Abstract
Background
Systematic reviews have shown a high prevalence of long-term persistent sequelae after COVID-19. The aim of this study was to describe the prevalence and risk factors associated with long‐lasting clinical symptoms (LLCS) in survivors on chronic dialysis at 6 months after the onset of acute COVID-19 infection in the pre-vaccination period.
Methods
This national cohort study included all French patients on dialysis who had SARS-Cov-2 infection between March and December 2020 and who were alive and still on dialysis 6 months after infection. A form was filled in at 6 months concerning the presence of the following persistent symptoms: extreme fatigue, headache, muscle or weight loss of > 5%, respiratory sequelae, tachycardia, chest pain, joint or muscle pain, persistent anosmia or ageusia, diarrhea, sensory disorders, neuro-cognitive disorders, post-traumatic stress syndrome, depression, and anxiety.
Results
Complete survey results were available for 1217 patients (25.2% of those included); 216 (17.7%) had some LLCS. Probability of 6-month LLCS was higher in patients who were hospitalized in a medical or intensive care unit: OR 1.64 (95% CI 1.16–2.33) and 5.03 (2.94–8.61), respectively. Younger patients had a lower probability of LLCS. Each year on dialysis, as well as diabetes, overweight or obesity were associated with a higher probability of LLCS by 1.03 (1.01–1.06), 1.53 (1.08–2.17), 1.96 (1.10–3.52) and 2.35 (1.30–4.26), respectively.
Conclusions
This national study shows that at least one in six patients on dialysis who have COVID-19 will have LLCS. Systematic screening in dialysis patients would allow us to identify those who need more careful prevention and long-term care and to address them towards a rehabilitation pathway.
Keywords: Dialysis, SARS-Cov-2, Sequelae, Cohort-registry
Introduction
Acute infection with SARS-CoV-2 virus is of variable severity, and for patients who survive, complete recovery is expected. However, some patients recovering from an acute episode complain of symptoms long after the infection. Data are accumulating on the existence of various sequelae, including their nature, intensity and duration. Systematic reviews have estimated the high prevalence of long-term persistent sequelae of COVID-19 [1–3]. The studies included in these reviews were highly heterogeneous; most were single-center, they often included the general hospitalized population, and had various follow-up times.
Some studies included more specific populations such as transplanted patients [4, 5] or patients on dialysis [6, 7]. In fact, SARS-Cov-2 infection is particularly dangerous for these patients because they are more vulnerable owing to their multiple pathologies associated with poor prognosis during COVID-19. In the French national end-stage kidney disease (ESKD) registry, the Renal Epidemiology and Information Network (REIN), from the beginning of the pandemic to December 2021, 17% of dialysis patients had contracted COVID-19, and 20% of them had died due to SARS-Cov-2 infection (weekly newsletter available at https://www.agence-biomedecine.fr/Les-chiffres-du-R-E-I-N).
The aim of this study by the French REIN registry was to describe the prevalence and risk factors associated with long‐lasting clinical symptoms of COVID-19 survivors on chronic dialysis 6 months after the acute onset of COVID-19 in the pre-vaccination period.
Methods
Study design and population
This national cohort study included all patients on dialysis who had SARS-Cov-2 infection between March 1, 2020 and December 31, 2020 (before the vaccination period), who were alive and still on dialysis 6 months after infection, in all centers and regions of France. Patients were identified from the French national REIN registry. The goal of the French ESKD registry is to collect data on all end-stage kidney disease patients on renal replacement therapy who are living in France, including the overseas territories. The details of the organizational principles and quality control are described elsewhere [8]. This registry, whose strength lies in its national network and expertise, rapidly set up epidemiological monitoring of chronic dialysis patients with COVID-19 in March 2020. With the help of the REIN clinical research assistants, the nephrologists reported infection by SARS-CoV-2 in the registry in the presence of suspicious clinical symptoms, characteristic signs on CT chest scan or a positive RT-PCR result for SARS-CoV-2 on a nasopharyngeal swab. A form reporting their initial condition and outcome was filled in for each patient [9, 10].
Information
All dialysis patients who were diagnosed with SARS-Cov-2 infection were included, regardless of the clinical presentation. The clinical and care status was reported according to 7 groups of illness over time: asymptomatic, mild disease treated at home, moderate disease treated in a hospital, severe disease treated in an intensive care unit, death, healing, and incidental diagnosis. The date of the first report to the registry was the date of diagnosis. Each modification to the clinical status was reported upon occurrence. The highest degree of severity was used in the present study to characterize the infection and was defined according to 3 groups of gravity: mild (asymptomatic, treated at home or incidental diagnosis), moderate (hospitalization in a medical unit), and severe (hospitalization in an intensive care unit).
Six months after SARS-Cov-2 infection, the clinical research assistant of the registry in close collaboration with the nephrologists filled in a form for each survivor. The information collected concerned the absence or presence of the following persistent clinical symptoms: extreme fatigue, headache, muscle loss or weight loss > 5%, not recovered, respiratory sequelae, tachycardia, chest pain, joint or muscle pain, persistent anosmia or ageusia, diarrhea, sensory disorders, neuro-cognitive disorders, post-traumatic stress syndrome, depression, anxiety, or other sequelae.
Clinical characteristics collected at last follow-up included age, sex, comorbidities, mobility status (walks without help, needs assistance for transfers, or totally dependent for transfers) and body mass index (BMI).
Statistical analysis
The clinical characteristics of patients with or without long‐lasting clinical symptoms are expressed as frequencies (percentage) for qualitative variables and median (interquartile range) or mean (SD) for quantitative variables. Fisher’s exact and Wilcoxon tests were used to compare groups. Risk factors associated with the presence of long‐lasting clinical symptoms were analyzed by logistic regression. Results are reported as odds ratios (ORs) with their 95% confidence interval (CI). P < 0.05 (two-sided) was considered statistically significant. SAS software was used for all analyses.
Results
Among 6733 dialysis patients (hemodialysis or peritoneal dialysis) reported as being infected with SARS-Cov-2 between March 1, 2020 and December 31, 2020, 1907 were no longer on dialysis at 6 months (death, renal transplantation, recovery, lost to follow-up) (Fig. 1). Complete survey results were available for 1217 of 4826 (25.2%) patients. In 82% of cases, the first diagnosis was by RT-PCR on a nasopharyngeal swab, 11% by characteristic signs on a CT chest scan and 4% by suspicious clinical symptoms. Finally, a positive RT-PCR result was available for 92% of patients.
Fig. 1.
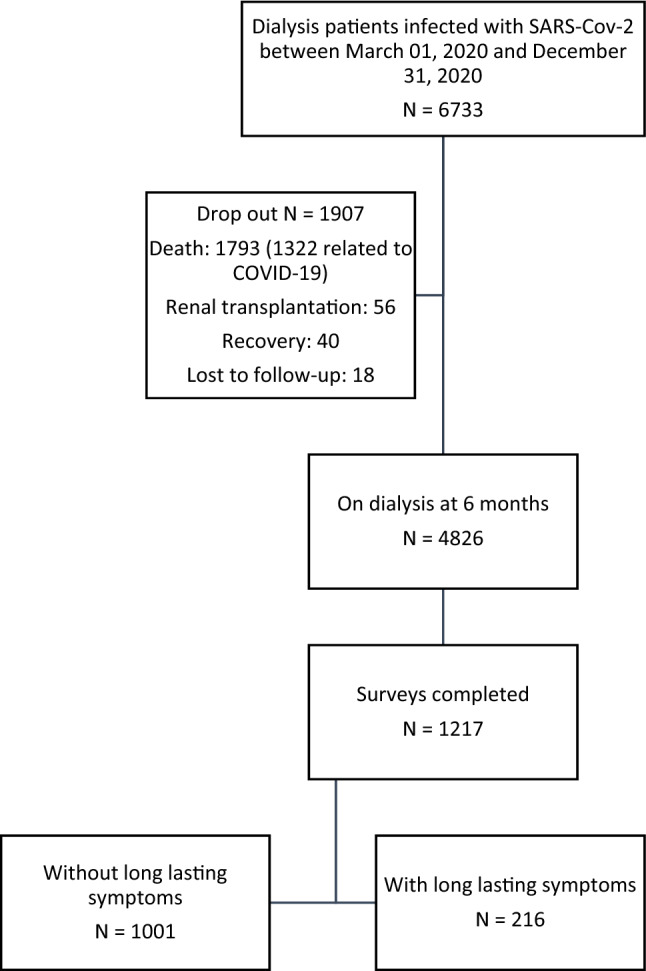
Study flow chart
At 6 months, 216 (17.7%) patients reported having some long‐lasting clinical symptoms. The characteristics of patients according to the presence of long‐lasting clinical symptoms at 6 months are shown in Table 1. Patients with long‐lasting clinical symptoms were older and had higher BMI and more frequently associated comorbidities (diabetes, coronary vascular disease, peripheral vascular disease) than others. They more frequently had severe COVID-19 as well.
Table 1.
Characteristics of patients on dialysis according to the presence of long‐lasting clinical symptoms at 6 months
| COVID-19 population (6-month follow-up) N = 4826 |
Population with completed survey N = 1217 |
Without long‐lasting clinical symptoms N = 1001 |
With long‐lasting clinical symptoms N = 216 |
p value* | |
|---|---|---|---|---|---|
| COVID-19 max severity state | |||||
| n obs. (missing) | 4768 (58) | 1216 (1) | 1000 (1) | 216 (0) | < 0.0001 |
| Mild | 2592 (54.4%) | 642 (52.8%) | 558 (55.8%) | 84 (38.9%) | |
| Moderate | 1914 (40.1%) | 492 (40.5%) | 394 (39.4%) | 98 (45.4%) | |
| Severe | 262 (5.5%) | 82 (6.7%) | 48 (4.8%) | 34 (15.7%) | |
| Sex | |||||
| Female | 1875 (38.9%) | 471 (38.7%) | 386 (38.6%) | 85 (39.4%) | 0.87 |
| Male | 2951 (61.1%) | 746 (61.3%) | 615 (61.4%) | 131 (60.6%) | |
| Age (years) | |||||
| 00–44 | 442 (9.2%) | 140 (11.5%) | 126 (12.6%) | 14 (6.5%) | 0.01 |
| 45–64 | 1327 (27.5%) | 344 (28.3%) | 289 (28.9%) | 55 (25.5%) | |
| 65–74 | 1366 (28.3%) | 337 (27.7%) | 266 (26.5%) | 71 (32.9%) | |
| 75–84 | 1160 (24.0%) | 266 (21.8%) | 220 (22.0%) | 46 (21.3%) | |
| 85 + | 531 (11.0%) | 130 (10.7%) | 100 (10.0%) | 30 (13.9%) | |
| BMI (kg/m2) | |||||
| n obs. (missing) | 4380 (446) | 1142 (75) | 930 (71) | 212 (4) | 0.04 |
| < 18.5 | 223 (5.1%) | 53 (4.6%) | 44 (4.7%) | 9 (4.2%) | |
| 18.5–23 | 926 (21.1%) | 275 (24.1%) | 230 (24.7%) | 45 (21.2%) | |
| 23–25 | 608 (13.9%) | 162 (14.2%) | 143 (15.4%) | 19 (9.0%) | |
| 25–30 | 1385 (31.6%) | 343 (30.0%) | 273 (29.4%) | 70 (33.0%) | |
| > 30 | 1238 (28.3%) | 309 (27.1%) | 240 (25.8%) | 69 (32.6%) | |
| Diabetes | |||||
| n obs. (missing) | 4790 (36) | 1212 (5) | 996 (5) | 216 (0) | < 0.0001 |
| Yes | 2449 (51.1%) | 607 (50.1%) | 478 (48.0%) | 129 (59.7%) | |
| No | 2341 (48.9%) | 605 (49.9%) | 518 (52.0%) | 87 (40.3%) | |
| Respiratory disease | |||||
| n obs. (missing) | 4691 (135) | 1191 (26) | 975 (26) | 216 (0) | 0.14 |
| Yes | 444 (9.5%) | 115 (9.7%) | 92 (9.4%) | 23 (10.7%) | |
| Yes with O2 | 346 (7.4%) | 82 (6.9%) | 61 (6.3%) | 21 (9.7%) | |
| No | 3901 (83.1%) | 994 (83.4%) | 822 (84.3%) | 172 (79.6%) | |
| Heart failure | |||||
| n obs. (missing) | 4631 (195) | 1185 (32) | 973 (28) | 212 (4) | 0.08 |
| Yes, stage 1–2 | 670 (14.5%) | 168 (14.2%) | 129 (13.3) | 39 (18.4%) | |
| Yes, stage 3–4 | 329 (7.1%) | 75 (6.3%) | 59 (6.0%) | 16 (7.5%) | |
| No | 3632 (78.4%) | 942 (79.5%) | 785 (80.7%) | 157 (74.1%) | |
| Coronary vascular disease | |||||
| n obs. (missing) | 4693 (133) | 1187 (30) | 973 (28) | 214 (2) | 0.01 |
| Yes | 794 (16.9%) | 182 (15.3%) | 140 (14.4%) | 42 (19.6%) | |
| Yes with myocardial infarction | 461 (9.8%) | 122 (10.3%) | 93 (9.6%) | 29 (13.6%) | |
| No | 3438 (73.3%) | 883 (74.4%) | 740 (76.0%) | 143 (66.8%) | |
| Arrhythmia or conduction disorders | |||||
| n obs. (missing) | 4716 (110) | 1196 (21) | 980 (21) | 216 (0) | 0.46 |
| Yes | 1051 (22.3%) | 265 (22.2%) | 213 (21.7%) | 52 (24.1%) | |
| No | 3665 (77.7%) | 931 (77.8%) | 767 (78.3%) | 164 (75.9%) | |
| Peripheral vascular disease | |||||
| n obs. (missing) | 4606 (220) | 1176 (41) | 965 (36) | 211 (5) | 0.02 |
| Yes, stage 1–2 | 582 (12.6%) | 131 (11.1%) | 96 (9.9%) | 35 (16.6%) | |
| Yes, stage 3–4 | 456 (9.9%) | 122 (10.4%) | 101 (10.5%) | 21 (9.9%) | |
| No | 3568 (77.5%) | 923 (78.5%) | 768 (79.6%) | 155 (73.5%) | |
| Cerebrovascular accident | |||||
| n obs. (missing) | 4732 (94) | 1198 (19) | 982 (19) | 216 (0) | 0.11 |
| Yes | 527 (11.1%) | 133 (11.1%) | 104 (10.6%) | 29 (13.4%) | |
| Yes with para/hemiplegia | 67 (1.4%) | 23 (1.9%) | 22 (2.2%) | 1 (0.5%) | |
| No | 4138 (87.5%) | 1042 (87.0%) | 856 (87.2%) | 186 (86.1%) | |
| Active cancer | |||||
| n obs. (missing) | 4744 (82) | 1203 (14) | 988 (13) | 215 (1) | 0.17 |
| Yes | 428 (9.0%) | 100 (8.3%) | 77 (7.8%) | 23 (10.7%) | |
| No | 4316 (91.0%) | 1103 (91.7%) | 911 (92.2%) | 192 (89.3%) | |
| Walking | |||||
| n obs. (missing) | 4579 (247) | 1162 (55) | 953 (48) | 209 (7) | 0.73 |
| Total disability | 238 (5.2%) | 55 (4.7%) | 45 (4.7%) | 10 (4.8%) | |
| Assistance 1/3 person | 643 (14.0%) | 149 (12.8%) | 119 (12.5%) | 30 (14.3%) | |
| Autonomous | 3698 (80.8%) | 958 (82.4%) | 789 (82.8%) | 169 (80.9%) | |
| Location of dialysis treatment at COVID diagnosis | |||||
| Home | 115 (2.4%) | 29 (2.4%) | 25 (2.5%) | 4 (1.9%) | 0.80 |
| Facility-based | 4711 (97.6%) | 1188 (97.6%) | 976 (97.5%) | 212 (98.1%) | |
| Time on dialysis before infection (years) | |||||
| Mean (SD) | 5.3 (6.7) | 5.4 (7.0) | 5.2 (6.7) | 6.2 (8.3) | 0.13 |
| Median (IQR) | 3 (0–53.5) | 3 (0–53.5) | 2.9 (0–45.8) | 3.5 (0–53.5) | |
obs. Observations, IQR interquartile range
*Comparing groups with and without long-lasting symptoms
The most frequent long‐lasting clinical symptoms were extreme fatigue (52.8%) and muscle or weight loss (31.5%) (Table 2). Symptoms were isolated in 63.4% of patients, and 21.8% had two, 8.3% three, 6% four and 0.5% five cumulative symptoms.
Table 2.
Frequency of persistent symptoms
| Muscle loss or weight loss > 5% not recovered at 6 months | 52.8% |
| Extreme fatigue | 31.5% |
| Respiratory symptoms or chest pain | 14.8% |
| Post-traumatic distress syndrome, depression, anxiety | 13.0% |
| Joint or muscle pain | 9.3% |
| Sensory disorders | 8.8% |
| Diarrhea | 6.0% |
| Neuro-cognitive disorders | 5.1% |
| Headache | 8.8% |
| Tachycardia | 2.8% |
| Persistent anosmia or ageusia | 2.3% |
| Other | 3.2% |
Factors associated with the probability of having 6-month, long‐lasting clinical symptoms are reported in Table 3. As compared to asymptomatic patients or those with mild symptoms treated at home, the probability of long‐lasting clinical symptoms in subjects with moderate or severe disease with hospitalization in a medical or intensive care unit was increased by 1.64 (95% CI 1.16–2.33) and 5.03 (95% CI 2.94–8.61), respectively. As compared to patients in the oldest age group, taken as reference (≥ 85 years old), those with younger age had reduced probability of long‐lasting clinical symptoms (OR 0.40, 95% CI 0.19–0.83 for age < 45 years, 0.49 95% CI 0.28–0.85 for age 45–64 years). Each year on dialysis increased the probability by 1.03 (95% CI 1.01–1.06). Having diabetes, overweight or obesity increased the probability by 1.53 (95% CI 1.08–2.17), 1.96 (1.10–3.52) and 2.35 (1.30–4.26), respectively. The probability of long-lasting impaired general condition (extreme fatigue or weight loss, n = 160 [13.1%]) was associated with hospitalization in an intensive care unit, increased age, and low or high BMI as compared with the reference group (23–25 kg/m2), and with history of myocardial infarction.
Table 3.
Factors associated with long‐lasting clinical symptoms or impaired general condition (extreme fatigue or weight loss)
| All long‐lasting clinical symptoms N = 216 |
Impaired general condition N = 160 |
|||
|---|---|---|---|---|
| OR | 95% Wald CI | OR | 95% Wald CI | |
| Highest degree of severity | ||||
| Mild | 1 | |||
| Moderate | 1.64 | 1.16–2.33 | 1.47 | 0.99–2.18 |
| Severe | 5.03 | 2.94–8.61 | 4.30 | 2.36–7.84 |
| Age (years) | ||||
| 0–44 | 0.40 | 0.19–0.83 | 0.26 | 0.11–0.62 |
| 45–64 | 0.49 | 0.28–0.85 | 0.41 | 0.22–0.76 |
| 65–74 | 0.71 | 0.42–1.22 | 0.60 | 0.33–1.10 |
| 75–84 | 0.65 | 0.37–1.14 | 0.58 | 0.31–1.09 |
| ≥ 85 | 1 | 1 | ||
| Time on dialysis before infection (years) | 1.03 | 1.01–1.06 | 1.03 | 1.00–1.05 |
| Diabetes | 1.53 | 1.08–2.17 | ||
| BMI (kg/m2) | ||||
| < 18.5 | 1.70 | 0.68–4.24 | 3.57 | 1.23–10.33 |
| 18.5–23 | 1.70 | 0.92–3.12 | 2.74 | 1.24–6.05 |
| 23–25 | 1 | |||
| 25–30 | 1.96 | 1.10–3.52 | 3.47 | 1.61–7.49 |
| ≥ 30 | 2.35 | 1.30–4.26 | 4.99 | 2.30–10.83 |
| Coronary artery disease | 1.31 | 0.80–2.14 | ||
| Myocardial infarction | 2.05 | 1.18–3.57 | ||
OR odds ratio, CI confidence interval
Discussion
To our knowledge, this study of long‐lasting clinical symptoms related to COVID-19 is the first of a national cohort with the longest follow-up for patients on chronic dialysis recovering from COVID-19. At least one in six dialysis patients with COVID has long‐lasting clinical symptoms.
Other studies have described such long-term persistent symptoms in patients with chronic kidney disease, but these studies involved small single-center cohorts. Among 104 adults with transplantation and SARS-CoV-2 infection, 47 (45.2%) showed prolonged symptom duration and clinical complications at a median follow-up of 64 days, and 22 at 6 months [4]. In this study, diabetes and estimated glomerular filtration rate predicted the development of post–COVID-19 clinical complications. In another study of 67 kidney transplant patients, post-COVID syndrome was diagnosed in 70% at 6 months, and 26% reported at least 3 persistent symptoms [5]. In this study, persistent symptoms were more frequent in older patients and in those with more comorbidities. In this same department, 81% of hemodialysis patients reported at least one persistent symptom at 6 months [6]. In a French multicenter study, 25/189 (13%) patients exhibited post–COVID-19 cachexia, which was more frequent in patients with low initial albuminemia [7].
The most common symptom in our patients was weight or muscle loss (53%), consequences that have seldom been explored in other studies. As in other studies, extreme fatigue and weakness was frequently observed: 32% in our dialysis patients versus 61% in the Gdansk hemodialysis population [6] or 43% in the Gdansk transplanted population [5], and 39% to 73% in a systematic review [1]. Breathlessness is often recorded in the general population (39–74%) [1] but is less common in dialysis patients: 15% in our study and 34% in the Gdansk study. Overall, 13% of our patients exhibited depression and/or anxiety.
The severity of the initial SARS-Cov-2 infection was associated with long-lasting symptoms, especially for patients who were admitted to an intensive care unit. This finding may not be specific to COVID-19 dialysis patients because survivors of critical illness frequently have long-term physical, neuropsychiatric, and quality-of-life impairments [11]. Similar to other studies, we found persistent symptoms in frail patients such as older patients or in those with comorbidities or long duration on dialysis.
Long lasting symptoms in CKD patients tend to be similar to those of the general population. In a recent systematic review that included 6 studies with more than 6 months of follow-up (2112 patients), fatigue (25–61%), dyspnea (13–48%), cough (2–13%), arthralgia (15%), thoracic pain (9%), and smell and taste disorders (3–24%) were the most frequent persisting symptoms over 6 months [12]. The high prevalence of long-lasting COVID fatigue can be compared to other outcomes after acute systemic infections, such as mononucleosis, and underlying medical and mental health conditions should be excluded [13]. Research has made substantial progress, and some medical interventions can be now proposed [14, 15].
The strength of our study is that the data were obtained from a national registry. However, our results should be interpreted with caution since, first of all, defining symptoms as sequelae 6 months after infection should be done with great caution. Some symptoms are frequent in the dialysis population and could be part of the natural disease evolution. Moreover, patients may have changed their treatment unit, so assessing a change, or lack thereof in comparison to the previous 6 months is difficult. Second, given the number of surveys that were returned, we cannot exclude some selection bias. However, this bias could be in favor of fewer sequelae (the survey is easier to answer when there is a lack of sequelae, or perhaps the staff in centers with a great burden of COVID-19 patients could be exhausted and not willing to answer the survey), or nephrologists who were more aware of the possibility of sequelae may have been more willing to complete the survey. Third, the number of patients limited our analysis of factors associated with long‐lasting clinical symptoms, and the lack of power could explain the non-identification of some associations found in the general population. Fourth, our survey was completed by nephrologists and research assistants and was not based on a standardized form directly filled in by the patients. Finally, the low rate of return can be explained by the difficult working conditions in this time of crisis and the priority given to the registration of all COVID-19 cases.
Systematic screening of long‐lasting clinical symptoms in dialysis patients would allow us to identify those who need more careful prevention and long-term care and to address them towards rehabilitation pathways [16, 17]. Older people are at increased risk of having poorer general conditions and require a greater amount of care. We need studies investigating the protective effect of vaccination on the incidence of long‐lasting clinical symptoms after SARS-Cov-2 infection in dialysis patients.
Acknowledgements
We gratefully acknowledge all participants of the REIN registry, nephrologists and research assistants alike, especially at this very particular time. The centers participating in the registry are listed in the REIN annual reporthttp://www.agence-biomedecine.fr/Le-programme-REIN.
Declarations
Conflict of interest
No authors have any conflict of interest to declare.
Ethical statement
The results presented in this paper have not been published previously in whole or part.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Willi S, Lüthold R, Hunt A, Hänggi NV, Sejdiu D, Scaff C et al (2021) COVID-19 sequelae in adults aged less than 50 years: a systematic review. Travel Med Infect Dis 40:101995 [DOI] [PMC free article] [PubMed]
- 2.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR et al (2021) Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 4(10):e2128568 [DOI] [PMC free article] [PubMed]
- 3.Nasserie T, Hittle M, Goodman SN (2021) Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 4(5):e2111417 [DOI] [PMC free article] [PubMed]
- 4.Basic-Jukic N, Juric I, Furic-Cunko V, Katalinic L, Radic J, Bosnjak Z, et al. Follow-up of renal transplant recipients after acute COVID-19—A prospective cohort single-center study. Immun Inflamm Dis. 2021;9(4):1563–1572. doi: 10.1002/iid3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malinowska A, Muchlado M, Ślizień Z, Biedunkiewicz B, Heleniak Z, Dębska-Ślizień A, et al. Post-COVID-19 sydrome and decrease in health-related quality of life in kidney transplant recipients after SARS-COV-2 infection—a cohort longitudinal study from the north of Poland. J Clin Med. 2021;10(21):5205. doi: 10.3390/jcm10215205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Och A, Tylicki P, Polewska K, Puchalska-Reglińska E, Parczewska A, Szabat K, et al. Persistent post-COVID-19 syndrome in hemodialyzed patients—a longitudinal cohort study from the north of Poland. J Clin Med. 2021;10(19):4451. doi: 10.3390/jcm10194451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakhi H, Chawki S, Buchard A, Dardim K, Boulanger H, Mokhtar C, et al. Impact à long terme du COVID-19 chez les patients dialysés. Néphrologie Thérapeutique. 2021;17(5):269–270. doi: 10.1016/j.nephro.2021.07.273. [DOI] [Google Scholar]
- 8.Couchoud C, Stengel B, Landais P, Aldigier JC, de CF, Dabot C et al (2006) The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant 21(0931–0509 (Print)):411–418 [DOI] [PubMed]
- 9.Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int. 2020;98(6):1519–1529. doi: 10.1016/j.kint.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapalu S, Izaaryene G, Honoré N, Couchoud C. Le rôle du registre national REIN en France dans la veille sanitaire des patients en insuffisance rénale chronique terminale infectés par le SARS-CoV-2: organisation et premières données. Néphrologie Thérapeutique. 2021;17(4):218–225. doi: 10.1016/j.nephro.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39(2):371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NN, Hoang VT, Dao TL, Dudouet P, Eldin C, Gautret P (2022) Clinical patterns of somatic symptoms in patients suffering from post-acute long COVID: a systematic review. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol [DOI] [PMC free article] [PubMed]
- 13.Sandler CX, Wyller VBB, Moss-Morris R, Buchwald D, Crawley E, Hautvast J, et al. Long COVID and Post-infective Fatigue Syndrome: A Review. Open Forum Infect Dis. 2021 Oct;8(10):ofab440. [DOI] [PMC free article] [PubMed]
- 14.Yong SJ, Liu S (2021) Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev Med Virol e2315 [DOI] [PubMed]
- 15.Vance H, Maslach A, Stoneman E, Harmes K, Ransom A, Seagly K, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med JABFM. 2021;34(6):1229–1242. doi: 10.3122/jabfm.2021.06.210254. [DOI] [PubMed] [Google Scholar]
- 16.Barker-Davies RM, O’Sullivan O, Senaratne KPP, Baker P, Cranley M, Dharm-Datta S, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54(16):949–959. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):89. doi: 10.2340/16501977-2727. [DOI] [PubMed] [Google Scholar]


