Abstract
Background
Non-operative management of Osteoarthritis (OA) can be challenging. The intra-articular injection with hyaluronic acid (IAHA), corticosteroids and Platelet rich plasma are some of the popular modalities which are increasingly being employed as a stop-gap strategy before considering any surgical intervention for OA management. Among these, the intra-articular HA (IAHA) has been widely studied with variable and conflicting results.
Method
This was a prospective observational study conducted in adults with knee OA. Suitable patients were given IAHA (Synvisc-One®) on an out-patient basis. They were assessed in terms of VAS score, WOMAC score and SF36 scores at successive follow-up visits at 8, 24 and 52 weeks.
Results
50 patients were recruited in this study and followed for 52 weeks post injection of HA. Mean and SD values of VAS, WOMAC and SF36 scores were on a decreasing trend in each follow up visit. Percentage change between the visits was also statistically significant. The improvement in pain scores at successive visits was significant in KL grade 1 OA than grade 2 or 3 indicating strong association between them.
Conclusion
Short-term (up to one year) beneficial effects of intra-articular viscosupplementation with HA in early primary knee OA can be seen with a decreasing trend in the intensity of pain and an increasing trend in improving the physical functioning and health-related quality of life.
Keywords: Knee, Osteoarthritis, Viscosupplementation, Intra-articular, Hyaluronic acid, Synvisc-one®
1. Introduction
OA is an age-related chronic degenerative joint disorder of multifactorial cause. It is mainly characterized by wear and tear of joint articular cartilage, joints marginal hypertrophy of bone, reduction in joint space and subchondral sclerosis. Several factors such as mechanical, biochemical, and genetic are involved in the pathogenesis of OA, which ultimately causes morphological alterations in synovial membrane and joint capsule. In developing countries like India, where substantial physical activity is required for the livelihood, the increasing burden of the disease leads to increased morbidity and reduced quality of life.
Conservative management options for OA include analgesics, steroids, and non-steroidal anti-inflammatory drugs, glucosamine/chondroitin supplementation, physical therapy, education, lifestyle modification, and intra-articular injections. Cardiovascular and gastrointestinal adverse events are more frequently encountered with the chronic use of oral drugs. Chronicity of the disease mandate more suitable agents to give long relief and fewer side effects.
Intra-articular administration of drugs has been considered as a preferred treatment modality due to their promising long-term efficacy.1 At present, there are numerous non-invasive treatment approaches with emphasis on pain management, improvement in function, and a potential to modify the disease process and progress of cartilage degeneration.
With the improvement in health care system, life expectancy has improved. On the flip side, this has also increased the incidence of OA in elderly individuals. Therefore, the requirement of effective treatment options for this disorder is increasing day by day. There are no potent pharmacological drugs which would stop the disease progression without causing side effects.
In OA, there is a gradual decrease in the concentration of hyaluronic acid in the joint space leading to degenerative changes in the joint with low-grade inflammation. The overall prevalence of Knee OA in India is 28.7%.2 It was found that OA knee had female, obese, and elderly age group preponderance. In an epidemiological study done recently, it was found that the prevalence of knee OA in females was 31.6% and was associated with obesity, old age, and sedentary lifestyle.2
There are several modalities for treating knee OA. Conservative management strategies are considered in the initial stages of the disease process. Conservative treatments include mainly education, creating awareness, extra-articular functional devices, physiotherapy, weight reduction, exercises, lifestyle modifications, avoiding excessive weight bearing on the knee. In further stages of the disease, along with these non-pharmacological modalities, pharmacological treatment helps in effective control of symptoms. The pharmacological modalities include oral Non-steroidal anti-inflammatory drugs (NSAID's) and Opioid analgesics. Long term use of these drugs not only produces pain reduction but also causes multiple systemic side effects involving the cardiovascular system, gastrointestinal system, and renal system increasing morbidity.3
Patients with a severe form of OA, not responding to conservative management would ultimately end up in some form of surgical treatment. Surgical treatment has its complications such as infections, implant failures, peri-prosthetic fractures, financial constraints, with added anaesthesia-related complications, especially in an old patient with multiple comorbidities. Hence, it is better to delay the surgical treatment as much as possible, to avoid such complications unless needed.
This led to the development of a new approach to the treatment of OA, which is comparatively safer and has fewer complications, i.e., intra-articular (IA) injections. Intra-articular injection of certain substances decreases the progression of the disease and hence delays the need for surgical treatment.4 Most commonly used IA substances include corticosteroids, platelet-rich plasma (PRP), and hyaluronic acid, with PRP showing better outcomes among all these components.5 In contrast, others have noted that among the intra-articular injection therapy for knee OA, the evidence was strongly favouring corticosteroids with promising results for HA and PRP.1 When it comes to the effectiveness of intra-articular injections of Hyaluronic acid in OA knee, several study trials have been done, but the results have been variable.6
2. Method
The study was conducted after obtaining approval from departmental dissertation committee and Institutional Ethics Committee (IEC No: 448/2018). The study was also registered under Clinical Trial Registry of India (CTRI/2019/01/01687). This was a prospective observational study conducted in adults with primary OA knee patients undergoing IAHA injection treatment.
Patients with clinically and radiographically diagnosed knee OA, patients with Kellgren and Lawrence grade 1 to 3 who were not responding to other conservative forms of treatment for at least last six months, patients with no contraindication or reactions to viscosupplementation injection, male and female patients aged above 45 years and patients who had minimum follow-up of 52weeks from the first presentation were included in the study.
Patients with other forms of knee arthritis (inflammatory/post-traumatic OA/Septic), patients who underwent total knee replacement surgery of the same knee within the follow-up period, OA of other joints, OA injection treatment with other modalities/drugs (steroids/PRP) and patients who received more than one dose of IAHA injection within the study period were excluded from the study.
2.1. Procedure
The study was designed to be an observational study. It was a 52 week long, one group, repeated measures design which evaluated the clinical efficacy and safety of a single intra-articular hyaluronic acid (IAHA) injection.
Principal investigator-assessed all patients undergoing intra-articular viscosupplementation in orthopaedic OPD in Arthroscopy and Sports Injuries unit. All the injection to the patients were given by single experienced surgeon. A detailed history was taken, and clinical examination was conducted under the following headings.
History: Detailed history regarding knee pain, early morning stiffness, the involvement of other joint pains, reduced functioning and causing limitations in daily activities was assessed.
Local Examination: Detailed examination of the involved knee was done. Presence of Quadriceps muscle wasting, swelling, and deformity of the knee joint, any bony tenderness along the medial or lateral joint lines, patellofemoral joint tenderness by Grid test, Range of movements and presence of crepitus was checked. Diagnosis of OA knee was made based on the EULAR Diagnostic Criteria for Knee OA (2010).7 It included three symptoms (Persistent knee pain, limited morning stiffness, reduced function), and three signs (crepitus, restricted movements, bony enlargement). If all signs and symptoms are present, then the probability of having radiographic OA in an adult ≥45 years is estimated to be 99%. Radiographically presence of OA knee was graded by Kellgren-Lawrence (KL) grading system.8 Each patient was explained about their participation in the study involving the intra-articular injection of HA into the knee joint. Written informed consent before being enlisted in the study was taken from each patient. Clinical and radiological findings of each patient included in the study along with their baseline Visual Analogue Scale (VAS), Western Ontario and McMaster Universities OA Index (WOMAC) and SF36 (Short Form 36 Health Survey) scores were documented.
Standard IAHA protocols were observed by the principal investigator, done on an out-patient department basis. All patients received single shot of intraarticular injection of 10 ml of Hylan G-F 20 (Synvisc-One® (Hylan G-F 20)/(Hylan Polymer A & B G-F 20)9 under strict aseptic precautions. Post-procedure, the patients were advised to take rest, ice the affected area, and elevate the limbs for 48 h. Post injection information regarding potential risks and complications of the procedure was provided to each patient. Light stretching exercises and physiotherapy were also prescribed. Short course oral analgesics (<1 week) were given as per the requirement of the patient. Any side effects observed after injection and during follow-up were also documented.
Patients were followed as per the standard protocol, i.e., at 8 weeks, 24 weeks, and 52weeks after the IAHA injection. At each follow-up visit, re-assessments were done using Visual Analogue Scale (VAS), Western Ontario And McMaster Universities OA Index (WOMAC), and SF36 scoring systems.
Visual Analogue Scale (VAS): VAS scoring is a unidimensional measure of the intensity of pain. In a horizontal line of 10 cm on a paper, with markings at each centimetre, the intensity of pain was graded ranging from no pain (score 0) to worst pain imaginable (score 10). Patients were asked to grade from 0 to 10 as per their pain intensity. This was used for pain assessment before injection and at 8, 24, 52 weeks after injection.
Western Ontario And Mcmaster Universities OA Index (WOMAC): It determines the course of the disease over a period and tells about the effectiveness of intra-articular injection. It is a set of standard questionnaires having 24 parameters subdivided into three subscales, pain (5), stiffness (2) and physical function (17).
Each one of the components is tested on a scale ranging from 0 to 4, which corresponds to None (0), Mild (1), Moderate (2), Severe (3), and Extreme (4). Pain has five parameters, with a minimum score of 0 and a maximum score of 20. Two parameters in stiffness with a minimum score of 0 and a maximum score of 8. The physical function has 17 parameters, with a minimum score of 0 and a maximum score of 68. Sum of all 24 parameters, gives a total WOMAC score. High WOMAC score indicates the worst pain, stiffness, and functional limitation.
Short Form [36] Health Survey (SF36): It is a measure of health-related quality of life. It helps in evaluating individual patient's health status and cost-effectiveness of treatment. The commercial version of the original SF36 is known as RAND SF36 which is used here. Pain and general health scales are different in both these versions.
RAND SF 36 consists of 8 scaled scores namely: Physical function (10), Role limitation due to physical health problem (4), Role limitation due to emotional problem (3), Energy/Fatigue (4), Emotional well-being (5), Social functioning (2), Bodily Pain (2), General Health Perceptions (5) and Health change (1). It has a total of 36 questionnaires which will be pooled to form these eight domains. Each domain is directly transformed into a 0–100 range on the assumption that each question carries equal weight. A low score indicates a high disability, and a high score indicates less impairment. Hence score of 0 means profoundly disabled, and a score of 100 means no disability at all.
Sample Size: The sample size was estimated using repeated-measures ANOVA, based on the primary objective, using the formula
| n= (Z1-α/2 + Z1-β)2 σ2 [1+ (m-1) ρ] ÷ md | 2 |
Where n = sample size
| Z1-α/2 = 1.96 at α = | 0.05 |
| Z1-β = 0.84 at 80% power |
σ = standard deviation
m = number of assessments = 4
ρ = interclass correlation (0.3)
d = clinically significant difference = 7
Thus, upon calculation, for 80% power of study with a confidence interval of 95%, accounting for a dropout rate of 15%, the minimum sample size was estimated to be 25. We have recruited 50 patients.
2.2. Statistical analysis
Data were analysed using SPSS Statistics Version 20. Change in VAS, SF36, WOMAC Scores after injection were calculated as the baseline -post-injection follow up at 8 weeks, 24 weeks, 52 weeks. To account for patient providing a repeated response to each outcome measure throughout the study, Repeated measure ANOVA was used to compare the effect of viscosupplementation for VAS, WOMAC, SF36 at 8 weeks, 24 weeks, 52 weeks vs. baseline. Categorical data were represented by frequency, and continuous data were analysed using parametric tests. A value of p < 0.05 was considered significant. All data were compared as mean ± standard deviation. Within the group, an association was analysed by Post HOC test.
3. Results
Table 1 shows the demographic characteristics of the study patients and Table 2 shows the demographic details of the disease.
Table 1.
Shows the demographic characteristics of the study patients.
| PARAMETERS | FREQUENCY (n = 50) | PERCENT (%) | |
|---|---|---|---|
| GENDER | MALE | 21 | 42 |
| FEMALE | 29 | 58 | |
| AGE in years | (45–55) Years | 27 | 54 |
| (55–65) Years | 12 | 24 | |
| >65 Years | 11 | 22 | |
| AGE (MEAN ± SD) in years | 55.9 ± 10.29 | ||
| BMI (MEAN ± SD) in kg/m2 | 26.03 ± 3.88 | ||
Table 2.
Shows the demographic details of the disease.
| PARAMETERS | FREQUENCY (n = 50) | PERCENT (%) | |
|---|---|---|---|
| SIDE OF KNEE | Left | 23 | 46 |
| Right | 25 | 50 | |
| Bilateral | 2 | 4 | |
| KL GRADE | Grade 1 | 18 | 36 |
| Grade 2 | 21 | 42 | |
| Grade 3 | 11 | 22 | |
| DURATION OF PAIN (MEAN ± SD) in years | 3.7 ± 3.5971 | ||
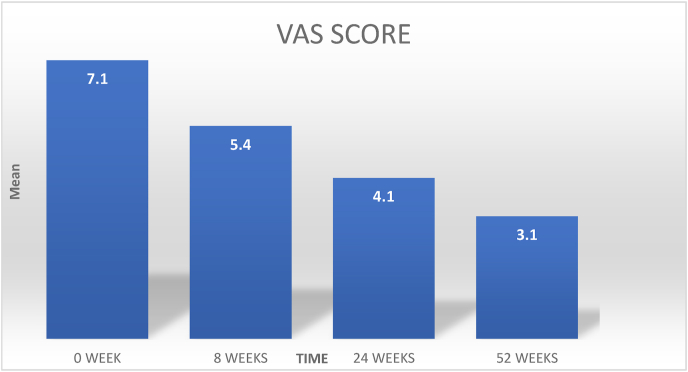
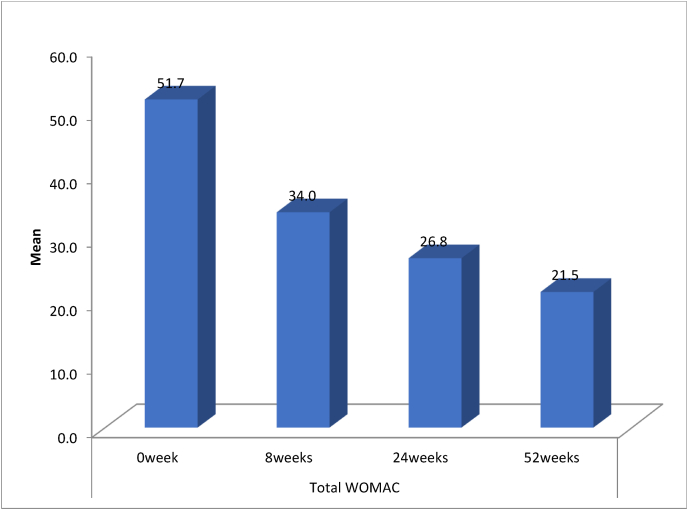
Fig. 1, Fig. 2 shows the mean VAS scores and the mean total WOMAC scores at baseline (0 weeks) and 8, 24, 52 weeks post-injection respectively. We found that the mean VAS score was decreasing at successive intervals and the mean values of WOMAC scores in all subscales were also reducing at successive intervals.(Fig. 4)
Fig. 1.
Shows the mean VAS scores at different time points at baseline (0 weeks) and 8, 24, 52 weeks post-injection.
Fig. 2.
Shows the mean total WOMAC scores at baseline (0 weeks) and 8, 24, 52 weeks post-injection.
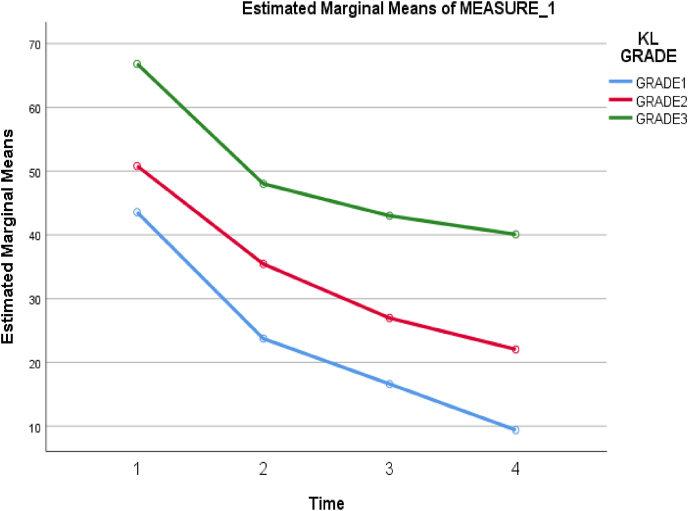
Fig. 4.
Association between KL grade and Total WOMAC scores.
Table 3: shows mean and standard deviation values of SF36 scores for each subscale at baseline (0 weeks) and 8, 24, 52 weeks post-injection. Mean SF36 scores in each component improved from baseline to 52 weeks post-injection. Post hoc analysis of each sub-scale showed significant improvement at all time points (p-value <0.005).
Table 3.
Shows mean and standard deviation values of SF36 scores for each subscale at baseline (0 weeks) and 8, 24, 52 weeks post-injection.
| SF-36 | 0 WEEK (mean ± SD) | 8 WEEKS (mean ± SD) | 24 WEEKS (mean ± SD) | 52 WEEKS (mean ± SD) |
|---|---|---|---|---|
| Physical Functioning | 27.2 ± 25.27 | 57.4 ± 25.27 | 66.9 ± 26.24 | 71.8 ± 28.26 |
| Limitation due to physical health | 8.5 ± 24.54 | 59 ± 47.04 | 74 ± 44.30 | 80 ± 40.40 |
| Limitation due to an emotional problem | 16.66 ± 35.15 | 66 ± 47.85 | 76 ± 43.14 | 80.66 ± 39.32 |
| Energy/Fatigue | 44.2 ± 28.43 | 60.8 ± 21.38 | 69.8 ± 20.55 | 74.2 ± 21.83 |
| Emotional well-being | 46.64 ± 27.35 | 63.04 ± 17.97 | 69.84 ± 18.11 | 73.68 ± 19.53 |
| Social functioning | 32.65 ± 26.27 | 53.5 ± 22.02 | 63 ± 23.54 | 67.75 ± 26.61 |
| Pain | 44.8 ± 23.12 | 66.05 ± 22.13 | 75.65 ± 21.20 | 78.2 ± 21.94 |
| General Health | 47.6 ± 29.24 | 67.3 ± 20.40 | 74.6 ± 20.96 | 78.2 ± 23.83 |
| Health change | 28 ± 26.06 | 66 ± 20.05 | 75 ± 22.01 | 78.9 ± 25.95 |
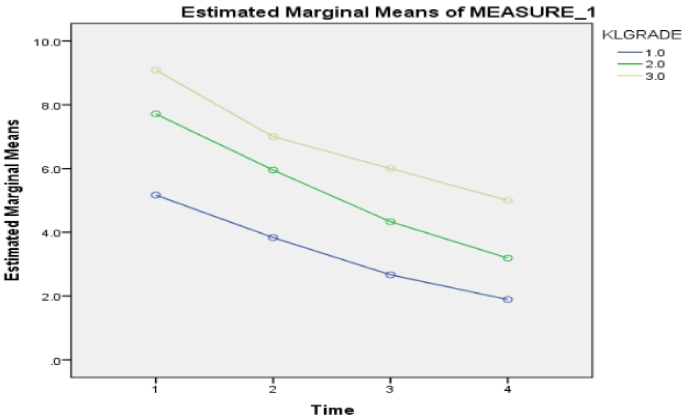
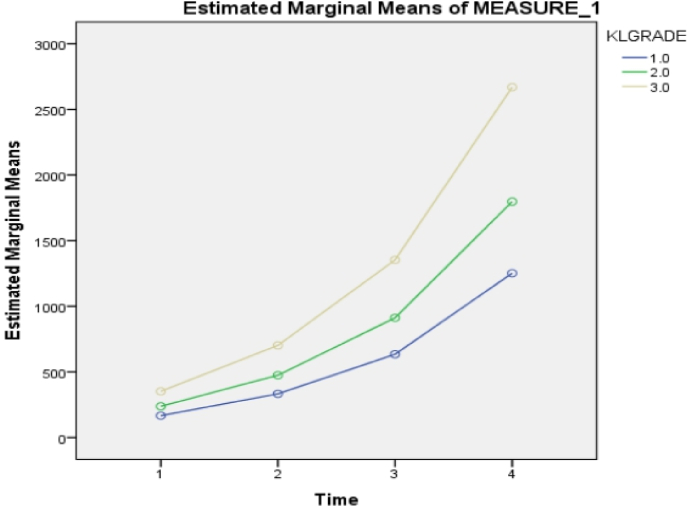
A significant association between KL grade 1, 2, 3, and all three clinical scores were noted (Table 4, Table 5, Table 6) (P < 0.05). Further, it was noted that VAS Score reduced in all three KL grades of patients with a maximum reduction of the score in Grade 1 (Fig. 3). Likewise, maximum improvement in SF36score was noted in Grade 1 followed by Grade 2 and grade 3 (Fig. 5).
Table 4.
Shows an association between KL grade 1, 2, 3, and VAS scores.
| KL Grade | VAS Score (Mean ± SD) | P-value | |||
| 0 WEEK | 8 WEEKS | 24 WEEKS | 52 WEEKS | P < 0.01∗ | |
| 1 (n=18) | 5.16 ± 1.15 | 3.83 ± 1.15 | 2.66 ± 1.37 | 1.88 ± 1.52 | |
| 2 (n=21) | 7.71 ± 1.48 | 5.95 ± 1.39 | 4.33 ± 1.49 | 3.19 ± 1.99 | |
| 3 (n=11) | 9.09 ± 0.94 | 7 ± 1.54 | 6 ± 1.78 | 5 ± 2.36 | |
Table 5.
Shows the association between KL grades 1, 2, 3, and total WOMAC scores.
| KL Grade | WOMAC Score (mean ± SD) | P value | |||
| 0 WEEK | 8 WEEKS | 24 WEEKS | 52 WEEKS | P < 0.05∗ | |
| 1 (n=18) | 43.55 ± 17.99 | 23.72 ± 17.24 | 16.61 ± 16.34 | 9.38 ± 13.31 | |
| 2 (n=21) | 50.81 ± 19.1 | 35.42 ± 18.15 | 26.95 ± 18.17 | 22.04 ± 18.84 | |
| 3 (n=11) | 66.81 ± 19.48 | 48 ± 21.06 | 43 ± 25.77 | 40.09 ± 28.85 | |
Table 6.
Shows the association between KL grades 1, 2, 3, and total WOMAC scores.
| KL Grade | SF-36 (mean ± SD) | P-value | ||||
| 0 WEEK | 8 WEEKS | 24 WEEKS | 52 WEEKS | P < 0.05∗ | ||
| 1 (n=18) | 166.72 ± 102.96 | 333.06 ± 205.45 | 634.50 ± 400.26 | 1252 ± 787.61 | ||
| 2 (n=21) | 237.81 ± 120.21 | 474.95 ± 240.01 | 912.19 ± 467.33 | 1797.71 ± 921.37 | ||
| 3 (n=11) | 351.64 ± 152.42 | 702.36 ± 304.05 | 1353.45 ± 596.97 | 2669.82 ± 1179.95 | ||
Fig. 3.
Association between KL grades and VAS score.
Fig. 5.
Association between KL grade and SF36 scores.
None of the patients experienced any serious adverse reactions (such as anaphylactic shock, anaphylactoid reaction or angioedema) following the drug administration. Transient reaction at the injection site in the form of redness was noted in only three cases that resolved completely within three days without any further intervention.
4. Discussion
This study was mainly conducted to know the efficacy of single intra-articular IAHA injection in terms of pain, physical functioning and quality of life in patients with primary early OA knee (KL Grade<3). We found that a single IAHA injection was effective in reducing the intensity of pain, improving physical functioning and quality of life in patients with early primary OA knee.
Synovial fluid in the joint space is required for the normal healthy functioning of the joint. The main component of synovial fluid is a polysaccharide chain known as hyaluronic acid, made up of repeating units of N-acetyl glucosamine and glucuronic acid with a molecular weight of 4–10 million Da. Normal joint space contains 2 ml of fluid with 2.5–4 mg/ml of HA concentration. The protective effect of HA is directly attributed to its concentration, molecular weight, and mechanical force exerted on the joint.10
There are multiple mechanisms of action of IAHA. Firstly, it allows lubrication and shock absorption, thus causing mechanical viscosupplementation to the joint. Secondly, it increases endogenous hyaluronic acid production and re-establishes joint homeostasis, the effect of which continues long even after the exogenous injection has left the joint space.11 Further, it is also believed to inhibit the pain receptors, prevent enzymatic cartilage degradation and also act like a free radical scavenger.4 It has been shown to prevent the breakdown of joint matrix by inhibiting the proinflammatory factors (PGE2 and NFkB).12
There are different products of HA used for intra-articular injections. The extent of benefit obtained from each product is different, because of the different molecular weights of HA. There are no proven studies for this, and hence it is controversial. In the index study, we had used single use agent (10 mL of Synvisc-One® containing 6 mL of Hylan G-F 20) for all cases. Hylan G-F 20 is an HA preparation consisting of hylan A, a 6000 kDa HA, and hylan B, a cross-linked derivative of natural HA.13 This cross linking of the formulation is intended to improve the long-term efficacy of the preparation by resisting its degradation inside the knee joint.12
Several studies have been done across the world to evaluate the efficacy of this modality of treatment for OA knee over the more common pharmacological treatment such as NSAIDs.14 Several Meta analytical studies have demonstrated a positive effect of intra-articular injection of HA in reducing pain and improving the quality of life.15 In contrast to these, there are few studies which have proven that HA in the treatment of OA knee has “small and clinically irrelevant benefit” and an increased risk for serious adverse events.16 Although previous studies have shown conflicting results for viscosupplementation in the management of OA, more recent evidence appears to be in favour of this modality of treatment.12
HA does not cause rapid effects, rather its clinical effect on pain and functional improvement shows a carryover effect which extends for a long time after initial administration. In Indian patients, a multicentric phase-4 study - OASIS (Osteoarthritis Synvisc-One® Indian Post-Marketing Study) was undertaken. The study concluded that at one-year, single dose of 6 ml Hylan GF 20 was safe and effective in treatment of symptomatic OA. The present study concurs with the findings of OASIS and noted statistically significant improvements SF-36, WOMAC and VAS scores.17
There are two types of Hylan G-F 20 formulations available in the market: a single-shot (wherein a higher volume (6 ml) is administered – Synvisc-One®) and the once weekly x 3 approach (wherein a lower volume (2 ml) is administered across multiple injections - Synvisc®3 × 2).13 A previous RCT reported that patients receiving more than one dose of Hylan GF-20 are likely to experience increased frequency of local adverse reactions.18 A recent systematic review and meta-analysis was conducted to analyse the long-term (one year) efficacy and safety of single or 1–3 weekly injections of Hylan G-F 20. It was observed that there was no difference in level of efficacy based on injection schedule, nor between randomized and non-randomized studies. The meta-analysis revealed that there was statistically significant improvement in WOMAC (pain, physical function and stiffness), VAS, SF-36 scores (both mental and physical component summary). Furthermore, the drug is well tolerated with low rates of adverse events.13
Since many clinical trials have demonstrated a differing response in the effectiveness of intra-articular HA in the treatment of OA, the level of recommendations afforded to HA by different international and national societies varies.19
Despite the use of non-steroidal anti-inflammatory drugs (NSAIDs), in patients with OA knee who remain symptomatic, The European Society for Clinical and Economic Aspects of Osteoporosis and OA (ESCEO) treatment algorithm recommends intra-articular hyaluronic acid (HA) for pharmacological management.11 Similarly, The European League Against Rheumatism (EULAR) guidelines recommend Intra-articular HA based upon level 1B evidence for both joint functional improvement and pain reduction.20 Both the American College of Rheumatology (ACR) guidelines and ESCEO algorithm recommend IA HA, especially in patients whose symptoms persist even after prior treatments.20
In contrast to these, OA Research Society International (OARSI) Guidelines recommend uncertainty in the use of IA HA. The use of HA should be individualized based on the patient's characteristics, type of OA, risk: benefit ratio, and need of the patient. The OARSI guidelines also attributed risk score of IA HA as superior to the risk score of IA corticosteroid in patients with comorbidities.19
Though considered safer than the pharmacological treatment,21 intra-articular HA also has few adverse effects such as local reactions at the site of injection, and non-septic arthritis especially with the high molecular weight HA products. The exact incidence of IAHA drug reactions is somewhat unclear and a diverse rate have been reported in the literature. Despite the presence of small amounts of avian proteins in the preparation, overall safety profile seems to be satisfactory as serious adverse reactions are seldom seen. Even in the index series, besides a transient local reaction in three cases, none had any serious adverse reactions.
Limitations: There were several limitations in this study. We did not make any comparison of responses to different dose and different source of HA. Further, the effect of HA in the presence of comorbidities in the older population and late stages of OA can be quite variable. The LOBRAS study group noted that with increase in age, the beneficial effects of the Hylan GF20 also decreased.22 This was a prospective observational study with no control group and no comparison of HA with other agents were made. We also looked into functional scores as the outcome of the treatment and the cartilage changes or change in the severity of OA was not investigated in the follow-up period. Further, we did look at the cost benefit analysis of treatment with Hylan GF20. However, a recent study from Italy noted that compared with NSAIDs, Hylan G-F 20 1 × 6 mL appeared to be cost effective in treatment of knee OA.23
5. Conclusion
Short-term (up to one year) beneficial effects of intra-articular viscosupplementation with HA (Synvisc-One®) in early primary knee OA can be seen with a decreasing trend in the intensity of pain and an increasing trend in improving the physical functioning and health-related quality of life.
Source of funding
None.
CRediT authorship contribution statement
Kiran Acharya: Conceptualization, concept & design, manuscript preparation and revision and final approval. Vinaykumar Si: Data curation, design, data collection, manuscript preparation and revision and final approval. Sandesh Madi: Data curation, design, data collection, manuscript preparation and revision and final approval.
Declaration of competing interest
None.
Acknowledgement
None.
Contributor Information
Kiran Acharya, Email: kirankatte@gmail.com.
Vinaykumar Si, Email: docvinaysi@gmail.com.
Sandesh Madi, Email: sandesh.madi@gmail.com.
References
- 1.Ding J B., Hu K. Injectable therapies for knee osteoarthritis. Reumatologia. 2021;59(5):330–339. doi: 10.5114/reum.2021.110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pal C.P., Singh P., Chaturvedi S., Pruthi K.K., Vij A. Epidemiology of knee osteoarthritis in India and related factors. Indian J Orthop. 2016;50(5):518–522. doi: 10.4103/0019-5413.189608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wongrakpanich S., Wongrakpanich A., Melhado K., Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9(1):143–150. doi: 10.14336/AD.2017.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Testa G., Giardina S.M.C., Culmone A., et al. Intra-articular injections in knee osteoarthritis: a review of literature. J Funct Morphol Kinesiol. 2021;6(1):15. doi: 10.3390/jfmk6010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migliorini F., Driessen A., Quack V., et al. Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a Bayesian network meta-analysis. Arch Orthop Trauma Surg. 2021;141(9):1473–1490. doi: 10.1007/s00402-020-03551-y. [DOI] [PubMed] [Google Scholar]
- 6.Uson J., Rodriguez-Garciá S.C., Castellanos-Moreira R., et al. EULAR recommendations for intra-articular therapies. Ann Rheum Dis. 2021;80(10):1299–1305. doi: 10.1136/annrheumdis-2021-220266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W., Doherty M., Peat G., et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69(3):483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- 8.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://www.sanofi.in/-/media/Project/One-Sanofi-Web/Websites/Asia-Pacific/Sanofi-IN/Home/science-and-innovation/for-healthcare-professionals/product-information/Synvisc-One-India.pdf?la=en.
- 10.Moreland L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5(2):54–67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruyère O., Cooper C., Pelletier J.P., et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4):S3–S11. doi: 10.1016/j.semarthrit.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Peck J., Slovek A., Miro P., et al. A comprehensive review of viscosupplementation in osteoarthritis of the knee. Orthop Rev (Pavia) 2021 doi: 10.52965/001c.25549. Published online July 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lucia O., Jerosch J., Yoon S., Sayre T., Ngai W., Filippou G. One-year efficacy and safety of single or one to three weekly injections of hylan G-F 20 for knee osteoarthritis: a systematic literature review and meta-analysis. Clin Rheumatol. 2021;40(6):2133–2142. doi: 10.1007/s10067-020-05477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller L.E., Fredericson M., Altman R.D. Hyaluronic acid injections or oral nonsteroidal anti-inflammatory drugs for knee osteoarthritis: systematic review and meta-analysis of randomized trials. Orthop J Sports Med. 2020;8(1) doi: 10.1177/2325967119897909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellamy N., Campbell J., Welch V., Gee T.L., Bourne R., Wells G.A. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2006(2) doi: 10.1002/14651858.CD005321.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutjes A.W.S., Jüni P., da Costa B.R., Trelle S., Nüesch E., Reichenbach S. Viscosupplementation for osteoarthritis of the knee. Ann Intern Med. 2012;157(3):180. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 17.Pal S., Thuppal S., Reddy K.J., et al. Long-term (1-year) safety and efficacy of a single 6-mL injection of hylan G-F 20 in Indian patients with symptomatic knee osteoarthritis. Open Rheumatol J. 2014;8(1):54–68. doi: 10.2174/1874312901408010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leopold S.S., Warme W.J., Pettis P.D., Shott S. Increased frequency of acute local reaction to intra-articular hylan GF-20 (Synvisc) in patients receiving more than one course of treatment. J Bone Joint Surg Am. 2002;84(9):1619–1623. doi: 10.2106/00004623-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Altman R.D., Schemitsch E., Bedi A. Assessment of clinical practice guideline methodology for the treatment of knee osteoarthritis with intra-articular hyaluronic acid. Semin Arthritis Rheum. 2015;45(2):132–139. doi: 10.1016/j.semarthrit.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Maheu E., Rannou F., Reginster J.Y. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4):S28–S33. doi: 10.1016/j.semarthrit.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Honvo G., Reginster J.Y., Rannou F., et al. Safety of intra-articular hyaluronic acid injections in osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging. 2019;36:101–127. doi: 10.1007/s40266-019-00657-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearey P., Popple A.E., Warren J., Davis T., Bellamy N. Improvement in condition-specific and generic quality of life outcomes in patients with knee osteoarthritis following single-injection Synvisc: results from the LOBRAS study. Curr Med Res Opin. 2017;33(3):409–419. doi: 10.1080/03007995.2016.1260533. [DOI] [PubMed] [Google Scholar]
- 23.Migliore A., Integlia D., Pompilio G., di Giuseppe F., Aru C., Brown T. Cost-effectiveness and budget impact analysis of viscosupplementation with hylan G-F 20 for knee and hip osteoarthritis. Clinicoecon Outcomes Res. 2019;11:453–464. doi: 10.2147/CEOR.S194669. [DOI] [PMC free article] [PubMed] [Google Scholar]