007/s11064-007-9485-8. [DOI] [PubMed] [Google Scholar]
- Bebawy M., Chetty M. Differential pharmacological regulation of drug efflux and pharmacoresistant schizophrenia. Bioessays. 2008;30:183–188. doi: 10.1002/bies.20706. [DOI] [PubMed] [Google Scholar]
- Becker L., et al. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67:827–836. doi: 10.1136/gutjnl-2016-312940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Gray J.A., Roth B.L. The expanded Biology of serotonin. Annu. Rev. Med. 2009:60 355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogush M., Heldt N.A., Persidsky Y. Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J. Neuroimmune Pharmacol. 2017;12:593–601. doi: 10.1007/s11481-017-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin C.V., et al. Insulin resistance and outcome in bipolar disorder. Br. J. Psychiatry. 2015;206:52–57. doi: 10.1192/bjp.bp.114.152850. [DOI] [PubMed] [Google Scholar]
- Carloni S., et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science. 2021;374:439–448. doi: 10.1126/science.abc6108. [DOI] [PubMed] [Google Scholar]
- Carvalho A.F., Firth J., Vieta E. Bipolar disorder. N. Engl. J. Med. 2020;383:58–66. doi: 10.1056/NEJMra1906193. [DOI] [PubMed] [Google Scholar]
- Cerullo M.A., Strakowski S.M. The prevalence and significance of substance use disorders in bipolar type I and II disorder. Subst. Abuse Treat. Prev. Pol. 2007;2:29. doi: 10.1186/1747-597X-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.L., Bersig J., Felix B., Fiala M., House S.D. Chronic cocaine alters hemodynamics and leukocyte-endothelial interactions in rat mesenteric venules. Life Sci. 2000;66:2357–2369. doi: 10.1016/s0024-3205(00)00566-x. [DOI] [PubMed] [Google Scholar]
- Chengappa K.N., Levine J., Gershon S., Kupfer D.J. Lifetime prevalence of substance or alcohol abuse and dependence among subjects with bipolar I and II disorders in a voluntary registry. Bipolar Disord. 2000;2:191–195. doi: 10.1034/j.1399-5618.2000.020306.x. [DOI] [PubMed] [Google Scholar]
- Chuang D.-M., et al. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disord. 2002;4:129–136. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- Coelho-Santos V., et al. The TNF-α/Nf-κB signaling pathway has a key role in methamphetamine–induced blood–brain barrier dysfunction. J. Cerebr. Blood Flow Metabol. 2015;35:1260–1271. doi: 10.1038/jcbfm.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klerk O.L., et al. Regional increase in P-glycoprotein function in the blood-brain barrier of patients with chronic schizophrenia:: a PET study with [11C] verapamil as a probe for P-glycoprotein function. Psychiatr. Res. Neuroimaging. 2010;183:151–156. doi: 10.1016/j.pscychresns.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Elmorsy E., Elzalabany L.M., Elsheikha H.M., Smith P.A. Adverse effects of antipsychotics on micro-vascular endothelial cells of the human blood–brain barrier. Brain Res. 2014;1583:255–268. doi: 10.1016/j.brainres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Emmert D., et al. Reversible dimers of the atypical antipsychotic quetiapine inhibit p-glycoprotein-mediated efflux in vitro with increased binding affinity and in situ at the blood-brain barrier. ACS Chem. Neurosci. 2014;5:305–317. doi: 10.1021/cn4002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., et al. Alterations in cerebrospinal fluid in patients with bipolar syndromes. Front. Psychiatr. 2016;7:194. doi: 10.3389/fpsyt.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis S.R., Keep R.F. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J. Cerebr. Blood Flow Metabol. 2007;27:1573–1582. doi: 10.1038/sj.jcbfm.9600454. [DOI] [PubMed] [Google Scholar]
- Falcone T., Lovell R., Janigro D., Anand A. The impact of childhood trauma on the blood-brain barrier and the risk of Suicide. Biol. Psychiatr. 2019;85:S65. 156. [Google Scholar]
- Feinstein D.L., Galea E., Reis D.J. Norepinephrine suppresses inducible nitric oxide synthase activity in rat astroglial cultures. J. Neurochem. 1993;60:1945–1948. doi: 10.1111/j.1471-4159.1993.tb13425.x. [DOI] [PubMed] [Google Scholar]
- Fries G.R., Walss-Bass C., Bauer M.E., Teixeira A.L. Revisiting inflammation in bipolar disorder. Pharmacol. Biochem. Behav. 2019;177:12–19. doi: 10.1016/j.pbb.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Fukui S., Schwarcz R., Rapoport S.I., Takada Y., Smith Q.R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Futtrup J., et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav. Immun. Health. 2020;6:100102. doi: 10.1016/j.bbih.2020.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giridharan V.V., et al. Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol. Psychiatr. 2020;25:94–113. doi: 10.1038/s41380-019-0448-7. [DOI] [PubMed] [Google Scholar]
- Greene C., et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol. Psychiatr. 2018;23:2156–2166. doi: 10.1038/mp.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C., Hanley N., Campbell M. Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS. 2019;16 doi: 10.1186/s12987-019-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C., Hanley N., Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry. 2020;10:373. doi: 10.1038/s41398-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C., Hanley N., Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry. 2020;10 doi: 10.1038/s41398-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze H., Schaefer M., Scherk H., Born C., Preuss U.W. Comorbid bipolar and alcohol use disorder—a therapeutic challenge. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.660432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch F., et al. Multiplex immunoassay analysis of plasma shows differences in biomarkers related to manic or mixed mood states in bipolar disorder patients. J. Affect. Disord. 2015;185:12–16. doi: 10.1016/j.jad.2015.05.065. [DOI] [PubMed] [Google Scholar]
- Haorah J., Knipe B., Leibhart J., Ghorpade A., Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J. Leukoc. Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Hawkins B.T., et al. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Hsuchou H., Kastin A.J., Mishra P.K., Pan W. C-reactive protein increases BBB permeability: implications for obesity and neuroinflammation. Cell. Physiol. Biochem. 2012;30:1109–1119. doi: 10.1159/000343302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A., Makadia V., Valicherla G.R., Riyazuddin M., Gayen J.R. Approaches to minimize the effects of P-glycoprotein in drug transport: a review. Drug Dev. Res. 2022 doi: 10.1002/ddr.21918. [DOI] [PubMed] [Google Scholar]
- Ji Y.-B., et al. Lithium alleviates blood-brain barrier breakdown after cerebral ischemia and reperfusion by upregulating endothelial Wnt/β-catenin signaling in mice. Neuropharmacology. 2021;186:108474. doi: 10.1016/j.neuropharm.2021.108474. [DOI] [PubMed] [Google Scholar]
- Jones G.H., Vecera C.M., Pinjari O.F., Machado-Vieira R. Inflammatory signaling mechanisms in bipolar disorder. J. Biomed. Sci. 2021;28:45. doi: 10.1186/s12929-021-00742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar M.A., Prasad S., Cucullo L. Protecting the BBB endothelium against cigarette smoke-induced oxidative stress using popular antioxidants: are they really beneficial? Brain Res. 2015;1627:90–100. doi: 10.1016/j.brainres.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamintsky L., et al. Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. Neuroimage Clin. 2020;26:102049. doi: 10.1016/j.nicl.2019.102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczinski F., et al. Peripheral biomarkers and illness activity in bipolar disorder. J. Psychiatr. Res. 2011;45:156–161. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Kessing L.V., Ziersen S.C., Andersen P.K., Vinberg M. A nation-wide population-based longitudinal study on life expectancy and cause specific mortality in patients with bipolar disorder and their siblings. J. Affect. Disord. 2021;294:472–476. doi: 10.1016/j.jad.2021.07.065. [DOI] [PubMed] [Google Scholar]
- Kılıç F., Işık Ü., Demirdaş A., Doğuç D.K., Bozkurt M. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J. Affect. Disord. 2020;266:37–42. doi: 10.1016/j.jad.2020.01.117. [DOI] [PubMed] [Google Scholar]
- Kuhlmann C.R.W., et al. Mechanisms of C-reactive protein-induced blood–brain barrier disruption. Stroke. 2009;40:1458–1466. doi: 10.1161/STROKEAHA.108.535930. [DOI] [PubMed] [Google Scholar]
- la Cour Karottki N.F., et al. Sleep and physical activity in patients with newly diagnosed bipolar disorder in remission, their first-degree unaffected relatives and healthy controls. Int. J. Bipolar Disord. 2020;8:16. doi: 10.1186/s40345-020-00181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-Q., Chen P., Haimovitz-Friedman A., Reilly R.M., Wong C.S. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003;63:5950–5956. [PubMed] [Google Scholar]
- Lin K., Liu T. Exercise on bipolar disorder in humans. Int. Rev. Neurobiol. 2019:189–198. doi: 10.1016/bs.irn.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Liu X., et al. Clozapine affects the pharmacokinetics of risperidone and inhibits its metabolism and P-glycoprotein-mediated transport in vivo and in vitro: a safety attention to antipsychotic polypharmacy with clozapine and risperidone. Toxicol. Appl. Pharmacol. 2021;422:115560. doi: 10.1016/j.taap.2021.115560. [DOI] [PubMed] [Google Scholar]
- Lizano P., et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am. J. Psychiatr. 2019;176:564–572. doi: 10.1176/appi.ajp.2019.18070825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizano P., Pong S., Santarriaga S., Bannai D., Karmacharya R. TNFα and MMP1 in brain microvascular endothelial cells regulate blood-brain barrier dysfunction in psychotic disorders. Res. Square. 2022 doi: 10.21203/rs.3.rs-1162029/v1. [DOI] [PubMed] [Google Scholar]
- Luo H., et al. The role of brain barriers in the neurokinetics and pharmacodynamics of lithium. Pharmacol. Res. 2021;166:105480. doi: 10.1016/j.phrs.2021.105480. [DOI] [PubMed] [Google Scholar]
- Ma Q., et al. Effect of dolutegravir and sertraline on the blood brain barrier (BBB) J. Neuroimmune Pharmacol. 2020;15:7–9. doi: 10.1007/s11481-020-09904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFabe D.F. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012;23 doi: 10.3402/mehd.v23i0.19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S.D., et al. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008;1203:133–148. doi: 10.1016/j.brainres.2008.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S.D., et al. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J. Clin. Immunol. 2008;28:528–541. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- Mahmood T., Silverstone T. Serotonin and bipolar disorder. J. Affect. Disord. 2001;66:1–11. doi: 10.1016/s0165-0327(00)00226-3. [DOI] [PubMed] [Google Scholar]
- Małkiewicz M.A., Małecki A., Toborek M., Szarmach A., Winklewski P.J. Substances of abuse and the blood brain barrier: interactions with physical exercise. Neurosci. Biobehav. Rev. 2020;119:204–216. doi: 10.1016/j.neubiorev.2020.09.026. [DOI] [PubMed] [Google Scholar]
- Mann Brukner A., Ben-Hur T., Honig A., Ekstein D., Eyal S. Effects of valproic acid on cerebral nutrient carriers' expression in the rat. Front. Pharmacol. 2018;9:1054. doi: 10.3389/fphar.2018.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N., et al. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci. 2003;21:109–121. [PMC free article] [PubMed] [Google Scholar]
- Mark K.S., Miller D.W. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci. 1999;64:1941–1953. doi: 10.1016/s0024-3205(99)00139-3. [DOI] [PubMed] [Google Scholar]
- McKee A.C., Daneshvar D.H., Alvarez V.E., Stein T.D. The neuropathology of sport. Acta Neuropathol. 2014;127:29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C., et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017;20:1752–1760. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer T., Lammers G., Müller-Siecheneder F., Schmidt R.-F., Latifi S. Substance abuse in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatr. Res. 2017;253:338–350. doi: 10.1016/j.psychres.2017.02.067. [DOI] [PubMed] [Google Scholar]
- Monda V., et al. Exercise modifies the gut microbiota with positive Health effects. Oxid. Med. Cell. Longev. 2017:3831972. doi: 10.1155/2017/3831972. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra J.A., Alao A.O. Role of quetiapine in protection of neurodegeneration after traumatic brain injury. Int. J. Psychiatr. Med. 2020;55:67–73. doi: 10.1177/0091217419838105. [DOI] [PubMed] [Google Scholar]
- Mudgal J., et al. Possible involvement of metformin in downregulation of neuroinflammation and associated behavioural changes in mice. Inflammopharmacology. 2019;27:941–948. doi: 10.1007/s10787-019-00638-w. [DOI] [PubMed] [Google Scholar]
- Muneer P.M.A., Abdul Muneer P.M., Alikunju S., Szlachetka A.M., Haorah J. Inhibitory effects of alcohol on glucose transport across the blood–brain barrier leads to neurodegeneration: preventive role of acetyl-l-carnitine. Psychopharmacology. 2011;214:707–718. doi: 10.1007/s00213-010-2076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S.A., Pan Y., Short J.L., Nicolazzo J.A. Assessing the impact of lithium chloride on the expression of P-glycoprotein at the blood-brain barrier. J. Pharmaceut. Sci. 2017;106:2625–2631. doi: 10.1016/j.xphs.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Ng F., Dodd S., Berk M. The effects of physical activity in the acute treatment of bipolar disorder: a pilot study. J. Affect. Disord. 2007;101:259–262. doi: 10.1016/j.jad.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Nicolae A.C., et al. In vitro P-GP expression after administration of CNS active drugs. FARMACIA. 2016;64:844–850. [Google Scholar]
- Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral E., Halici Z., Cinar I., Ozcan E., Kutlu Z. Evaluation of endothelial dysfunction in bipolar affective disorders: serum endocan and urotensin-II levels. Clin. Psychopharmacol. Neurosci. 2019;17:211–221. doi: 10.9758/cpn.2019.17.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovska-Waast S., et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol. Psychiatr. 2019;24:869–887. doi: 10.1038/s41380-018-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien F.E., Dinan T.G., Griffin B.T., Cryan J.F. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: clinical significance of in vitro and in vivo findings. Br. J. Pharmacol. 2012;165:289–312. doi: 10.1111/j.1476-5381.2011.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V.H. Stress primes microglia to the presence of systemic inflammation: implications for environmental influences on the brain. Brain Behav. Immun. 2007:21 45–46. doi: 10.1016/j.bbi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Petersen A.M.W., Pedersen B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Pong S., Lizano P., Karmacharya R. Investigating blood-brain barrier dysfunction in schizophrenia using brain microvascular endothelial cells derived from patient-specific stem cells. Biol. Psychiatr. 2020;87:S189–S190. [Google Scholar]
- Robinson B.D., et al. Quetiapine protects the blood-brain barrier in traumatic brain injury. J. Trauma Acute Care Surg. 2018;85:968–976. doi: 10.1097/TA.0000000000002011. [DOI] [PubMed] [Google Scholar]
- Sajja R.K., Rahman S., Cucullo L. Drugs of abuse and blood-brain barrier endothelial dysfunction: a focus on the role of oxidative stress. J. Cerebr. Blood Flow Metabol. 2016;36:539–554. doi: 10.1177/0271678X15616978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum I.M., Brown E.S. Management of comorbid bipolar disorder and substance use disorders. Am. J. Drug Alcohol Abuse. 2017;43:366–376. doi: 10.1080/00952990.2017.1292279. [DOI] [PubMed] [Google Scholar]
- Schmidt A.J., et al. Effects of quetiapine, risperidone, 9-hydroxyrisperidone and ziprasidone on the survival of human neuronal and immune cells in vitro. J. Psychopharmacol. 2010;24:349–354. doi: 10.1177/0269881108096506. [DOI] [PubMed] [Google Scholar]
- Schmitz S.L., Abosi O.J., Persons J.E., Sinkey C.A., Fiedorowicz J.G. Impact of mood on endothelial function and arterial stiffness in bipolar disorder. Heart Mind (Mumbai) 2018;2:78–84. doi: 10.4103/hm.hm_20_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Abdul-Khaliq H., Krebs M., Diefenbacher A., Blasig I.E. Serum markers support disease-specific glial pathology in major depression. J. Affect. Disord. 2008;111:271–280. doi: 10.1016/j.jad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Solár P., Zamani A., Kubíčková L., Dubový P., Joukal M. Choroid plexus and the blood–cerebrospinal fluid barrier in disease. Fluids Barriers CNS. 2020;17:1–29. doi: 10.1186/s12987-020-00196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar S.S., et al. Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct. Funct. 2016;221:4319–4335. doi: 10.1007/s00429-015-1163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza P.S., et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood-brain barrier disruption. Mol. Neurobiol. 2017;54:4723–4737. doi: 10.1007/s12035-016-0014-0. [DOI] [PubMed] [Google Scholar]
- Sublette M.E., et al. Bipolar disorder and the gut microbiome: a systematic review. Bipolar Disord. 2021 doi: 10.1111/bdi.13049. [DOI] [PubMed] [Google Scholar]
- Sylvia L.G., et al. Pilot study of a lifestyle intervention for bipolar disorder: Nutrition exercise wellness treatment (NEW Tx) J. Affect. Disord. 2019;250:278–283. doi: 10.1016/j.jad.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taler M., et al. Regulatory effect of lithium on hippocampal blood-brain barrier integrity in a rat model of depressive-like behavior. Bipolar Disord. 2021;23:55–65. doi: 10.1111/bdi.12962. [DOI] [PubMed] [Google Scholar]
- Tong B., et al. Bipolar disorder and related mood states are not associated with endothelial function of small arteries in adults without heart disease. Gen. Hosp. Psychiatr. 2018;51:36–40. doi: 10.1016/j.genhosppsych.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M.-C., Huang T.-L. Decreased S100B serum levels after treatment in bipolar patients in a manic phase. Compr. Psychiatr. 2017;74:27–34. doi: 10.1016/j.comppsych.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Turgut G., et al. Association of MDR1 C3435T polymorphism with bipolar disorder in patients treated with valproic acid. Mol. Biol. Rep. 2009;36:495–499. doi: 10.1007/s11033-007-9206-z. [DOI] [PubMed] [Google Scholar]
- Turkheimer F.E., et al. Increased serum peripheral C-reactive protein is associated with reduced brain barriers permeability of TSPO radioligands in healthy volunteers and depressed patients: implications for inflammation and depression. Brain Behav. Immun. 2021;91:487–497. doi: 10.1016/j.bbi.2020.10.025. [DOI] [PubMed] [Google Scholar]
- Valvassori S.S., et al. Sodium butyrate has an antimanic effect and protects the brain against oxidative stress in an animal model of mania induced by ouabain. Psychiatr. Res. 2016:235 154–159. doi: 10.1016/j.psychres.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Van Dyken P., Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front. Neurosci. 2018;12:930. doi: 10.3389/fnins.2018.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S., et al. Childhood trauma in bipolar disorder. Aust. N. Z. J. Psychiatr. 2014;48:564–570. doi: 10.1177/0004867413516681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J., Markie D., Fitches A. Cholecystokinin system genes: associations with panic and other psychiatric disorders. J. Affect. Disord. 2012;136:902–908. doi: 10.1016/j.jad.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Ying G.-Y., et al. Neuroprotective effects of valproic acid on blood-brain barrier disruption and apoptosis-related early brain injury in rats subjected to subarachnoid hemorrhage are modulated by heat shock protein 70/matrix metalloproteinases and heat shock protein 70/AKT pathways. Neurosurgery. 2016;79:286–295. doi: 10.1227/NEU.0000000000001264. [DOI] [PubMed] [Google Scholar]
- Yu F., et al. Posttrauma cotreatment with lithium and valproate: reduction of lesion volume, attenuation of blood-brain barrier disruption, and improvement in motor coordination in mice with traumatic brain injury. J. Neurosurg. 2013;119:766–773. doi: 10.3171/2013.6.JNS13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., et al. Valproate sodium protects blood brain barrier integrity in intracerebral hemorrhage mice. Oxid. Med. Cell. Longev. 2020;2020:8884320. doi: 10.1155/2020/8884320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Fu Z., Chen X., Wang M., Zhu R. Effects of ABCB1 gene polymorphism on the efficacy of antidepressant drugs: a protocol for systematic review and meta-analysis. Medicine. 2021;100 doi: 10.1097/MD.0000000000026411. [DOI] [PMC free article] [PubMed] [Google Scholar]