Abstract
If the tendency to discount rewards reflects individuals' general level of impulsiveness, then the discounting of delayed and probabilistic rewards should be negatively correlated: The less a person is able to wait for delayed rewards, the more they should take chances on receiving probabilistic rewards. It has been suggested that damage to the ventromedial prefrontal cortex (vmPFC) increases individuals' impulsiveness, but both intertemporal choice and risky choice have only recently been assayed in the same patients with vmPFC damage. Here, we assess both delay and probability discounting in individuals with vmPFC damage (n = 8) or with medial temporal lobe (MTL) damage (n = 10), and in age- and education-matched controls (n = 30). On average, MTL-lesioned individuals discounted delayed rewards at normal rates but discounted probabilistic rewards more shallowly than controls. In contrast, vmPFC-lesioned individuals discounted delayed rewards more steeply but probabilistic rewards more shallowly than controls. These results suggest that vmPFC lesions affect the weighting of reward amount relative to delay and certainty in opposite ways. Moreover, whereas MTL-lesioned individuals and controls showed typical, nonsignificant correlations between the discounting of delayed and probabilistic rewards, vmPFC-lesioned individuals showed a significant negative correlation, as would be expected if vmPFC damage increases impulsiveness more in some patients than in others. Although these results are consistent with the hypothesis that vmPFC plays a role in impulsiveness, it is unclear how they could be explained by a single mechanism governing valuation of both delayed and probabilistic rewards.
INTRODUCTION
People must often make decisions involving future and/or risky outcomes that require them to choose between smaller-immediate and larger-delayed rewards or between smaller-certain and larger-probabilistic rewards. Such decisions are modeled in the laboratory using tasks that measure delay and probability discounting, where the terms delay discounting and probability discounting, respectively, refer to the finding that increasing the time to a future reward and/or decreasing the likelihood of a probabilistic reward decrease the reward's subjective value.
It has been proposed that a common mechanism (impulsive decision-making) underlies both delay discounting and probability discounting. After all, rewards available after longer delays are actually less certain than immediate rewards, and both types of discounting are well described by a hyperboloid function (Green & Myerson, 2004). However, previous research has shown that delay and probability discounting respond in opposite ways to manipulations of reward amount, and also reflect relatively independent traits in healthy adults, as evidenced by the finding that the tendency to discount delayed rewards is often uncorrelated with the tendency to discount probabilistic rewards (for a review, see Green & Myerson, 2013). As a result, exactly how these two types of discounting are related remains a matter of dispute.
Examining neural mechanisms of decision-making could shed light on the relation between delay and probability discounting. Functional neuroimaging experiments on delay discounting (e.g., Benoit, Gilbert, & Burgess, 2011; Peters & Büchel, 2010) show that the effects of episodic imagining on the value of future rewards are mediated by increased activity and coordination between the ventromedial prefrontal cortex (vmPFC) and the medial temporal lobes (MTLs). Direct support for a vmPFC role in value-based decisions comes from studies showing that lesions to the vmPFC may impact subjective valuation and weighing of key visual attributes pertinent to the process involved in reward-driven decision-making (Vaidya, Sefranek, & Fellows, 2018; Vaidya & Fellows, 2015). Indeed, Seaman et al. (2018) found that subjective valuation of different decision types—delay, probability, and effort-based discounting—share overlapping activity in the medial PFC after differences in participants' discount rates across the three tasks are taken into consideration.
Activation of the vmPFC has further been shown to occur during value comparison and when evaluating differences between outcomes (i.e., magnitude, immediate availability; Hare, Hakimi, & Rangel, 2014; Boorman, Rushworth, & Behrens, 2013) and various categories or perceptual inputs of rewards (Bartra, McGuire, & Kable, 2013; Levy & Glimcher, 2012; see Clithero & Rangel, 2014). The vmPFC/orbitofrontal cortex has been implicated in reward sensitivity and greater subjective risk-taking tendencies (Blankenstein, Peper, Crone, & van Duijvenvoorde, 2017; Engelmann & Tamir, 2009). In separate work, Luhmann, Chun, Yi, Lee, and Wang (2008) found that vmPFC/orbitofrontal cortex contributes to the valuation of both delayed and probabilistic reward types, suggesting that activation in this region leads to the value of both kinds of rewards being represented in a common “neural currency” and domain-general subjective valuation system (Bartra et al., 2013; Peters & Büchel, 2009; Montague & Berns, 2002; see Weber & Huettel, 2008, for a review).
Previous studies have reported that patients with lesions to the vmPFC show steeper discounting of future rewards compared to healthy and brain-damaged controls (Peters & D'Esposito, 2016; Sellitto, Ciaramelli, & di Pellegrino, 2010; but see Fellows & Farah, 2005), consistent with the view that vmPFC is critical for reward valuation. As noted by Stuss and Levine (2002), patients with vmPFC lesions also have difficulties imagining detail-rich future events, and this may relate to their steep delay discounting (see also Bertossi, Tesini, Cappelli, & Ciaramelli, 2016). However, patients with MTL lesions also have impaired future thinking, yet they are indistinguishable from matched controls in delay discounting (Kwan, Craver, Green, Myerson, & Rosenbaum, 2013; Kwan et al., 2012), at least in the absence of episodic cues (Kwan et al., 2015; Palombo, Keane, & Verfaellie, 2015). This warrants further inquiry into the relation between future thinking and delay discounting in vmPFC (and MTL) patients. For example, the future is inherently less certain than the past. Is this the reason why, on delay discounting tasks, vmPFC patients tend to choose smaller, immediate rewards available now, over larger rewards not available until later? Does this suggest that such patients bypass more deliberate consideration informed by reward utility? If this is the case, we would expect steep delay discounting in vmPFC patients to be accompanied by steep probability discounting. That is, as much as an individual with vmPFC damage will choose smaller, immediate rewards over larger, delayed ones, so, too, will these patients be expected to choose smaller, certain rewards as opposed to gambling on larger, probabilistic ones. This dual pattern has been observed in rats with lesions in homologous regions (Mobini et al., 2002).
Notably, however, vmPFC patients are not classically, nor consistently, described as risk averse. In seminal studies using the Iowa Gambling Task, vmPFC patients were significantly more likely than controls to choose from “bad” decks that result in large, immediate gains but even larger losses overall than “good” decks (Hochman, Yechiam, & Bechara, 2010; Bechara, Tranel, & Damasio, 2000; Bechara, Tranel, Damasio, & Damasio, 1996), suggesting greater risk-taking. This pattern of results also is observed in MTL patients (Rosenbaum et al., 2016; Gupta et al., 2009; Gutbrod et al., 2006). However, performance on gambling tasks may be confounded because probabilities must be learned, placing greater demands on working memory and declarative memory (Mata, Josef, Samanez-Larkin, & Hertwig, 2011; Floden, Alexander, Kubu, Katz, & Stuss, 2008), and because reward delivery and contingencies have differed markedly across studies.
A more recent study with vmPFC patients showed increased risk-taking only under “hot” decision-making conditions in which immediate reward feedback was provided after each choice, requiring the online integration of affective states with other sources of information (e.g., reward probability or magnitude), and not under “cold” conditions where feedback was provided cumulatively at the end of the task, thus minimizing integration demands and leading to more deliberate decision-making (Spaniol, Di Muro, & Ciaramelli, 2019). Probability discounting tasks like the one used in this study represent cold conditions, as no feedback is provided, and, because they are quite different from card tasks like the one used by Spaniol et al., they provide a test of the robustness of their findings.
To date, only one other study has investigated both probability discounting and delay discounting in patients with focal lesions to vmPFC (Peters & D'Esposito, 2020), and the results were consistent with shallower probability discounting and steeper delay discounting than in control participants. However, a separate patient-lesion study comparing choice between sure and fixed risky gambles of gains and losses (50% certainty) that used a smaller patient group was unable to establish clear differences in risky reward choice selection between vmPFC patients and controls; a significant difference was found only when vmPFC patients were compared to a nonspecific group of patient controls (Pujara, Wolf, Baskaya, & Koenigs, 2015). Thus, this study will be one of the first to formally investigate whether vmPFC patients discount probabilistic, risky rewards more or less steeply than controls using an established iterative choice-adjusting procedure (Green & Myerson, 2004). Inclusion of MTL-lesion patients as a comparison group will further help to determine if delay and probability discounting are affected similarly when episodic future thinking is compromised (Bertossi, Aleo, Braghittoni, & Ciaramelli, 2016; Bertossi, Tesini, et al., 2016).
Here, we test the idea that focal lesions that affect one type of discounting necessarily affect the other type. Importantly, we test this idea at both the group level as well as at the level of the individual patient. For example, if a lesion group's discounting of probabilistic rewards is, on average, shallower than that of controls, reflecting greater risk-taking, will their discounting of delayed rewards also differ from that of controls, and if so, will it be shallower or steeper? And at the individual level, if a patient's probability discounting is shallower than average for their group, will their delay discounting be shallower as well, or will it be steeper, as would be predicted if their lesion has increased their impulsiveness? If delay and probability discounting share a component process supported by vmPFC, as we suspect, then lesions of vmPFC should affect both types of discounting. Moreover, if that process contributes to an individual's impulsiveness, then delay and probability discounting should be affected in opposite ways at both the group and the individual level. In this study, we take a patient-lesion approach inspired by Donald Stuss and colleagues to shed light on the precise neural computation supported by vmPFC when rewards are evaluated as well as on the very nature of impulsiveness.
METHODS
Participants
Focal Lesion Patients
All patients were recruited from Baycrest Health Sciences. Patients were in the stable phase of recovery and had no additional diagnosis that would affect cognitive abilities other than those pertaining to their brain injuries.
vmPFC
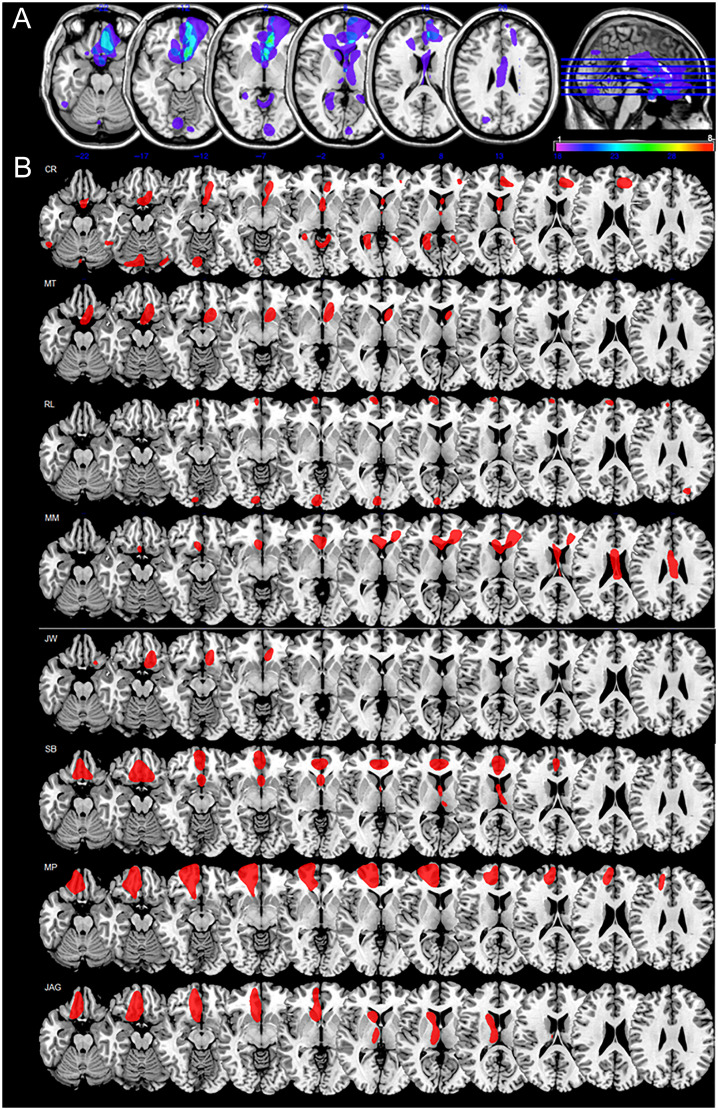
Eight individuals (four men) with vmPFC lesions were tested, seven of whom acquired focal brain lesions following rupture of an anterior communicating artery (ACoA) aneurysm (M age = 57.5 years, SD = 9.5 years). The eighth, R. L. (76 years old), was identified as having a focal vmPFC lesion following an anterior cerebral artery stroke. All patients were tested between 2015 and 2019, at least 12 months post-lesion (range: 12–96 months). Inclusion of patients was based on the location of their lesion evident on MRI or computerized tomography scans (see Figure 1).
Figure 1. .
Lesion location and extent in vmPFC patients. (A) Axial slice template illustrating lesion overlap across vmPFC patients. Slices are 8 mm apart at z = −30, −22, −14, −6, +2, and +10, with level of slice depicted in the sagittal reference image. The color bar indicates the number of patients with damage to a particular area, with purple representing regions damaged in only one patient and red representing regions damaged in all eight patients. The image was created using MRIcro software (Chris Rorden; www.psychology.nottingham.ac.uk/staff/cr1/mricro.html). (B) Axial slice templates illustrating the lesion location and extent for each of the vmPFC patients. Slices are 8 mm apart at z = −22, −17, −12, −7, −2, +3, +8, +13, +18, +23, +28. Neurological convention is followed (left hemisphere presented on the left). Details of lesion location and size are provided in the article, and etiology, demographic information, and neuropsychological profiles are presented in Table 1.
Individual vmPFC lesions were manually drawn on each slice of normalized T1-weighted template MRI scans from the Montreal Neurological Institute using MRIcro software (Rorden & Brett, 2000), based on the most recent MRI or computerized tomography scan available. This manual procedure combines segmentation (identification of lesion boundaries) and registration (to a standard template) into a single step, with no additional transformation required (Kimberg, Coslett, & Schwartz, 2007). Figure 1 shows the location, extent, and overlap of the vmPFC patients' lesions. Lesions were bilateral in six of the eight cases and left-lateralized in the other two cases, largely affecting Brodmann's areas (BAs) 10, 11, 32, 24, and 25. Four patients had minimal damage to lateral PFC (BAs 9, 46, 47), constituting ∼5% of their lesion volume, whereas their vmPFC lesions were on average 10 times larger. Patients C. R. and R. L. had damage to visual cortex (BAs 17, 18, 19, 37) that constituted ∼41% and ∼32% of their lesion volume, respectively. These patients did not have visual problems precluding their participation in the study. They attained normal scores on the Rey–Osterrieth Complex Figure test (percentile scores: 66 and 68; Spreen & Strauss, 1998) and on the Wechsler Test of Adult Reading (percentile scores: 55 and 47; Holdnack, 2001), and showed a good understanding of the discounting test instructions.
MTL
Ten individuals (all men, M age = 55.3 years, SD = 5.9 years) with MTL lesions also were tested. The etiology of brain damage for these cases included anoxia (n = 4), encephalitis (n = 2), stroke (n = 2), temporal lobe resection (n = 1), and traumatic brain injury (n = 1). All of the patients have been described previously (Robin, Rivest, Rosenbaum, & Moscovitch, 2019; Keven, Kurczek, Rosenbaum, & Craver, 2017; Kwan, Kurczek, & Rosenbaum, 2016; Kwan et al., 2013, 2015), with the exception of two patients (R. V. and J. M.). K. C.'s and L. D.'s lesions were bilateral and included the hippocampus and surrounding MTL cortices. K. C. had widespread lesions beyond the MTL including small lesions to left and right posteromedial orbitofrontal cortex (Gao et al., 2020). D. A.'s lesions were also widespread, extending beyond the MTL bilaterally (though primarily right) and into ventral frontal, anterior cingulate, and occipital cortices. B. L. experienced bilateral lesions to his hippocampus that selectively affected the dentate gyrus and part of the CA3 subfield. He also experienced volume loss within the left superior parietal lobe and right precuneus. S. N.'s hippocampal damage was greater on the left, with additional volume loss to left occipital lobe and basal nuclei. M. H. contracted herpes simplex encephalitis, resulting in bilateral MTL atrophy as well as damage along the right medial occipital and inferotemporal cortices (Keven et al., 2017). D. G., J. D., and J. M. suffered anoxia secondary to cardiac arrest and could not be scanned because of medical contraindications. MTL pathology in these cases was inferred based on etiology and neuropsychological profiles (Table 1).
Table 1. .
Demographic and Neuropsychological Data for vmPFC and MTL Participants
| Case | Etiology | Age | Sex | Edu | IQ/PF | WCST | LF | Word List Learning | ROCF | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AQ | LDFR | Recog | Copy | DR | ||||||||
| vmPFC | ||||||||||||
| C. R. | ACoA | 54 | M | 17 | 99 | – | – | 1% | < 0.7% | < 0.7% | 68–70% | 1–2% |
| M. T. | ACoA | 50 | M | 12 | 98 | > 16% | 20% | 4% | < 0.7% | 84–86% | 13% | |
| R. L. | ACA | 76 | F | 16 | 102 | – | 40% | 81% | 50% | 66–68% | 61–63% | |
| M. M. | ACoA | 58 | M | 18 | 98 | > 16% | < 2% | 8% | 6–7% | < 0.7% | 22–23% | 18–19% |
| J. W. | ACoA | 58 | F | 15 | 99 | > 16% | 30–40% | 1% | < 0.02% | 30–32% | 1–2% | 13% |
| S. B. | ACoA | 45 | M | 12 | 116 | – | 50% | 1% | < 0.03% | < 0.02% | 58–61% | < 1% |
| M. P. | ACoA | 54 | F | 13 | 103 | 11–16% | 30% | 2–3% | 2–3% | – | 8% | 42% |
| J. A. G. | ACoA | 65 | F | 15 | – | 50–60% | 1% | < 0.7% | 3–9% | 70% | 2–3% | |
| MTL | ||||||||||||
| D. A. | Encephalitis | 62 | M | 17 | 117 | > 16% | 21–32% | < 1% | < 1% | < 0.02% | > 99% | < 1% |
| D. G. | Anoxia | 48 | M | 16 | 92 | > 16% | 7–13% | 3–8%% | 3–6% | < 0.02% | 21–32% | < 1% |
| L. D. | TLR | 61 | M | 19 | 111 | > 16% | 21–32% | < 1% | < 1% | – | 1% | 21–32% |
| S. N. | Stroke | 46 | M | 12 | 114 | 6–10% | 21–32% | 0.05% | < 0.02% | < 1% | 21–32% | 1% |
| B. L. | Anoxia | 52 | M | 13 | 92 | > 16% | 58–68% | 21–32% | 14–19% | < 0.7% | 14–19% | 3–6% |
| K. C. | TBI | 62 | M | 16 | 99 | > 16% | 7–13% | < 1% | < 1% | < 0.02% | > 99% | < 1% |
| R. V. | Stroke | 51 | M | 16 | 104 | 11–16% | 7–13% | 1% | < 1% | < 0.02% | 14–19% | < 1% |
| M. H. | Encephalitis | 56 | M | 13 | 110 | > 16% | 21–32% | 7–13% | 2–3% | < 0.02% | 70–81% | 3–6% |
| J. M. | Anoxia | 51 | M | 16 | 95 | > 16% | 2–3% | < 1% | < 1% | < 0.02% | < 1% | < 1% |
| J. D. | Anoxia | 64 | M | 19 | 240 | > 16% | 7–13% | 13–14% | < 1% | 50% | – | – |
Age = age in years; Edu = education in years; IQ = full scale IQ; P. F. = premorbid functioning, based on National Adult Reading Test for M. T. and M. M.; Wechsler Test of Adult Reading for R. L. and J. A. G. (vmPFC); FSIQ based on Wechsler Adult Intelligence Scale–Revised for D. A., D. G., and K. C., Wechsler Adult Intelligence Scale–III for L. D. and S. N., Wechsler Adult Intelligence Scale–IV for B. L., R. V., and M. H. (MTL); The following were reported in percentiles compared to normative samples: WCST = Wisconsin Card Sorting Task; L. F. = Letter Fluency; for Word List Learning, learning based on Wechsler Memory Scale Verbal Paired Associates for M. P. and J. A. G., Hopkins Verbal Learning Test–Revised for L. D., Kaplan Baycrest Neurocognitive Assessment, word-list Learning for S. N., California Verbal Learning Test–II for all others; for Stories (WMS): LM I/II = Logical Memory I/II; ROCF = Rey–Osterrieth Complex Figure Test; DR = Delay Recall; ACA = anterior cerebral artery.
Six of the participants with MTL lesions had been previously tested on the delay discounting task, and their data were included for comparison in this study: K. C. (Kwan et al., 2012), D. A. and D. G. (Kwan et al., 2013), L. D., B. L., and S. N. (Kwan et al., 2015). Data for three of the patients (K. C., D. A., and D. G.) who had been tested on the probability discounting task (Kwan et al., 2013) were also included for comparison. See Table 1 for additional demographic information and neuropsychological test performance for both patient groups.
Importantly, most of the vmPFC and MTL patients have documented deficits in episodic prospection, producing fewer internal (episodic) details than healthy controls on a Galton-Crovitz cue-word test. Results for 5 of the 10 MTL patients (D. A., D. G., L. D., S. N., and B. L.) were previously reported (Kwan et al., 2016). Results of the episodic prospection abilities of three additional MTL patients and six of eight vmPFC patients are listed in Table 2, with comparisons made with the control group from Kwan et al. (2016).
Table 2. .
Performance on a Galton-Crovitz Cue Word Test of Episodic Prospection in vmPFC and MTL Patients
| Case | Internal Details | External Details | ||||
|---|---|---|---|---|---|---|
| z-Score | % Rank | Descriptive Label | z-Score | % Rank | Descriptive Label | |
| vmPFC | ||||||
| C. R. | −2.48 | < 0.9th | Severely Impaired | −1.26 | < 12th | Low Average |
| M. T. | – | – | – | – | – | – |
| R. L. | 0.38 | > 63rd | Average | −0.90 | < 19th | Low Average |
| M. M. | −2.02 | < 3rd | Borderline | −1.03 | < 16th | Low Average |
| J. W. | – | – | – | – | – | – |
| S. B. | −1.97 | < 3rd | Mild–Moderately Impaired | 0.92 | 82nd | High Average |
| M. P. | −2.40 | < 0.9th | Severely Impaired | −1.76 | < 4th | Borderline |
| J. A. G. | −1.79 | < 4th | Borderline | −1.42 | < 8th | Borderline |
| MTL | ||||||
| D. A. | −1.65 | < 5th | Borderline | −0.72 | < 25th | Low Average |
| D. G. | −2.46 | < 0.8th | Severely Impaired | −1.85 | < 4th | Borderline |
| L. D. | −0.89 | < 19th | Low Average | 0.40 | > 63rd | Average |
| S. N. | −2.07 | < 2nd | Moderately Impaired | 1.23 | > 88th | High Average |
| B. L. | −1.43 | < 8th | Borderline | 1.46 | > 92nd | Superior |
| K. C. | −2.68 | < 0.4th | Severely Impaired | −2.20 | < 2nd | Moderately Impaired |
| R. V. | – | – | – | – | – | – |
| M. H. | −2.28 | < 2nd | Moderately Impaired | −1.47 | < 8th | Low Average |
| J. M. | −2.28 | < 2nd | Moderately Impaired | −1.91 | < 3rd | Mild–Moderately Impaired |
| J. D. | – | – | – | – | – | – |
Internal details refer to episodic information (e.g., time, place, people, objects, thoughts, and emotions) specific to a central event that a person might experience in the future. External details refer to details that are not specific to the central event and/or that are semantic (factual) in nature and not specific to time and place, repetitions, commentary on the event, or other metacognitive statements. Scoring of the Galton-Crovitz Task Episodic Prospection Task is based on internal and external details of the Autobiographical Interview (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002). “High Average” and “Superior” performance indicate an excess of details. Patients' scores are compared to scores of a demographically matched control group reported in Kwan et al. (2016).
Controls
Performance of 30 age-matched control participants (16 men; age range: 46–67 years, M age = 58.4 years, SD = 6.3 years) was compared to that of each patient group on the delay and probability discounting tasks. Control participants were screened for variables associated with steeper than average discounting (Madden & Bickel, 2010), including smoking, significant alcohol and drug use, and gambling problems according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (American Psychiatric Association, 2000); DSM-5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (APA, 2013) criteria. Although 31 participants were tested, one proved to be a significant outlier on the probability discounting task and was excluded from all subsequent analyses. All participants were fluent in English. Participants gave informed written consent and received monetary compensation in accordance with the Human Research Ethics Committees of York University and Baycrest Health Sciences.
Delay Discounting
All participants completed the same computerized delay discounting task that had been used previously to test several of the participants with MTL lesions. Over a series of trials, participants were presented with pairs of hypothetical monetary amounts and made choices between a smaller, immediate reward and a larger, delayed reward. They were told that the task assessed their preferences and that there were no correct or incorrect choices. An immediate reward amount, which changed depending on participants' previous choices, was presented along with a larger delayed reward amount ($100 or $2000) that was available after one of seven delays (1 week, 1 month, 3 months, 6 months, 1 year, 3 years, 10 years), presented in random order. Across six trials, an iterative, adjusting-amount procedure converged on the estimate of the amount of immediate reward that the participant judged to be subjectively equal in value to the delayed reward. For example, in the condition where a future hypothetical reward of $2000, if chosen, would be received in 3 months, the first choice presented to the participants was “$1000 right now or $2000 in 3 months?” If the participant chose “$1000 right now,” the choice on the second trial would be between “$500 right now” and “$2000 in 3 years.” If the participant chose “$2000 in 3 months,” the choice on the third trial would be “$750 right now or $2000 in 3 months.” Thus, adjustments in the amount of immediate reward were made such that the first adjustment was half the difference between the immediate and delayed reward amounts presented on the first trial, with each subsequent adjustment being half the preceding adjustment. Following the sixth and final trial of each delay condition, the subjective value of the delayed reward was estimated as the amount of immediate reward that would be presented if there were a seventh trial (see Green & Myerson, 2004).
Probability Discounting
Patients and controls also completed a probability discounting task previously described in Kwan et al. (2013). For patients, the probability discounting task was completed on the same day as the delay discounting task, which was completed first, with a 30- to 45-min interval between discounting tasks, during which the participants completed questionnaires and other behavioral tasks unrelated to the discounting tasks (not reported in the current study). Control participants completed both discounting tasks in a counterbalanced order on separate days, with 1–3 weeks between the tasks.
Participants were told that the task assessed their preferences and that there were no correct or incorrect choices. Over a series of trials, participants were presented with pairs of hypothetical monetary amounts and made choices between a smaller, certain reward and a larger, probabilistic reward. For each of two probabilistic amounts ($250 and $2,000), participants were asked to make choices between certain rewards and probabilistic rewards with a 90%, 75%, 50%, 20%, 10%, or 5% chance of receiving the reward, with the probabilities presented in random order. As in the delay discounting task, an iterative, adjusting-amount procedure was used in which the amount of the certain reward changed depending on the participant's previous selection. Across six trials, this procedure converged on an estimate of the amount of certain reward that the participant judged to be subjectively equal in value to the probabilistic reward. For example, in the condition where a reward of $2000 had a 50% chance of being received, the first choice was “$1000 for sure or $2000 with a 50% chance?” Adjustments in the amount of the certain reward were made such that the first adjustment was half of the difference between the certain and probabilistic amount presented on the first trial, with each subsequent adjustment being half the preceding adjustment. Following the sixth and final trial of each probability condition, the subjective value of the probabilistic reward was estimated as the amount of certain reward that would be presented if there were a seventh trial.
Experimental Design and Statistical Analysis
The degree to which a participant discounted delayed and probabilistic rewards was assessed using the area-under-the-curve (AuC) discounting measure. The AuC is theoretically neutral in that it represents the area under observed subjective values rather than under a curve representing a particular theoretical model fit to those subjective values (Myerson, Green, & Warusawitharana, 2001). The “curve” is actually a series of lines on a graph with the delays until or odds against receiving a reward expressed as a proportion of the maximum delay or odds against and the subjective values expressed as a proportion of the maximum delayed or probabilistic amount. Note that the odds against a probabilistic reward are equal to [(1 − p)/p], where p is the probability of receiving the reward.
AuCs are calculated by first normalizing the delays, odds against, and subjective values to make it easier to compare the discounting of different reward amounts. The area under the discounting curve is then subdivided into trapezoids, and the area of each trapezoid is calculated as A = (x2 − x1)(y1 + y2) / 2, where values of x represent successive delays or odds against and values of y represent subjective values associated with these delays or odds against. The AuC represents the sum of the areas of all the trapezoids and can range from 0.0 (maximal discounting) to 1.0 (no discounting).
Because our plan was to compare each patient group's discounting rate to baseline measures based on a single, larger control group, we began our analyses by assessing the representativeness of our participant groups and the reliability of our discounting measures and procedures. We did this by examining the internal consistency of the AuC data for our participant groups on each type of discounting task and the degree to which their performances met benchmarks established based on previous studies of discounting in healthy adults (for a review, see Green & Myerson, 2010).
We then subjected all of the AuC data to a single 3 (Group) × 2 (Task) × 2 (Amount) mixed ANOVA, with Task (delay, probability) and Amount (smaller, larger) as repeated-measures factors. We were primarily interested in possible differences in discounting between the lesion groups, although differences would likely have to be interpreted in light of any observed interactions. Based on previous reports that amount has opposite effects on delay and probability discounting in healthy participants, we predicted that Type of Task would interact with Amount, perhaps even cancelling out the effects of Amount. However, the effects of Amount might also be different for different groups and/or types of task, leading to a three-way interaction between Group, Task, and Amount that might well cancel out the effects of Group.
Accordingly, our three-way ANOVA was followed by four planned comparisons, each of which compared the control group to a specific lesion group performing a specific task (i.e., delay discounting by MTL patients, delay discounting by vmPFC patients, probability discounting by MTL patients, and probability discounting by vmPFC patients) of both reward amounts in order to explicate the observed pattern of interactions. Finally, because the hypothesis of individual differences in a general impulsiveness trait predicts that individuals who show steep delay discounting will also show shallow probability discounting, we examined the correlation between delay and probability discounting for each group separately.
RESULTS
We begin our analyses of the AuC data by assessing the internal consistency of the discounting measures in our patient groups. This is especially important for the vmPFC group because previous studies have noted increased vmPFC activity during irregular preference judgments (Kurtz-David, Persitz, Webb, & Levy, 2019). In patient studies, more erratic judgments and greater inconsistencies in choice selections under conditions of uncertainty (e.g., risky or ambiguous decisions) have been observed for patients with focal damage to the vmPFC compared to age- and education-matched controls (Henri-Bhargava, Simioni, & Fellows, 2012; Fellows, 2011; Fellows & Farah, 2007). In fact, additional research has shown that the vmPFC may even be more specifically involved in value-based decision-making for risky choices across both human and animal models (Spaniol et al., 2019; Abela & Chudasama, 2013; Weber & Huettel, 2008).
We first identified whether inconsistent preferences were observed for each individual participant across both discounting tasks, separately for the smaller and larger reward amount conditions. For delay discounting, a monotonically decreasing discounting curve is expected when the subjective value (R1) of the future outcome R at a given delay (t1) is greater than the subjective value (R2) at the immediately following delay (t2; where t2 > t1; Johnson & Bickel, 2008). In accordance with the method proposed by Sellitto et al. (2010), we accounted for variability in the data by counting the number of “inconsistent choices” where the subjective value R2 was greater than the subjective value R1 at the preceding delay by more than 10% of the amount of the future outcome (i.e., R2 > R1 + R/10). Neither the small amount (vmPFC = 0.75; MTL = 0.80; control = 0.33), F(2, 45) = 2.83, p = .07, ηp2 = .11, nor the large amount (vmPFC = 0.75; MTL = 0.40; control = 0.33), F(2,45) = 0.84, p = .43, ηp2 = .04, conditions revealed significant differences in the mean number of inconsistent choices across participant groups for the delay discounting task, replicating previous findings (Sellitto et al., 2010).
The same procedure also was applied for the probability discounting task. Unlike delay discounting, one-way ANOVA revealed a significant Group effect for the small amount condition, F(2, 45) = 5.71, p < .01, ηp2 = .20. Post hoc comparisons revealed a significant difference between the mean number of inconsistent choices for the vmPFC group (M = 0.875) compared to the MTL group (M = 0.00), t = 3.36, p < .01, d = 1.34, after Bonferroni correction. For the large amount condition, one-way ANOVA also revealed a significant Group difference, F(2, 45) = 8.89, p < .001, ηp2 = .28. Post hoc comparisons revealed significant differences between the vmPFC group (M = 1.25) and the MTL group (M = 0.30), t = 3.26, p < .01, d = 1.12, as well as the vmPFC group with the control group (M = 0.23), t = 4.16, p < .001, d = 1.70, after Bonferroni correction. These inconsistencies observed in probability discounting but not delay discounting support our inclusion of the “control” MTL patient-lesion group and agrees with the notion that impaired preferences may be the result of vmPFC's contribution more to conditions of uncertainty and risk (Abela & Chudasama, 2013; Weber & Huettel, 2008; Fellows & Farah, 2007).
We also looked at the correlation between the degree of discounting of smaller and larger amounts for each of our participant groups. As expected, the vmPFC group showed strong correlations for both delay discounting (r = .87, p ≤ .001) and probability discounting (r = .73, p < .05). The MTL group did not show significant correlations between amounts for delay discounting (r = .27, p = .45), but showed strong correlations for probability discounting, which is the task of primary interest in the current study (r = .92, p ≤ .001), supporting the inclusion of the MTL patients as a patient comparison group.
We also assessed the control group in the same manner. Correlations between the degree of discounting of smaller and larger rewards were equally strong for our controls, regardless of whether the rewards were delayed (r = .88, p < .001) or probabilistic (r = .89, p < .001). In contrast, the correlation between measures of delay and probability discounting was not significant, regardless of whether the correlation between delay and probability discounting was assessed for each amount condition separately or whether the AuC measures for the control group were averaged across the smaller and larger reward conditions (r = .163, p = .389). The finding of a nonsignificant positive correlation between delay and probability discounting is consistent with the benchmark set by many previous studies of discounting in healthy adults (Green & Myerson, 2010). Finally, the control group showed both of the benchmark magnitude effects commonly observed in healthy adults: shallower discounting of larger delayed rewards (t = 3.87, p < .001, d = 0.71) but steeper discounting of larger probabilistic rewards (t = 5.78, p < .001, d = 1.06).
With the patient and neurotypical control groups established as appropriate comparison groups, the AuC data for all three groups were submitted to a 3 (Group) × 2 (Task) × 2 (Amount) mixed ANOVA. Although the main effect of Group was not significant, F(2, 45) = 1.97, p = .151, Group strongly interacted with Task, F(2, 45) = 6.32, p = .004, ηp2 = .219, consistent with the fact that the vmPFC group showed steeper delay discounting and shallower probability discounting than the other two groups. A three-way Group × Task × Amount interaction also was observed, F(2, 45) = 4.10, p = .023, ηp2 =.154, consistent with the fact that larger group differences were observed in the larger amount condition. Finally, there was a significant Task × Amount interaction, consistent with the fact that, overall, the amount effects on the probability discounting task tended to be larger than those on the delay discounting task: F(1, 45) = 5.73, p = .021, ηp2 =.113.
Delay Discounting
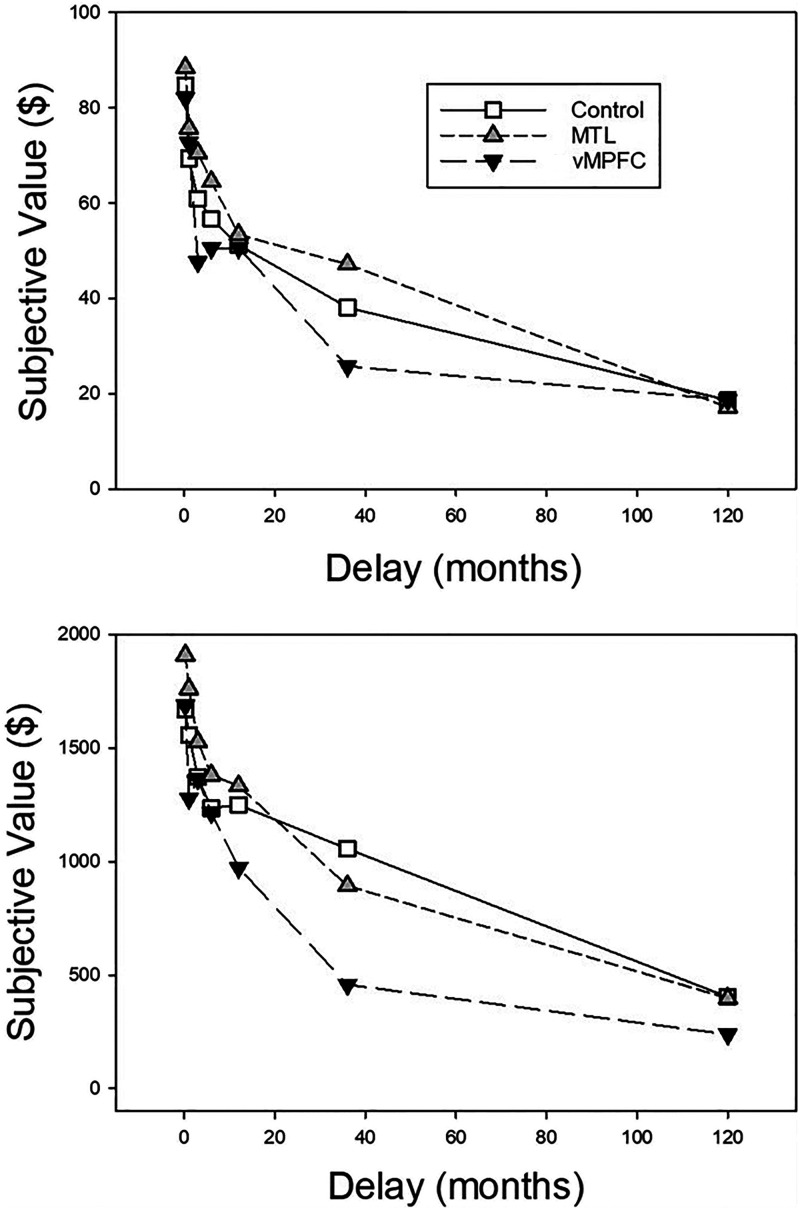
Figure 2 presents group mean subjective values of the delayed rewards plotted as a function of the delay until their receipt. Both patient groups and control participants showed clear evidence of delay discounting as indicated by decreases in subjective value as the delay until the reward increased.
Figure 2. .
Mean subjective value as a function of delay until receiving the reward for the vmPFC, MTL, and control groups. The top and bottom present the data from the smaller ($100) and larger ($2000) delayed reward amount conditions, respectively.
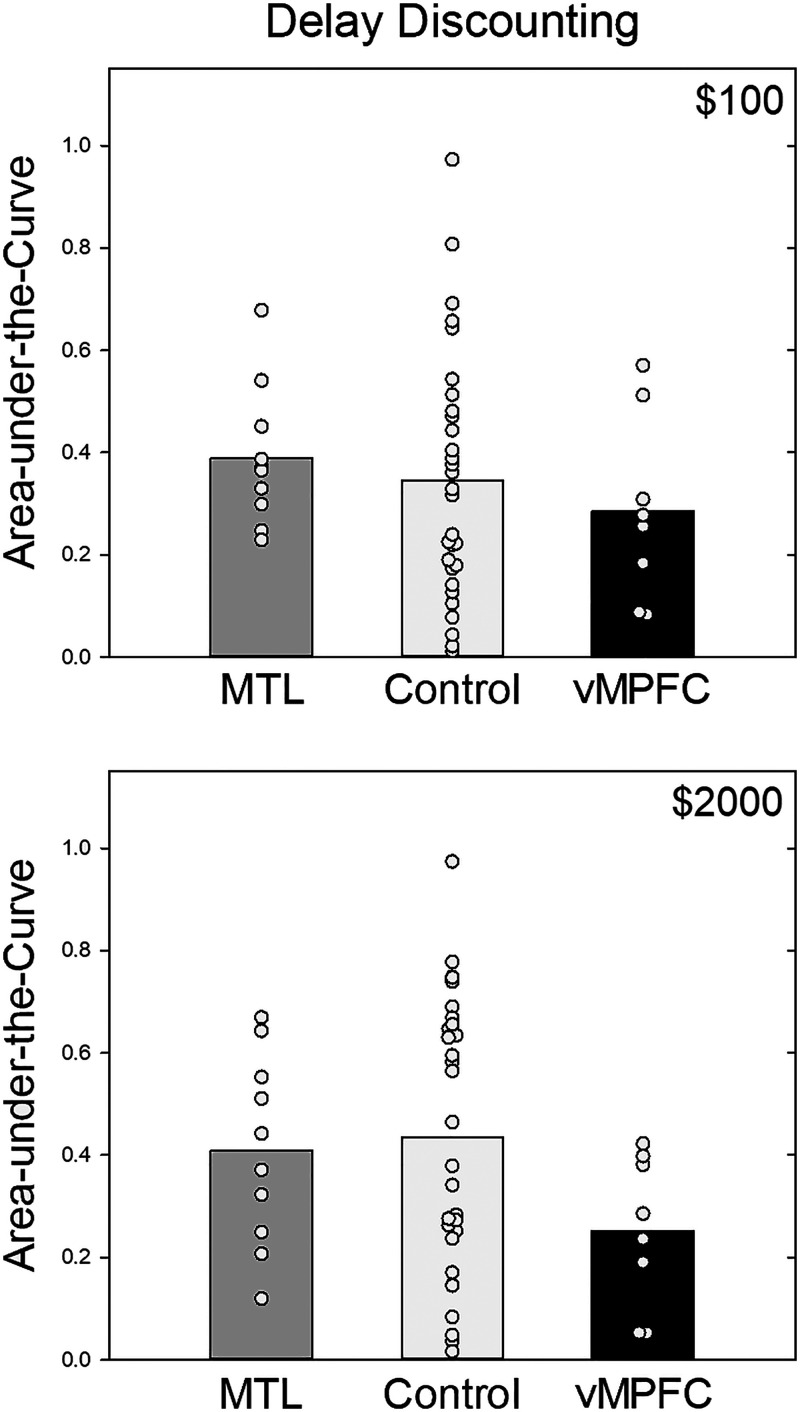
Group mean AuC scores are presented in Figure 3. The vmPFC patients appear to have discounted delayed rewards more steeply than the participants in the control group, as indicated by their smaller AuCs. Only the control group appears to show the usual magnitude effect in which smaller delayed rewards are discounted more steeply than larger ones (Green, Myerson, & McFadden, 1997).
Figure 3. .
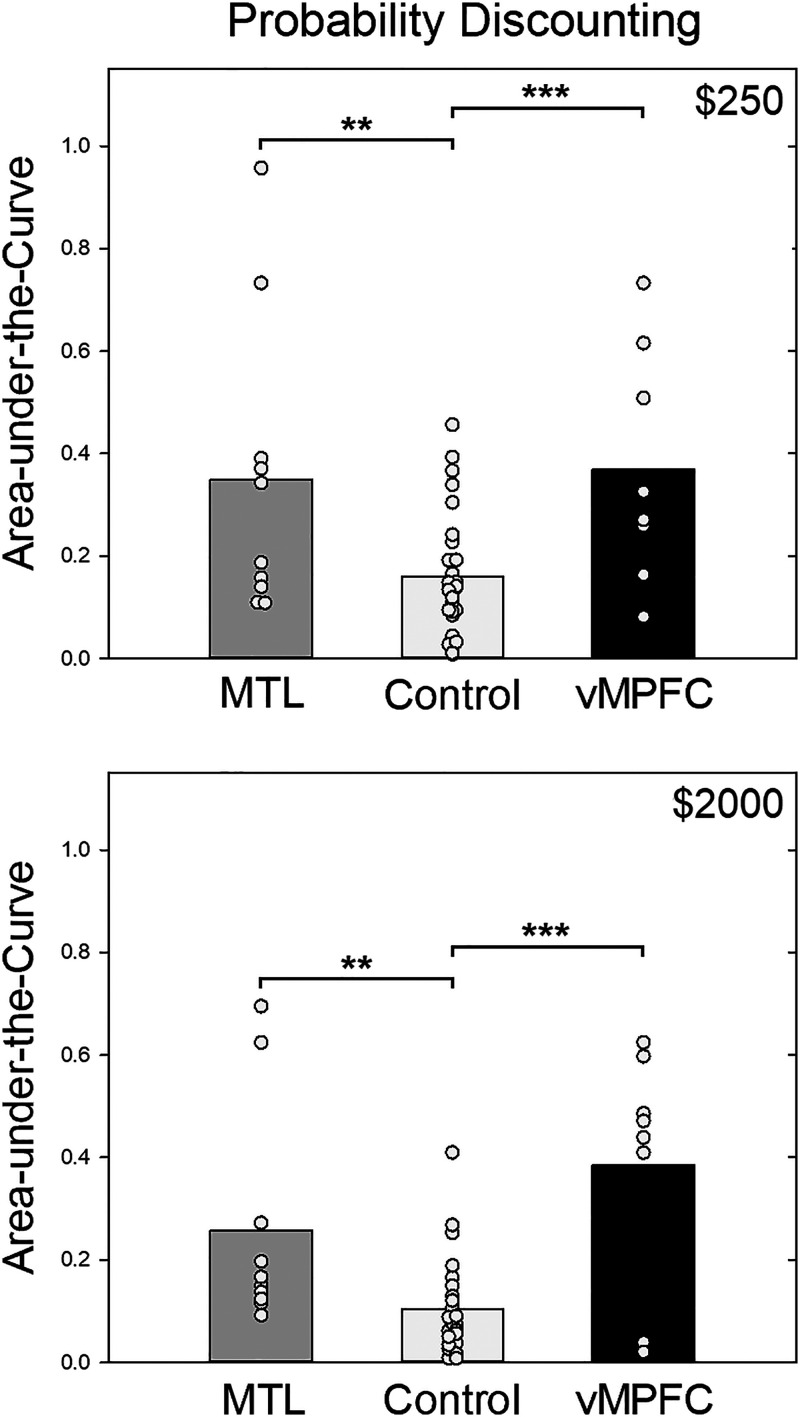
Mean AuC for the vmPFC, MTL, and control groups. The top and bottom present the data from the smaller and larger delayed reward amount conditions, respectively. The circles represent individual participants' data from each group overlaid on their respective bars.
Our first planned comparison was conducted on the AuCs of the MTL and control groups for the delay discounting task. Neither the main effect of Group nor the effect of Amount was significant: F(1, 38) = 0.011, p = .917, and F(1, 38) = 4.08, p = .051, respectively. The interaction between Amount and Group also failed to reach significance, F(1, 38) = 1.73, p = .196, although as noted previously, there was a significant effect of Amount (shallower discounting of larger delayed rewards) in the control group.
Our second planned comparison, conducted on the delay discounting AuCs of the vmPFC and control groups, also failed to reveal significant effects of Group or Amount: F(1, 36) = 1.74, p = .196 and F(1, 36) = 1.43, p = .239. However, these results must be interpreted in light of the significant interaction between Group and Amount, F(1, 36) = 6.50, p = .015, ηp2 = .038, which suggests that the magnitude of the differences between the groups are significantly different between the two amount conditions. This is consistent with the fact that the difference between the patients and control participants (i.e., steeper discounting by vmPFC patients) was larger for the larger delayed reward than for the smaller delayed reward condition, although neither was significant (ps = .07 and .51, respectively; see Figure 3).
Probability Discounting
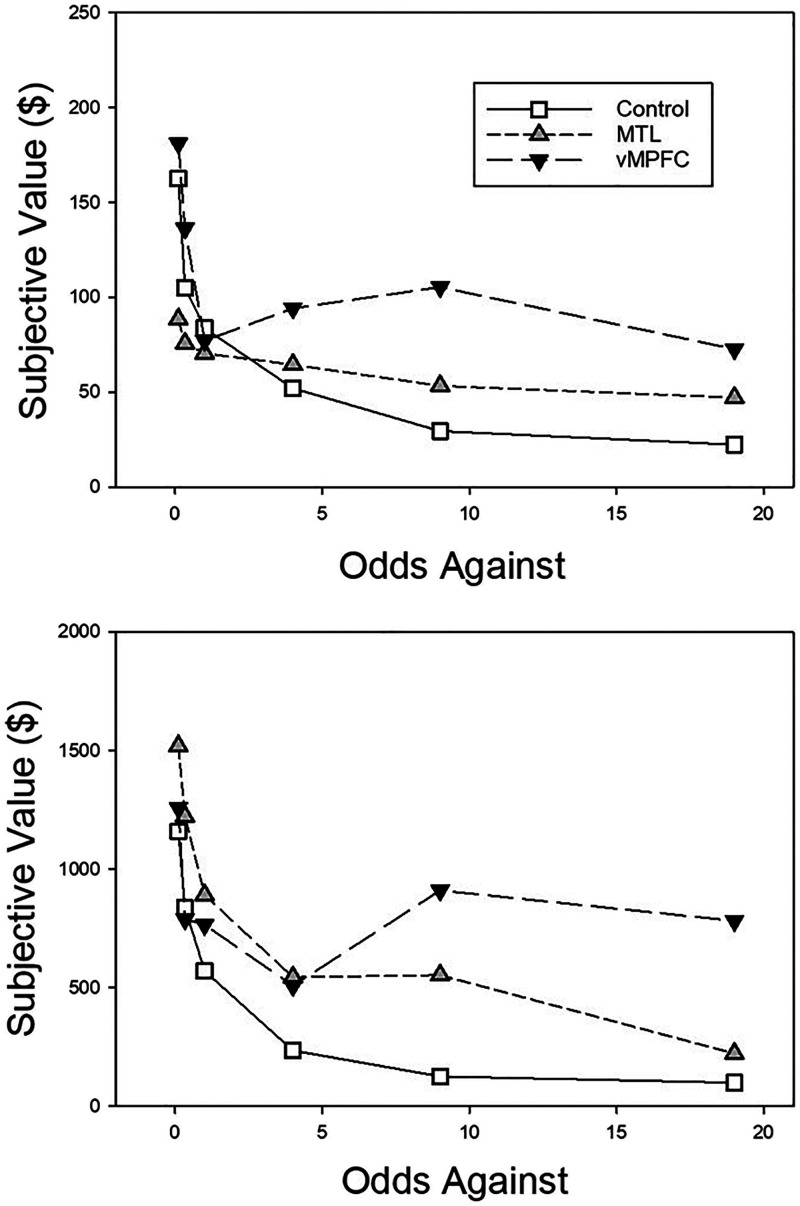
Figure 4 presents group mean subjective values of the probabilistic rewards as a function of the odds against their receipt. Again, both patient groups, as well as the controls, exhibited discounting of both the smaller and larger rewards. In contrast to the delay discounting curves seen in Figure 2, however, the vmPFC patients tended to show higher subjective values for probabilistic rewards than MTL patients and participants in the control group, particularly when the probability of reward was low (and the odds against were high).
Figure 4. .
Mean subjective value as a function of the odds against receiving the reward for the vmPFC, MTL, and control groups. The top and bottom present the data from the smaller ($250) and larger ($2000) probabilistic reward amount conditions, respectively.
Group mean AuC scores on the probability discounting task are presented in Figure 5. The vmPFC patients appear to have discounted probabilistic rewards less steeply than the participants in the control group in both amount conditions, and less steeply than the MTL group in the large amount condition. Whereas both the MTL patients and the control participants appear to have discounted smaller probabilistic rewards less steeply than larger ones, the vmPFC group discounted both smaller and larger amounts to approximately the same degree.
Figure 5. .
Mean AuC for the vmPFC, MTL, and control groups. The top and bottom present the data from the smaller and larger probabilistic reward amount conditions, respectively. The circles represent individual participants' data from each group overlaid on their respective bars. **p < .01. ***p < .001.
Our third planned comparison was conducted on the AuCs of the MTL and control groups for the probability discounting task. This analysis revealed a significant effect of Group, reflecting shallower discounting by the MTL group: F(1, 38) = 10.13, p = .003, ηp2 = .211. There also was a significant effect of Amount, F(1, 38) = 29.60, p < .001, ηp2 = .438, consistent with the magnitude effect for probabilistic rewards usually observed in healthy participants. The interaction of group and amount was not significant, F(1, 38) = 1.70, p = .200.
Finally, our fourth planned comparison focused on the vmPFC and control groups' probability discounting AuCs. There was a significant main effect of Group, F(1, 36) = 23.10, p < .001, ηp2 = .391, which may reflect the shallower discounting of probabilistic rewards by the vmPFC group compared to the controls. Although the main effect of Amount was not significant, F(1, 36) = 1.28, p = .265, there was a statistically significant interaction between Amount and Group, F(1, 36) = 4.27, p = .046, ηp2 = .106, which may reflect the fact that, whereas the vmPFC group's discounting showed little effect of reward amount, the control group showed the magnitude effect for probability discounting (steeper discounting of larger probabilistic rewards) usually observed in healthy participants. Consistent with this interpretation, tests for simple main effects revealed group differences for both smaller and larger reward amounts: F(1, 36) = 13.54, and 30.40, ps < .001, respectively.
Relations between Delay and Probability Discounting
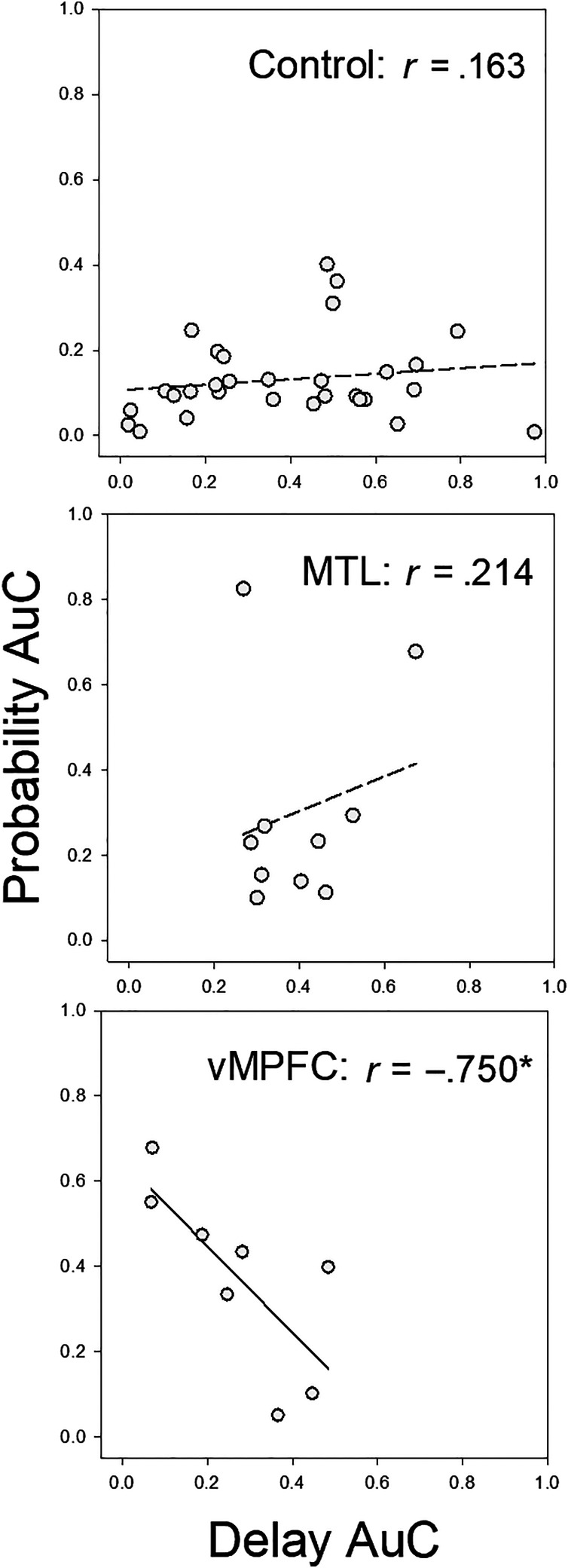
Our final set of analyses examined the relations between individuals' performance on the two types of discounting task. As already noted with respect to the control group, the correlation between participants' delay and probability discounting was not significant: r = .163, p = .389 (see the top of Figure 6). A similar lack of significant correlation between delay and probability discounting was observed for the MTL group: r = .214, p = .553 (see the middle of Figure 6). Contrary to the idea that a unitary impulsiveness trait underlies both the ability to delay gratification and risk aversion, these correlations not only failed to reach significance; they also were in the direction opposite to that predicted.
Figure 6. .
Individual mean AuCs (averaged across the two amount conditions) for the delay discounting task, plotted as a function of their mean AuCs for the probability discounting task. Data for the control group are shown in the top, data for the MTL group are shown in the middle, and data for the vmPFC group are shown in the bottom.
The correlation between the vmPFC patients' delay and probability discounting was not only significant, r = −.750, p = .032; it also was negative (see the bottom of Figure 6), in contrast to the nonsignificant correlations observed for participants in both the MTL and neurotypical control groups. That is, the correlation for the vmPFC group was in the direction expected if these patients varied in impulsiveness, such that those who were less willing to wait (as indicated by their lower delay discounting AuCs) were also more willing to take risks (as indicated by their higher probability discounting AuCs).
DISCUSSION
Financial choices are sensitive to the temporal proximity, likelihood, and amount of each option (Rangel, Camerer, & Montague, 2008), as is evident from the way neurotypical individuals tend to discount the value of delayed and probabilistic outcomes (Green & Myerson, 2010). However, a neurotypical individual's tendency to discount delayed rewards more or less steeply is relatively independent of their tendency to discount probabilistic rewards (Green & Myerson, 2013). The current study tested whether focal lesions that affect one type of discounting necessarily affect the other type, with the goal of revealing component processes shared by different forms of discounting. Importantly, we tested this idea at both the group level as well as at the level of the individual patient.
In the current study, the vmPFC and MTL patient and control groups all showed systematic discounting of both delayed and probabilistic rewards, although important quantitative differences in degree of discounting were observed. On average, vmPFC-lesioned individuals discounted delayed rewards more steeply but discounted probabilistic rewards less steeply than controls, whereas MTL-lesioned individuals discounted delayed rewards at normal rates but discounted probabilistic rewards less steeply than neurotypical controls. These results suggest that vmPFC lesions affect the weighting of reward amount relative to delay and certainty in opposite ways.
Notably, patients with MTL lesions did not differ from controls in the degree to which they discounted delayed rewards, but compared to the controls, they discounted probabilistic rewards to a significantly lesser extent, suggesting that they put less weight on the likelihood of actually getting a reward. That is, they were more likely to gamble on the possibility of getting a reward, whereas the controls were significantly more risk averse. Importantly, some of these patients have extensive lesions affecting the hippocampus and surrounding MTL cortices (e.g., K. C., D. A.) and yet these patients are indistinguishable from controls in delay discounting despite impaired episodic memory and episodic future thinking. In contrast, patients with vmPFC lesions did tend to discount delayed rewards more steeply than the control group, with the size of the difference increasing with the amount of reward; like MTL patients, they discounted probabilistic rewards less steeply than controls. Differences between the vmPFC and controls were amplified with larger reward amounts, reflecting the control participants' tendency to be more risk averse when the stakes were higher, whereas the vmPFC patients were unaffected in this regard.
As Green and Myerson (2013) and others have noted, delay and probability discounting are similar in that, in both cases, subjective value shows systematic, negatively accelerated decreases that are well described by hyperboloid functions (see Figures 2 and 4). They are different, however, in that, in healthy adults, varying the amount of reward has opposite effects on delay and probability discounting (Myerson, Green, Hanson, Holt, & Estle, 2003; Green, Myerson, & Ostaszewski, 1999). Myerson et al. (2003) further showed that the degree to which an individual discounts delayed rewards is, at most, weakly positively related to the degree to which that individual discounts probabilistic rewards. This finding, which was replicated in our analyses of participants in the control group, argues against the hypothesis that delay and probability discounting represent a unitary underlying trait of impulsiveness in healthy adults. The unitary trait hypothesis implies that individuals' delay and probability discounting should be negatively correlated with one another. According to this view, impulsive individuals have both a strong preference for immediate rewards over delayed ones and a strong tendency to gamble on getting a large reward rather than a smaller, certain one, although with the former, they risk getting no reward at all.
As already noted, the correlation between delay and probability discounting in the control group was weakly positive, but not significantly so. Importantly, whereas a similar relation was observed in the MTL patients, the vmPFC patients showed a strong negative correlation between individual patients' delay and probability discounting (see Figure 6), a finding that would be expected if vmPFC lesions affected the degree of their “impulsiveness.” The fact that, as a group, vmPFC patients also showed both steeper delay discounting and shallower probability discounting is consistent with that interpretation.
Previously, researchers examining the effects of vmPFC lesions in humans have primarily studied intertemporal choice, which includes delay discounting (Peters & D'Esposito, 2016; Sellitto et al., 2010; Fellows & Farah, 2005). Lesion studies on the vmPFC have also considered risky choice but have relied for the most part on laboratory-based gambling tasks or animal models (e.g., Spaniol et al., 2019; St. Onge & Floresco, 2010; Bechara et al., 2000), or have not observed consistent findings supporting comparative differences between patients and matched controls in probability discounting (Pujara et al., 2015). The overall patterns (i.e., steeper discounting for delayed rewards or preference for risky rewards compared to controls) have been reported, but this study is among the first to observe both these patterns in the same patients (see also Peters & D'Esposito, 2020). Importantly, the present investigation did so by using analogous tasks specifically designed to facilitate direct comparisons of intertemporal and risky choice (Green & Myerson, 2004). It is also the first study to directly compare performance of vmPFC patients on both discounting tasks with that of MTL patients who have similar deficits in episodic future thinking. The results have important implications for the localization of function in decision-making and for the nature of the mechanisms involved.
Decision-making by Intact Brains
In neurotypical adults, there appear to be neural systems for which activity reflects valuation of both delayed and probabilistic rewards as well as other systems involved in domain-specific valuation (e.g., Seaman et al., 2018). Using fMRI to examine the neural bases of delay and probability discounting, Peters and Büchel (2009) found that activity in fronto-polar, lateral parietal, and posterior cingulate cortices correlated with the value of delayed, but not probabilistic, rewards, whereas activity in superior parietal cortex and middle occipital areas correlated with the value of probabilistic, but not delayed, rewards. Notably, activity in ventral striatum and vmPFC coded for subjective value in a domain-general manner, suggesting that these regions integrate results from the domain-specific valuation systems into a common neural currency that is involved in value computation across tasks and is utilized across different reward types and stages of decision-making (Clithero & Rangel, 2014; Bartra et al., 2013; Levy & Glimcher, 2012). Furthermore, activity in these regions can be dissociated from activity in other reward valuation regions (including anterior insula, other striatal regions, and dorsomedial PFC) involved in arousal, saliency of reward options, and meta-decision processes (e.g., confidence and deliberation time of choices; see also Clairis & Pessiglione, 2020). Studying individuals with focal lesions to these brain regions provides more definitive evidence regarding the localization of function in decision-making mechanisms, particularly with respect to the issue of domain-general versus domain-specific processes.
Decision-making by Brains with Focal Lesions
In this study, patients with vmPFC lesions not only showed steeper delay discounting than controls, but they also showed shallower probability discounting, and within the vmPFC group, those who showed the steepest delay discounting also showed the shallowest probability discounting. These findings are consistent with the results of previous studies that investigated each kind of decision-making in isolation (for reviews, see Bechara, 2011; Sellitto, Ciaramelli, & di Pellegrino, 2011). Sellitto et al. (2010) demonstrated that vmPFC lesions affect delay discounting (see also Peters & D'Esposito, 2016; but see Leland & Grafman, 2005), systematically increasing patients' preferences for immediate rewards even when those rewards are smaller than delayed ones. Although previous studies suggest vmPFC lesions also affect risky choice, the procedures usually required the participants to learn probabilities from experience (Iowa Gambling Task; Bechara et al., 1996; Bechara, Damasio, Damasio, & Anderson, 1994). As a result, findings from these procedures may be confounded, as studies comparing younger and older adults have shown (for a review, see Mata et al., 2011). That is, observed differences could reflect deficits in either decision-making, learning, or both.
Fortunately, the present probability discounting procedures do not require new learning by participants, and therefore our finding that vmPFC patients show shallower probability discounting than controls strongly support previous conclusions regarding the effects of vmPFC lesions on risky choice. Our results are consistent with those of a recent study of decision-making by Peters and D'Esposito (2020) that also found both steeper delay discounting and shallower probability discounting in patients with focal lesions to vmPFC/orbitofrontal than in controls. Consistent with previous work is the current finding that MTL patients' probability discounting was shallower than that of controls (Rosenbaum et al., 2016; Gupta et al., 2009; Gutbrod et al., 2006) and that their delay discounting did not differ from that of controls (e.g., Kwan et al., 2012, 2013). Notably, MTL patients' pattern of impaired probability discounting with preserved delay discounting provides additional evidence that the two kinds of discounting involve at least some separate processes.
Nevertheless, there still could be a cognitive process or processes common to both delay and probability discounting that might be affected by lesions of the vmPFC. The present findings provide an answer to Peters' (2011) question of whether vmPFC/orbitofrontal cortex damage affects only the valuation of delayed rewards, or whether it leads to “a more general impairment in cost-benefit decision-making that extends beyond the domain of intertemporal choice.” The present findings support the latter view: Patients with focal vmPFC lesions differed from controls in both the intemporal and risky choice domains, as indicated by performance on both delay and probability discounting tasks. The question that remains is why this might be so. Intuitively, it would seem likely that delay and probability discounting would involve a common process because the future is inherently risky, but this intuition is not borne in the data: Were this the case, vmPFC patients should have evinced steep probability discounting and should be risk averse just as they are attracted to immediate rewards. In this study, we found the opposite pattern.
One common process that may underlie performance on delay and probability discounting is prospection (see Szpunar, Spreng, & Schacter, 2014; Gilbert & Wilson, 2007), a hypothesis we can test, as the current study involves two patient populations with important, yet qualitatively different, prospection deficits (Verfaellie, Wank, Reid, Race, & Keane, 2019; Bertossi, Tesini, et al., 2016; Rosenbaum et al., 2016; Kwan et al., 2013, 2015). Although prospection is more commonly associated with delay discounting (Boyer, 2008), some theories of risky choice posit that choices involving probabilistic outcomes also involve consideration of future outcomes (e.g., Loomes & Sugden, 1982). For example, regret theories of risky choice posit that gambles involve prospection in the form of imagining possible future outcomes (i.e., winning and losing the gamble and the regret that would follow a loss). The fact that both vmPFC patients and MTL patients show steep probability discounting, and that both groups fail to show consistent effects of reward magnitude, might support a link between discounting behavior and prospection. However, the results observed on delay discounting argue against this possibility.
Although previous studies of the effects of episodic cueing demonstrate that prospection can play a role in delay discounting (e.g., Mok et al., 2020; Bulley et al., 2019; O'Donnell, Daniel, & Epstein, 2017), patients with MTL lesions who have severe prospection deficits nevertheless exhibit typical, systematic discounting of delayed rewards. As an extension of our previous findings, this systematic discounting was observed at the group level and with a larger group of patients than previously described by Kwan et al. (2013). MTL patients also show the certainty and common ratio effects (i.e., the Allais paradox; Craver et al., 2014) benchmark characteristics of risky choice that have been attributed to anticipated regret (Bell, 1982; see also Klein, 2013).
Importantly, in this study, both patients with MTL lesions and patients with vmPFC lesions had deficits in prospection, but only the vmPFC group differed significantly from controls in delay discounting. One possibility is that, although prospection can contribute to effective decision-making, and may even compensate for cognitive deficits, it is not required. Another possibility is that vmPFC and MTL patients have qualitatively different prospection deficits, and it is the form of prospection affected in vmPFC—but not MTL—patients that has an impact on the valuation, or even the conception, of future rewards. What then might be the common process or mechanism that underlies the observed effects of vmPFC lesions on delay and probability discounting? It should be noted, of course, that there need not be one. That is, the vmPFC could contain some neurons contributing to intertemporal decision-making and other neurons contributing to decisions involving risky options, or it could contain neurons that do both.
Delay and probability discounting require complex decisions involving multiple cognitive processes. It is possible that performance on both tasks depends on schemas, which refer to knowledge structures extracted across multiple experiences. Schemas influence how new events (e.g., choice options) are perceived, and they have been linked to the vmPFC in neuroimaging and patient-lesion studies (Hebscher & Gilboa, 2016; Ghosh, Moscovitch, Colella, & Gilboa, 2014; for a review, see Gilboa & Marlatte, 2017). Reliance on schemas could explain the surprising finding by Kwan et al. (2013) that MTL patients' delay discounting does not differ from controls despite the patients' deficits in prospection. These results hold in the current study, even with the addition of seven new MTL patients to the patients described by Kwan et al. MTL patients may compensate for their episodic memory deficits by relying on schemas along with simple heuristics or even aphorisms that provide the basis for heuristics (e.g., sooner is better, a bird in the hand). Although vmPFC patients, like MTL patients, have prospection deficits, their schemas may be compromised, unlike those of MTL patients, leading to deficits in both delay and probability discounting for vmPFC (but not MTL) patients.
An explanation for the direction of the differences on the discounting tasks would not be necessary if impulsiveness were a basic behavioral tendency, as accounts that pit impulsiveness against self-control imply. If it were, then one could imagine a tendency toward impulsiveness being “unmasked” or disinhibited by brain damage that somehow weakened self-control. However, correlational data from healthy adults do not support this view (for a review, see Green & Myerson, 2010). This view is also not supported in light of previous findings by Donald Stuss et al. who have shown that risk-taking can be dissociated from impulsiveness within PFC (Floden et al., 2008). If anything, the data support the opposite view; other things being equal, the fundamental tendency may be to choose rewards that are both immediate and certain, because people generally prefer their rewards to come sooner and with greater certainty.
Reward size also matters. People tend to want more (i.e., larger rewards), and they want their rewards sooner and for sure. Reward amount is frequently pitted against immediacy and certainty in choice situations. In light of the present findings, the critical question no longer appears to be whether the vmPFC's contribution to decision-making concerns valuation or prospection, as Peters (2011) had suggested. Rather, the issue is how and why vmPFC lesions decrease the weight given to a reward's amount relative to its immediacy, as reflected in steeper delay discounting, but increase the weight given to a reward's amount relative to its likelihood, as reflected in shallower probability discounting. An adequate account of the effects of vmPFC lesions will need to explain the differential weighting of reward amount depending on whether a reward is delayed or probabilistic. It is unclear at this time how a single mechanism could underlie both of these effects given that this pattern of behavior is not at all observed in controls and likely plays no significant role in reward discounting in neurotypical individuals.
There is indeed an alternative way to approach this issue, as recently described by Hiser and Koenigs (2018). They view the vmPFC as an area containing at least three functionally specialized subregions, with each subregion characterized by a pattern of connections with cortical and subcortical structures appropriate to the subregion's function. Additional evidence is required to establish whether or not this view of vmPFC function(s) is correct, and, in any case, it is unclear how the proposed tripartite structure could account for the apparent paradox of differential weighting of reward amount following vmPFC lesions. Nevertheless, if the vmPFC is inherently multifunctional, a thesis that forms the backbone of Donald Stuss's general approach to the PFC (Stuss, 2006, 2017; Stuss, Rosenbaum, Malcolm, Christiana, & Keenan, 2005; Stuss & Levine, 2002), then rather than being two aspects of a single computational function, the integration of immediacy and amount might well be a separate function from the integration of likelihood and amount. In the absence of an alternative explanation for why focal lesions of the vmPFC would have opposite effects on the weighting of amount relative to other reward attributes (e.g., immediacy, likelihood), the present findings suggest that these are two distinct functions. Why these functions are differentially affected by vmPFC lesions remains to be understood.
Conclusions
Regardless of whether a multifunctional view or a more traditional view of vmPFC function holds the key to understanding the issues raised by the current findings, we believe that these issues are fundamental and have potentially important implications for our understanding of both the vmPFC and decision-making itself. The present findings suggest that, rather than being two aspects of a single attribute-integration function involved in the valuation of multi-attribute rewards, separate functions are involved in the integration of amount with reward immediacy and the integration of amount with reward likelihood. This view raises the possibility that integration of other outcome attributes also may involve separate functions, although it should be noted that separate functions do not require separate substrates (i.e., locations) or even separate neurons, just separate circuits. The puzzle of opposite effects of vmPFC lesions on the relative weighting of reward amount remains, but one implication is that consideration of the integration of other outcome attributes, perhaps most prominently losses (Estle, Green, & Myerson, 2019), may shed light on specialized integration functions in general. Although considerable effort, both experimental and theoretical, may be required to resolve these issues, we believe that their fundamental nature justifies the effort.
Discounting is an especially interesting aspect of decision-making for two reasons: first, because the outcomes of everyday choices typically have multiple attributes and discounting focuses specifically on the problem of attribute-integration, and second, because the relative weighting of attributes like immediacy and likelihood appears to underlie many important behavioral problems at both the individual (e.g., substance abuse) and societal (e.g., climate change, pandemic) levels. The functions of the vmPFC appear to be key to understanding such attribute-integration issues, and as this study shows, focal lesion patients can provide insight into these issues, particularly when the same patients are studied using tasks like delay and probability discounting that require integration of different attributes.
Acknowledgments
This article is dedicated to the memory of Dr. Donald T. Stuss, a pioneer in Clinical Neuropsychology and Cognitive Neuroscience who moved the fields forward by uncovering the complexity and fragility of the frontal lobes. His work continues to influence theoretical, methodological, and clinical approaches to understanding the basis of functions that may be at the core of what makes us human. We thank Dr. Asaf Gilboa for assistance in preparing images depicting lesion location and overlap.
Reprint requests should be sent to R. Shayna Rosenbaum, Department of Psychology, York University, 4700 Keele St., Toronto, ON, M3J 1P3, Canada, or via e-mail: shaynar@yorku.ca.
Author Contributions
Jenkin N. Y. Mok: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing—Original draft; Writing—Review & editing. Leonard Green: Formal analysis; Funding acquisition; Investigation; Methodology; Software; Writing—Original draft; Writing—Review & editing. Joel Myerson: Formal analysis; Funding acquisition; Investigation; Methodology; Software; Writing—Original draft; Writing—Review & editing. Donna Kwan: Conceptualization; Data curation; Investigation; Methodology; Project administration; Writing—Review & editing. Jake Kurczek: Conceptualization; Data curation; Investigation; Methodology; Writing—Original draft; Writing—Review & editing. Elisa Ciaramelli: Methodology; Writing—Review & editing. Carl F. Craver: Writing—Review & editing. R. Shayna Rosenbaum: Conceptualization; Funding acquisition; Investigation; Methodology; Resources; Supervision; Formal analysis; Writing—Original draft; Writing—Review & editing.
Funding Information
Research reported in this article was funded by the Natural Sciences and Engineering Research Council (NSERC; https://dx.doi.org/10.13039/501100000038), grant RGPIN-04238-2015, and Vision: Science to Applications (VISTA) York Research Chair in Cognitive Neuroscience of Memory to R. S. R. Preparation of the article was also supported by the National Institute on Aging of the National Institutes of Health (https://dx.doi.org/10.13039/100000049), under award R01AG058885 to L. G. and J. M.
Diversity in Citation Practices
A retrospective analysis of the citations in every article published in this journal from 2010 to 2020 has revealed a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .408, W(oman)/M = .335, M/W = .108, and W/W = .149, the comparable proportions for the articles that these authorship teams cited were M/M = .579, W/M = .243, M/W = .102, and W/W = .076 (Fulvio et al., JoCN, 33:1, pp. 3–7). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article's gender citation balance.
REFERENCES
- Abela, A. R., & Chudasama, Y. (2013). Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. European Journal of Neuroscience, 37, 640–647. DOI: https://doi.org/10.1111/ejn.12071, PMID: 23190048 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: APA. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Bartra, O., McGuire, J. T., & Kable, J. W. (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage, 76, 412–427. DOI: https://doi.org/10.1016/j.neuroimage.2013.02.063, PMID: 23507394, PMCID: PMC3756836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara, A. (2011) The somatic marker hypothesis and its neural basis: Using past experiences to forecast the future in decision making. In Bar M. (Ed.), Predictions in the brain using our past to generate a future (pp. 122–133). New York: Oxford University Press. DOI: 10.1093/acprof:oso/9780195395518.003.0048 [DOI] [Google Scholar]
- Bechara, A., Damasio, A. R., Damasio, H., & Anderson, S. W. (1994). Insensitivity to future consequences following damage to the human prefrontal cortex. Cognition, 50, 7–15. DOI: https://doi.org/10.1016/0010-0277(94)90018-3, PMID: 8039375 [DOI] [PubMed] [Google Scholar]
- Bechara, A., Tranel, D., Damasio, H., & Damasio, A. R. (1996). Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex, 6, 215–225. DOI: https://doi.org/10.1093/cercor/6.2.215, PMID: 8670652 [DOI] [PubMed] [Google Scholar]
- Bechara, A., Tranel, D., & Damasio, H. (2000). Characterization of the decision-making impairment of patients with bilateral lesions of the ventromedial prefrontal cortex. Brain, 123, 2189–2202. DOI: https://doi.org/10.1093/brain/123.11.2189, PMID: 11050020 [DOI] [PubMed] [Google Scholar]
- Bell, D. E. (1982). Regret in decision making under uncertainty. Operations Research, 30, 961–981. DOI: 10.1287/opre.30.5.961 [DOI] [Google Scholar]
- Benoit, R. G., Gilbert, S. J., & Burgess, P. W. (2011). A neural mechanism mediating the impact of episodic prospection on farsighted decisions. Journal of Neuroscience, 31, 6771–6779. DOI: https://doi.org/10.1523/JNEUROSCI.6559-10.2011, PMID: 21543607, PMCID: PMC6632845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi, E., Aleo, F., Braghittoni, D., & Ciaramelli, E. (2016). Stuck in the here and now: Construction of fictitious and future experiences following ventromedial prefrontal damage. Neuropsychologia, 81, 107–116. DOI: https://doi.org/10.1016/j.neuropsychologia.2015.12.015, PMID: 26707714 [DOI] [PubMed] [Google Scholar]
- Bertossi, E., Tesini, C., Cappelli, A., & Ciaramelli, E. (2016). Ventromedial prefrontal damage causes a pervasive impairment of episodic memory and future thinking. Neuropsychologia, 90, 12–24. DOI: https://doi.org/10.1016/j.neuropsychologia.2016.01.034, PMID: 26827916 [DOI] [PubMed] [Google Scholar]
- Blankenstein, N. E., Peper, J. S., Crone, E. A., & van Duijvenvoorde, A. C. (2017). Neural mechanisms underlying risk and ambiguity attitudes. Journal of Cognitive Neuroscience, 29, 1845–1859. DOI: https://doi.org/10.1162/jocn_a_01162, PMID: 28686139 [DOI] [PubMed] [Google Scholar]
- Boorman, E. D., Rushworth, M. F., & Behrens, T. E. (2013). Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. Journal of Neuroscience, 33, 2242–2253. DOI: https://doi.org/10.1523/JNEUROSCI.3022-12.2013, PMID: 23392656, PMCID: PMC3743024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, P. (2008). Evolutionary economics of mental time travel? Trends in Cognitive Sciences, 12, 219–224. DOI: https://doi.org/10.1016/j.tics.2008.03.003, PMID: 18468941 [DOI] [PubMed] [Google Scholar]
- Bulley, A., Miloyan, B., Pepper, G. V., Gullo, M. J., Henry, J. D., & Suddendorf, T. (2019). Cuing both positive and negative episodic foresight reduces delay discounting but does not affect risk-taking. Quarterly Journal of Experimental Psychology, 72, 1998–2017. DOI: https://doi.org/10.1177/1747021818819777, PMID: 30501578 [DOI] [PubMed] [Google Scholar]
- Clairis, N., & Pessiglione, M. (2020). Value, confidence and deliberation in preference tasks: A triple dissociation in the medial prefrontal cortex. bioRxiv. DOI: 10.1101/2020.09.17.301291 [DOI] [Google Scholar]
- Clithero, J. A., & Rangel, A. (2014). Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience, 9, 1289–1302. DOI: https://doi.org/10.1093/scan/nst106, PMID: 23887811, PMCID: PMC4158359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver, C. F., Cova, F., Green, L., Myerson, J., Rosenbaum, R. S., Kwan, D., et al. (2014). An Allais paradox without mental time travel. Hippocampus, 24, 1375–1380. DOI: https://doi.org/10.1002/hipo.22318, PMID: 24976273 [DOI] [PubMed] [Google Scholar]
- Engelmann, J. B., & Tamir, D. (2009). Individual differences in risk preference predict neural responses during financial decision-making. Brain Research, 1290, 28–51. DOI: https://doi.org/10.1016/j.brainres.2009.06.078, PMID: 19576868, PMCID: PMC4353645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estle, S. J., Green, L., & Myerson, J. (2019). When immediate losses are followed by delayed gains: Additive hyperboloid discounting models. Psychonomic Bulletin & Review, 26, 1418–1425. DOI: https://doi.org/10.3758/s13423-019-01599-5, PMID: 31012080, PMCID: PMC6710237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows, L. K. (2011). Orbitofrontal contributions to value-based decision making: Evidence from humans with frontal lobe damage. Annals of the New York Academy of Sciences, 1239, 51–58. DOI: https://doi.org/10.1111/j.1749-6632.2011.06229.x, PMID: 22145875 [DOI] [PubMed] [Google Scholar]
- Fellows, L. K., & Farah, M. J. (2005). Dissociable elements of human foresight: A role for the ventromedial frontal lobes for framing the future, but not in discounting future rewards. Neuropsychologia, 43, 1214–1221. DOI: https://doi.org/10.1016/j.neuropsychologia.2004.07.018, PMID: 15817179 [DOI] [PubMed] [Google Scholar]
- Fellows, L. K., & Farah, M. J. (2007). The role of ventromedial prefrontal cortex in decision making: Judgment under uncertainty or judgment per se? Cerebral Cortex, 17, 2669–2674. DOI: https://doi.org/10.1093/cercor/bhl176, PMID: 17259643 [DOI] [PubMed] [Google Scholar]
- Floden, D., Alexander, M. P., Kubu, C. S., Katz, D., & Stuss, D. T. (2008). Impulsivity and risk-taking behavior in focal frontal lobe lesions. Neuropsychologia, 46, 213–223. DOI: https://doi.org/10.1016/j.neuropsychologia.2007.07.020, PMID: 17854845 [DOI] [PubMed] [Google Scholar]
- Gao, A. F., Keith, J. L., Gao, F. Q., Black, S. E., Moscovitch, M., & Rosenbaum, R. S. (2020). Neuropathology of a remarkable case of memory impairment informs human memory. Neuropsychologia, 140, 107342. DOI: https://doi.org/10.1016/j.neuropsychologia.2020.107342, PMID: 31972232 [DOI] [PubMed] [Google Scholar]
- Ghosh, V. E., Moscovitch, M., Colella, B. M., & Gilboa, A. (2014). Schema representation in patients with ventromedial PFC lesions. Journal of Neuroscience, 34, 12057–12070. DOI: https://doi.org/10.1523/JNEUROSCI.0740-14.2014, PMID: 25186751, PMCID: PMC6608465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, D. T., & Wilson, T. D. (2007). Prospection: Experiencing the future. Science, 317, 1351–1354. DOI: https://doi.org/10.1126/science.1144161, PMID: 17823345 [DOI] [PubMed] [Google Scholar]
- Gilboa, A., & Marlatte, H. (2017). Neurobiology of schemas and schema-mediated memory. Trends in Cognitive Sciences, 21, 618–631. DOI: https://doi.org/10.1016/j.tics.2017.04.013, PMID: 28551107 [DOI] [PubMed] [Google Scholar]
- Green, L., & Myerson, J. (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin, 130, 769–792. DOI: https://doi.org/10.1037/0033-2909.130.5.769, PMID: 15367080, PMCID: PMC1382186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, L., & Myerson, J. (2010). Experimental and correlational analyses of delay and probability discounting. In Madden G. J. & Bickel W. K. (Eds.), Impulsivity: The behavioral and neurological science of discounting (pp. 67–92). American Psychological Association. DOI: 10.1037/12069-003 [DOI] [Google Scholar]
- Green, L., & Myerson, J. (2013). How many impulsivities? A discounting perspective. Journal of the Experimental Analysis of Behavior, 99, 3–13. DOI: https://doi.org/10.1002/jeab.1, PMID: 23344985, PMCID: PMC3893105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, L., Myerson, J., & McFadden, E. (1997). Rate of temporal discounting decreases with amount of reward. Memory & Cognition, 25, 715–723. DOI: https://doi.org/10.3758/BF03211314, PMID: 9337589 [DOI] [PubMed] [Google Scholar]
- Green, L., Myerson, J., & Ostaszewski, P. (1999). Amount of reward has opposite effects on the discounting of delayed and probabilistic outcomes. Journal of Experimental Psychology: Learning, Memory, and Cognition, 25, 418–427. DOI: https://doi.org/10.1037/0278-7393.25.2.418, PMID: 10093208 [DOI] [PubMed] [Google Scholar]
- Gupta, R., Duff, M. C., Denburg, N. L., Cohen, N. J., Bechara, A., & Tranel, D. (2009). Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia, 47, 1686–1693. DOI: https://doi.org/10.1016/j.neuropsychologia.2009.02.007, PMID: 19397863, PMCID: PMC2697903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbrod, K., Kroužel, C., Hofer, H., Müri, R., Perrig, W., & Ptak, R. (2006). Decision-making in amnesia: Do advantageous decisions require conscious knowledge of previous behavioural choices? Neuropsychologia, 44, 1315–1324. DOI: https://doi.org/10.1016/j.neuropsychologia.2006.01.014, PMID: 16513144 [DOI] [PubMed] [Google Scholar]
- Hare, T. A., Hakimi, S., & Rangel, A. (2014). Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Frontiers in Neuroscience, 8, 50. DOI: https://doi.org/10.3389/fnins.2014.00050, PMID: 24672421, PMCID: PMC3957025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebscher, M., & Gilboa, A. (2016). A boost of confidence: The role of the ventromedial prefrontal cortex in memory, decision-making, and schemas. Neuropsychologia, 90, 46–58. DOI: https://doi.org/10.1016/j.neuropsychologia.2016.05.003, PMID: 27150705 [DOI] [PubMed] [Google Scholar]
- Henri-Bhargava, A., Simioni, A., & Fellows, L. K. (2012). Ventromedial frontal lobe damage disrupts the accuracy, but not the speed, of value-based preference judgments. Neuropsychologia, 50, 1536–1542. DOI: https://doi.org/10.1016/j.neuropsychologia.2012.03.006, PMID: 22433288 [DOI] [PubMed] [Google Scholar]
- Hiser, J., & Koenigs, M. (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biological Psychiatry, 83, 638–647. DOI: https://doi.org/10.1016/j.biopsych.2017.10.030, PMID: 29275839, PMCID: PMC5862740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman, G., Yechiam, E., & Bechara, A. (2010). Recency gets larger as lesions move from anterior to posterior locations within the ventromedial prefrontal cortex. Behavioural Brain Research, 213, 27–34. DOI: https://doi.org/10.1016/j.bbr.2010.04.023, PMID: 20412820, PMCID: PMC3124685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdnack, H. A. (2001). Wechsler test of adult reading: WTAR. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Keven, N., Kurczek, J., Rosenbaum, R. S., & Craver, C. F. (2017). Narrative construction is intact in episodic amnesia. Neuropsychologia, 110, 104–112. DOI: https://doi.org/10.1016/j.neuropsychologia.2017.07.028, PMID: 28757002 [DOI] [PubMed] [Google Scholar]
- Kimberg, D. Y., Coslett, H. B., & Schwartz, M. F. (2007). Power in voxel-based lesion-symptom mapping. Journal of Cognitive Neuroscience, 19, 1067–1080. DOI: https://doi.org/10.1162/jocn.2007.19.7.1067, PMID: 17583984 [DOI] [PubMed] [Google Scholar]
- Klein, S. B. (2013). The complex act of projecting oneself into the future. Wiley Interdisciplinary Reviews: Cognitive Science, 4, 63–79. DOI: https://doi.org/10.1002/wcs.1210, PMID: 26304175 [DOI] [PubMed] [Google Scholar]
- Kurtz-David, V., Persitz, D., Webb, R., & Levy, D. J. (2019). The neural computation of inconsistent choice behavior. Nature Communications, 10, 1–14. DOI: https://doi.org/10.1038/s41467-019-09343-2, PMID: 30952855, PMCID: PMC6450930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, D., Craver, C. F., Green, L., Myerson, J., Boyer, P., & Rosenbaum, R. S. (2012). Future decision-making without episodic mental time travel. Hippocampus, 22, 1215–1219. DOI: https://doi.org/10.1002/hipo.20981, PMID: 21997930, PMCID: PMC3262098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, D., Craver, C. F., Green, L., Myerson, J., Gao, F., Black, S. E., et al. (2015). Cueing the personal future to reduce discounting in intertemporal choice: Is episodic prospection necessary? Hippocampus, 25, 432–443. DOI: https://doi.org/10.1002/hipo.22431, PMID: 25676022 [DOI] [PubMed] [Google Scholar]
- Kwan, D., Craver, C. F., Green, L., Myerson, J., & Rosenbaum, R. S. (2013). Dissociations in future thinking following hippocampal damage: Evidence from discounting and time perspective in episodic amnesia. Journal of Experimental Psychology: General, 142, 1355–1369. DOI: https://doi.org/10.1037/a0034001, PMID: 23978187 [DOI] [PubMed] [Google Scholar]
- Kwan, D., Kurczek, J., & Rosenbaum, R. S. (2016). Specific, personally meaningful cues can benefit episodic prospection in medial temporal lobe amnesia. British Journal of Clinical Psychology, 55, 137–153. DOI: https://doi.org/10.1111/bjc.12095, PMID: 26384730 [DOI] [PubMed] [Google Scholar]
- Leland, J. W., & Grafman, J. (2005). Experimental tests of the somatic marker hypothesis. Games and Economic Behavior, 52, 386–409. DOI: 10.1016/j.geb.2004.09.001 [DOI] [Google Scholar]
- Levine, B., Svoboda, E., Hay, J. F., Winocur, G., & Moscovitch, M. (2002). Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging, 17, 677–689. DOI: https://doi.org/10.1037/0882-7974.17.4.677, PMID: 12507363 [PubMed] [Google Scholar]
- Levy, D. J., & Glimcher, P. W. (2012). The root of all value: A neural common currency for choice. Current Opinion in Neurobiology, 22, 1027–1038. DOI: https://doi.org/10.1016/j.conb.2012.06.001, PMID: 22766486, PMCID: PMC4093837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes, G., & Sugden, R. (1982). Regret theory: An alternative theory of rational choice under uncertainty. Economic Journal, 92, 805–824. DOI: 10.2307/2232669 [DOI] [Google Scholar]
- Luhmann, C. C., Chun, M. M., Yi, D. J., Lee, D., & Wang, X. J. (2008). Neural dissociation of delay and uncertainty in intertemporal choice. Journal of Neuroscience, 28, 14459–14466. DOI: https://doi.org/10.1523/JNEUROSCI.5058-08.2008, PMID: 19118180, PMCID: PMC2742332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden, G. J., & Bickel, W. K. (2010). Impulsivity: The behavioral and neurological science of discounting. Washington: American Psychological Association. DOI: 10.1037/12069-000 [DOI] [Google Scholar]
- Mata, R., Josef, A. K., Samanez-Larkin, G. R., & Hertwig, R. (2011). Age differences in risky choice: A meta-analysis. Annals of the New York Academy of Sciences, 1235, 18–29. DOI: https://doi.org/10.1111/j.1749-6632.2011.06200.x, PMID: 22023565, PMCID: PMC3332530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini, S., Body, S., Ho, M. Y., Bradshaw, C., Szabadi, E., Deakin, J., et al. (2002). Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology, 160, 290–298. DOI: https://doi.org/10.1007/s00213-001-0983-0, PMID: 11889498 [DOI] [PubMed] [Google Scholar]
- Mok, J. N. Y., Kwan, D., Green, L., Myerson, J., Craver, C. F., & Rosenbaum, R. S. (2020). Is it time? Episodic imagining and the discounting of delayed and probabilistic rewards in young and older adults. Cognition, 199, 104222. DOI: https://doi.org/10.1016/j.cognition.2020.104222, PMID: 32092551, PMCID: PMC7152567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague, P. R., & Berns, G. S. (2002). Neural economics and the biological substrates of valuation. Neuron, 36, 265–284. DOI: https://doi.org/10.1016/S0896-6273(02)00974-1, PMID: 12383781 [DOI] [PubMed] [Google Scholar]
- Myerson, J., Green, L., Hanson, J. S., Holt, D. D., & Estle, S. J. (2003). Discounting delayed and probabilistic rewards: Processes and traits. Journal of Economic Psychology, 24, 619–635. DOI: 10.1016/S0167-4870(03)00005-9 [DOI] [Google Scholar]
- Myerson, J., Green, L., & Warusawitharana, M. (2001). Area under the curve as a measure of discounting. Journal of Experimental Analysis of Behavior, 76, 235–243. DOI: https://doi.org/10.1901/jeab.2001.76-235, PMID: 11599641, PMCID: PMC1284836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, S., Daniel, T. O., & Epstein, L. H. (2017). Does goal relevant episodic future thinking amplify the effect on delay discounting? Consciousness and Cognition, 51, 10–16. DOI: https://doi.org/10.1016/j.concog.2017.02.014, PMID: 28282631, PMCID: PMC5651988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo, D. J., Keane, M. M., & Verfaellie, M. (2015). The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus, 25, 345–353. DOI: https://doi.org/10.1002/hipo.22376, PMID: 25284804, PMCID: PMC4331231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, J. (2011). The role of the medial orbitofrontal cortex in intertemporal choice: Prospection or valuation? Journal of Neuroscience, 31, 5889–5890. DOI: https://doi.org/10.1523/JNEUROSCI.0268-11.2011, PMID: 21508212, PMCID: PMC6632962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, J., & Büchel, C. (2009). Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. Journal of Neuroscience, 29, 15727–15734. DOI: https://doi.org/10.1523/JNEUROSCI.3489-09.2009, PMID: 20016088, PMCID: PMC6666169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, J., & Büchel, C. (2010). Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron, 66, 138–148. DOI: https://doi.org/10.1016/j.neuron.2010.03.026, PMID: 20399735 [DOI] [PubMed] [Google Scholar]
- Peters, J., & D'Esposito, M. (2016). Effects of medial orbitofrontal cortex lesions on self-control in intertemporal choice. Current Biology, 26, 2625–2628. DOI: https://doi.org/10.1016/j.cub.2016.07.035, PMID: 27593380 [DOI] [PubMed] [Google Scholar]
- Peters, J., & D'Esposito, M. (2020). The drift diffusion model as the choice rule in inter-temporal and risky choice: A case study in medial orbitofrontal cortex lesion patients and controls. PLoS Computational Biology, 16, e1007615. DOI: https://doi.org/10.1371/journal.pcbi.1007615, PMID: 32310962, PMCID: PMC7192518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujara, M. S., Wolf, R. C., Baskaya, M. K., & Koenigs, M. (2015). Ventromedial prefrontal cortex damage alters relative risk tolerance for prospective gains and losses. Neuropsychologia, 79, 70–75. DOI: https://doi.org/10.1016/j.neuropsychologia.2015.10.026, PMID: 26597003, PMCID: PMC4679627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel, A., Camerer, C., & Montague, P. R. (2008). A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience, 9, 545–556. DOI: https://doi.org/10.1038/nrn2357, PMID: 18545266, PMCID: PMC4332708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin, J., Rivest, J., Rosenbaum, R. S., & Moscovitch, M. (2019). Remote spatial and autobiographical memory in cases of episodic amnesia and topographical disorientation. Cortex, 119, 237–257. DOI: https://doi.org/10.1016/j.cortex.2019.04.013, PMID: 31163312 [DOI] [PubMed] [Google Scholar]
- Rorden, C., & Brett, M. (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12, 191–200. DOI: https://doi.org/10.1155/2000/421719, PMID: 11568431 [DOI] [PubMed] [Google Scholar]
- Rosenbaum, R. S., Kwan, D., Floden, D., Levine, B., Stuss, D. T., & Craver, C. F. (2016). No evidence of risk-taking or impulsive behaviour in a person with episodic amnesia: Implications for the role of the hippocampus in future-regarding decision-making. Quarterly Journal of Experimental Psychology, 69, 1606–1618. DOI: https://doi.org/10.1080/17470218.2015.1090461, PMID: 26440609 [DOI] [PubMed] [Google Scholar]
- Seaman, K. L., Brooks, N., Karrer, T. M., Castrellon, J. J., Perkins, S. F., Dang, L. C., et al. (2018). Subjective value representations during effort, probability and time discounting across adulthood. Social Cognitive and Affective Neuroscience, 13, 449–459. DOI: https://doi.org/10.1093/scan/nsy021, PMID: 29618082, PMCID: PMC6007391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto, M., Ciaramelli, E., & di Pellegrino, G. (2010). Myopic discounting of future rewards after medial orbitofrontal damage in humans. Journal of Neuroscience, 30, 16429–16436. DOI: https://doi.org/10.1523/JNEUROSCI.2516-10.2010, PMID: 21147982, PMCID: PMC6634874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto, M., Ciaramelli, E., & di Pellegrino, G. (2011). The neurobiology of intertemporal choice: Insight from imaging and lesion studies. Reviews in the Neurosciences, 22, 565–574. DOI: https://doi.org/10.1515/RNS.2011.046, PMID: 21967518 [DOI] [PubMed] [Google Scholar]
- Spaniol, J., Di Muro, F., & Ciaramelli, E. (2019). Differential impact of ventromedial prefrontal cortex damage on “hot” and “cold” decisions under risk. Cognitive, Affective, & Behavioral Neuroscience, 19, 477–489. DOI: https://doi.org/10.3758/s13415-018-00680-1, PMID: 30535630 [DOI] [PubMed] [Google Scholar]
- Spreen, O., & Strauss, E. (1998). A compendium of neuropsychological tests: Administration, norms, and commentary (2nd ed.). New York: Oxford University Press. [Google Scholar]
- St. Onge, J. R., & Floresco, S. B. (2010). Prefrontal cortical contribution to risk-based decision making. Cerebral Cortex, 20, 1816–1828. DOI: https://doi.org/10.1093/cercor/bhp250, PMID: 19892787 [DOI] [PubMed] [Google Scholar]
- Stuss, D. T. (2006). Frontal lobes and attention: Processes and networks, fractionation and integration. Journal of the International Neuropsychological Society, 12, 261–271. DOI: https://doi.org/10.1017/S1355617706060358, PMID: 16573859 [DOI] [PubMed] [Google Scholar]
- Stuss, D. T. (2017). Frontal lobes. In Kreutzer J. S., DeLuca J., & Caplan B. (Eds.), Encyclopedia of clinical neuropsychology (2nd ed.). Cham, Switzerland: Springer. DOI: 10.1007/978-3-319-56782-2_318-2 [DOI] [Google Scholar]
- Stuss, D. T., & Levine, B. (2002). Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology, 53, 401–433. DOI: https://doi.org/10.1146/annurev.psych.53.100901.135220, PMID: 11752491 [DOI] [PubMed] [Google Scholar]
- Stuss, D. T., Rosenbaum, R. S., Malcolm, S., Christiana, W., & Keenan, J.P. (2005). The frontal lobes and self-awareness. In Feinberg T. E. & Keenan J. P. (Eds.), The lost self: Pathologies of the brain and identity (pp. 50–64). New York: Oxford University Press. DOI: 10.1093/acprof:oso/9780195173413.003.0005 [DOI] [Google Scholar]
- Szpunar, K. K., Spreng, R. N., & Schacter, D. L. (2014). A taxonomy of prospection: Introducing an organizational framework for future-oriented cognition. Proceedings of the National Academy of Sciences, U.S.A., 111, 18414–18421. DOI: https://doi.org/10.1073/pnas.1417144111, PMID: 25416592, PMCID: PMC4284580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya, A. R., & Fellows, L. K. (2015). Ventromedial frontal cortex is critical for guiding attention to reward-predictive visual features in humans. Journal of Neuroscience, 35, 12813–12823. DOI: https://doi.org/10.1523/JNEUROSCI.1607-15.2015, PMID: 26377468, PMCID: PMC6795201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya, A. R., Sefranek, M., & Fellows, L. K. (2018). Ventromedial frontal lobe damage alters how specific attributes are weighed in subjective valuation. Cerebral Cortex, 28, 3857–3867. DOI: https://doi.org/10.1093/cercor/bhx246, PMID: 29069371, PMCID: PMC6188550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaellie, M., Wank, A. A., Reid, A. G., Race, E., & Keane, M. M. (2019). Self-related processing and future thinking: Distinct contributions of ventromedial prefrontal cortex and the medial temporal lobes. Cortex, 115, 159–171. DOI: https://doi.org/10.1016/j.cortex.2019.01.028, PMID: 30826623, PMCID: PMC6513722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, B. J., & Huettel, S. A. (2008). The neural substrates of probabilistic and intertemporal decision making. Brain Research, 1234, 104–115. DOI: https://doi.org/10.1016/j.brainres.2008.07.105, PMID: 18710652, PMCID: PMC2629583 [DOI] [PMC free article] [PubMed] [Google Scholar]