This is the fifth and final update for the living, rapid review on remdesivir for adults with COVID-19. It is a major update that summarizes randomized controlled trials that were published before 19 October 2021.
Abstract
Background:
Remdesivir is approved for the treatment of adults hospitalized with COVID-19.
Purpose:
To update a living review of remdesivir for adults with COVID-19.
Data Sources:
Several electronic U.S. Food and Drug Administration, company, and journal websites from 1 January 2020 through 19 October 2021.
Study Selection:
English-language, randomized controlled trials (RCTs) of remdesivir for COVID-19.
Data Extraction:
One reviewer abstracted, and a second reviewer verified data. The Cochrane Risk of Bias Tool and GRADE (Grading of Recommendations Assessment, Development and Evaluation) method were used.
Data Synthesis:
Since the last update (search date 9 August 2021), 1 new RCT and 1 new subtrial comparing a 10-day course of remdesivir with control (placebo or standard care) were identified. This review summarizes and updates the evidence on the cumulative 5 RCTs and 2 subtrials for this comparison. Our updated results confirm a 10-day course of remdesivir, compared with control, probably results in little to no mortality reduction (5 RCTs). Updated results also confirm that remdesivir probably results in a moderate increase in the proportion of patients recovered by day 29 (4 RCTs) and may reduce time to clinical improvement (2 RCTs) and hospital length of stay (4 RCTs). New RCTs, by increasing the strength of evidence, lead to an updated conclusion that remdesivir probably results in a small reduction in the proportion of patients receiving ventilation or extracorporeal membrane oxygenation at specific follow-up times (4 RCTs). New RCTs also alter the conclusions for harms—remdesivir, compared with control, may lead to a small reduction in serious adverse events but may lead to a small increase in any adverse event.
Limitation:
The RCTs differed in definitions of COVID-19 severity and outcomes reported.
Conclusion:
In hospitalized adults with COVID-19, the findings confirm that remdesivir probably results in little to no difference in mortality and increases the proportion of patients recovered. Remdesivir may reduce time to clinical improvement and may lead to small reductions in serious adverse events but may result in a small increase in any adverse event.
Primary Funding Source:
U.S. Department of Veterans Affairs.
This is the fifth update of our living, rapid review on remdesivir for adults with COVID-19 (1). Remdesivir, a nucleotide analogue prodrug that inhibits viral RNA (2), is approved by the U.S. Food and Drug Administration for the treatment of adults hospitalized with COVID-19 (3). Our first update, which included randomized controlled trials (RCTs) published through 7 December 2020 (4–8), led to a major update (9). Our second update, including RCTs published through 8 February 2021, found no new evidence (10). Our third update (11) derived from RCTs published through 10 May 2021 included 1 new RCT (12), and our fourth update of RCTs published through 9 August 2021 (13) included 1 new add-on subtrial of the World Health Organization (WHO) Solidarity trial—the Norwegian Solidarity trial (14). On the basis of the results from these RCTs, we had previously concluded that a 10-day course of remdesivir probably results in little to no difference in mortality but probably reduces serious adverse events and may reduce time to recovery in hospitalized patients. Two RCTs found that a 10-day course was not more effective than a 5-day course for moderate and severe disease (6, 7).
This fifth quarterly update including RCTs published through 19 October 2021 is the final update for this living review according to the preplanned protocol. It summarizes information on remdesivir from 2 newly published RCTs by Ader and colleagues (DisCoVeRy [Trial of Treatments for COVID-19 in Hospitalized Adults]; subtrial) (15) and Abd-Elsalam and colleagues (16) alongside previous updates. We update previous analyses and certainty of evidence (COE) and conduct cumulative meta-analyses, where feasible. In addition, we report on results of SARS-CoV-2 clearance.
Methods
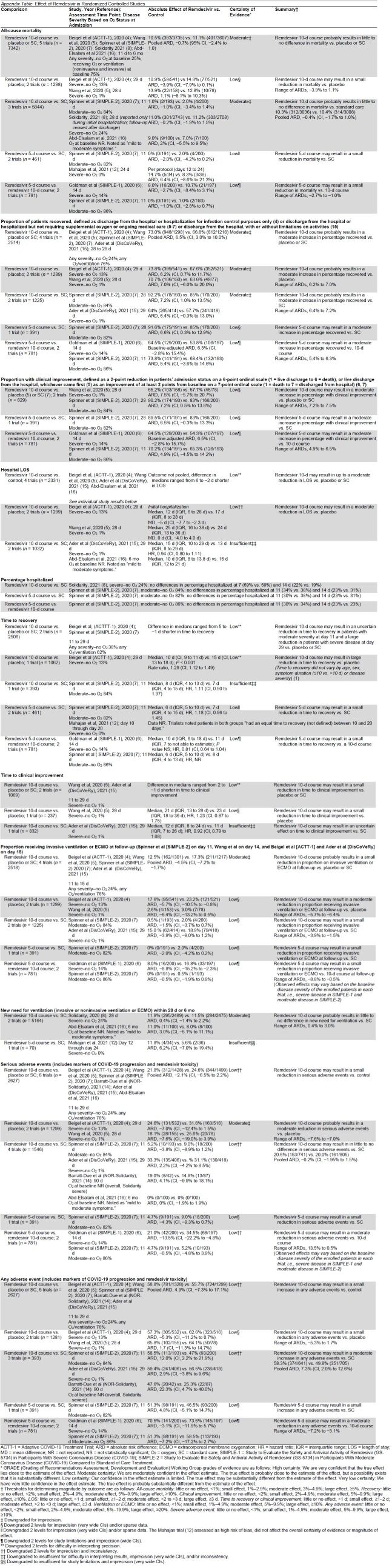
We included RCTs evaluating remdesivir for adults with COVID-19 using methods identical to those described previously (1, 9). Our literature search was updated to include RCTs published through 19 October 2021 and used the original search strategies and inclusion criteria (Supplement Table 1). DisCoVeRy (15) is a subtrial of Solidarity. Results on some patients were also included in the published Solidarity report (8). Hence, for outcomes that were reported by both DisCoVeRy and Solidarity (mortality and new need for ventilation), we did not include DisCoVeRy data in our main analyses to avoid double counting persons. The DisCoVeRy authors provided us data for DisCoVeRy participants who were not previously included in published Solidarity results for these end points (Mentré F. Personal communication.). We used these unpublished data to conduct sensitivity analyses. For outcomes not reported in Solidarity (proportion recovered, proportion on ventilation at follow-up, and adverse events), we included data of all DisCoVeRy patients in our main analyses. Tools to assess risk of bias (17) and estimate COE (18) were unchanged (Supplement Tables 2 and 9). The definitions of our a priori–defined outcomes, both critical (mortality, proportion recovered, hospital length of stay, and serious adverse events) and important (time to clinical improvement, need for ventilation or extracorporeal membrane oxygenation [ECMO], and any adverse event) and our a priori–established thresholds for estimating effect magnitude for these outcomes were also unchanged (Appendix Table, footnote) (1).
Appendix Table.
Effect of Remdesivir in Randomized Controlled Studies
Data Synthesis and Analysis
We conducted a cumulative meta-analysis combining data from previous updates with data from the newly identified RCTs when outcomes were reported in at least 3 trials and calculated relative and absolute measures of effect with corresponding 95% CIs. We used a fixed-effects model when outcomes were reported by fewer than 5 trials and a random-effects model (Hartung–Knapp–Sidik–Jonkman) when 5 or more trials reported on the outcome. Data were analyzed in R (R Foundation) (19). The magnitude of statistical heterogeneity was assessed with the I 2 statistic (I 2 > 75% may indicate substantial heterogeneity) (20).
We include updated meta-analyses, incorporating data from the newly published RCTs for the outcomes of mortality (all severity COVID-19), proportion recovered, proportion receiving mechanical ventilation at follow-up, serious adverse events, and any adverse event. We describe findings for SARS-CoV-2 clearance. Although this outcome was deemed an intermediate, nonclinical outcome, we include this information to address uncertainty about the effect of remdesivir on viral clearance and the potential implications on use of remdesivir on the basis of COVID-19 symptom duration.
Role of the Funding Source
This work is based on a living, rapid review done for the U.S. Department of Veterans Affairs (VA) Evidence Synthesis Program that concludes with this update (21). Funding for that review was provided by the Veterans Health Administration Office of Research and Development, Health Services Research and Development Service. The funding source assigned the topic but was not involved in data collection, analysis, manuscript preparation, or submission.
Results
The updated literature search identified 426 citations (Appendix Figure). We identified 2 new eligible publications: a subtrial by Ader and colleagues (DisCoVeRy) (15) and an RCT by Abd-Elsalam and colleagues (16). When added to the previous update that includes 6 RCTs and a subtrial, there are a total of 7 RCTs and 2 subtrials that assess effectiveness of remdesivir for COVID-19 (4–8, 12, 14–16).
Appendix Figure. Evidence search and selection.
RCT = randomized controlled trial.
Overview of All Randomized Trials (9 Trials)
Of the 7 RCTs and 2 subtrials evaluating remdesivir for COVID-19, the 2 new studies (1 RCT and 1 subtrial) add to the previous 5 studies (4 RCTs and 1 subtrial) comparing a 10-day course of remdesivir with control (placebo or standard care [SC]) (4, 5, 8, 14–16). Hence, our updated analyses focus on the 5 RCTs and 2 subtrials comparing a 10-day course of remdesivir with control (4, 5, 7, 8, 14–16). The remaining comparisons between 5-day course and either a 10-day course and/or SC (6, 7) did not have any new evidence. The previous summaries and conclusions from these are presented in summary tables. Details about study characteristics, outcomes, and harms are reported in Supplement Tables 3 to 8, and information on risk of bias is presented in Supplement Table 9.
New Findings From Ader and Colleagues (DisCoVeRy) and Abd-Elsalam and Colleagues
Ader and Colleagues (DisCoVeRy)
DisCoVeRy was a multicenter, open-label subtrial of Solidarity (15) done in Europe (Supplement Table 3). DisCoVeRy (n = 832) compared a 10-day course of remdesivir with SC for adults hospitalized with laboratory-confirmed COVID-19 with clinical hypoxia or need for oxygen supplementation (Supplement Table 10). Results from some DisCoVeRy participants (53%) had been included in the published Solidarity report (8). The primary outcome for the DisCoVeRy trial was clinical status at day 15 measured using the WHO 7-point ordinal scale, an outcome not reported in the Solidarity trial. Median symptom duration was 9 days. At baseline, nearly all patients were receiving at least supplemental oxygen, and 40% were receiving corticosteroids. Compared with SC, remdesivir did not result in a statistically significant improvement in clinical status on day 15 (odds ratio, 0.98 [95% CI, 0.77 to 1.25]). In addition, there was no statistically significant difference between remdesivir and SC in time to improvement, length of hospitalization, proportion needing ventilation on day 15, 28-day mortality, serious adverse events, or any adverse events. There was no effect of remdesivir on SARS-CoV-2 kinetics measured in the nasopharynx.
Abd-Elsalam and Colleagues
The study by Abd-Elsalam and colleagues was an open-label RCT done in Egypt (Supplement Table 3) (16). The study (n = 200) compared a 10-day course of remdesivir with SC for adults hospitalized with laboratory-confirmed COVID-19. The primary outcomes were length of hospital stay and mortality. The median symptom duration and patient stratification by baseline oxygen requirements were not reported. However, the mean baseline oxygen saturation, reported as 88.5%, was consistent with the National Institutes of Health and WHO definition of severe COVID-19 (Supplement Table 10). Remdesivir, compared with SC, resulted in a statistically significant reduction in median duration of hospitalization (10 vs. 16 days; P < 0.001) but did not reduce mortality (9% vs. 7%; P = 0.602). Remdesivir did not affect new need for ventilation. No serious adverse events were noted in either group.
Summary Findings
For summary findings, see Figures 1 and 2, the Table, the Appendix Table, and Supplement Tables 1 to 10.
Figure 1. Mortality for remdesivir 10-day course versus control (placebo or standard care).
ACTT-1 = Adaptive COVID-19 Treatment Trial; RR = risk ratio; SIMPLE-2 = Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment.
Figure 2. Nonmortality outcomes for remdesivir 10-day course versus control (placebo or standard care).
ACTT-1 = Adaptive COVID-19 Treatment Trial; DisCoVeRy = Trial of Treatments for COVID-19 in Hospitalized Adults; ECMO = extracorporeal membrane oxygenation; RR = risk ratio; SIMPLE-2 = Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment. Top. Proportion recovered. Middle. Need for invasive ventilation or ECMO. Bottom. Patients with ≥1 serious adverse events.
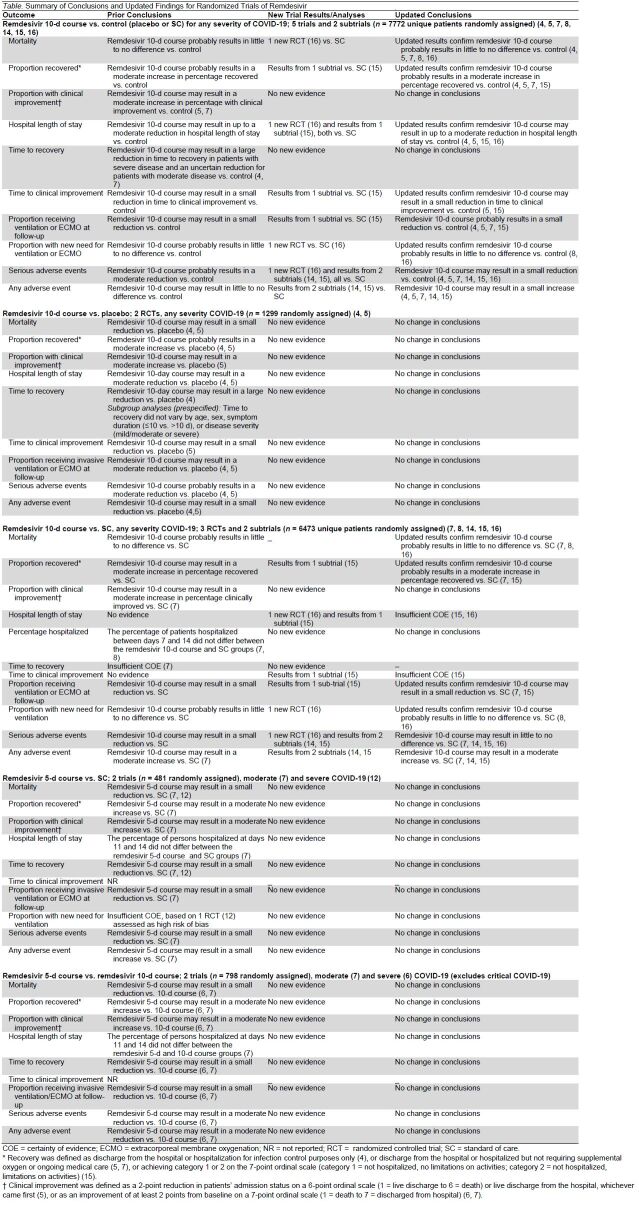
Table.
Summary of Conclusions and Updated Findings for Randomized Trials of Remdesivir
Remdesivir 10-Day Course Compared With Control (Placebo or SC) (7 Trials)
Of the 5 trials and 2 subtrials comparing a 10-day course of remdesivir with control, 2 used a placebo (4, 5) and 5 used SC as the control (7, 8, 14–16) (Supplement Table 3). Five RCTs included patients with severe and critical COVID-19 (4, 5, 8, 14, 15), 1 RCT included only patients with moderate disease (7), and 1 RCT included patients with unclear severity of disease (16). Six studies had a low risk of bias (4, 5, 7, 14–16), and 1 had a moderate risk of bias (8).
All-Cause Mortality
Our updated analyses, including new results from Abd-Elsalam and colleagues (16), confirm that remdesivir compared with control (placebo or SC) probably results in little to no difference in mortality (absolute risk difference [ARD], −0.7% [CI, −2.4% to 1.0%]) (moderate COE) (4, 5, 7, 8, 16) (Figure 1). A sensitivity analysis, with the addition of the results from the 392 patients from DisCoVeRy that were not previously included in the published Solidarity report, produced results similar to those of the primary analysis. Prior subgroup analyses for mortality by baseline oxygen support requirements remain unchanged (9) because the newly included RCT (16) did not stratify patients by baseline disease severity or oxygen needs.
Proportion of Patients Recovered
Updated analyses, including new results from DisCoVeRy (15), confirm that remdesivir, compared with control, probably results in a moderate increase in the percentage of patients who recovered by day 28 or 29 (ARD, 6.5% [CI, 3% to 10%]) (moderate COE) (4, 5, 7, 15) (Figure 2, A). Recovery was defined as not hospitalized (15), discharged from the hospital or hospitalization for infection control purposes only (4), or discharged from the hospital or hospitalized but not requiring supplemental oxygen or ongoing medical care (5, 7).
Proportion with Clinical Improvement
No new studies provided data on this outcome. Hence, our prior conclusion that remdesivir, compared with control, may result in a moderate increase in the proportion with clinical improvement by day 28 is unchanged (range of ARDs, 7.2% to 7.5%; 2 RCTs) (low COE) (5, 7).
Hospital Length of Stay
Updated analyses, including results from 2 new studies versus SC—DisCoVeRy and Abd-Elsalam and colleagues (15, 16)—show that remdesivir may result in up to a moderate reduction in hospital length of stay compared with control (low COE) (4, 5, 15, 16).
Percentage of Patients Hospitalized
No new studies provided data on this outcome. Our prior conclusion that remdesivir, compared with control, may not decrease the percentage of patients hospitalized between days 7 and 14 is unchanged (2 RCTs) (low COE) (7, 8).
Time to Recovery
No new studies provided data on this outcome. Our prior conclusion that remdesivir, compared with control, may result in a large reduction in patients with severe disease and an uncertain reduction in time to recovery in patients with moderate disease remains unchanged (2 RCTs) (low COE) (4, 7).
Time to Clinical Improvement
Updated analyses, including new results from DisCoVeRy (15), confirm that remdesivir, compared with control, may result in a small reduction in median time to clinical improvement (clinical improvement was defined as days to improvement of 2 categories of the 7-point ordinal scale, ranging from 1 = not hospitalized and no limitations on activities to 7 = death) or hospital discharge up to day 29 (low COE) (5, 15).
Need for Ventilation or ECMO
Proportion Receiving Ventilation or ECMO at Follow-up
Our updated analyses, including new results from DisCoVeRy (15), show that remdesivir, compared with control, probably results in a small reduction in the proportion of patients receiving ventilation or ECMO at specific time points between days 11 and 15 (ARD, −4.5% [CI, −7.2% to −1.7%]) (moderate COE) (Figure 2, B) (4, 5, 7, 15).
New Need for Ventilation or ECMO
The inclusion of results from Abd-Elsalam and colleagues' study (16) with Solidarity confirms our prior conclusion that remdesivir, compared with control, probably results in little to no difference in new need for ventilation or ECMO within 28 days or 6 months (range of ARDs, 0.4% to 3.0%; 2 RCTs) (moderate COE) (Figure 2, B) (8, 16). On the basis of a sensitivity analysis, which included information from DisCoVeRy patients not previously included in the published Solidarity report, we concluded (due to inconsistency between RCT results) that remdesivir, compared with control, may result in little to no difference in new need for ventilation between 28 days to 6 months (3 RCTs) (8, 15, 16).
Adverse Events
Updated meta-analyses, including results from additional RCTs (14–16), show that remdesivir, compared with control, may lead to a small reduction in serious adverse events (ARD, −2.1% [CI, −6.5% to 2.2%]) (low COE) (Figure 2, C) (4, 5, 7, 14–16). Our last update found that remdesivir versus control probably results in a moderate reduction in serious adverse events (9). There was variation in how the trials reported serious adverse events, often including a combination of direct remdesivir toxicity and clinical findings consistent with COVID-19 progression (such as respiratory failure and need for endotracheal intubation). Updated meta-analyses, including results from 2 new RCTs (14, 15), show that remdesivir, compared with control, may result in a small increase in any adverse event (ARD, 4.9% [CI, −7.3% to 17.1%]; 5 RCTs) (low COE) (4, 5, 7, 14, 15).
Viral Clearance
Three RCTs assessed the effect of remdesivir on SARS-CoV-2 kinetics in the respiratory tract (5, 14, 15)—an intermediate outcome not assessed for COE (Supplement Table 6). All studies measured SARS-CoV-2 viral loads sequentially for 14 to 28 days after randomization using a quantitative, real-time, reverse transcriptase polymerase chain reaction test. All 3 RCTs showed that regardless of specimen site or collection methods (upper or lower airways; nasopharyngeal or oropharyngeal swabs or expectorated sputum), there was no statistically significant difference in the kinetics of SARS-CoV-2 load with remdesivir compared with control. All 3 RCTs also showed that the effect of remdesivir on SARS-CoV-2 clearance did not vary by symptom duration (stratified as ≤10 or >10 days [5]; <7 or ≥7 days [14]; or ≤7 days, −14 days, or >14 days [15]) or by baseline oxygen requirements (15).
Duration of Remdesivir Therapy: 5 Versus 10 Days (2 Trials)
No new RCTs compared a 5-day with a 10-day course of remdesivir. Hence, our prior conclusions, based on 2 RCTs (6, 7), remain unchanged (9) (Table and Appendix Table).
Discussion
This final update of our living review updates some findings comparing the effect of a 10-day course of remdesivir with control (placebo or SC) (4, 5, 7, 8, 14–16). The newly included RCTs strengthen previous findings on the benefit of remdesivir on the proportion of patients receiving ventilation or ECMO at follow-up but decreases the strength of previous findings on the reduction of serious adverse events with remdesivir. Another major change for this update was the low certainty of an increase in any adverse event with remdesivir (compared with a previous finding of little or no change in any adverse event). Other findings of the effect of a 10-day course of remdesivir (intervention) compared with either placebo or SC (control) are confirmed or unchanged.
Despite the reported strong antiviral effect of remdesivir against SARS-CoV-2 in preclinical models (22), 3 RCTs consistently show that remdesivir does not accelerate viral clearance in upper or lower airways compared with control, regardless of symptom duration. Another study published after our search date reported similar findings among outpatients with COVID-19 with symptoms for 7 days or less (23). These results suggest that remdesivir's effectiveness is not related to viral load clearance and that using SARS-CoV-2 clearance in upper and lower airways is not a valid surrogate for clinical outcomes (24).
Cost-effectiveness models assume that remdesivir shortens duration of hospitalization for patients with COVID-19 (25). Contrary to this assumption, 1 large propensity-matched retrospective cohort study among veterans hospitalized at VA medical centers (n = 2344) found that remdesivir treatment was associated with prolonged hospitalization without improved survival (26). The clustering of discharges suggested that patients ready for medical discharge were hospitalized solely to complete the prescribed course of remdesivir—a practice inconsistent with RCT protocols and treatment guidelines (4, 27, 28).
Given that this is our last living review update, we note ongoing trials of remdesivir for COVID-19 evaluating formulations and populations not previously studied, which may alter practice and policy. These include inhaled and oral formulations of remdesivir and studies including previously excluded populations (pregnant women, children, and patients with renal dysfunction) (29). In addition, 1 placebo-controlled RCT was published after our last search date, which is the only study done among outpatient adults and assessing the effect on hospitalizations. The study evaluated remdesivir given intravenously daily for 3 days to high-risk, unvaccinated outpatients with COVID-19 with 7 days or less of symptoms (23). Compared with placebo, remdesivir reduced COVID-19–related hospitalization at day 28 (0.7% [2 of 279] vs. 5.3% [15 of 283]; P = 0.008). There were no deaths in either group. The study enrolled patients before the emergence of the Delta or Omicron variants of SARS-CoV-2 as the dominant strain and was terminated early due to “the changing epidemiology and adoption of additional treatment options at the time” (23, 30).
In conclusion, in hospitalized adults with COVID-19, remdesivir probably results in little to no difference in mortality. However, remdesivir probably increases the proportion of patients recovered and may reduce time to clinical improvement and length of hospitalization. Remdesivir may lead to a small reduction in serious adverse events but may lead to a small increase in any adverse event. Compared with a 5-day course of remdesivir, a 10-day course may have little to no benefit and has higher drug cost among patients not requiring mechanical ventilation or ECMO.
Supplementary Material
Footnotes
This article was published at Annals.org on 1 March 2022.
References
- 1. Wilt TJ , Kaka AS , MacDonald R , et al. Remdesivir for adults with COVID-19. A living systematic review for American College of Physicians practice points. Ann Intern Med. 2021;174:209-220. [PMID: ] doi: 10.7326/M20-5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sheahan TP , Sims AC , Leist SR , et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. [PMID: ] doi: 10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration. FDA's approval of Veklury (remdesivir) for the treatment of COVID-19—the science of safety and effectiveness. Accessed at www.fda.gov/drugs/news-events-human-drugs/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness on 14 November 2021.
- 4. Beigel JH , Tomashek KM , Dodd LE , et al; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. [PMID: ] doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y , Zhang D , Du G , et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [PMID: ] doi: 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldman JD , Lye DCB , Hui DS , et al; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827-1837. [PMID: ] doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spinner CD , Gottlieb RL , Criner GJ , et al; GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048-1057. [PMID: ] doi: 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan H , Peto R , , et al; WHO Solidarity Trial Consortium. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity Trial Results. N Engl J Med. 2021;384:497-511. [PMID: ] doi: 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaka AS , MacDonald R , Greer N , et al. Major update: remdesivir for adults with COVID-19. A living systematic review and meta-analysis for the American College of Physicians practice points. Ann Intern Med. 2021;174:663-672. [PMID: ] doi: 10.7326/M20-8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaka AS, MacDonald R, Greer N, et al. Surveillance update – 02/08/21. Accessed at www.acpjournals.org/doi/full/10.7326/M20-8148 on 11 August 2021.
- 11. Kaka AS , MacDonald R , Linskens EJ , et al. Update alert: remdesivir for adults with COVID-19 [Letter]. Ann Intern Med. 2021;174:W65. [PMID: ] doi: 10.7326/L21-0375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahajan L , Singh AP , . Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: a prospective randomised study. Indian J Anaesth. 2021;65:S41-S46. [PMID: ] doi: 10.4103/ija.IJA_149_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaka AS , MacDonald R , Linskens EJ , et al. Update alert 2: remdesivir for adults with COVID-19 [Letter]. Ann Intern Med. 2021;174:W114-W115. [PMID: ] doi: 10.7326/L21-0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barratt-Due A , Olsen IC , Nezvalova-Henriksen K , et al; NOR-Solidarity trial. Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19. A randomized trial. Ann Intern Med. 2021;174:1261-1269. [PMID: ] doi: 10.7326/M21-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ader F , Bouscambert-Duchamp M , Hites M , et al; DisCoVeRy Study Group. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2021. [PMID: ] doi: 10.1016/S1473-3099(21)00485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abd-Elsalam S , Ahmed OA , Mansour NO , et al. Remdesivir efficacy in COVID-19 treatment: a randomized controlled trial. Am J Trop Med Hyg. 2021. [PMID: ] doi: 10.4269/ajtmh.21-0606 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; 2011.
- 18. Schünemann H, Brożek J, Guyatt G, et al, eds. GRADE handbook. Accessed at https://gdt.gradepro.org/app/handbook/handbook.html on 29 October 2021.
- 19. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1-48. doi: 10.18637/jss.v036.i03 [DOI]
- 20. Higgins JP , Thompson SG , Deeks JJ , et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilt TJ, Kaka AS, MacDonald R, et al. Rapid response: COVID-19: remdesivir for hospitalized adults. Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs; 2020. VA ESP Project #09-009.
- 22. Bimonte S , Crispo A , Amore A , et al. Potential antiviral drugs for SARS-CoV-2 treatment: preclinical findings and ongoing clinical research. In Vivo. 2020;34:1597-1602. [PMID: ] doi: 10.21873/invivo.11949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gottlieb RL , Vaca CE , Paredes R , et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-315. [PMID: ] doi: 10.1056/NEJMoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sax PE. HIV and ID observations. Accessed at https://blogs.jwatch.org/hiv-id-observations/ on 8 November 2021.
- 25. Institute for Clinical and Economic Review. ICER provides second update to pricing models for remdesivir as a treatment for COVID-19. Accessed at https://icer.org/news-insights/press-releases/icer-provides-second-update-to-pricing-models-for-remdesivir-as-a-treatment-for-covid-19 on 9 November 2021.
- 26. Ohl ME , Miller DR , Lund BC , et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw Open. 2021;4:e2114741. [PMID: ] doi: 10.1001/jamanetworkopen.2021.14741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Institutes of Health. COVID-19 treatment guidelines. Accessed at www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/ on 9 November 2021. [PubMed]
- 28. Baracco GJ . Remdesivir use and hospital length of stay-the paradox of a clinical trial vs real-life use. JAMA Netw Open. 2021;4:e2116057. [PMID: ] doi: 10.1001/jamanetworkopen.2021.16057 [DOI] [PubMed] [Google Scholar]
- 29. U.S. National Library of Medicine. ClinicalTrials.gov. Accessed at https://clinicaltrials.gov/ct2/results?cond=remdesivir&term=&cntry=&state=&city=&dist= on 9 November 2021.
- 30. Gilead Sciences. Study to evaluate the efficacy and safety of remdesivir (GS-5734™) treatment of coronavirus disease 2019 (COVID-19) in an outpatient setting [clinical trial]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04501952 on 9 November 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Figure 1. Mortality for remdesivir 10-day course versus control (placebo or standard care). ACTT-1 = Adaptive COVID-19 Treatment Trial; RR = risk ratio; SIMPLE-2 = Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/31c2/8924790/0c8411c655b6/aim-olf-M214784-M214784ff1.jpg)
![Figure 2. Nonmortality outcomes for remdesivir 10-day course versus control (placebo or standard care). ACTT-1 = Adaptive COVID-19 Treatment Trial; DisCoVeRy = Trial of Treatments for COVID-19 in Hospitalized Adults; ECMO = extracorporeal membrane oxygenation; RR = risk ratio; SIMPLE-2 = Study to Evaluate the Safety and Antiviral Activity of Remdesivir [GS-5734] in Participants With Moderate Coronavirus Disease [COVID-19] Compared to Standard of Care Treatment. Top. Proportion recovered. Middle. Need for invasive ventilation or ECMO. Bottom. Patients with ≥1 serious adverse events.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/31c2/8924790/bd0d200a0bda/aim-olf-M214784-M214784ff2.jpg)