Abstract
We present a case of spontaneous osteomyelitis of the left ulna in a 25-year-old man. There was no history of trauma or haematogenous source identified. Bone biopsy found staphylococcus aureus on culture, sensitive to flucloxacillin, but antibiotic treatment was unsuccessful. He underwent excision of the osteomyelitic ulna and a vascularised free fibula graft (VFF graft) reconstruction of the bony defect (18 cm in length), using the ulnar artery at the wrist as recipient vessel. Six months later he was found to have radiological evidence of bony resorption at the proximal fibula-ulnar junction. He underwent resection of a 5 cm segment of the fibula flap and insertion of an antibiotic-impregnated cement spacer in preparation for the placement of bone graft as per Masquelet technique. Following bone graft placement, he united 4 months later. This case demonstrates that the Masquelet technique can be used successfully as an adjunct to VFF graft when reconstructing very long bony defects.
Abbreviations: VFF, Vascularised Free Fibula
Keywords: Vascularised free fibula graft, Osteomyelitis, Masquelet, Induced membrane
Introduction
The incidence of osteomyelitis in the general population is reported as 0.2% [1] Treatment requires debridement that leads to bone defect. The objective is to convert a septic to an aseptic environment and then reconstruct the bone that is missing. Options for defect reconstruction include VFF graft [2], Masquelet [3] and bone transport [2]. Herein, we report on a case of ulna osteomyelitis which was treated originally with a VFF graft but required subsequent application of the Masquelet technique to achieve bone restoration and limb function.
Case report
A 25year-old man was referred to our bone reconstruction unit with an 18 month-2 year history of left forearm pain. Haematological investigations revealed an elevated CRP (26) and an elevated WBC (15,420). Radiological investigations including an MRI scan revealed the presence of left ulna spontaneous osteomyelitis (Fig. 1.1). Bone biopsy revealed staphylococcus aureus on culture, sensitive to flucloxacillin, but antibiotic treatment was unsuccessful. There was no history of trauma or haematogenous source identified, but he was later found to have Lupus anticoagulant, and be heterozygous for Factor V Leiden deficiency. No other prothrombotic state was identified.
Fig. 1.
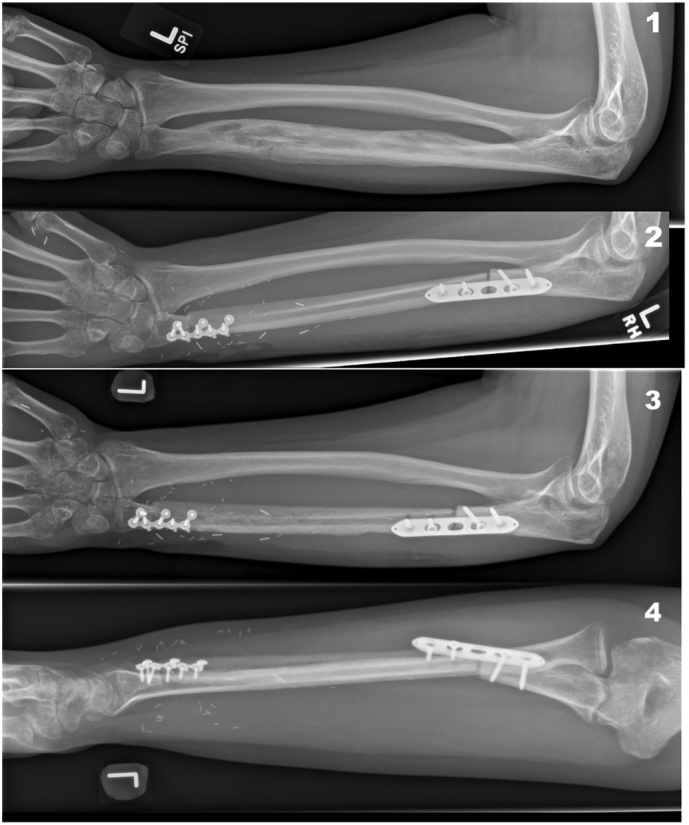
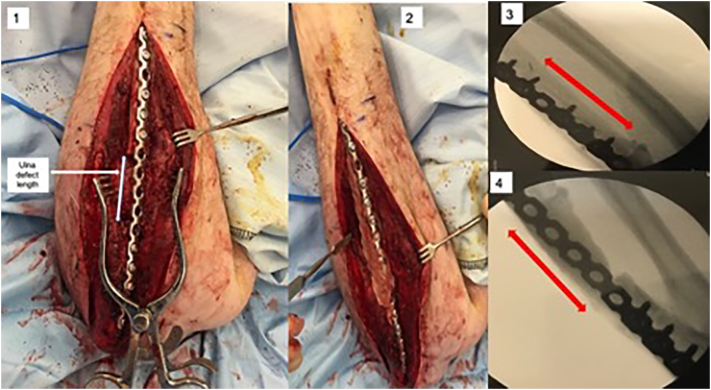
Spontaneous osteomyelitis of the left ulna (1), resection of 16 cm of osteomyelitic ulna and VFF graft reconstruction (2), non-union at the proximal end of the VFF graft and failure of metalwork (3–4).
He underwent excision of the osteomyelitic ulna and a free fibula flap-based reconstruction of the bony defect (18 cm), using the ulnar artery and a superficial vein at the wrist as recipient vessels (Fig. 1.2).
His post-operative recovery was complicated by pulmonary emboli- with subsequent discovery of the Factor V Leiden deficiency- managed with Apixaban (a direct Factor Xa inhibitor). Six months later he was found to have radiological evidence of non-union with metal work failure at the proximal fibula-ulnar junction (Figs. 1.3-4).
Fig. 4.
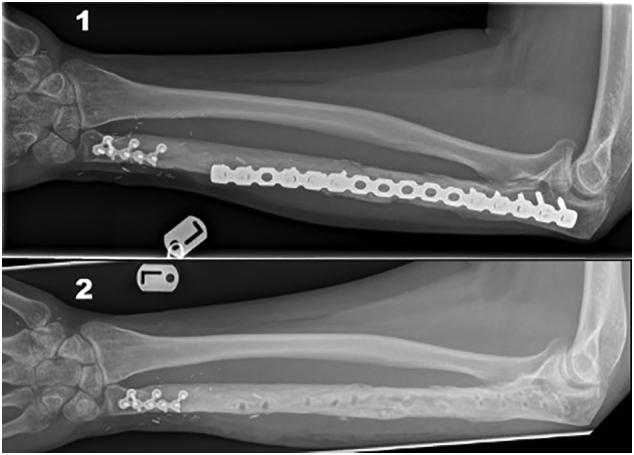
AP Forearm radiographs: The bone graft has consolidated and incorporated into the ulna proximally and the fibula distally 6 months after grafting (1) and after removal of bridging plate 4 years after grafting (2).
He underwent resection of a 5 cm segment of the fibula and insertion of an antibiotic-impregnated cement spacer as the bone looked avascular and presence of infection could not be ruled out. A reconstruction plate was used to stabilise the ulna (Fig. 2). Bone biopsies sent to microbiology revealed the presence of Klebsiella pneumoniae pathogen sensitive to ciprofloxacin, which was prescribed for a period of 6 weeks.
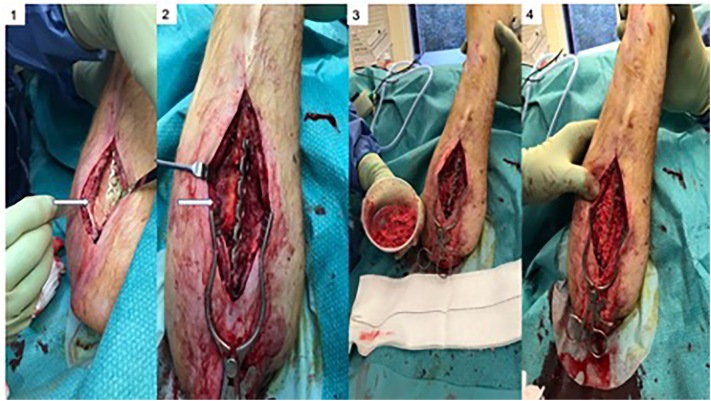
Fig. 2.
Resection of the necrotic fibula and placement of reconstruction plate (1), insertion of antibiotic-impregnated cement spacer (2), radiographs of plate and cement spacer in place (3–4).
Five months later, second stage of the Masquelet technique was performed. Autologous bone graft was harvested from the ipsilateral femur using the RIA device. Bone marrow aspirate (BMA) (60 mls) was also harvested from his left iliac crest which was concentrated with the BIOCUE bone marrow aspiration concentration system (ZimmerBiomet, Warsow, Indiana, USA) to a volume of 7 mls. In addition, 60 mls of peripheral blood was harvested and a volume of 7 mls platelet rich plasma (PRP) was obtained following concentration and filtration with the BIOCUE concentration system (ZimmerBiomet, Warsow, Indiana, USA). The RIA graft was mixed with the BMA and PRP to increase the biological potency of the graft material which was implanted in the defect area following removal of the cement spacer (Fig. 3). There was no need for revision of the osteosynthesis as the fixation was stable.
Fig. 3.
Opening of the induced membrane envelope (1), removal of cement spacer (2), preparation of bone graft (3), and packing of bone graft into cavity (4).
Six months after grafting there was radiological evidence of the graft consolidating and incorporating itself into the ulna and fibula proximally and distally (Fig. 4.1), respectively. The reconstruction plate was removed nearly 4 years later as it was causing soft tissue irritation and had become symptomatic for the patient. Both the fibula the bone graft were found to have fully consolidated and there was clinical and radiological union (Fig. 4.2).
Discussion
Treatment of bone infections remains challenging with often unfavourable outcomes. The principles of bone infections remain the same irrespectively of anatomical site involvement. Radical debridement of the affected necrotic-avascular bone with soft tissue reconstruction as indicated remains the mainstay of treatment. Systemic antibiotics represent adjunctive treatment usually for a period of 6–8 weeks. Successful eradication of the infection is followed by bone reconstruction. Different techniques have been employed to regenerate the bone missing with the 3 most popular being bone transport, VFF grafting and the Masquelet technique.
In the herein study following successful eradication of the infection a 18 cm VFF graft was harvested from the ipsilateral fibula. However, the proximal fibula-ulnar coaptation failed to unite and the patient developed non-union. In a series of 28 VFF grafts for oncological reconstruction of the upper limb, primary union was achieved in 71% of cases, increasing to 96% following supplemental bone-grafting [4].
Treatment of this complication is influenced by the length of the underlying defect. In our case following debridement a 5 cm defect was present. As we were concerned by ongoing infection, we decided to apply the Masquelet technique. We loaded the cement spacer with vancomycin. Indeed, infection was present which was treated successfully with ciprofloxacin.
While it has been suggested that the second stage should be performed after a period of 6–8 weeks [5] due to the optimum secretion of growth factors at this time point, in this case, due to patient's travelling away for family reasons, the second stage was carried out 5 months later. However, in our practice the timing of second stage reconstruction is not detrimental as we always augment the bone graft (RIA) with progenitor cells (BMA) and a growth factor (PRP) to improve its biological potency. Indeed, consolidation was observed after a period of 6 months. We believe that this practice of ours improves the results of treatment as previously reported in a larger series [3].
This case highlights the versatility of the Masquelet technique. A systematic review of technique found only 18 cases of it being employed in the ulna in the whole included published literature [6], with the largest series of ulnas comprising 6 cases, none of which had had a previous vascularised bone reconstruction [7]. In this case, the initial surgery required the resection of a very long segment of ulna and reconstruction with vascularised bone in the form of a free fibula, and although long segment reconstructions >20 cm in length have been described in the tibia [8], the mean length is similar to this case of 5.56 cm [6], and ranged from 4 to 8 cm in the largest series of ulnas [7].
The part of the fibula distal most to the vascular anastomosis, but proximal in the forearm, progressed to non-union, possibly reflecting an insufficiently rigid bony fixation at that end. A second vascularised bony reconstruction would have required the harvest of either the contralateral fibula, or the deep circumflex iliac artery (DCIA) flap. The added risks associated with the newly-diagnosed Factor V Leiden deficiency could have resulted in complications for both the flap in terms of vessel thrombosis, and for the patient in terms of further venous thromboembolism if the donor leg was immobilised, or bleeding from anticoagulation. It is possible that the Factor V Leiden deficiency played a role in the partial necrosis of the free fibula [9].
This case also reports spontaneous long-bone osteomyelitis in a patient subsequently found to be heterozygous for Factor V Leiden deficiency, and Lupus anticoagulant, but without a diagnosis of Lupus itself that carries an increased risk of osteomyelitis [1]. Hypercoagulable states have been reported in patients- including children [10] - with musculoskeletal sepsis, but no haematological predisposition. However, there are no published cases of musculoskeletal sepsis possibly resulting from a prothrombotic condition, although causality cannot be established.
Summary
In summary, this case describes a rare presentation of spontaneous osteomyelitis of the ulna in a patient subsequently found to have Factor V Leiden deficiency, which may have been a causative factor. A vascularised bony reconstruction with a VFF graft was performed, but was complicated by non union, again possibly related to the Factor V Leiden deficiency. This was successfully salvaged with the Masquelet technique, with union of the graft and normal forearm function. It highlights the versatility of these combined techniques in challenging cases such the one presented in this study.
Declaration of competing interest
The authors declare no conflict of interest.
Contributor Information
Edmund Hugh Wright, Email: edmundhughwright@gmail.com.
Grainne Bourke, Email: grainnebourke@nhs.net.
Peter V. Giannoudis, Email: pgiannoudi@aol.com.
References
- 1.Huang Y.F., Chang Y.S., Chen W.S., Tsao Y.P., Wang W.H., Liao H.T., Tsai C.Y., Lai C.C. Incidence and risk factors of osteomyelitis in adult and pediatric systemic lupus erythematosus: a nationwide, population-based cohort study. Lupus. 2019;28:19–26. doi: 10.1177/0961203318811601. [DOI] [PubMed] [Google Scholar]
- 2.Gan A.W.T., Puhaindran M.E., Pho R.W.H. The reconstruction of large bone defects in the upper limb. Injury. 2013;44:313–317. doi: 10.1016/j.injury.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Giannoudis P.V., Harwood P.J., Tosounidis T., Kanakaris N.K. Restoration of long bone defects treated with the induced membrane technique: protocol and outcomes. Injury. 2016;47:S53–S61. doi: 10.1016/S0020-1383(16)30840-3. [DOI] [PubMed] [Google Scholar]
- 4.Claxton M.R., Shirley M.B., Bakri K., Rose P.S., Moran S.L., Houdek M.T. Utility of the free vascularized fibula flap to reconstruct oncologic defects in the upper extremity. Anticancer Res. 2020;40:2751–2755. doi: 10.21873/anticanres.14246. [DOI] [PubMed] [Google Scholar]
- 5.Masquelet A., Kanakaris N.K., Obert L., Stafford P., Giannoudis P.V. Bone repair using the Masquelet technique. J. Bone Joint Surg. (Am. Vol.) 2019;101:1024–1036. doi: 10.2106/JBJS.18.00842. [DOI] [PubMed] [Google Scholar]
- 6.Morelli I., Drago L., George D.A., Gallazzi E., Scarponi S., Romanò C.L. Masquelet technique: myth or reality?A systematic review and meta-analysis. Injury. 2016;47:S68–S76. doi: 10.1016/S0020-1383(16)30842-7. [DOI] [PubMed] [Google Scholar]
- 7.Luo T.D., Nunez F.A., Lomer A.A., Nunez F.A. Management of recalcitrant osteomyelitis and segmental bone loss of the forearm with the Masquelet technique. J. Hand Surg. Eur. 2017;42:640–642. doi: 10.1177/1753193416650171. [DOI] [PubMed] [Google Scholar]
- 8.Moghaddam A., Zietzschmann S., Bruckner T., Schmidmaier G. Treatment of atrophic tibia non-unions according to “diamond concept”: results of one- and two-step treatment. Injury. 2015;46(Suppl 4):S39–S50. doi: 10.1016/S0020-1383(15)30017-6. [DOI] [PubMed] [Google Scholar]
- 9.Olsson E., Höijer P. Activated protein C resistance due to factor V Leiden, elevated coagulation factor VIII and postoperative deep vein thrombosis in late breast reconstruction with a free TRAM flap: a report of two cases. Br. J. Plast. Surg. 2005;58:720–723. doi: 10.1016/j.bjps.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Walsh S., Phillips F. Deep vein thrombosis associated with paediatric musculoskeletal sepsis. J. Pediatr. Orthop. 2002;22:329–332. http://www.ncbi.nlm.nih.gov/pubmed/11961448 http://www.ncbi.nlm.nih.gov/pubmed/11961448. [PubMed] [Google Scholar]