Abstract
Purpose:
To determine the risk of stroke, transient ischemic attack (TIA), and transient monocular vision loss (TMVL) before and after a central retinal artery occlusion (CRAO).
Design:
Population-based retrospective case series.
Subjects:
Patients diagnosed with a CRAO in Olmsted County, Minnesota from 1976 to 2016
Methods:
Patients living in Olmsted County with a diagnosis code of CRAO from 1976 to 2016 were reviewed. New CRAOs were confirmed and stroke, TIA, and TMVL events in the 15 days before and after CRAO were recorded.
Main Outcome Measures:
Incidence of stroke, TIA, and TMVL events in the 15 days before and after CRAO.
Results:
Eighty-nine patients with a CRAO were identified providing an annual incidence of 2.58/100,000 (95% CI 2.04–3.11). Median age at the time of CRAO was 76 years old (range 46 to 100 years); 56.2% were male and 89.9% of the cohort was white. In the 15 days before and after CRAO, there were 2 ischemic strokes (2.2%), 1 hemorrhagic stroke (1.1%), 2 TIAs (2.2%), and 9 patients with TMVL (10.1%). Starting in 1999, 15 of 45 patients had magnetic resonance imaging within 2 months of CRAO. One (6.7%) had evidence of asymptomatic diffusion restriction and 9 (60%) had a remote infarct.
Conclusions:
This population-based study demonstrated that the risk of symptomatic ischemic stroke is 2.2% in the 15 days before and after a CRAO, which is slightly lower than most studies from tertiary centers. These data should be considered as practice recommendations are developed regarding the urgency of neurovascular workup in patients with acute CRAO.
Keywords: central retinal artery occlusion, stroke, cerebrovascular disease, population-based study
Introduction:
Occlusion of the central retinal artery and its branches can produce severe acute vision loss in the affected eye due to inner retinal ischemia. The incidence of central retinal artery occlusion (CRAO) has been estimated at 1–2/100,000 people per year with 80% of patients having a final visual acuity (VA) of 20/400 or worse.1,2
In addition to vision loss, there has been recent increased recognition of stroke complications temporally related to CRAO. International studies in Taiwan and Korea have demonstrated that retinal artery occlusions (RAOs) are associated with an increased risk of ischemic stroke around the time of RAO.3,4 Other investigators have found that diffusion weighted imaging (DWI) sequences on magnetic resonance imaging (MRI) were abnormal in a significant percentage of patients.5–7 With increasing evidence of neurovascular complications, content experts have proposed that RAOs are an emergency that necessitate cerebrovascular evaluation within 24 hours.8–10
In a previous publication, our group has reported that 5.3% of patients had a stroke within 15 days before or after a CRAO based on institution-wide Mayo Clinic data.11 This data; however, was subject to referral bias. The Taiwan and Korean studies on RAO and stroke were population-based, but relied on diagnosis codes that have the potential for errors in coding.3,4 The present study was undertaken to evaluate the association of stroke in the 15 days before and after CRAO using population-based data from the Rochester Epidemiology Project (REP) that allowed for manual review of patient information rather than relying solely on diagnosis codes in order to provide an accurate risk assessment of neurovascular events associated with CRAO.
Methods:
Patients living in Olmsted County, MN diagnosed with a CRAO between January 1, 1976 and September 9, 2016 were reviewed. Patient charts were identified for screening using diagnosis codes including central and branch retinal artery occlusions (BRAO). Previously reviewed patient data from Leavitt, et al. 2011 was incorporated into the final dataset, which included all CRAOs in Olmsted County from 1976 to 2005. Patient charts were selected and reviewed using the REP. The REP is a medical records linkage system established to study the epidemiology of disease for residents of Olmsted County in Southeastern Minnesota. It links and indexes diagnostic and procedure information from Mayo Clinic in Rochester, MN and other facilities that provide health care to residents in this community.12,13
Patients were eligible for inclusion in this study if they were over the age of 18 and had a confirmed, new, diagnosis of CRAO while a resident in Olmsted County between January 1, 1976 and September 9, 2016. In cases where the diagnosis of CRAO was in question, a staff neuro-ophthalmologist reviewed the available data to make a final determination. Patients were excluded if they were under the age of 18 years when diagnosed, the original diagnosis was made outside the study window, the CRAO occurred while the patient was not a resident of Olmsted County, MN, or the CRAO diagnosis was incorrect.
Charts of patient’s were reviewed retrospectively to note the following information: patient gender, CRAO laterality, age at diagnosis, cause of CRAO, ischemic or hemorrhagic stroke within 15 days before or after CRAO, TIA within 15 days before or after CRAO, and TMVL within 15 days before or after CRAO. Care was taken to determine the time course of stroke and CRAO, including if the stroke and CRAO occurred simultaneously (same day). Systemic comorbidities were also identified, including hypertension, hyperlipidemia, diabetes mellitus, history of stroke, and smoking (prior or active). Visual acuity was noted at initial presentation for CRAO and as close as possible to 1 year after initial presentation. Attempted treatment of the visual loss, the presence of a relative afferent pupillary deficit, and the presence of a visible retinal embolus were also documented. In patients whose workup included an MRI, asymptomatic diffusion restriction, indicating clinically silent cerebral ischemia, was recorded. Evidence of remote cerebral ischemia on MRI was also noted.
Stroke was defined as neurologic deficits correlating to ischemia or hemorrhage on computed tomography (CT) scan or diffusion restriction on MRI. If patients were diagnosed before the era of readily available neuroimaging, a clinical diagnosis of stroke was used. Asymptomatic diffusion restriction was defined as diffusion restriction on MRI without any neurologic deficits (outside of CRAO). Transient ischemic attack was defined as neurologic deficits lasting <24 hours with negative CT or MRI, if imaging was obtained. The following causes of CRAO were considered: embolic, thrombotic, vasculitic, central retinal vein occlusion (CRVO) causing CRAO, or unknown. Embolic was coded if the patients had 70% or greater occlusion of their ipsilateral internal carotid artery or mobile or ulcerated atheroma was identified on ultrasound, CT angiogram, or MR angiogram, thrombus was identified in the left side of the heart or distally, atrial fibrillation was documented, or emboli were noted on fundus examination. Central retinal vein occlusion causing CRAO was coded if the patient was diagnosed with both disorders simultaneously or if the patient was recently (within approximately 1 month) diagnosed with CRVO, and the diagnoses were not otherwise explainable by another cause previously described above.
Overall comparison of strokes before, simultaneous with, and after CRAO diagnosis was performed using McNemar’s test. Comparisons of the strokes between groups were completed using Fisher’s exact test. Yearly incidence rates of CRAO for each age and sex group were determined by dividing the number of cases within each group by the estimated total Olmsted County resident population for that year. Incidence of stroke was determined and compared to an age-matched control group. Population figures for 1990, 2000, and 2010 came from United States (US) census data, and populations for the inter-census years were estimated by using linear interpolation. Incidence rates were also age- and sex-adjusted to the 2010 census figures for the US white population. The 95% confidence intervals (CI) were calculated by assuming a Poisson distribution. Trends over time, across age groups, and between genders were assessed using Poisson regression models. The probability of peri-CRAO stroke was estimated using the Kaplan-Meier method. Olmsted County age- and sex-specific stroke incidence was used to construct an estimate of the expected stroke rate in the population; the expected stroke rate was compared to our cohort using a one-sample log-rank test. Data analysis was performed using Statistical Analysis System software version 9.4 (SAS; SAS Institute, Cary, NC).
Mayo Clinic Institutional Review Board Committee approval and informed consent was obtained; the described research adhered to the tenets of the Declaration of Helsinki.
Results:
Eighty-nine patients living in Olmsted County, MN had a confirmed, new CRAO from 1976–2016, which provides an overall age- and sex-adjusted annual incidence of 2.58/100,000 (95% CI 2.04–3.11). The median age at the time of the CRAO was 76 years (range 46–100 years) and 56.2% were men (Table 1). White patients comprised 89.9% of the CRAO cohort. The male age-adjusted incidence was 3.43/100,000 (95% CI 2.47–4.39) and the female age-adjusted incidence was 1.87/100,000 (95% CI 1.28–2.46); this difference was not significant (P = 0.15). The age- and sex-adjusted incidence from 1976 to 1988 was 3.02 (95% CI 1.80–4.25); from 1989–2002 the incidence was 2.40 (95% CI 1.45–3.34); from 2003–2016 the incidence was 2.44 (95% CI 1.68–3.20). There was no significant difference between these time periods (P = 0.54). The incidence rate rose in each 10-year age range starting at 0.15/100,000 in subjects age 40–49, and peaked at 20.68/100,000 in subjects age 80–89, before decreasing in the 90–110 year-old age range (Figure 1). There was a significant difference in the incidence between age ranges (P < 0.001).
Table 1:
Demographic data of central retinal artery occlusion patients
| CRAO (n = 89) | |
|---|---|
| Age (y): Mean; Median (Range) | 74.6; 76.2 (46–100) |
| Gender (male): n (%) | 50 (56.2) |
| CRAO laterality (right): n (%) | 44 (49.4) |
| History of smoking: n (%) | 45 (50.6) |
| Diabetes mellitus: n (%) | 26 (29.2) |
| Hypertension: n (%) | 82 (92.1) |
| Hyperlipidemia: n (%) | 47 (52.8) |
| History of stroke: n (%) | 28 (31.5) |
| Unknown | 5 (5.6) |
| Embolus visible on exam: n (%) | 22 (24.7) |
| Peri-CRAO stroke: n (%) | 3 (3.4) |
| Stroke 15 days before CRAO: n (%) | 0 |
| Stroke simultaneous with CRAO: n (%) | 1 (1.1) |
| Stroke 15 days after CRAO: n (%) | 2 (2.2) |
CRAO = Central retinal artery occlusion
Figure 1:
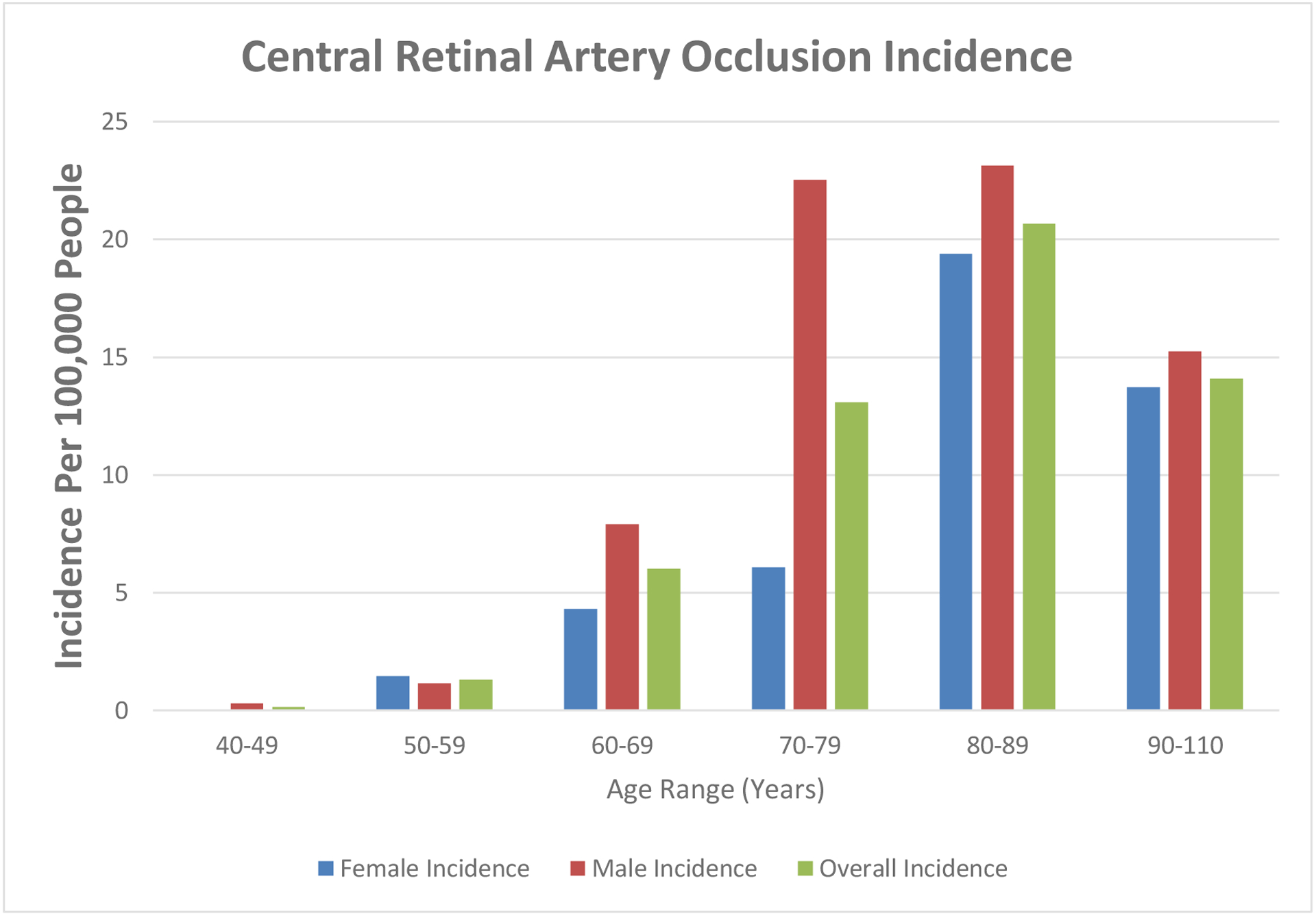
Incidence of central retinal artery occlusion by age and gender
Three patients (3.4%) suffered a stroke in the 15 days before and after CRAO, with 2 (2.2%) ischemic strokes and 1 (1.1%) hemorrhagic stroke. One ischemic stroke occurred the same day as the CRAO; the other ischemic stroke occurred 4 days after CRAO. The hemorrhagic stroke occurred 4 days after CRAO. Two patients with stroke were male (1 ischemic, 1 hemorrhagic); 1 ischemic stroke patient was female. Using historical Olmsted county age- and sex-specific stroke incidence rates, a cohort of this size would be expected to have 0.08 strokes in a given 30-day period.14 The 3 strokes found is a significant increase (P < 0.001). Eliminating the 28 patients with a history of prior stroke resulted in 1 stroke in the 15 days after CRAO out of 61 patients. Historical Olmsted County data in patients with no stroke history gives an expected number of strokes of 0.05 in a 30-day period. The 1 stroke found is a significant increase (P < 0.001). In the 30-day peri-CRAO period there were 2 cases of TIA (2.2%) and 9 patients (10.1%) with TMVL. The TIA cases were split with 1 each before and after CRAO; all the TMVL events occurred before the CRAO. Of the 2 patients with TIAs, one was in the patient that went on to develop a hemorrhagic stroke.
The patients were categorized into the following causes of CRAO: unknown (46, 51.7%), embolic (40, 44.9%), and CRVO causing CRAO (3, 3.4%). No patients had a CRAO secondary to a thrombotic or vasculitic etiology. All patients who had a peri-CRAO stroke or TIA had an embolic etiology (Table 2). The embolic category was further characterized into cardiac (15, 37.5%), carotid (10, 25%), and visible emboli on examination without another cause identified (15, 37.5%). The cardiac etiology included 9 patients with atrial fibrillation (including 1 with a thrombus identified on trans-esophageal echocardiogram), 4 patients with cardiac valve disease, and 2 patients with a patent foramen ovale. The 2 patients with ischemic strokes were considered to have a cardiac etiology to their CRAO and both had atrial fibrillation (including one patient with visualized thrombus on cardiac echocardiogram). The 1 patient with a hemorrhagic stroke and a TIA had a CRAO related to carotid disease with 70–99% ipsilateral internal carotid artery stenosis. The second TIA patient had an embolic CRAO with a visible embolus on examination without an identified cause. From 1976 to 1999, there were 45 CRAOs, of which 48.9% had carotid imaging and 60% had cardiac echocardiography. From 2000 to 2016, there were 44 CRAOs, of which 90.1% had carotid imaging and 75% had cardiac echocardiography. The change in frequency of carotid ultrasound is significant (P < 0.001) while the change in frequency of cardiac echocardiography was not (P = 0.13)
Table 2:
Stroke, transient ischemic attack, and transient monocular vision loss by cause of central retinal artery occlusion
| CRAO Cause (n, %) | CRAO | Simultaneous Stroke | Stroke 15d after | TIA 15d prior | TIA 15d after | Transient monocular vision loss 15d prior |
|---|---|---|---|---|---|---|
| Unknown | 46 (51.7) | 0 | 0 | 0 | 0 | 4 (4.5) |
| Embolic | 40 (44.9) | 1 (1.1) | 2 (2.2) | 1 (1.1) | 1 (1.1) | 5 (5.6) |
| CRVO cause CRAO | 3 (3.4) | 0 | 0 | 0 | 0 | 0 |
| All causes | 89 (100) | 1 (1.1) | 2 (2.2) | 1 (1.1) | 1 (1.1) | 9 (10.1) |
CRAO = Central retinal artery occlusion; TIA = Transient ischemic attack; CRVO = Central retinal vein occlusion
No strokes occurred prior to CRAO. No transient monocular vision loss occurred after CRAO.
Presenting VA was counting fingers (CF) or worse in 82% of patients. Of the 86 patients with available follow up VA data, 78% had a 1-year VA of CF or worse. The 1-year VA data was collected at a median time of 310 days (range 2–3441 days) after initial presentation. Defining a VA change as improvement or worsening ≥3 lines or steps of vision, 74.4% of patients had no change, 16.3% improved, and 9.3% worsened.
Myriad combinations of treatments were attempted for the CRAOs (Table 3), while 36 (40.4%) patients were observed without treatment. For those where treatment was attempted, the most common intervention (either alone or in concert with other treatments) was ocular massage, which was performed on 32 patients (36.0%). Of the 86 patients with follow-up data, 60.5% had empiric treatment and 39.5% were observed. Of those treated, 69.2% had no change in VA, 21.2% improved, and 9.6% worsened. Of those observed, 82.4% showed no change in VA, 8.8% improved, and 8.8% worsened. There was no statistical difference between these groups (P = 0.3)
Table 3:
Attempted acute treatments for central retinal artery occlusion
| Attempted Treatment (n = 89) | N (%)* |
|---|---|
| None | 36 (40.4) |
| Ocular massage | 32 (36.0) |
| Intraocular pressure lowering drops | 23 (25.8) |
| Anterior chamber paracentesis | 14 (15.7) |
| Intravenous heparin | 13 (14.6) |
| Hyperbaric oxygen | 10 (11.2) |
| Oral intraocular pressure medication | 9 (10.1) |
| High flow oxygen | 7 (7.9) |
| Supraorbital artery cannulation with injection of heparin and papaverine | 6 (6.7) |
| Carbogen inhalation | 6 (6.7) |
| Intravenous tissue plasminogen activator | 2 (2.2) |
| Intravenous Methylprednisolone | 1 (1.1) |
| Intra-arterial tissue plasminogen activator | 1 (1.1) |
Numbers exceed 100% as many patients received several treatments
Starting in 1999, 15 out of 45 CRAO patients had brain MRI within 2 months of CRAO diagnosis. Two patients (13.3%) had diffusion restriction, one of which was asymptomatic. Nine patients (60%) had evidence of remote infarction on MRI.
Discussion:
This study was completed to provide population-based data regarding the incidence of stroke associated with CRAO. Among the 89 patients with CRAO, 3 patients (3.4%) suffered a stroke the same day or within 15 days after the CRAO, with 2 (2.2%) ischemic strokes and 1 (1.1%) hemorrhagic stroke. Notably, the 3 patients with stroke and 2 patients with TIA all had an embolic etiology of their CRAO, underscoring the prudence of evaluating for a nidus of embolic disease. Compared to the expected number of strokes over a given 30-day period using age- and sex-specific stroke incidence rates for Olmsted County, MN, the 3 strokes observed are a significant increase. It is notable, however, that the historical Olmsted County stroke incidence is calculated using the entire population of Olmsted County, which is not likely to match the exact co-morbidity profile of the CRAO cohort. This may lead to an underestimation of the expected number of strokes. Using Mayo Clinic institutional data from 2001–2016, our group showed that out of 300 CRAOs seen, 5.3% had a symptomatic stroke (all ischemic) within the 15 days before or after CRAO.11 The present study eliminates the referral bias inherent in evaluating patients seen at a tertiary care referral center, which might increase patients referred for coinciding stroke and CRAO. We posit that the 2.2% of patients with peri-CRAO ischemic strokes found in the current population-based study is closer to the true percentage of patients with this complication. The overall peri-CRAO stroke risk from the present study is similar to prior international studies. In Korea, identifying all incident CRAOs from 2009–2010 found a stroke rate of 9.18% within one year of CRAO and a stroke rate of 3.08% within 14 days before and after CRAO (0.73% pre-CRAO; 2.36% post-CRAO).4 In Taiwan, researchers found that 4.96% of patients suffered a stroke within 1 month after a CRAO.3 In a US based cohort, a retrospective review identified that 1% of patients had a stroke or TIA within 3 months after a CRAO.15 Another US cohort identified a stroke rate of 9.3% in the year after CRAO.16 French et al., conducted a US study using the national Medicare dataset to find all CRAOs coded in 2013 and compared the incidence of ischemic stroke around the time of CRAO using patients with hip fractures as a control group. They found that 0.24% of patients had an ischemic stroke in the 14 days before CRAO and 2.2% of patients had an ischemic stroke in the 14 days after CRAO. Compared to the rate of stroke in hip fracture patients, they found a significant 28-fold and 33-fold increase in ischemic stroke in the first and second week after CRAO, respectively. This study has an identical percentage of ischemic strokes in the 2 weeks after CRAO compared to the present study. Methodological differences should be noted, however, with the French, et al. study relying solely on diagnosis codes in Medicare benificiaries.17
While most studies have demonstrated an increased risk of stroke around the time of a RAO, Laczynski and colleagues contend that the risk is comparable to that of all at-risk adults.18 The authors used an institution-based cohort identifying 221 patients with a confirmed diagnosis of RAO. They found 5 patients (2.3%) had a stroke across the entire cohort and follow-up period, which included 4 patients with ‘concurrent’ ischemic stroke events. The remaining stroke occurred 1.2 years after RAO. The authors appear to minimize the importance of the concurrent strokes, commenting that with their exclusion the risk of stroke is <1%. However, these patients with concurrent stroke are precisely the patients that would benefit the most from emergent evaluation and potential intervention. The assertion that the stroke risk is comparable to that of all at-risk adults is not consistent with the data in our study, showing statistically higher strokes compared to historical Olmsted County data, the Park study (70-fold increase in ischemic stroke within 1 week of CRAO), the Chang study (9.5-fold increase in stroke within 1 month of RAO), or the French, et al. study detailed above.3,4,17 The recent American Heart Association Scientific Statement indicates that acute CRAO is a medical emergency that requires an urgent etiological workup because CRAO is felt to be a TIA/stroke equivalent.19 There is evidence that the early risk of stroke after TIA is underestimated with some research indicating a 7-day stroke risk after first ever TIA of 8.6%.20 Additionally, data from the United Kingdom shows decreasing the time from primary care visit to specialty evaluation in an out-patient setting from 3 days to <1 day after TIA or minor stroke reduced the risk of recurrent stroke within 90 days by 80%.21 While these studies in a broad sense indicate a potential benefit for emergent neurovascular evaluation of patients with CRAO, it is important to recognize that our study was not designed to show that outcomes are improved with an emergent workup. The field would benefit from further comprehensive research to compare different care models and rapidity of workup incorporating short- and long-term outcomes as well as cost and quality of life analysis.
Starting in 1999, MRI was obtained in 33% of CRAO patients within 2 months of the diagnosis. This identified 2 patients (13.3%) with diffusion restriction (one patient was asymptomatic). This is less than what we found in our prior institutional data, in which 22.7% of patients had diffusion restriction, of which 9.1% were asymptomatic. The decrease is not unexpected again owing to the population-based data in the present study compared to institution-wide data. Other radiologic investigations around the time of RAO have shown DWI pathology on MRI in 23 to 24% of patients at the time of RAO, with symptomatic strokes in 2.3 to 15%.5–7 Lavin and colleagues obtained MRI on 66% of a CRAO cohort that demonstrated radiologic evidence of stroke in 37.3% of patients.16 This is higher than that found in our study likely owing to a combination of referral bias with a tertiary care center, inclusion of only acute CRAOs within 7 days of symptom onset, and a possible selection bias for those who had an MRI among the admitted patients.
This study found an overall age- and sex-adjusted incidence of CRAO of 2.58/100,000 people. While CRAOs are thought of as uncommon, the peak incidence in the 80–89 year-old age range is over 8 times higher than the overall incidence. While the overall male and female incidence did not significantly differ, it is interesting to note that the incidence of CRAO in men jumps sharply in the 70–79 year-old age range, compared to the more gradual increase seen in women (Figure 1).
Strengths of this study include near complete medical record access and manual review of charts to confirm CRAO and stroke diagnoses. The population-based design of the study also helps to limit bias. However, the generalizability of the results is limited given that 89.9% of our CRAO cohort was white. It is possible there are racial demographic discrepancies that were not captured in this study. This study is also not immune to the inherent difficulties of retrospective studies, especially those that span several decades, including variable diagnostic evaluations, treatment, follow-up, and technological advancements.
Overall, the findings presented add to the body of literature with evidence of a significant increase in stroke complications around the time of CRAO. This population-based study, mitigating referral bias, provides a 2.2% risk of symptomatic ischemic stroke around the time of a CRAO, which is slightly lower than most studies from tertiary centers. These data should be considered as standards of care are developed for the workup and management of acute CRAOs.
Financial Support:
This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic. This work was supported by the Leonard and Mary Lou Hoeft Career Development Award in Ophthalmology Research. (This funding organization had no role in the design or conduct of this research)
Legend:
- CRAO
Central retinal artery occlusion
- VA
Visual acuity
- ICA
Internal carotid artery
- TEE
Trans-esophageal echocardiography
- TTE
Trans-thoracic echocardiography
- MRI
Magnetic resonance imaging
- DWI
Diffusion-weighted imaging
- RAO
Retinal artery occlusion
- TMVL
Transient monocular vision loss
- CT
Computed tomography
- BRAO
Branch retinal artery occlusion
- REP
Rochester Epidemiology Project
- US
United States
- CI
Confidence interval
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
Meeting Presentation: American Academy of Ophthalmology Annual Meeting, San Francisco, 2019
References
- 1.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):376–391. [DOI] [PubMed] [Google Scholar]
- 2.Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152(5):820–823 e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang YS, Jan RL, Weng SF, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol. 2012;154(4):645–652 e641. [DOI] [PubMed] [Google Scholar]
- 4.Park SJ, Choi NK, Yang BR, et al. Risk and Risk Periods for Stroke and Acute Myocardial Infarction in Patients with Central Retinal Artery Occlusion. Ophthalmology. 2015;122(11):2336–2343 e2332. [DOI] [PubMed] [Google Scholar]
- 5.Helenius J, Arsava EM, Goldstein JN, et al. Concurrent acute brain infarcts in patients with monocular visual loss. Ann Neurol. 2012;72(2):286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauda F, Neugebauer H, Reiber L, Juttler E. Acute Silent Brain Infarction in Monocular Visual Loss of Ischemic Origin. Cerebrovasc Dis. 2015;40(3–4):151–156. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Kim SW, Lee SC, Kwon OW, Kim YD, Byeon SH. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol. 2014;157(6):1231–1238. [DOI] [PubMed] [Google Scholar]
- 8.Biousse V, Nahab F, Newman NJ. Management of Acute Retinal Ischemia: Follow the Guidelines! Ophthalmology. 2018;125(10):1597–1607. [DOI] [PubMed] [Google Scholar]
- 9.Olsen TW, Pulido JS, Folk JC, Hyman L, Flaxel CJ, Adelman RA Retinal and ophthalmic artery occlusions preferred practice pattern. 2016:120–143. [DOI] [PubMed] [Google Scholar]
- 10.Stuart A. CRAO: Harbinger of Ischemic Stroke. In. EyeNet Magazine. 2016:29–31. [Google Scholar]
- 11.Chodnicki KD, Pulido JS, Hodge DO, Klaas JP, Chen JJ. Stroke Risk Before and After Central Retinal Artery Occlusion in a US Cohort. Mayo Clin Proc. 2019;94(2):236–241. [DOI] [PubMed] [Google Scholar]
- 12.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. [DOI] [PubMed] [Google Scholar]
- 14.Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27(3):373–380. [PubMed] [Google Scholar]
- 15.Hayreh SS, Zimmerman MB. Ocular Arterial Occlusive Disorders and Carotid Artery Disease. Ophthalmol Retina. 2017;1(1):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavin P, Patrylo M, Hollar M, Espaillat KB, Kirshner H, Schrag M. Stroke Risk and Risk Factors in Patients With Central Retinal Artery Occlusion. Am J Ophthalmol. 2018;196:96–100. [DOI] [PubMed] [Google Scholar]
- 17.French DD, Margo CE, Greenberg PB. Ischemic Stroke Risk in Medicare Beneficiaries with Central Retinal Artery Occlusion: A Retrospective Cohort Study. Ophthalmol Ther. 2018;7(1):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laczynski DJ, Gallop J, Lyden SP, et al. Retinal artery occlusion does not portend an increased risk of stroke. J Vasc Surg. 2020;72(1):198–203. [DOI] [PubMed] [Google Scholar]
- 19.Mac Grory BSM, Biousse V, Furie KL, Gerhard-Herman M, Lavin PJ, Sobrin L, Tjoumakaris SI, Weyand CM, Yaghi S; on behalf of the American Heart Association Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; and Council on Peripheral Vascular Disease. Management of central retinal artery occlusion: a scientific statement from the American Heart Association. Stroke. 2021;52:e1–e13. [DOI] [PubMed] [Google Scholar]
- 20.Lovett JK, Dennis MS, Sandercock PA, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke. 2003;34(8):e138–140. [DOI] [PubMed] [Google Scholar]
- 21.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432–1442. [DOI] [PubMed] [Google Scholar]


