Abstract
Background/Aims
Nonalcoholic fatty liver disease (NAFLD) and obesity are independently associated with an increased risk for atherosclerotic cardiovascular disease (ASCVD), the leading cause of mortality in patients with NAFLD. Many NAFLD patients are lean, but their ASCVD risk compared to obese subjects with NAFLD is unclear.
Methods
Data from the 2008 to 2011 Korea National Health and Nutrition Examination Surveys database were analyzed (n=4,786). NAFLD was defined as a comprehensive NAFLD score ≥40 or a liver fat score ≥–0.640. ASCVD risk was evaluated using the American College of Cardiology/American Heart Association guidelines.
Results
The frequency of subjects without NAFLD, with obese NAFLD, and with lean NAFLD was 62.4% (n=2,987), 26.6% (n=1,274), and 11.0% (n=525), respectively. Subjects with lean NAFLD had a significantly higher ASCVD score and prevalence of a high ASCVD risk (mean 15.6±14.0, 51.6%) than those with obese NAFLD and without NAFLD (mean 11.2±11.4, 39.8%; mean 7.9±10.9, 25.5%; all p<0.001). Subjects with lean NAFLD and significant liver fibrosis showed a significantly higher odds ratio for a high risk for ASCVD than those with obese NAFLD with or without significant liver fibrosis (odds ratio, 2.60 vs 1.93; p=0.023).
Conclusions
Subjects with lean NAFLD had a significantly higher ASCVD score and prevalence of high risk for ASCVD than those with obese NAFLD. Similarly, lean subjects with significant liver fibrosis had a higher probability of ASCVD than obese subjects in the subpopulation with NAFLD.
Keywords: Nonalcoholic fatty liver disease, Lean, Liver fibrosis, Fatty liver, Cardiovascular risk
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide. Although its prevalence varies geographically, overall it is around 25% globally.1-3 Its increasing prevalence is linked to the alarming increase in obesity, insulin resistance, and diabetes mellitus (DM), which are risk factors for both NAFLD and cardiovascular disease (CVD). Therefore, there is a higher prevalence of CVD among individuals with NAFLD compared to those without NAFLD, and CVD is the leading cause of mortality in individuals with NAFLD.4-7
NAFLD is associated with obesity and its related comorbidities, but it can also develop in individuals with a body mass index (BMI) <25 kg/m2 in Caucasians and <23 kg/m2 in Asians; these are defined as having lean NAFLD.8 This sub-phenotype of patients with NAFLD has been described in various ethnicities, particularly in Asia, and in 10% to 20% of nonobese Americans and Caucasians.9,10 The pathophysiological mechanisms are unclear and may include dysfunctional adipose tissue, altered body composition, genetic mutations, epigenetic changes early in life, and a different gut microbiota composition.10-12 Although this phenotype has a more favorable metabolic profile than obese NAFLD, patients with lean NAFLD can develop the full spectrum of liver damage that characterizes non-lean NAFLD.13,14 The natural history and long-term prognosis of patients with lean NAFLD are unclear, but lean NAFLD is not a benign condition. Some data suggest it is associated with a worse mortality rate and accelerated disease progression despite a more favorable metabolic risk profile.15,16
NAFLD and obesity are independently associated with increased CVD risk.17-19 However, the relative risks for patients with obese and lean NAFLD have not been analyzed. Thus, we investigated whether CVD risk differs according to obese versus lean NAFLD and NAFLD with significant liver fibrosis, using data from the Korea National Health and Nutrition Examination Survey (KNHANES).
MATERIALS AND METHODS
1. Subjects
The KNHANES is a nationwide, population-based, cross-sectional health examination and survey that is annually conducted by the Division of Chronic Disease Surveillance of the Korea Disease Control and Prevention Agency of the Ministry of Health and Welfare to monitor the general health and nutritional status of the general civilian population of South Korea.20 Subjects are randomly selected from 600 randomly selected districts in cities and provinces of South Korea to provide a representative sample of the Korean population.
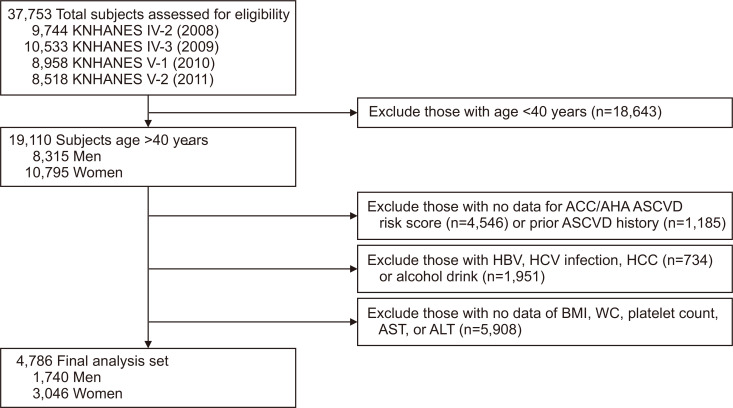
As shown in Fig. 1, of the 37,753 subjects in the KNHANES 2008 to 2011, we initially selected 19,110 subjects aged ≥40 years (8,315 men and 10,795 women). Of these, 14,324 were excluded based on insufficient data to calculate the risk for atherosclerotic cardiovascular disease (ASCVD) or a history of ASCVD; positivity for serologic markers of hepatitis B virus or hepatitis C virus, or hepatocellular carcinoma at enrolment or a history thereof; heavy alcohol consumption (>210 g/week for men and >140 g/week for women); or insufficient clinical and laboratory information to calculate BMI or the magnitude of liver fibrosis or steatosis.
Fig. 1.
Flow diagram of subject inclusion and exclusion in the Korea National Health and Nutrition Examination Surveys (KNHANES IV and V). Of 37,753 subjects, 4,786 were ultimately included (1,740 men and 3,046 women).
ACC/AHA, American College of Cardiology/American Heart Association; ASCVD, atherosclerotic cardiovascular disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; BMI, body mass index; WC, waist circumference; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Written informed consent was secured from all subjects before the study began, and the KNHANES was conducted after approval by the Institutional Review Board of the Korea Disease Control and Prevention Agency (IRB numbers: 2008–04EXP-01-C, 2009–01CON-03–2C, 2010–02CON-21-C, and 2011–02CON-06C). The study protocol was also approved by the Institutional Review Board of Yonsei University Health System (IRB number: Y-2020-0133).
2. NAFLD and liver fibrosis
NAFLD was defined using previously validated fatty liver prediction models (Supplementary Table 1): the comprehensive NAFLD score (CNS) and the NAFLD liver fat score (LFS); a CNS of ≥40 or an LFS of ≥–0.640 were considered indicative of NAFLD.21,22 We assessed the fibrotic burden of subjects with NAFLD (n=1,799) using validated liver fibrosis prediction models: the NAFLD fibrosis score (NFS) and BARD score (Supplementary Table 1).23 Significant liver fibrosis was defined as NFS ≥0.676 or BARD score ≥2. Firstly, CNS and NFS were used to define NAFLD and significant liver fibrosis, respectively. Then, LFS and BARD were used for validation.
3. Assessment of ASCVD risk and cardiometabolic disease components
ASCVD risk was evaluated using the 10-year ASCVD risk score from the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 Subjects with an ACC/AHA ASCVD risk >10% were considered to have a high probability of ASCVD. Based on the criteria for the Asian-Pacific region,25 subjects were considered obese at BMI ≥25 kg/m2. Central obesity was defined based on the waist circumference criteria of the Korean Society for the Study of Obesity (≥90 cm for men and ≥85 cm for women).25 DM was defined based on use of insulin or oral hypoglycemic agents, fasting plasma glucose ≥126 mg/dL, or glycated hemoglobin ≥6.5%. Subjects were diagnosed as hypertensive at systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg, or if they were taking antihypertensive medications. The estimated glomerular filtration rate was calculated using the epidemiology collaboration equation26 and chronic kidney disease (CKD) was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2.27 Hyper-low-density lipoprotein (LDL) cholesterolemia was defined according to the 2004 update of the Adult Treatment Panel III guidelines, or current use of an anti-dyslipidemia drug (e.g., statin) for both sexes.28 Hypo-high-density lipoprotein (HDL) cholesterolemia was defined as HDL <40 mg/dL for men and <50 mg/dL for women. Hypertriglyceridemia was defined as serum levels of triglyceride ≥150 mg/dL or use of triglyceride-lowering agents. Proteinuria was defined as more than one positive dip-stick test or urine albumin-to-creatinine ratio >30 mg/g, according to the guidelines of the Kidney Disease Outcomes Quality Initiative.27 Proteinuria was assessed in 4,588 subjects via urinalysis.
4. Clinical parameters and biochemical analyses
The KNHANES data include medical history, smoking habit, alcohol consumption, exercise level, and disease diagnosis and/or treatment, based on direct interviews and self-reporting using standardized health questionnaires.29 Smoking status was categorized as non- or current smoker. Regular exercise was defined as engaging in moderate or vigorous exercise on a regular basis (≥20 minutes at a time, at least three times per week).30
After an overnight fast (≥8 hours), blood specimens were collected, processed, and transported in cold storage to the Central Testing Institute (Neodin Medical Institute, Seoul, Korea). Blood samples were analyzed within 24 hours of transportation, as described previously.30 The homeostasis model assessment of insulin resistance (HOMA-IR) was assessed as reported elsewhere.31
5. Statistical analyses
The characteristics of the subjects were analyzed according to NAFLD and obesity status using one-way analysis of variance for continuous variables and chi-square tests for categorical variables, followed by post hoc analyses using the Bonferroni method. The association between ASCVD risk and obesity in NAFLD was evaluated using a chi-square test after transforming the variables into quartiles.
Multivariate logistic regression analyses were performed to determine the independent association between high ASCVD risk (ACC/AHA ASCVD risk >10%) and obesity status in NAFLD after adjusting for age and sex in model 1. The variables in model 1 (smoking, exercise, waist circumference, hypertension, DM, HOMA-IR, CKD, hyper-LDL cholesterolemia, and hypo-HDL cholesterolemia) were adjusted in model 2. In addition, to assess the effects of a high ASCVD risk, the adjusted model 2 was applied to NAFLD, obesity, hypertension, DM, smoking, exercise, CKD, hyper-LDL cholesterolemia, and hypo-HDL cholesterolemia. Finally, we evaluated whether NAFLD with liver fibrosis was independently associated with a high ASCVD risk in the subpopulation with NAFLD.
Because the total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, insulin, and HOMA-IR values were not normally distributed, analyses were performed using log-transformed data to achieve approximately symmetrical distributions. Continuous and categorical variables are expressed as means±standard deviations and numbers (%), respectively. A p<0.05 was considered indicative of statistical significance. Statistical analyses were performed using Statistical Package for the Social Sciences version 23.0 for Windows (IBM Corp., Armonk, NY, USA).
RESULTS
1. Baseline characteristics of the study population
After excluding 14,324 subjects according to the exclusion criteria, 4,786 subjects (1,740 men and 3,046 women) were included in the statistical analyses (Fig. 1). Baseline characteristics according to NAFLD and obesity status are shown in Table 1. The frequencies of subjects without NAFLD, obese subjects with NAFLD, and lean subjects with NAFLD were 62.4% (n=2,987), 26.6% (n=1,274), and 11.0% (n=525), respectively.
Table 1.
Baseline Characteristics
| Variable | Subjects without NAFLD (n=2,987, 62.4%) |
Obese subjects with NAFLD (n=1,274, 26.6%) |
Lean subjects with NAFLD (n=525, 11.0%) |
p-value |
|---|---|---|---|---|
| Demographic variables | ||||
| Age, yr | 56.6±11.7 | 58.2±10.6† | 60.5±10.8†,‡ | <0.001 |
| Male sex | 924 (30.9) | 513 (40.3)† | 303 (57.7)†,‡ | <0.001 |
| Waist circumference, cm | 77.4±7.0 | 91.9±6.4† | 85.4±6.1†,‡ | <0.001 |
| Body mass index, kg/m2 | 22.3±2.2 | 27.5±2.2† | 23.7±1.1†,‡ | <0.001 |
| Systolic blood pressure, mm Hg | 118.4±17.6 | 125.8±16.4† | 124.8±17.0† | <0.001 |
| Diastolic blood pressure, mm Hg | 73.8±10.0 | 78.7±10.0† | 76.6±10.5†,‡ | <0.001 |
| Hypertension | 824 (27.6) | 684 (53.7)† | 251 (47.8)† | <0.001 |
| Metabolic syndrome | 541 (18.1) | 942 (73.9)† | 377 (71.8)† | <0.001 |
| Diabetes mellitus | 153 (5.1) | 291 (22.8)† | 171 (32.6)†,‡ | <0.001 |
| Current smoker | 365 (12.2) | 184 (14.4) | 118 (22.5)†,‡ | <0.001 |
| Central obesity | 710 (23.8) | 1,090 (85.6)† | 242 (11.8)†,‡ | <0.001 |
| Obesity | 337 (11.3) | 1,274 (100)† | 0†,‡ | <0.001 |
| Exercise | 472 (15.8) | 191 (15.0) | 64 (12.2) | 0.046 |
| Laboratory variables | ||||
| Fasting blood glucose, mg/dL | 93.8±12.7 | 108.3±28.7† | 117.3±40.6†,‡ | <0.001 |
| Insulin, µIU/mL* | 8.6±3.2 | 12.6±5.8† | 10.9±5.8†,‡ | <0.001 |
| Homeostatic model assessment of insulin resistance* | 2.0±0.9 | 3.4±2.0† | 3.1±2.0†,‡ | <0.001 |
| Total cholesterol, mg/dL | 191.2±34.5 | 202.4±37.5† | 199.4±38.0† | <0.001 |
| Triglyceride, mg/dL* | 104.3±54.6 | 175.2±95.7† | 219.4±128.4†,‡ | <0.001 |
| High density lipoprotein cholesterol, mg/dL* | 54.5±12.4 | 47.0±10.5† | 43.9±9.6†,‡ | <0.001 |
| Low density lipoprotein cholesterol, mg/dL* | 119.8±30.7 | 125.8±34.0† | 119.0±34.2† | <0.001 |
| Serum creatinine, mg/dL | 0.8±0.2 | 0.9±0.2† | 0.9±0.2‡ | <0.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 89.8±15.4 | 86.1±16.1† | 85.1±14.8‡ | <0.001 |
| Aspartate aminotransferase, IU/L* | 20.7±6.8 | 25.0±11.5† | 25.4±10.8†,‡ | <0.001 |
| Alanine aminotransferase, IU/L* | 16.7±7.4 | 28.0±18.8† | 29.6±18.2‡ | <0.001 |
| Platelet count, 109/L* | 255.2±58.7 | 257.4±58.9 | 251.5±25.5 | 0.950 |
| Gamma glutamyl-transpeptidase, IU/L* | 24.0±41.9 | 39.6±44.4† | 50.6±69.2†,‡ | <0.001 |
| Proteinuria | 28 (1.0) | 36 (2.9)† | 17 (3.3)† | <0.001 |
| Liver steatosis | ||||
| Comprehensive NAFLD score | 13.7±11.5 | 73.4±17.2† | 59.4±14.6†,‡ | <0.001 |
| NAFLD liver fat score | 16.1±12.3 | 56.1±19.4† | 44.2±16.9†,‡ | <0.001 |
Data are presented as mean±SD or number (%).
NAFLD, nonalcoholic fatty liver disease.
*Log-transformed; †p<0.05 by post hoc analyses compared without NAFLD; ‡p<0.05 by post hoc analyses compared with obese NAFLD.
Obese subjects with NAFLD were significantly older (mean 58.2 years vs 56.6 years) and there was a significantly higher proportion of males’ sex those without NAFLD (40.3% vs 30.9%) (all p<0.05; hereafter, all p<0.05 unless otherwise noted); the same was true among lean subjects (mean 60.5 years vs 56.6 years; 57.7% vs 30.9%). Subjects with NAFLD, regardless of obese or lean status, showed unfavorable characteristics, such as larger waist circumference, higher BMI, higher blood pressure, and higher frequencies of hypertension, metabolic syndrome, DM, central obesity, and obesity compared to those without NAFLD.
Compared to subjects with obese NAFLD, those with lean NAFLD were significantly older (mean 60.5 years vs 58.2 years) and there were significantly more males (57.7% vs 40.3%). They had significantly lower waist circumferences (mean 85.4 cm vs 91.9 cm), BMIs (mean 23.7 kg/m2 vs 27.5 kg/m2), and diastolic blood pressures (mean 76.6 mm Hg vs 78.7 mm Hg) but higher rates of diabetes (32.6% vs 22.8%) and current smoking (22.5% vs 14.4%). In addition, they had significantly higher levels of fasting blood glucose (mean 117.3 mg/dL vs 108.3 mg/dL), triglycerides (mean 219.4 mg/dL vs 175.2 mg/dL), aspartate aminotransferase (mean 25.4 IU/L vs 25.0 IU/L), alanine aminotransferase (mean 29.6 IU/L vs 28.0 IU/L), and gamma glutamyl-transpeptidase (mean 50.6 IU/L vs 39.6 IU/L) levels, but significantly lower levels of insulin (mean 10.9 µIU/mL vs 12.6 µIU/mL), HOMA-IR (mean 3.1 vs 3.4), HDL (mean 43.9 mg/dL vs 47.0 mg/dL), LDL (mean 119.0 mg/dL vs 125.8 mg/dL), and estimated glomerular filtration rate (mean 85.1 mL/min/1.73 m2 vs 86.1 mL/min/1.73 m2). Finally, they had a significantly lower CNS (mean 59.4 vs 73.4) and LFS (mean 44.2 vs 56.1). The frequencies of hypertension, metabolic syndrome, and regular exercise were similar between the groups. As expected, CNS and LFS were significantly higher in subjects NAFLD than in those without NAFLD (mean 73.4 and 59.4 vs 13.7 for CNS; mean 56.1 and 44.2 vs 16.1 for LFS).
2. Association between ASCVD risk and NAFLD/obesity status
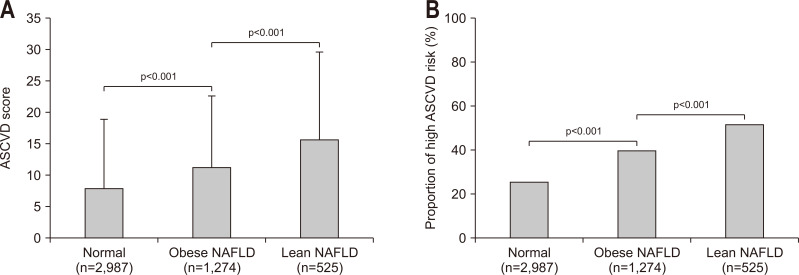
ASCVD scores and the relative risk of ASCVD according to NAFLD/obesity status are shown in Fig. 2. Subjects with lean NAFLD had a significantly higher ASCVD score and prevalence of a high ASCVD risk (mean 15.6±14.0, 51.6%), followed by subjects with obese NAFLD and those without NAFLD (mean 11.2±11.4, 39.8%; mean 7.9±10.9, 25.5%; respectively, all p<0.001). Similar findings were obtained when NAFLD was defined using the LFS (Supplementary Fig. 1). When obesity was defined as BMI ≥30 kg/m2, ASCVD scores and the proportion of high ASCVD risk among obese NAFLD subjects were significantly lower than those among lean NAFLD subjects (mean 11.2±11.4, 39.8% vs mean 15.6±14.0, 51.6%; all p<0.001) (Supplementary Fig. 2).
Fig. 2.
ASCVD score and proportion of patients with high ASCVD risk according to the CNS-defined NAFLD/obesity status. Subjects with lean NAFLD had a significantly higher ASCVD score (A) and prevalence of a high ASCVD risk (B) than subjects with obese NAFLD and those without NAFLD (all p<0.001).
ASCVD, atherosclerotic cardiovascular disease; NAFLD, nonalcoholic fatty liver disease; CNS, comprehensive NAFLD score.
3. Relative risks for cardiometabolic risk factors
The adjusted relative risks for cardiometabolic factors were analyzed when NAFLD was defined based on the CNS (Table 2). Risks for hypertension, DM, hypo-HDL cholesterolemia, hypertriglyceridemia, and proteinuria were significantly higher in the lean group whereas the risks of CKD and hyper-LDL cholesterolemia were significantly higher in the obese group. Next, we repeated the analysis after defining NAFLD based on the LFS (Supplementary Table 2).
Table 2.
Cardiometabolic Risk Factors Stratified by Obesity and NAFLD Status Using the Comprehensive NAFLD Score
| Variable | Subjects without NAFLD OR (95% CI) | Obese subjects with NAFLD | Lean subjects with NAFLD | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Hypertension | 1.00 (reference) | 3.23 (2.78–3.73) | <0.001 | 5.40 (4.37–6.68) | <0.001 | |
| Diabetes mellitus | 1.00 (reference) | 5.40 (4.37–10.29) | <0.001 | 8.00 (6.22–10.29) | <0.001 | |
| Chronic kidney disease | 1.00 (reference) | 1.85 (1.37–2.50) | <0.001 | 1.50 (1.01–2.23) | 0.043 | |
| Hyper-LDL cholesterolemia | 1.00 (reference) | 2.24 (1.94–2.59) | <0.001 | 2.09 (1.71–2.55) | <0.001 | |
| Hypo-HDL cholesterolemia | 1.00 (reference) | 2.94 (2.55–3.39) | <0.001 | 4.29 (3.48–5.31) | <0.001 | |
| Hypertriglyceridemia | 1.00 (reference) | 5.46 (4.72–6.30) | <0.001 | 10.29 (8.29–12.76) | <0.001 | |
| Proteinuria | 1.00 (reference) | 2.90 (1.76–4.77) | <0.001 | 3.01 (1.61–5.61) | 0.001 | |
Adjusted for age and sex.
NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
4. Association between a high probability of ASCVD and NAFLD/obesity status
The associations between a high probability of ASCVD and NAFLD/obesity status with multistep adjustments are shown in Table 3. When CNS was used to define NAFLD and the relative risk of ASCVD was assessed after sufficient adjustment (model 2), lean subjects had a higher OR for a high probability of ASCVD than those with obese NAFLD (odds ratio 2.63 vs 2.05; all p<0.001) or subjects without NAFLD. We also repeated the analysis after defining NAFLD based on the LFS (Supplementary Table 3).
Table 3.
High Probability of ASCVD According to Obesity and NAFLD Based on the Comprehensive NAFLD Score
| Model | Subjects without NAFLD OR (95% CI) | Obese subjects with NAFLD | Lean subjects with NAFLD | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Crude | 1.00 (reference) | 1.93 (1.68–2.22) | <0.001 | 3.12 (2.58–3.77) | <0.001 | |
| Model 1 | 1.00 (reference) | 3.68 (2.89–4.69) | <0.001 | 3.71 (2.68–5.14) | <0.001 | |
| Model 2 | 1.00 (reference) | 2.05 (1.37–3.07) | 0.001 | 2.63 (1.61–3.58) | <0.001 | |
ASCVD, atherosclerotic cardiovascular disease; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval.
Model 1: adjusted for age and sex and model 2: adjusted for age, sex, smoking, exercise, waist circumference, hypertension, diabetes, homeostasis model assessment of insulin resistance, chronic kidney disease, and hyper-low-density lipoprotein cholesterolemia.
5. Association between ASCVD risk and significant liver fibrosis
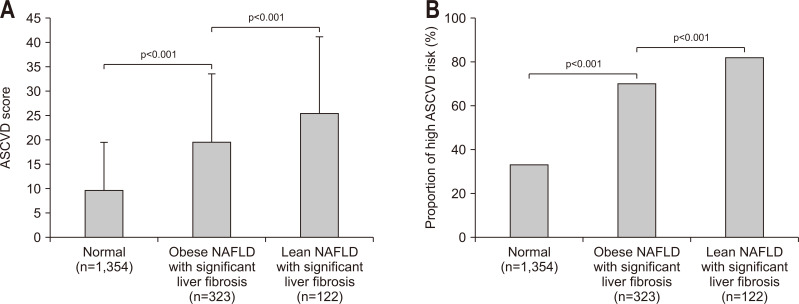
Because fibrosis progression is significantly associated with an increased risk for ASCVD among subjects with NAFLD,32 we selected subjects with NAFLD for further statistical analyses (n=1,799). Lean subjects with NFS-defined significant liver fibrosis had a significantly higher ASCVD score and prevalence of a high ASCVD risk (mean 25.6±15.7, 82.0%), followed by obese subjects with NFS-defined significant liver fibrosis and those without NFS-defined significant liver fibrosis (mean 19.5±14.1, 70.3%; mean 9.7±9.8, 33.3%; respectively, all p<0.001) (Fig. 3). When BARD was used to define significant liver fibrosis, similar findings were obtained (Supplementary Fig. 3).
Fig. 3.
ASCVD score and proportion of patients with high ASCVD risk according to NFS-defined significant liver fibrosis stratified by the CNS-defined NAFLD/obesity status. Lean subjects with NFS-defined significant liver fibrosis had a significantly higher ASCVD score (A) and prevalence of a high ASCVD risk (B) than obese subjects with significant liver fibrosis and those without significant liver fibrosis (all p<0.001).
ASCVD, atherosclerotic cardiovascular disease; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD fibrosis score; CNS, comprehensive NAFLD score.
6. Association between a high probability of ASCVD and significant liver fibrosis
The associations between a high probability of ASCVD and significant liver fibrosis stratified by obesity among subjects with NAFLD according to multistep adjustments are shown in Table 4. When NFS was used to define significant fibrosis and the relative risk of ASCVD was assessed after sufficient adjustment (model 2), subjects with lean NAFLD and significant liver fibrosis showed a significantly higher OR for the risk of ASCVD than subjects with obese NAFLD with or without significant liver fibrosis (odds ratio, 2.60 vs 1.93; p=0.023). When BARD was used to define significant liver fibrosis, similar findings were obtained (Supplementary Table 4).
Table 4.
High Probability of ASCVD According to Obesity and Significant Fibrosis Based on the NAFLD Fibrosis Score
| Model | NAFLD with no fibrosis OR (95% CI) | Obese NAFLD subjects with significant fibrosis | Lean NAFLD subjects with significant fibrosis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Crude | 1.00 (reference) | 1.66 (1.33–2.08) | <0.001 | 2.61 (1.98–3.43) | <0.001 | |
| Model 1 | 1.00 (reference) | 2.20 (1.59–3.06) | <0.001 | 2.53 (1.40–4.56) | 0.002 | |
| Model 2 | 1.00 (reference) | 1.93 (1.28–3.63) | 0.002 | 2.60 (1.14–5.91) | 0.023 | |
ASCVD, atherosclerotic cardiovascular disease; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval.
Model 1: adjusted for age and sex and model 2: adjusted for age, sex, smoking, exercise, waist circumference, hypertension, diabetes, homeostasis model assessment of insulin resistance, chronic kidney disease, and hyper-low-density lipoprotein cholesterolemia.
DISCUSSION
A close association between NAFLD and obesity has consistently been reported.3,19 In this context, most subjects with NAFLD are overweight/obese and have varying components of metabolic syndrome.4,33 Nonetheless, a significant proportion of subjects with NAFLD are lean, although the clinical implications are unclear.34 Accordingly, using nationwide, population-based, cross-sectional data, we investigated whether ASCVD risk differs according to obesity in patients with NAFLD and checked whether similar results can be reproduced in subgroups with significant liver fibrosis.
Subjects with lean NAFLD had significantly higher ASCVD scores and a greater chance of having high ASCVD risk than those with obese NAFLD. Even if obesity was defined as BMI ≥30 kg/m2, our main findings were nearly the same. Hypertension, DM, hypo-HDL cholesterolemia, hypertriglyceridemia, and albuminuria significantly increased the risk of ASCVD in lean compared to obese subjects with NAFLD. After appropriate adjustment, lean subjects with NAFLD had a significantly higher ASCVD risk than obese subjects with or without NAFLD. Furthermore, after adjustment, lean subjects with significant liver fibrosis had the highest ASCVD risk, followed by obese subjects with significant liver fibrosis and those without significant liver fibrosis.
This study had several strengths. First, the selected cohort was large (n>4,000), ensuring statistical reliability and robust results. Furthermore, the proportion of lean subjects with NAFLD in the cohort (11.0%) was similar to that in previous Asian studies (9% to 23.5%).13,35 This suggests that the subjects were selected appropriately and that the results are applicable to other Asian populations, although further validation is required for other ethnic groups. In addition, the prevalence of subjects with NAFLD (37.6%) was similar to that in a recent Asian study.36 Therefore, our study population, a nationwide representative cohort, was selected appropriately based on noninvasive surrogates for statistical analyses.
Second, to the best of our knowledge, this is the first report of an independent association between ASCVD risk and NAFLD according to obesity status. NAFLD and obesity are significant risk factors for cardiovascular events.17-19 However, after controlling for important confounders, lean NAFLD was associated with a higher ASCVD score and an increased prevalence of a high ASCVD risk compared to obese NAFLD; moreover, this finding was reproduced in the subgroup with significant liver fibrosis. In addition, our results provide evidence on the prognosis of lean NAFLD. Unlike the prognosis and complications of overall NAFLD, the data on lean NAFLD are scarce. Two studies have reported a higher mortality rate for lean compared to obese NAFLD.37,38 Moreover, CVD accounted for approximately one quarter of the causes of death.37 In our study, subjects with lean NAFLD were at markedly increased risk for individual cardiometabolic components and ASCVD compared to those with obese NAFLD. These data indicate that lean subjects with NAFLD should be counselled about the risk for unfavorable cardiovascular outcomes and managed accordingly.39
Third, the influence of NAFLD on ASCVD might have been biased because most subjects with NAFLD had simple hepatic steatosis, which has a very favorable prognosis. Thus, we selected subjects with NAFLD to determine whether the co-existence of significant liver fibrosis is significantly associated with ASCVD risk, because liver fibrosis can be considered a sequela of the inflammatory process of NAFLD. In addition, liver fibrosis is the single most important factor as well as a clinically relevant issue that correlates with poor outcomes.40 In the subgroup with NAFLD, around 20% of subjects had significant liver fibrosis, indicating that lean subjects had higher ASCVD risk than obese subjects among those with NAFLD and significant fibrosis.
We are also aware of several unresolved issues that should be addressed. First, although we used well-validated liver fibrosis and steatosis prediction models, liver imaging and histological information was not available because of the high cost of ultrasonography and ethical concerns regarding screening of a large national population-based cohort. KNHANES participants who gave informed consent underwent only serum tests. In addition, because cancer diagnosis in the KNHANES was based on a questionnaire, not ultrasonography, subjects with hepatocellular carcinoma were excluded.
Second, because of the cross-sectional nature of the study, we could not assess the longitudinal dynamic association between changes in NAFLD, obesity status, and ASCVD risk. We were also unable to assess the effects of therapeutic interventions, such as lifestyle modification, exercise, weight loss, medications, nutritional support, and protein supplements, on NAFLD, obesity, and ASCVD risk. Nevertheless, our results reveal the need to screen patients with NAFLD, particularly lean patients, to identify those at high risk for ASCVD requiring intensive medical therapy.
Third, we used a pooled cohort risk equation to assess ASCVD risk and did not examine the risk for real clinical events during follow-up. The 10-year ASCVD risk with primary prevention is estimated in the blood cholesterol guidelines of the ACC/AHA. However, because the equation for calculating ASCVD risk might have been in the study population, our findings should be interpreted with caution.
Finally, several serum markers, such as HbA1c, were available for only a small proportion of the subjects. Thus, the incremental influence on the final results, not simply the presence of DM, could not be assessed. In addition, detailed information regarding antihypertensive and antidiabetic drugs was not available, preventing analyses of their influence. Furthermore, although we excluded subjects known to have chronic liver diseases (such as viral hepatitis and alcoholic liver disease), those with other types of such diseases (such as Wilson disease, autoimmune liver disease, or primary biliary hepatopathy) might have been included, which may have biased the results. Moreover, as a result of the limitations of general medical examination, we could not adjust for dietary preference and genetic risk factors (including patatin-like phospholipase domain-containing 3 and transmembrane 6 superfamily member 2), which could affect the risk for lean NAFLD.41
Although the characteristics and underlying pathophysiological mechanism of lean NAFLD/nonalcoholic steatohepatitis are unclear, individuals with a low BMI are likely to have reduced lean body mass, particularly muscle mass, which could lead to unfavorable traits related to cardiovascular outcomes.42 In addition, fat tissue in a distinct depot may have protective functions, particularly in people with chronic diseases.43 Subcutaneous fat can act as a metabolic reservoir, protecting other organs from lipotoxicity and ectopic fat formation, and leg fat is associated with lower ASCVD risk and cardiometabolic risk factors.44
In conclusion, this nationwide survey of a representative sample of Korean individuals demonstrated that, despite a more favorable metabolic profile, subjects with lean NAFLD had a significantly higher ASCVD score and prevalence of a high ASCVD risk than those with obese NAFLD. Similarly, lean subjects with significant liver fibrosis were at higher risk for ASCVD than obese subjects in the subpopulation with NAFLD. In this context, the presence of fatty liver should prompt clinicians to address metabolic conditions that could modify the long-term outcomes, irrespective of body weight. Prospective, well-designed, longitudinal studies are needed to elucidate the complex relationships among NAFLD, obesity, and cardiovascular risk.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl210084.
ACKNOWLEDGEMENTS
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2019R1A2C4070136), by the Ministry of Education (2018R1D1A1B07050005), and by the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare (HI17C0913), Republic of Korea. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST
S.H.A. and S.U.K are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
Conception and design: S.U.K., Y.H.L., Y.K., E.H. Development of methodology: S.U.K., Y.H.L., Y.K., E.H. Analysis and interpretation of data: S.U.K., Y.H.L., Y.K., E.H. Writing, review, and/or revision of the manuscript: S.U.K., Y.H.L., Y.K., E.H. Administrative, technical, or material support: S.U.K., Y.H.L., J.S.L., H.W.L., B.K.K., M.K.K., H.S.K., J.Y.P., D.Y.K., S.H.A., B.W.L., E.S.K., B.S.C., Y.K., E.H. Study supervision: S.U.K., Y.H.L.
REFERENCES
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Yoo JJ, Kim W, Kim MY, et al. Recent research trends and updates on nonalcoholic fatty liver disease. Clin Mol Hepatol. 2019;25:1–11. doi: 10.3350/cmh.2018.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotundo L, Persaud A, Feurdean M, Ahlawat S, Kim HS. The association of leptin with severity of non-alcoholic fatty liver disease: a population-based study. Clin Mol Hepatol. 2018;24:392–401. doi: 10.3350/cmh.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20:13306–13324. doi: 10.3748/wjg.v20.i37.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma J, Hwang SJ, Pedley A, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390–397. doi: 10.1016/j.jhep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golabi P, Fukui N, Paik J, Sayiner M, Mishra A, Younossi ZM. Mortality risk detected by atherosclerotic cardiovascular disease score in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2019;3:1050–1060. doi: 10.1002/hep4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das K, Chowdhury A. Lean NASH: distinctiveness and clinical implication. Hepatol Int. 2013;7 Suppl 2:806–813. doi: 10.1007/s12072-013-9477-5. [DOI] [PubMed] [Google Scholar]
- 9.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Albhaisi S, Chowdhury A, Sanyal AJ. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019;1:329–341. doi: 10.1016/j.jhepr.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. 2017;46:85–95. doi: 10.1111/apt.14112. [DOI] [PubMed] [Google Scholar]
- 12.Ando W, Yokomori H, Tsutsui N, et al. Serum matrix metalloproteinase-1 level represents disease activity as opposed to fibrosis in patients with histologically proven nonalcoholic steatohepatitis. Clin Mol Hepatol. 2018;24:61–76. doi: 10.3350/cmh.2017.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung JC, Loong TC, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54–64. doi: 10.1002/hep.28697. [DOI] [PubMed] [Google Scholar]
- 14.Younes R, Bugianesi E. NASH in lean individuals. Semin Liver Dis. 2019;39:86–95. doi: 10.1055/s-0038-1677517. [DOI] [PubMed] [Google Scholar]
- 15.Hagström H, Nasr P, Ekstedt M, et al. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019;39:197–204. doi: 10.1111/liv.13973. [DOI] [PubMed] [Google Scholar]
- 16.Chen F, Esmaili S, Rogers GB, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71:1213–1227. doi: 10.1002/hep.30908. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed MH, Barakat S, Almobarak AO. Nonalcoholic fatty liver disease and cardiovascular disease: has the time come for cardiologists to be hepatologists? J Obes. 2012;2012:483135. doi: 10.1155/2012/483135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 19.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y. The Korea National Health and Nutrition Examination Survey (KNHANES): current status and challenges. Epidemiol Health. 2014;36:e2014002. doi: 10.4178/epih/e2014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YH, Bang H, Park YM, et al. Non-laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: development, validation and comparison with other scores. PLoS One. 2014;9:e107584. doi: 10.1371/journal.pone.0107584.c5f9af726afb41d395dcf4d62709f641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Cichoż-Lach H, Celiński K, Prozorow-Król B, Swatek J, Słomka M, Lach T. The BARD score and the NAFLD fibrosis score in the assessment of advanced liver fibrosis in nonalcoholic fatty liver disease. Med Sci Monit. 2012;18:CR735–CR740. doi: 10.12659/MSM.883601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 25.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Kidney Foundation, author. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 28.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 29.Lee YH, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Kim JE, Roh YH, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008-2011. J Clin Endocrinol Metab. 2014;99:3879–3888. doi: 10.1210/jc.2013-3764. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330–9337. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curcic IB, Berkovic MC, Kuna L, et al. Obesity paradox in chronic liver diseases: product of bias or a real thing? J Clin Transl Hepatol. 2019;7:275–279. doi: 10.14218/JCTH.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon YM, Oh SW, Hwang SS, Lee C, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012;107:1852–1858. doi: 10.1038/ajg.2012.314. [DOI] [PubMed] [Google Scholar]
- 36.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 37.Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med. 2020;288:139–151. doi: 10.1111/joim.13069. [DOI] [PubMed] [Google Scholar]
- 38.Golabi P, Paik JM, Arshad T, Younossi Y, Mishra A, Younossi ZM. Mortality of NAFLD according to the body composition and presence of metabolic abnormalities. Hepatol Commun. 2020;4:1136–1148. doi: 10.1002/hep4.1534.93d73d367a8c4d48b2780ee4cbb5d1aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golabi P, Paik J, Fukui N, Locklear CT, de Avilla L, Younossi ZM. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes. 2019;37:65–72. doi: 10.2337/cd18-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 41.Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15:474–485. doi: 10.1016/j.cgh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Han E, Lee YH, Kim YD, et al. Nonalcoholic fatty liver disease and sarcopenia are independently associated with cardiovascular risk. Am J Gastroenterol. 2020;115:584–595. doi: 10.14309/ajg.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 43.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the "obesity paradox". J Am Coll Cardiol. 2012;60:1374–1380. doi: 10.1016/j.jacc.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 44.Han E, Lee YH, Lee BW, Kang ES, Lee IK, Cha BS. Anatomic fat depots and cardiovascular risk: a focus on the leg fat using nationwide surveys (KNHANES 2008-2011) Cardiovasc Diabetol. 2017;16:54. doi: 10.1186/s12933-017-0536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.