Abstract
Dementia and mortality rates rise inexorably with age and consequently interact. However, because of the major logistical difficulties in accounting for both outcomes in a defined population, very little work has examined how risk factors and biomarkers for incident dementia are influenced by competing mortality. The objective of this study was to examine long-term associations between amyloid PET, APOE ɛ4, sex, education and cardiovascular/metabolic conditions, and hazard and absolute risk of dementia and mortality in individuals without dementia at enrolment. Participants were enrolled in the Mayo Clinic Study of Aging, a population-based study of cognitive ageing in Olmsted County, MN, USA. All were without dementia and were age 55–92 years at enrolment and were followed longitudinally. Predictor variables were amyloid PET, APOE ɛ4 status, sex, education, cardiovascular/metabolic conditions and age. The main outcomes were incident dementia and mortality. Multivariable, multi-state models were used to estimate mortality and incident dementia rates and absolute risk of dementia and mortality by predictor variable group. Of the 4984 participants in the study, 4336 (87%) were cognitively unimpaired and 648 (13%) had mild cognitive impairment at enrolment. The median age at enrolment was 75 years; 2463 (49%) were women. The median follow-up time was 9.4 years (7.5 years after PET). High versus normal amyloid (hazard ratio 2.11, 95% confidence interval 1.43–2.79), APOE ɛ4 (women: hazard ratio 2.24, 95% confidence interval 1.80–2.77; men: hazard ratio 1.37, 95% confidence interval 1.09–1.71), older age and two additional cardiovascular/metabolic conditions (hazard ratio 1.37, 95% confidence interval 1.22–1.53) were associated with the increased hazard of dementia (all P < 0.001). Among APOE ɛ4 carriers with elevated amyloid, remaining lifetime risk of dementia at age 65 years was greater in women [74% (95% confidence interval 65–84%) high and 58% (95% confidence interval 52–65%) moderate amyloid], than men [62% (95% confidence interval 52–73%) high and 44% (95% confidence interval 35–53%) moderate amyloid]. Overall, the hazard and absolute risk of dementia varied considerably by predictor group. The absolute risk of dementia associated with predictors characteristic of Alzheimer’s disease was greater in women than men while at the same time the combination of APOE ɛ4 non-carrier with normal amyloid was more protective in women than men. This set of findings may be attributed in part to different biological effects and in part to lower mortality rates in women.
Keywords: amyloid PET, dementia, mortality, APOE, sex
The objective of this study was to examine long-term associations between amyloid PET, APOE ɛ4, sex, education and cardiovascular/metabolic conditions, and hazard and absolute risk of dementia and mortality in individuals without dementia at enrolment. Overall, the hazard and absolute risk of dementia varied considerably by predictor group.
Graphical Abstract
Introduction
Dementia in elderly individuals is typically due to combinations of ageing-related brain pathologies which often, but not necessarily, include Alzheimer’s disease.1–3 Alzheimer’s disease is defined by the presence of both β-amyloid plaques and tau neurofibrillary tangles,4 which can be ascertained in vivo by PET imaging or biofluid biomarkers.5
The objective of this study was to examine the long-term relationships between amyloid PET, APOE, sex, education and cardiovascular/metabolic conditions (CMC), and two clinically meaningful outcomes—incident dementia and mortality. Prior work has demonstrated that the predictor variables we evaluated—amyloid PET, APOE, age, sex, education and CMC—are related to the risk of dementia.6–15 These predictors were accurately captured in this study cohort.
Because both incident dementia and mortality increase with advancing age, failure to account for the competing risk of death impacts interpretation of the effects of risk factors and biomarkers on dementia incidence. The Mayo Clinic Study of Aging (MCSA) is a longitudinal observational study that is uniquely positioned to address this.
First, we were able to capture the two primary outcomes in both active participants and those who discontinued in person follow-up visits. In the latter group, clinical status could be determined by review of their medical records, owing to the unique design of the Rochester Epidemiology Project. Longer observation periods lead to greater cumulative withdrawal which in turn introduces greater selection bias in the remaining cohort. Therefore, this unique feature mitigated selection bias.16
Second, because we ascertained both incident dementia and death in the same defined population, we were able to calculate absolute risks for dementia, which are more interpretable, and more relevant to patients, than relative rates [i.e. hazard ratios (HRs)], the norm in Alzheimer’s biomarkers studies. Further, we applied analytic methods—multivariable, multi-state models—that are unique in their ability to portray competing outcomes.
A third distinguishing feature was a long-term follow-up after enrolment (median 9.4 years overall, 7.5 years post amyloid PET, maximum 15.7 years). Much of the existing literature relating amyloid PET to clinical outcomes in individuals without dementia has focused on cognitive change over short-to-medium observation periods (∼1–5 years).7,17–27 However, neuropathological changes leading to dementia evolve slowly8 and long-term follow-up is needed to fully capture associations between outcomes and upstream predictor variables.
Materials and methods
Participants
This study was approved by the Mayo Clinic and the Olmsted Medical Center Institutional Review Boards. All participants provided written informed consent.
All study participants were enrolled in the MCSA, a population-based epidemiological cognitive ageing study among a stratified random sample of a geographically defined population, Olmsted County, MN, USA (see Supplementary material).28 A clinical diagnosis was determined for each participant at enrolment and for all subsequent visits using clinical criteria alone.28 All participants in the present study were 55 years of age or older and without dementia at enrolment, including both mild cognitive impairment (MCI)29 and cognitively unimpaired (defined as not MCI and without dementia)30 participants.
Predictor variables
Predictor variables were age, sex, education, APOE genotype, the first available amyloid PET and the first available composite CMC score. CMC score is the sum of the presence or absence of seven vascular-health-related conditions. A higher score indicates worse cardiometabolic health (see Supplementary material).31,32 All participants had an initial study visit including at minimum a clinical evaluation and blood draw between November 2004 and September 2020. MCSA participants without a medical contraindication were invited to participate in imaging studies.
Amyloid PET imaging was performed with Pittsburgh Compound B33 using previously described methods (see Supplementary material).34 The continuous range of amyloid PET values was divided into normal, moderately elevated (referred to as moderate) and highly elevated (referred to as high) ranges on the Centiloid scale.35,36 The cut point separating normal and moderate amyloid was Centiloid 22 [standardized uptake value ratio (SUVR) 1.48] which is the value beyond which rates of amyloid PET reliably increase.34 The value separating moderate from high amyloid was Centiloid 68 (SUVR 2.0) which corresponds to the global maxima of the amyloid PET SUVR by delta amyloid curve.37,38
Outcomes
The two main outcomes were incident dementia, which was based on DSM IV criteria,30 and mortality. Participants were followed from enrolment through all MCSA visits until an event or censoring occurred (both incident dementia and mortality occurred in some individuals). Outcomes in study participants who had previously discontinued in person study follow-up visits were ascertained through semiannual reviews of the electronic medical record39 using the Rochester Epidemiology Project medical records-linkage system (see Supplementary material).40
Statistical analysis
Overall death and incident dementia rates by age and sex
This analysis was performed to facilitate comparisons with epidemiological literature and was based on a person-years analysis, using Poisson models to calculate confidence intervals (CIs) and P-values.41
Multi-state model
The primary analysis was based on a multi-state intensity model,42 illustrated in Fig. 1A. All participants started in the without dementia state, and could undergo transitions directly to dementia or death, or undergo sequential transitions to dementia and then to death during follow-up. We used age as the time scale for modelling which seemed more clinically relevant than time in study (see Supplementary material). The multi-state model is parameterized by the transition rates between each pair of states, the estimated future probability of being in each state at each age based on a set of initial conditions and the remaining lifetime risk of ever experiencing dementia.
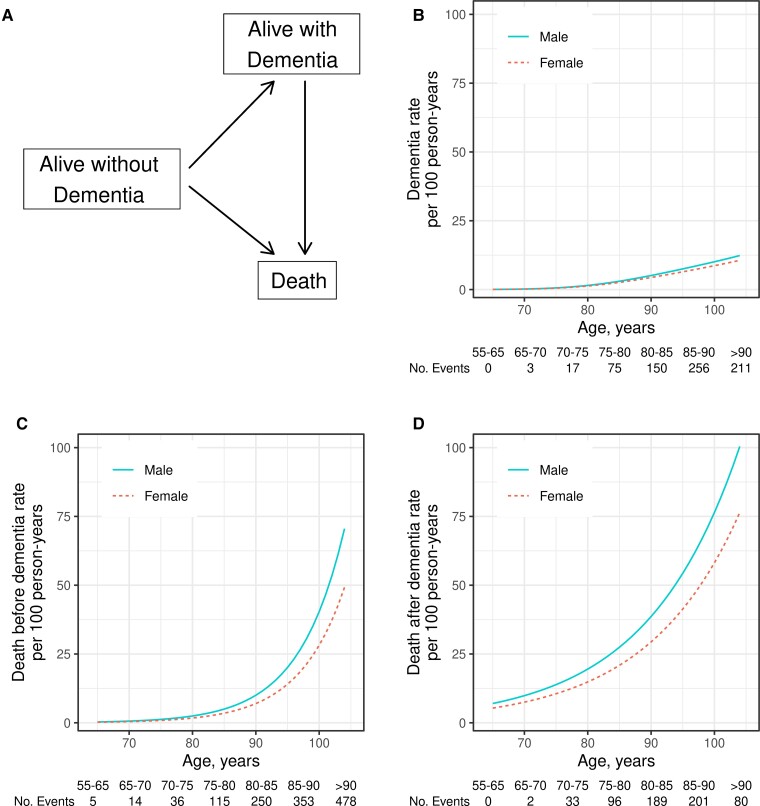
Figure 1.
Multi-state transition model and numbers of mortality and incident dementia events per 100 person-years by sex and age group. (A) Multi-state transition model. The three states in this model are denoted by boxes: alive without dementia, alive with dementia and deceased. The three possible transition paths are denoted by arrows: progression from without dementia to dementia, from without dementia to death and from dementia to death. (B–D) Estimated event rates for each transition type by continuous-age separately for men and women; (B) incident dementia events per 100 person-years by sex and age, (C) death events without dementia per 100 person-years by sex and age and (D) death events with dementia per 100 person-years by sex and age. In the tables below the plots in (B–D), we show actual event counts by age bin. In (B-D), males are denoted by a solid blue line and females by a dashed red line. Estimates are based on the person-years analysis using Poisson models.
HRs of mortality and incident dementia associated with predictor variables
Each of the three transition rates (represented by the arrows in Fig. 1A) is represented by a separate intensity model, equivalent to a Cox model focused on the end-point of the arrow; HRs and CIs can be interpreted in the same way as a Cox model. Each of the three models included the predictor variables sex, education, APOE status (ɛ4 carrier versus non-carrier), amyloid PET group (normal, moderate, high) and CMC score. Two-way interactions were examined for each of the three transitions (see Supplementary material). For the without dementia to dementia transition, the model included the two interactions with sex that were significant (APOE × sex and amyloid PET × sex). Therefore, we summarize results in the resulting 12 sex × APOE × amyloid predictor variable groups. HRs and CIs for these variables are reported using the overall study group as the reference, i.e. how each group differs from the overall mean (see Supplementary material). Tests for the comparison of two HR estimates were based on a Wald test.
Absolute risk predictions
For an individual without dementia at a given age with certain predictor variables, the fitted rate model also leads to estimates of the probability of being in each of the three states at a future age (i.e. predicted state curves) and the probability of ever passing through the dementia state before death (i.e. remaining lifetime risk). Tests comparing two predicted state curves were based on the area under the curve with jackknife standard errors. Tests for the comparison of two remaining lifetime risk estimates were based on a jackknife standard error.
Data availability
The MCSA makes data available to qualified researchers via an online request form at https://ras-rdrs.mayo.edu/Request/IndexRequest.
Results
Demographics
Of the 4984 participants in the study, 4336 (87%) were diagnosed as cognitively unimpaired and 648 (13%) as MCI at enrolment (Table 1). The participants had a median age at enrolment of 75 years [inter-quartile range (IQR) 69–81], median education of 14 years (IQR 12–16) and median CMC score of 2 (IQR 1–3) (Table 1 and Supplementary Table 1A); 2463 (49%) were women, 1342 (27%) were APOE ɛ4 carriers and 1786 (36%) underwent amyloid PET imaging. Age, education and diagnosis are also shown by sex, APOE ɛ4 and amyloid PET level in Supplementary Table 1B. In the subset with amyloid PET, the median age was 5 years younger; and, a slightly smaller proportion was MCI at enrolment (10 versus 15%). This subset was otherwise like those that did not undergo amyloid PET (Table 1). The median follow-up time was 9.4 years (maximum 15.7) from enrolment, and 7.5 years from the first available PET.
Table 1.
Demographics
| Overall (N = 4984) | Subset with amyloid PET (N = 1786) | Subset without amyloid PET (N = 3198) | |
|---|---|---|---|
| Diagnosis, no. (%) | |||
| CU | 4336 (87%) | 1604 (90%) | 2732 (85%) |
| MCI | 648 (13%) | 182 (10%) | 466 (15%) |
| Age, years | |||
| Median (Q1, Q3) | 75 (69, 81) | 72 (65, 77) | 77 (72, 83) |
| Range | 55–92 | 55–90 | 55–92 |
| Sex, no. (%) | |||
| Male | 2521 (51%) | 955 (53%) | 1566 (49%) |
| Female | 2463 (49%) | 831 (47%) | 1632 (51%) |
| Education, years | |||
| Median (Q1, Q3) | 14 (12, 16) | 14 (12, 16) | 14 (12, 16) |
| APOE ɛ4 genotype, no. (%) | |||
| Carrier | 1342 (27%) | 510 (29%) | 832 (26%) |
| Non-carrier | 3642 (73%) | 1276 (71%) | 2366 (74%) |
| CMC a | |||
| Median (Q1, Q3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) |
| Range | 0–7 | 0–7 | 0–7 |
| Centiloid group at initial amyloid PET, no. (%) | |||
| Highly elevated (68+) | 241 (13%) | ||
| Moderately elevated (22–68) | 350 (20%) | ||
| Normal (<22) | 1195 (67%) | ||
| Total dementia events by years after enrolment, no. (%) | |||
| 0–5 | 278 (39%) | 57 (31%) | 221 (42%) |
| 5–10 | 326 (46%) | 95 (51%) | 231 (44%) |
| 10+ | 108 (15%) | 34 (18%) | 74 (14%) |
| Total death events by years after enrolment, no. (%) | |||
| 0–5 | 493 (27%) | 54 (14%) | 439 (30%) |
| 5–10 | 843 (46%) | 191 (51%) | 652 (44%) |
| 10+ | 516 (28%) | 130 (35%) | 386 (26%) |
| Follow-up, years b | |||
| Median (95% CI) | 9.4 (8.9, 9.7) | 8.2 (8.1, 8.3) | 10.4 (10.1, 11.0) |
Median (Q1, Q3) refers to the median, first and third quartile.
CU, cognitively unimpaired; MCI, mild cognitive impairment; CMC, cardiovascular/metabolic conditions.
CMC score is the sum of the presence or absence of seven vascular-health-related conditions. A higher score indicates worse cardiometabolic health.
Median follow-up was estimated using the reverse Kaplan–Meier (see Supplementary material).
Overall incident dementia and mortality rates by age and sex
Mortality rates, both with and without dementia, increased exponentially with age and were higher in men than women, 1.31-fold (95% CI 1.12–1.54) for those without dementia and 1.43-fold (95% CI 1.28–1.60) for those with dementia (Fig. 1 and Supplementary Fig. 1). Rates of incident dementia increased exponentially with age and were 16% greater in men than women (95% CI 1.01–1.35).
Of the 712 incident dementia events and the 1852 deaths, 418 (59%) and 1066 (58%), respectively, occurred in participants who had previously discontinued in person study follow-up visits and thus were identified through medical record abstraction. Additionally, 434 (61%) incident dementia events and 1359 (73%) deaths occurred 5 or more years after enrolment.
Associations between hazards and different predictor variables
Using the overall study population average as the reference, the HRs associated with different predictor variables are shown in Fig. 2 and Supplementary Table 2 for the three possible state-to-state transitions. Two-way interactions were examined for each of the three transitions and only sex × amyloid and sex × APOE for the without dementia to dementia transition were significant (see Supplementary material).
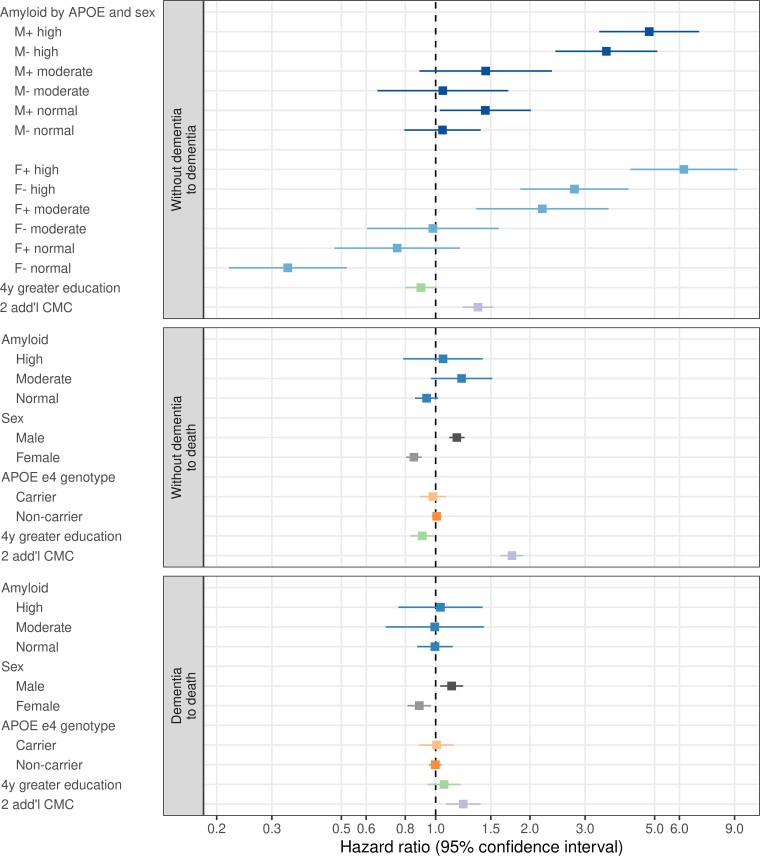
Figure 2.
HRs for state-to-state transitions associated with different subgroups. Three state-to-state transitions are illustrated: progression from without dementia to dementia, from without dementia to death and from dementia to death. We summarize the association between predictor variables and incidence rates in our multi-state model in the form of HRs where the reference group is the overall study population average for the amyloid, sex and APOE variables. This presentation allows for direct comparison of the rate in a predictor variable subgroup compared to the population average and allows visual comparisons of all possible pairwise comparisons since each HR estimate in the figure shares a common anchor point. The HRs for education and CMC are shown for a specified contrast. + and − symbols in the top panel represent APOE ɛ4 status: + refers to carrier, −refers to non-carrier. HRs estimates are from the multi-state intensity model.
Without dementia to dementia transitions
Among women, the hazard of incident dementia increased monotonically with increasing amyloid level; 2.91 (95% CI 1.37–6.17) for moderate versus normal amyloid and 2.83 (95% CI 1.61–4.99) for high versus moderate amyloid. Among men, the hazards of incident dementia were similar for moderate versus normal amyloid (HR 1.00, 95% CI 0.57–1.76), but were greater for high versus moderate amyloid (HR 3.33, 95% CI 1.92–5.75). Averaging across men, women and APOE, the HR associated with high versus normal amyloid was 2.11 (95% CI 1.43–2.79).
Within each amyloid group, the hazard of incident dementia was higher for APOE ɛ4 carriers than non-carriers for women (HR 2.24, 95% CI 1.80–2.77) and men (HR 1.37, 95% CI 1.09–1.71). The APOE ɛ4 effect was 1.63 (95% CI 1.20–2.23) times greater for women than men, averaged over all three amyloid groups.
Two additional CMC increased the hazard of incident dementia by a factor of 1.37 (95% CI 1.22–1.53). While greater education was mildly protective, the association was not significant.
Without dementia to death
The hazard of death among those without dementia was not associated with amyloid level or APOE ɛ4 status but was higher in men than women (HR 1.37, 95% CI 1.22–1.54). More education was slightly protective (HR 0.90, 95% CI 0.83–0.99). Two additional CMC increased the hazard by 1.75 (95% CI 1.61–1.91).
Dementia to death
The hazard of death among those with dementia was not associated with amyloid level, APOE ɛ4 status or education but was higher in men than women (HR 1.27, 95% CI 1.07–1.51). Two additional CMC increased the hazard by 1.22 (95% CI 1.08–1.39).
Absolute risk
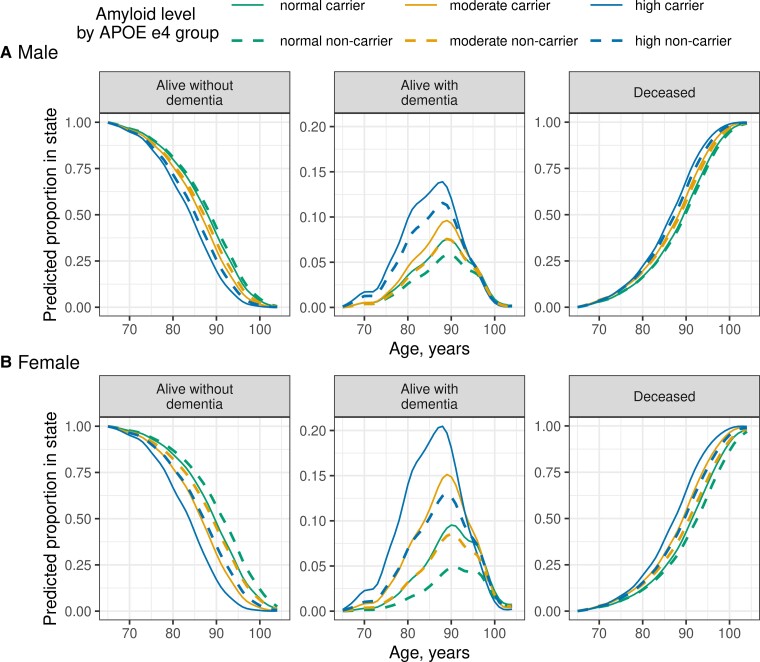
In Fig. 3, we show predicted state (alive without dementia, alive with dementia or deceased) by age for 12 groups defined by combinations of amyloid PET, sex and APOE based on an exemplar cohort of individuals without dementia at age 65. Figure 3 graphically illustrates associations between different predictor variables and the competing risks of death and dementia. Predicted average time with dementia (equivalent to area under the curve) varied considerably across the 12 predictor variable groups from a maximum of 3.35 (95% CI 2.64–4.06) years in APOE ɛ4 carrier high amyloid women to 0.75 (95% CI 0.51–0.99) years in APOE ɛ4 non-carrier normal amyloid women (Supplementary Table 3).
Figure 3.
Absolute predictions by age associated with different predictor variable subgroups. The two rows illustrate different risk predictor variable subgroups, amyloid PET level by APOE ɛ4 group among (A) men and (B) women. Other predictor variable effects were weighted to the frequencies observed in the overall study population. Amyloid PET level is indicated by colour (green = normal, gold = moderate, blue = high) while line type indicates APOE e4 group (solid = carrier, dashed = non-carrier). The columns are arranged by the three possible states in the multi-state model illustrated in Fig. 1A: alive without dementia, alive with dementia or deceased. The columns are also arranged from left to right to reflect group-level change over time in a cohort beginning at age 65 years: progression from the alive without dementia category into the alive with dementia or the deceased category. The y-axis scale of the middle column (i.e. predicted proportion of original cohort alive with dementia) is limited to a smaller range compared to the other columns to better show the alive with dementia curves. Estimates are based on predicted state curves obtained from the multi-state intensity model.
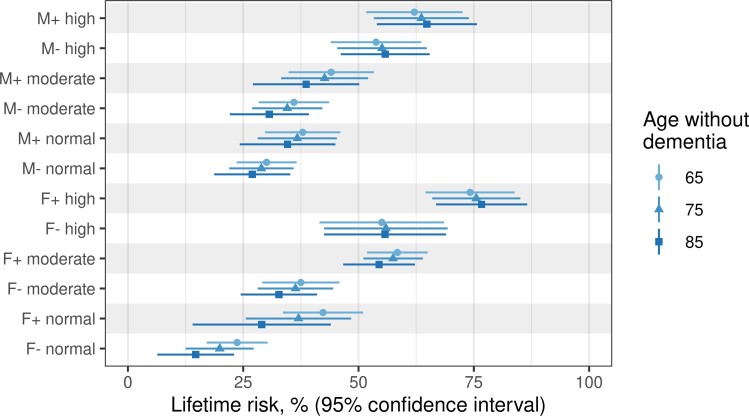
Figure 4 illustrates the estimated remaining lifetime risk of experiencing dementia for groups defined by combinations of amyloid PET, sex and APOE who were alive and without dementia at 65, 75 and 85 years. The remaining lifetime risk was constant across different starting ages for high amyloid men and women but declined slightly with advancing age for lower risk groups (Fig. 4 and Supplementary Table 4). Patterns of remaining lifetime risk of dementia varied considerably by APOE/sex/amyloid group (Fig. 4) and mirrored those for HRs (Fig. 2). However, the remaining lifetime risk was greater for women than men in the predictor variable groups most characteristic of Alzheimer’s disease. For example, among APOE ɛ4 carriers with moderate amyloid levels, the remaining lifetime risk of dementia at age 65 years was 58% (95% CI 52–65%) for women compared to 44% (95% CI 35–53%) among men. Among APOE ɛ4 carriers with high levels of amyloid, the risk increased to 74% (95% CI 65–84%) for women compared to 62% (95% CI 52–73%) for men (Supplementary Table 4).
Figure 4.
Remaining lifetime risk of dementia by sex, APOE genotype, and amyloid group for a person without dementia at starting ages 65, 75 or 85 years. The remaining lifetime risk for all 12 combinations of amyloid group, sex and APOE ɛ4 comes from the competing risks model and is averaged over all the combinations of education and CMC. The standard deviation for the remaining lifetime risk was computed using a grouped jackknife with 20 groups. Estimates are based on remaining lifetime risk estimates obtained from the multi-state intensity model.
Sensitivity analyses
We performed sensitivity analyses of HRs and lifetime risk (referenced to Figs 2 and 4, respectively, in the main analyses) in the subset of individuals with amyloid PET. As expected due to larger sample size, the CIs were narrower for non-amyloid PET covariates in the full sample. There were no important differences in either the HRs (Supplementary Fig. 2) or the lifetime risk estimates (Supplementary Fig. 3) when the subset with amyloid PET was compared with the full sample—i.e. CIs overlap considerably for each variable between the two sample sets.
Discussion
Most Alzheimer’s biomarker studies have focused on relative hazards (i.e. HRs, or relative rates); however, here we also estimated the absolute risk of dementia (Figs 3 and 4). HRs represent the ratio of rates of an event in individuals with a predictor variable pattern relative to a reference group. While informative, they do not provide individuals with probabilistic estimates of the likelihood that they will experience an outcome. In contrast, the remaining lifetime risk reflects how likely an individual with a predictor variable pattern is to experience the event in their remaining lifetime. To our knowledge, only one prior Alzheimer’s biomarker study9 has addressed remaining lifetime risk, and none have done so within a defined population.
Figure 3 illustrates the importance of the competing risk of mortality on the absolute risk of dementia. Knowledge of both dementia and death rates is needed to estimate the remaining lifetime risk of dementia.9,43–45 Prior studies examining age and sex alone as predictors most often observed that the remaining lifetime risk of incident dementia was constant or declined modestly with increasing age in both sexes.43–45 We examined remaining lifetime risk associated with various predictor variables beyond age and sex and found that remaining lifetime risk remained relatively constant across different starting ages for the highest risk groups, particularly those with high amyloid, but declined slightly with older starting age for lower risk groups (Fig. 4 and Supplementary Table 4). A likely explanation is that among persons with high amyloid, older individuals are as likely to experience dementia before death as younger individuals. In contrast, among persons with normal amyloid, older individuals are more likely to die without dementia than younger individuals because younger individuals have greater opportunity to develop abnormal amyloid and dementia in their remaining lifetime.
While absolute risk estimates are more clinically meaningful, it is also useful to compare HRs (i.e. relative rates) from our study with prior epidemiological literature. Therefore, we first estimated overall incident dementia and death rates by age and sex alone. Reported dementia incidence rates vary46,47; however, consistently reported findings include exponential increases in mortality and dementia rates with age, and higher mortality rates in men than women.46–48 We found slightly greater dementia incidence rates in men than women overall (Fig. 1 and Supplementary Fig. 1) which is consistent with most studies conducted in the USA, but not with all studies from other countries.10,46,47,49–54 Consistent with prior literature,55 we also found that mortality rates were higher in those with dementia prior to death versus those without dementia.
HRs of incident dementia varied considerably by predictor variable group (Fig. 2 and Supplementary Table 2). Higher amyloid levels and APOE ɛ4 most strongly increased the hazard of incident dementia in women and men. This was anticipated based on prior work showing both higher levels of amyloid PET7,11,12,17–24,56–61 and APOE ɛ413,24,57,62–69 are associated with cognitive decline and dementia. More interesting were the complex relationships between sex and both amyloid and APOE ɛ4.
While not replicated universally,10,14,70 some studies have identified a stronger association between APOE ɛ4 and dementia or cognitive decline in women than men.15,27,71 However, β-amyloid and APOE ɛ4 are closely related to each other. APOE ɛ4 increases the likelihood of and lowers the age of onset of both amyloidosis and dementia.13,72,73 To gain more comprehensive understanding, it is necessary to examine the separate sex × amyloid and sex × APOE ɛ4 interactions. Doing so, we found that APOE ɛ4 had a stronger association with incident dementia in women than men across all amyloid levels. At the same time, the combination of APOE ɛ4 non-carrier with low amyloid was selectively more protective in women than men (Fig. 2 and Supplementary Table 2). This set of findings implies different biological effects in women versus men.15,27,71,74,75
We also found a sex difference in the relationship between amyloid PET level and the hazard of incident dementia. Among women the relationship was a monotonic increase; in contrast, normal and moderate amyloid men had nearly equal hazards (Fig. 2 and Supplementary Table 2). One possible explanation for different associations by sex may be that a normal amyloid level is not as protective against dementia in men as in women. Dementia in elderly persons is typically not due to Alzheimer’s disease alone but rather due to more than one disease process.1–3 Both vascular risk factors76 and Lewy body disease77,78 are more common in men.
Of the predictor variables examined only male sex, less education and increased CMC were associated with an increased hazard of mortality. Prior studies report mixed conclusions on the association between APOE ɛ4 and mortality.63,79–81 It may seem counterintuitive that the two predictor variables in our model that are characteristic of Alzheimer’s disease, APOE ɛ4 and β-amyloid, had no direct association with the hazard of mortality (nor were their interactions with sex significant for mortality) (Fig. 2 and Supplementary Table 2). One possible explanation is that while predictor variables characteristic of Alzheimer’s disease increase the hazard of dementia, once an individual has dementia, mortality rates are not highly dependent on the specific aetiology.
The fact that higher amyloid PET levels increased the hazard, and more importantly, the remaining lifetime risk of incident dementia (Figs 2–4 and Supplementary Tables 2–4) is relevant to current Alzheimer’s disease clinical trials which often target β-amyloid.82,83 Monoclonal antibodies that target fibrillar forms of β-amyloid can effectively decrease amyloid load.84,85 While our data are observational and therefore cannot prove that removing amyloid would reduce incidence rates or absolute risk of dementia, the results show that those with higher amyloid progress to dementia at faster rates and have significantly higher lifetime risk for dementia than those with normal levels. At present, anti-amyloid clinical trials require an abnormal amyloid biomarker study for inclusion. The fact that hazard and remaining lifetime risk of incident dementia varied dramatically by the subgroups examined suggests that it might be useful to take a more granular approach to inclusion and stratification based on combinations of sex, APOE ɛ4 and amyloid level.
The US Food and Drug Administration recently approved aducanumab for individuals in the MCI or mild dementia phases of Alzheimer’s disease. Most individuals in this study were cognitively unimpaired at baseline and therefore results of this study are only somewhat relevant to current clinical care considerations. Information in this study nonetheless has practical clinical value in life planning for elderly individuals without dementia, value for assessing the utility of combined biomarker and genetic screening of individuals without dementia, and value for assessing the potential public health impact of interventions.
This study has some limitations. Including the entire MCSA cohort over age 55 years rather than only those with amyloid PET studies allowed us to strengthen estimates of the associations between other predictor variables and outcomes as shown in the sensitivity analyses (Supplementary Figs 2 and 3). However, only 36% of participants underwent amyloid PET which is not ideal.
This cohort is from a population-based sample and so by design reflects the de facto demographics of Olmsted County, MN, USA of which the majority is non-Hispanic White. Results may differ in populations with different patterns of social determinants of health86–88; however, the predictor variables examined in this study do exist in all populations.
Ascertainment of amyloidosis was based on amyloid PET which may not be available in some settings. However, recent reports indicate high correlation between amyloid PET and plasma biomarkers.89–91 Future work should assess if similar associations are found between plasma biomarkers and the absolute risk of dementia reported here with amyloid PET.
Supplementary Material
Acknowledgements
The Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic.
Glossary
Abbreviations
- CMC =
cardiovascular/metabolic conditions
- CI =
confidence interval
- HR =
hazard ratio
- MCSA =
Mayo Clinic Study of Aging
- MCI =
mild cognitive impairment
- SUVR =
standardized uptake value ratio
Funding
Funding was provided by the National Institutes of Health (R37 AG011378, RO1 AG041851, R01 AG056366, R01 NS097495, U01 AG06786, R01 AG034676), the GHR Foundation. Funders had no role in design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript and decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
C.R.J. has served as a consultant for Eisai and Biogen and serves on an independent data monitoring board for Roche but he receives no personal compensation from any commercial entity; receives funding from the NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. T.M.T. reports no disclosures. E.S.L. reports no disclosures. H.J.W. reports no disclosures. M.M.M. receives research support from the NIH and DOD and has consulted for Biogen, Brain Protection Company and LabCorp. D.S.K. serves on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety monitoring Board for a tau therapeutic for Biogen but receives no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California. He has served as a consultant for Roche, Samus Therapeutics, Magellan Health and Alzeca Biosciences but receives no personal compensation. He receives funding from the NIH. J.G.-R. receives funding from the NIH. V.J.L. consults for Bayer Schering Pharma, Piramal Life Sciences, Eisai, Inc., AVID Radiopharmaceuticals and Merck Research, and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI). P.V. receives funding from the NIH. C.G.S. receives funding from the NIH. M.L.S. at the time of manuscript submission, owned shares of the following medical-related stocks, unrelated to the current work: Align Technology, Inc., LHC Group, Inc., Mesa Laboratories, Inc., Natus Medical Incorporated, Varex Imaging Corporation. J.L.G. reports no disclosures. R.C.P. has consulted for Roche, Inc.; Merck, Inc.; Biogen, Inc. and Eisai, Inc.; a DSMB for Genentech, Inc. and receives royalties from Oxford University Press for Mild Cognitive Impairment and from UpToDate. His research funding is from NIH/NIA.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Power MC, Mormino E, Soldan A, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84(1):10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85(6):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20(1):66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging – Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-beta PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 7. Rowe CC, Bourgeat P, Ellis KA, et al. Predicting Alzheimer disease with beta-amyloid imaging: Results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol. 2013;74(6):905–913. [DOI] [PubMed] [Google Scholar]
- 8. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013;12(4):357–367. [DOI] [PubMed] [Google Scholar]
- 9. Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018;14:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolk DA, Sadowsky C, Safirstein B, et al. Use of flutemetamol F 18-labeled positron emission tomography and other biomarkers to assess risk of clinical progression in patients with amnestic mild cognitive impairment. JAMA Neurol. 2018;75(9):1114–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez OL, Klunk WE, Mathis C, et al. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology. 2014;83(20):1804–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts. PLoS Med. 2017;14(3):e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsiung GY, Sadovnick AD, Feldman H. Apolipoprotein E epsilon4 genotype as a risk factor for cognitive decline and dementia: Data from the Canadian Study of Health and Aging. CMAJ. 2004;171(8):863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carone M, Asgharian M, Jewell NP. Estimating the lifetime risk of dementia in the Canadian elderly population using cross-sectional cohort survival data. J Am Stat Assoc. 2014;109(505):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donohue MC, Sperling RA, Petersen R, et al. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA. 2017;317(22):2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knopman DS, Lundt ES, Therneau TM, et al. Entorhinal cortex tau, amyloid-beta, cortical thickness and memory performance in non-demented subjects. Brain. 2019;142(4):1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen RC, Wiste HJ, Weigand SD, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80(19):1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bertens D, Tijms BM, Vermunt L, Prins ND, Scheltens P, Visser PJ. The effect of diagnostic criteria on outcome measures in preclinical and prodromal Alzheimer’s disease: Implications for trial design. Alzheimers Dement. 2017;3(4):513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mormino EC, Papp KV, Rentz DM, et al. Early and late change on the preclinical Alzheimer’s cognitive composite in clinically normal older individuals with elevated amyloid beta. Alzheimers Dement. 2017;13(9):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lim YY, Kalinowski P, Pietrzak RH, et al. Association of beta-amyloid and apolipoprotein E epsilon4 with memory decline in preclinical Alzheimer disease. JAMA Neurol. 2018;75(4):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubois B, Epelbaum S, Nyasse F, et al. Cognitive and neuroimaging features and brain beta-amyloidosis in individuals at risk of Alzheimer’s disease (INSIGHT-preAD): A longitudinal observational study. Lancet Neurol. 2018;17(4):335–346. [DOI] [PubMed] [Google Scholar]
- 26. Farrell ME, Kennedy KM, Rodrigue KM, et al. Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: Evidence for a dose–response relationship. JAMA Neurol. 2017;74(7):830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOE ɛ4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement. 2018;14(9):1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic study of aging: Design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 30. Diagnostic and Statistical Manual of Mental Disorders, DSM-IV. 4th edn.American Psychiatric Association; 1994. [Google Scholar]
- 31. Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: Patterns by age, sex, and race/ethnicity. Mayo Clin Proc. 2014;89(10):1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vemuri P, Lesnick TG, Przybelski SA, et al. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol. 2017;82(5):706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. [DOI] [PubMed] [Google Scholar]
- 34. Jack CR, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13(3):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwarz CG, Tosakulwong N, Senjem ML, et al. Considerations for performing level-2 centiloid transformations for amyloid PET SUVR values. Sci Rep. 2018;8(1):7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jack CR Jr, Wiste HJ, Lesnick TG, et al. Brain beta-amyloid load approaches a plateau. Neurology 2013;80(10):890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Knopman DS, Lundt ES, Therneau TM, et al. Alzheimer's disease neuroimaging initiative. Association of initial beta-amyloid levels with subsequent flortaucipir positron emission tomography changes in persons without cognitive impairment. JAMA Neurol. 2021;78(2):217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knopman DS, Petersen RC, Rocca WA, Larson EB, Ganguli M. Passive case-finding for Alzheimer’s disease and dementia in two U.S. communities. Alzheimers Dement. 2011;7(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, Rocca WA. Generalizability of epidemiological findings and public health decisions: An illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berry G. The analysis of mortality by the subject-years method. Biometrics. 1983;39(1):173–184. [PubMed] [Google Scholar]
- 42. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: Competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. [DOI] [PubMed] [Google Scholar]
- 43. Fishman E. Risk of developing dementia at older ages in the United States. Demography. 2017;54(5):1897–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498–1504. [DOI] [PubMed] [Google Scholar]
- 45. Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A.. Incidence and risk of dementia. The Rotterdam Study. Am J Epidemiol. 1998;147(6):574–580. [DOI] [PubMed] [Google Scholar]
- 46. Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimers Dement. 2008;4(5):316–323. [DOI] [PubMed] [Google Scholar]
- 47. Fiest KM, Roberts JI, Maxwell CJ, et al. The prevalence and incidence of dementia due to Alzheimer’s disease: A systematic review and meta-analysis. Can J Neurol Sci. 2016;43(Suppl 1):S51–S82. [DOI] [PubMed] [Google Scholar]
- 48. Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: The Baltimore longitudinal study of aging. Neurology. 2000;54(11):2072–2077. [DOI] [PubMed] [Google Scholar]
- 49. Plassman BL, Langa KM, McCammon RJ, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70(3):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County Study. Neurology. 2002;58(2):209–218. [DOI] [PubMed] [Google Scholar]
- 52. Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol. 2002;59(10):1589–1593. [DOI] [PubMed] [Google Scholar]
- 53. Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018;14(9):1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Niu H, Alvarez-Alvarez I, Guillen-Grima F, Aguinaga-Ontoso I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia. 2017;32(8):523–532. [DOI] [PubMed] [Google Scholar]
- 55. Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol. 2002;59(11):1764–1767. [DOI] [PubMed] [Google Scholar]
- 56. Roberts RO, Aakre JA, Kremers WK, et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 2018;75(8):970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mormino EC, Betensky RA, Hedden T, et al. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82(20):1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burnham SC, Bourgeat P, Dore V, et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: A longitudinal study. Lancet Neurol. 2016;15(10):1044–1053. [DOI] [PubMed] [Google Scholar]
- 59. Dumurgier J, Hanseeuw BJ, Hatling FB, et al. Alzheimer’s disease biomarkers and future decline in cognitive normal older adults. J Alzheimers Dis. 2017;60(4):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jansen WJ, Ossenkoppele R, Tijms BM, et al. Association of cerebral amyloid-beta aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry. 2018;75(1):84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ebenau JL, Timmers T, Wesselman LMP, et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology. 2020;95(1):e46–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beydoun MA, Beydoun HA, Kaufman JS, et al. Apolipoprotein E epsilon4 allele interacts with sex and cognitive status to influence all-cause and cause-specific mortality in U.S. older adults. J Am Geriatr Soc. 2013;61(4):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: The Cache County Study. Neurology. 1999;53(2):321–331. [DOI] [PubMed] [Google Scholar]
- 65. Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195–204. [DOI] [PubMed] [Google Scholar]
- 66. Lipnicki DM, Crawford JD, Dutta R, et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: A collaborative cohort study. PLoS Med. 2017;14(3):e1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peloso GM, Beiser AS, Satizabal CL, et al. Cardiovascular health, genetic risk, and risk of dementia in the Framingham Heart Study. Neurology. 2020;95(10):e1341–e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Absolute 10-year risk of dementia by age, sex and APOE genotype: A population-based cohort study. CMAJ. 2018;190(35):E1033–E1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vermunt L, Sikkes SAM, van den Hout A, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15(7):888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. A Twin study of sex differences in genetic risk for all dementia, Alzheimer’s Disease (AD), and Non-AD Dementia. J Alzheimers Dis. 2020;76(2):539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 72. Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA. 2015;313(19):1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 74. Davis EJ, Broestl L, Abdulai-Saiku S, et al. A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci Transl Med. 2020;12(558):eaaz5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Koran MEI, Wagener M, Hohman TJ, Alzheimer’s Neuroimaging Initiative . Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017;11(1):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pinho-Gomes AC, Peters SAE, Thomson B, Woodward M. Sex differences in prevalence, treatment and control of cardiovascular risk factors in England. Heart. 2020;107:462–467. [DOI] [PubMed] [Google Scholar]
- 77. Ferman TJ, Aoki N, Boeve BF, et al. Subtypes of dementia with Lewy bodies are associated with alpha-synuclein and tau distribution. Neurology. 2020;95(2):e155–e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nelson PT, Schmitt FA, Jicha GA, et al. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol. 2010;257(11):1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wolters FJ, Tinga LM, Dhana K, et al. Life expectancy with and without dementia: A population-based study of dementia burden and preventive potential. Am J Epidemiol. 2019;188(2):372–381. [DOI] [PubMed] [Google Scholar]
- 80. Wolters FJ, Yang Q, Biggs ML, et al. The impact of APOE genotype on survival: Results of 38,537 participants from six population-based cohorts (E2-CHARGE). PLoS One. 2019;14(7):e0219668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lima-Costa MF, Peixoto SV, Taufer M, Moriguchi EH. Apolipoprotein e genotype does not predict 9-year all-cause mortality in Brazilian older adults: The Bambui Cohort Study. J Am Geriatr Soc. 2008;56(12):2366–2368. [DOI] [PubMed] [Google Scholar]
- 82. Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. 2019;5:272–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. [DOI] [PubMed] [Google Scholar]
- 85. Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: A phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9(4):363–372. [DOI] [PubMed] [Google Scholar]
- 86. Stokes AC, Weiss J, Lundberg DJ, et al. Estimates of the association of dementia with US mortality levels using linked survey and mortality records. JAMA Neurol. 2020;77:1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hunt JFV, Buckingham W, Kim AJ, et al. Association of neighborhood-level disadvantage with cerebral and hippocampal volume. JAMA Neurol. 2020;77(4):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement 2021;17:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26(3):379–386. [DOI] [PubMed] [Google Scholar]
- 90. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422–433. [DOI] [PubMed] [Google Scholar]
- 91. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MCSA makes data available to qualified researchers via an online request form at https://ras-rdrs.mayo.edu/Request/IndexRequest.