Abstract
Background/Aims
Small rectal neuroendocrine tumors (NETs) are often managed with local resection (endoscopic or transanal excision) owing to their low risk of metastasis and recurrence. However, the clinical significance of lymphovascular invasion in resected specimens remains controversial. In this study, we aimed to analyze the frequency of and risk factors for lymph node metastasis proven by histopathologic examination after radical resection.
Methods
We retrospectively reviewed the records of 750 patients diagnosed with a rectal NET at four academic medical centers in South Korea between 2001 and 2019. The frequency of histopathologically proven lymph node metastasis and the associated risk factors were analyzed for small tumors (≤1.5 cm) with lymphovascular invasion.
Results
Among 750 patients, 75 had a small tumor (≤1.5 cm) with lymphovascular invasion, of whom 31 patients underwent endoscopic resection only and 44 patients underwent additional radical surgery. Among the 41 patients who underwent surgery and had available data, the rate of regional lymph node metastasis was 48.8% (20/41). In multivariate analysis, the Ki-67 index (odds ratio, 6.279; 95% confidence interval, 1.212 to 32.528; p=0.029) was an independent risk factor for lymph node metastasis. During the mean follow-up period of 37.7 months, only one case of recurrence was detected in the surgery group. The overall survival was not significantly different between radical resection and local resection (p=0.332).
Conclusions
Rectal NETs with lymphovascular invasion showed a significantly high rate of regional lymph node metastasis despite their small size (≤1.5 cm).
Keywords: Rectum, Neuroendocrine tumors, Lymphovascular invasion, Metastasis
INTRODUCTION
The incidence of rectal neuroendocrine tumors (NETs) has increased worldwide.1-3 The rectum is the most common site of gastroenteropancreatic NETs in Asian patients and the second most common site in Western patients.1,3-6 Most rectal NETs are incidentally discovered during screening colonoscopy, and are small and localized within the mucosa or submucosa. Current guidelines recommend that tumors <1–2 cm and confined to the submucosa (T1) can be endoscopically managed with various resection techniques (e.g., endoscopic mucosal resection or endoscopic submucosal dissection).7-12 However, the optimal management of small tumors with lymphovascular invasion is controversial. Conventionally, lymphovascular invasion is known to be associated with lymph node metastasis; thus, radical resection with lymph node dissection has been considered in clinical practice.13 Radical resection has a risk of adverse events, and several reports have questioned the necessity of surgical management in this setting, with endoscopic resection providing a favorable outcome for patients with small tumors with lymphovascular invasion.14-16 However, these studies are limited by the inclusion of cases with radiologically or clinically defined lymph node metastases rather than pathologically proven metastases. Lymph node metastasis cannot be clearly identified with radiology, and rectal NETs are very slow-growing tumors; thus, a 3- to 5-year follow-up period is not sufficient for the clinical evaluation of lymph node status, leading to the justification of local resection.17 Conversely, some studies that analyzed surgical specimens with histologically proven lymph node metastasis provided a warning about endoscopic treatment, emphasizing the existence of a relatively high rate of lymph node metastasis even in tumors <1 cm.18-21 However, these studies are limited by the small number of included patients. Current guidelines do not clearly describe the status of lymphovascular invasion after the local excision of small rectal NETs.7,9,10
In this study, we aimed to analyze the frequency and risk factors of histopathologically proven lymph node metastasis, using the data of patients with small rectal NETs (<1.5 cm) with lymphovascular invasion treated with surgical resection. The secondary objective was to estimate the clinical and oncologic outcomes of small rectal NETs with lymphovascular invasion.
MATERIALS AND METHODS
1. Patients
We retrospectively reviewed the medical records of patients diagnosed with a rectal NET between December 2001 and September 2019 at the National Cancer Center, National Health Insurance Service Ilsan Hospital, Kangwon National University Hospital, and Wonju Severance Christian Hospital in South Korea. The institutional review board at each hospital approved the research protocol (approval number: KWNUH 2020-01-001). This study has been granted an exemption from requiring written informed consent.
The clinical and pathologic data extracted from the medical records included age, sex, date of diagnosis, carcinoid symptoms (facial flushing, diarrhea, asthma/wheezing, pellagra, and carcinoid heart disease), familial history of NET, biomarkers (5‐hydroxyindoleacetic acid and chromogranin A), diagnostic method, and histopathologic findings.
All patients were recommended to undergo additional radical surgery if lymphovascular invasion was identified, regardless of other tumor characteristics. However, a considerable number of patients preferred close follow-up rather than surgery. For those who preferred local therapy only, we chose to perform follow-up without radical resection based on several previous reports.14,15,22 We analyzed the frequency of histopathologically proven lymph node metastases among radically resected small rectal NETs showing lymphovascular invasion, and compared the clinical and pathologic characteristics between patients with and without lymph node metastases.
2. Histopathologic evaluation
Rectal NETs were diagnosed and classified according to the World Health Organization criteria and the American Joint Committee on Cancer manual for staging of cancer.23,24 NETs were assessed according to tumor location, histologic type, size, invasion depth, resection margin status, and lymphovascular invasion. The histologic grading was made using the Ki-67 labeling index according to the World Health Organization 2010 classification and the North American Neuroendocrine Tumor Society guidelines.8,24 Lymphovascular invasion was evaluated using hematoxylin and eosin staining and immunohistochemical staining with primary antibodies for anti-CD31 or anti-podoplanin (1:100; clone JC70A, Dako, Glostrup, Denmark or clone D2-40, Signet Laboratory, Dedham, MA, USA). Lymphovascular invasion was deemed present when synaptophysin-positive tumor cells were detected within vascular spaces lined by podoplanin or CD31-positive endothelial cells. In surgically resected cases, the depth of submucosal (SM) invasion was determined according to Kudo classification,25 as follows: sm1, infiltration into the upper third of the SM layer; sm2, infiltration into the middle third of the SM layer; and sm3, infiltration into the lower third of the SM layer. For endoscopically resected cases, the cutoff limit between sm1 and sm2 was 1,000 µm according to the Paris classification, and a depth of SM invasion exceeding 2,000 µm was defined as sm3.
3. Statistical analysis
Categorical variables are expressed as numbers with percentages, and continuous variables are expressed as means with standard deviations. For the univariate analysis, the Pearson chi-square test or the Fisher exact test was performed for categorical variables and the unpaired t-test for continuous variables. For the analysis of associated factors of locoregional lymph node metastasis, univariate and multivariate analyses using the logistic regression were performed. Variables with a p-value of <0.1 in the univariate analysis were included in the multivariate analysis. The results are expressed as odds ratios with 95% confidence intervals. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. A p-value of <0.05 was considered statistically significant. Data were analyzed using SPSS version 21 (IBM Corp., Armonk, NY, USA).
RESULTS
1. Patient characteristics
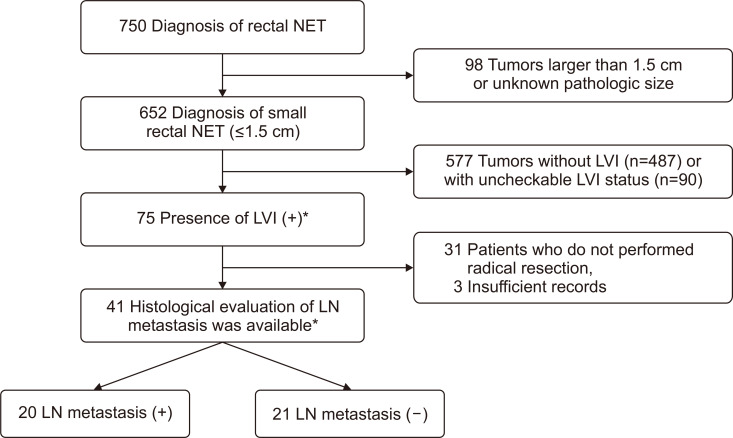
A total of 750 patients diagnosed with a rectal NET were reviewed in this study. Among them, 652 patients had small rectal NETs <1.5 cm in size, of whom 75 patients had a lymphovascular invasion determined with various resection methods (e.g., endoscopic mucosal resection, endoscopic submucosal dissection, or transanal excision). Among 75 patients with small tumors (≤1.5 cm) showing lymphovascular invasion, 44 patients underwent radical resection (i.e., low anterior resection), enabling histopathologic evaluation of the perirectal lymph node status, and three patients were excluded from the analysis owing to insufficient medical records (Fig. 1). Among 41 patients with available histologic lymph node status, the regional lymph node metastasis rate was 48.8% (20/41) (Fig. 1). Except for the tumor size and Ki-67 index, the clinicopathologic characteristics were similar between patients with and without lymph node metastasis (Table 1). Larger tumors (mean 9.60±2.78 mm vs 7.19±2.64 mm) and tumors with a higher Ki-67 index tended to have lymph node metastasis (Table 1). Various carcinoid symptoms were evaluated (facial flushing, diarrhea, asthma/wheezing, pellagra, and carcinoid heart disease), and most patients did not show any carcinoid symptoms. Only two patients complained of diarrhea (4.9%). However, it is not clear whether this is a carcinoid symptom or nonspecific gastrointestinal symptom, as almost all patients did not perform the assays for serum or urine biomarkers and we could not check out the symptom improvement after treatment. A few patients (8/42, 19.0%) showed other presenting symptoms, including hematochezia, low abdominal discomfort, weight loss, and constipation. Most tumors arose in the mid-rectum (5–10 cm from the anal verge, 80.5%), were 6–10 mm in size (68.3%), and were located in the submucosa (92.7%).
Fig. 1.
Flowchart of patient selection.
NET, neuroendocrine tumor; LVI, lymphovascular invasion; LN, lymph node. *Among 75 patients with LVI, five patients showed equivocal or suspicious LVI. Among 41 patients who underwent radical resection with available data, one patient showed equivocal LVI.
Table 1.
Clinicopathological Characteristics of Rectal Neuroendocrine Tumors at Diagnosis
| Characteristics | Study population (n=41) | LN (–) (n=21) | LN (+) (n=20) | p-value* |
|---|---|---|---|---|
| Age, mean±SD, yr | 48.73±12.64 | 52.05±12.29 | 45.25±12.34 | 0.085† |
| Sex | 0.062‡ | |||
| Male | 27 (65.9) | 11 (52.4) | 16 (80.0) | |
| Female | 14 (34.1) | 10 (47.6) | 4 (20.0) | |
| Carcinoid symptoms | 0.488§ | |||
| Present | 2 (4.9) | 2 (9.5) | 0 | |
| Absent | 39 (95.1) | 19 (90.5) | 21 (100) | |
| Tumor size, mean±SD, mm | 8.26±2.98 | 7.19±2.64 | 9.60±2.78 | 0.007† |
| Tumor size, mm | 0.008§ | |||
| 0–5 | 6 (14.6) | 6 (28.6) | 0 | |
| 6–10 | 28 (68.3) | 14 (66.7) | 14 (70.0) | |
| 11–15 | 7 (17.1) | 1 (4.8) | 6 (30.0) | |
| Tumor location (distance from anal verge), mean±SD, mm | 6.46±2.15 | 6.24±2.14 | 6.70±2.18 | 0.498† |
| Depth of invasion | 0.107§ | |||
| Mucosa | 1 (2.4) | 0 | 1 (5.0) | |
| Submucosa | 38 (92.7) | 21 (100) | 17 (85.0) | |
| Muscularis propria | 2 (4.9) | 0 | 2 (10.0) | |
| Ki-67 index, % | 0.027§ | |||
| <3 | 22 (53.7) | 15 (71.4) | 7 (35.0) | |
| 3–20 | 16 (39.0) | 6 (28.6) | 10 (50.0) | |
| >20 | 3 (7.3) | 0 | 3 (15.0) | |
| Tumor grade (%) | 0.027§ | |||
| G1 (low) | 22 (53.7) | 15 (71.4) | 7 (35.0) | |
| G2 (intermediate) | 16 (39.0) | 6 (28.6) | 10 (50.0) | |
| G3 (high) | 3 (7.3) | 0 | 3 (15.0) | |
| No. of harvested LN, mean±SD (range) | 19.39±12.04 (5–55) | 18.38±11.67 (5–53) | 20.45±12.63 (6–60) | 0.589† |
| No. of metastatic LN, mean±SD (range) | 0.90±1.39 (0–6) | 0 | 1.85±1.50 (1–6) | NA |
| Resection margin status, %‖ | NA | |||
| R0 | 41 (100) | 21 (100) | 20 (100) | |
| R1/R2 | 0 | 0 | 0 |
Data are presented as number (%) unless otherwise indicated.
LN, lymph node; NA, not applicable.
*p-value is calculated for comparing two groups, LN metastasis (–) versus LN metastasis (+) group; †Unpaired t-test; ‡Pearson chi-square test; §Fisher exact test; ‖R0: complete resection grossly and microscopically; R1: microscopic residual lesions; R2: gross residual tumors.
2. Risk factors associated with lymph node metastasis
The factors associated with lymph node metastasis in patients with lymphovascular invasion were tumor size and Ki-67 index in univariate logistic regression analysis. Only Ki-67 ≥3% was a significant risk factor in the multivariate analysis (Table 2). We also performed subgroup analysis for tumors located in the submucosa according to the degree of SM involvement. The depth of SM involvement was classified as sm1 (upper third), sm2 (middle third), and sm3 (lower third) in the surgically resected specimen. No significant relationship was observed between the degree of SM involvement and lymph node metastasis (Table 3).
Table 2.
Factors Associated with Locoregional Lymph Node Metastasis
| Factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age | 0.955 (0.905–1.007) | 0.090 | 0.965 (0.907–1.027) | 0.263 | |
| Sex (male) | 3.636 (0.905–14.609) | 0.069 | 4.946 (0.851–28.730) | 0.075 | |
| Tumor size | 1.427 (1.064–1.914) | 0.018 | 1.327 (0.972–1.811) | 0.075 | |
| Ki-67 index (≥3%) | 4.643 (1.241–17.368) | 0.023 | 6.279 (1.212–32.528) | 0.029 | |
p-value was calculated by univariable and multivariable logistic regression analysis.
OR, odds ratio; CI, confidence interval.
Table 3.
Degree of Submucosal Involvement for Tumors with Submucosal Invasion*
| Degree of submucosal involvement† | LN metastasis (–) (n=21) | LN metastasis (+) (n=16) | p-value‡ |
|---|---|---|---|
| sm1 | 1 (4.8) | 2 (12.5) | 0.456 |
| sm2 | 8 (38.1) | 3 (18.8) | |
| sm3 | 12 (57.1) | 11 (68.8) |
Data are presented as number (%).
LN, lymph node.
*One patient was excluded from the analysis owing to the lack of information on the degree of submucosal involvement; †For lesions confined to the submucosa, degree of submucosal involvement was classified as sm1 (upper third), sm2 (middle third) or sm3 (lower third); ‡p-value is calculated by Fisher exact test.
3. Long-term clinical outcomes
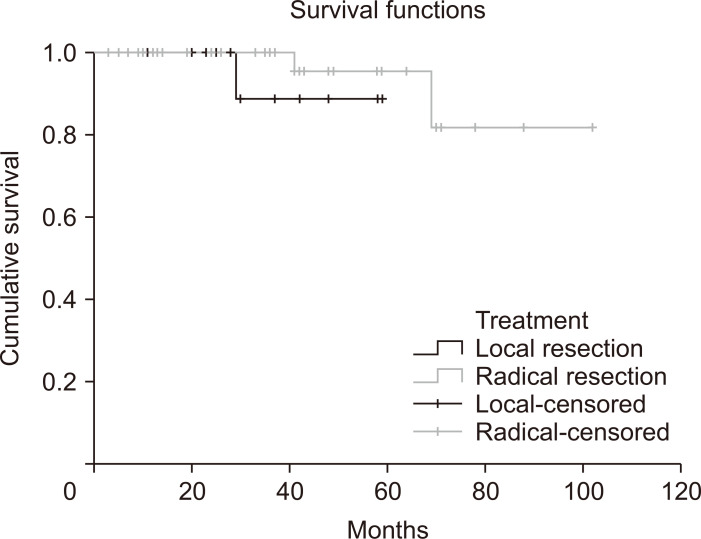
For the secondary study outcomes, we investigated recurrence and survival during the follow-up period of patients with small rectal NETs showing lymphovascular invasion. We compared the oncologic outcomes between patients who underwent local resection and those who underwent radical resection. Among 75 patients, 60 (80%) were followed up at least once (mean follow-up period 37.7 months, standard deviation 23.3 months). During the follow-up period, three mortality cases (one in the local resection group and two in the radical resection group) and one case of local recurrence (in the radical resection group) were detected (Supplementary Table 1). No NET-specific mortality was recorded, and all three patients died of other malignancies (stomach cancer, lung cancer, and acute leukemia). Survival curves according to the treatment method (local resection vs radical resection) are shown in Fig. 2. The Kaplan-Meier curve and the log-rank test showed no significant statistical difference between the two groups (p=0.332); however, the analysis was limited by the small number of events observed during the follow-up (n=3).
Fig. 2.
Kaplan-Meier curves showing the survival probability stratified by treatment method (local resection versus radical resection).
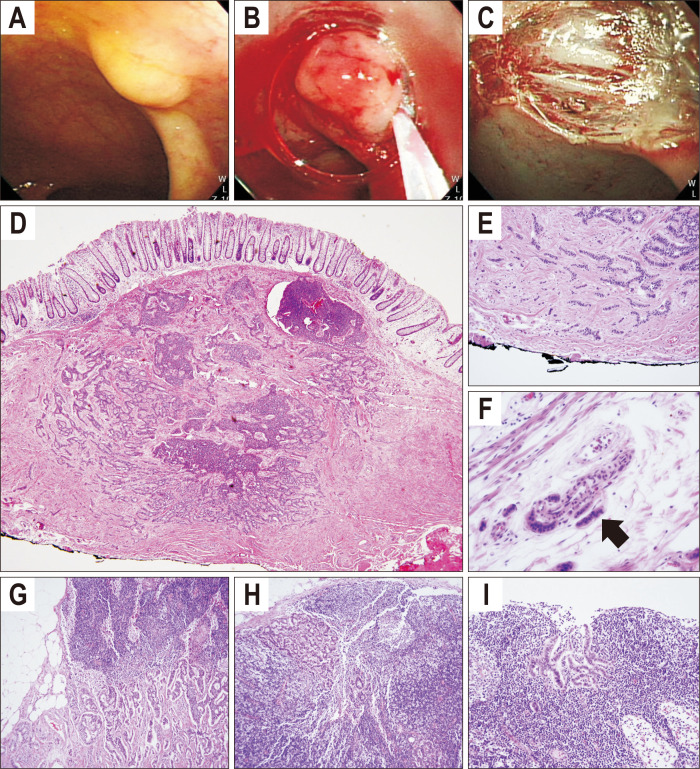
We present one typical case showing pathologically proven lymph node metastasis from small rectal NET (Fig. 3). In this patient, screening colonoscopy revealed small yellowish subepithelial tumor in the rectum. Pathologic diagnosis of endoscopically resected specimen was 6 mm sized low-grade NET with clear resection margin but presence of lymphovascular invasion. Abdominopelvic computed tomography showed no remarkable anorectal and retroperitoneal lymphadenopathy. Laparoscopic low anterior resection was performed. There was no residual tumor at the resected intestinal specimen. Nineteen perirectal lymph nodes were harvested and three of them had metastatic neuroendocrine cells (Fig. 3).
Fig. 3.
Case of small rectal neuroendocrine tumor with multiple lymph node metastases. Endoscopic (A-C) and histopathologic findings of rectal neuroendocrine tumor (D-F) and perirectal lymph nodes (G-I). A small, yellowish subepithelial tumor was located at the lower rectum (A). The lesion was completely removed by endoscopic mucosal resection (B, C). Microscopic findings showed monotonous small round cells arranged in a solid and pseudoglandular pattern (D, H&E, ×1; E, H&E, ×60). Angiolymphatic invasion was observed with monotonous small cell clusters (black arrow) (F, H&E, ×200). Monotonous cell clusters were also observed in three perirectal lymph nodes (G-I, H&E, ×200).
DISCUSSION
Small, well-differentiated rectal NETs are considered to have a good prognosis with local excision and are usually treated with endoscopic resection or transanal excision rather than radical resection. Traditionally, tumor size and depth of invasion are considered risk factors associated with poor prognosis and metastasis.26,27 Current guidelines (European Neuroendocrine Tumor Society, North American Neuroendocrine Tumor Society, and National Comprehensive Cancer Network guidelines) recommend local excision of tumors <1 or 2 cm in size if they lack regional lymphadenopathy in an imaging study (i.e., rectal magnetic resonance imaging or endoscopic ultrasound).7-10,28 After local excision, additional surgical resection may be considered according to high-risk histologic features such as tumor size, invasion depth, and tumor grade. However, lymphovascular invasion is not mentioned in these guidelines, and clinicians often face the dilemma of recommending additional radical resection versus the wait and see approach, especially in cases with no other high-risk features and for patients who are reluctant to undergo invasive treatment. Controversies remain concerning the optimal management of small rectal NETs with lymphovascular invasion.6,16 Previous studies have raised doubts about the necessity of radical resection for completely but locally resected tumors showing lymphovascular invasion.14,15,22,29,30 However, most studies reporting on the clinical significance of lymphovascular invasion had limitations of including a small number of patients, having a limited follow-up period, or including cases of non-surgically confirmed regional lymph node metastasis.
In this study, we included only patients who underwent radical resection to histopathologically confirm the regional lymph node status because rectal NETs can recur after a very long period, making clinical or radiologic evaluation of lymph node status difficult.17,31,32 We collected 41 patients who underwent radical resection for small rectal NETs (≤1.5 cm) with lymphovascular invasion. To our knowledge, this is the largest study to histopathologically analyze the rate of regional lymph node metastasis in small rectal NETs with lymphovascular invasion. Notably, nearly half of the patients showed regional lymph node metastasis (20/41). This result is in sharp contrast to the 0%–5% rate of clinically proven lymph node metastasis reported in other studies.14,15,29 We suppose that this difference can be explained by the different definitions of lymph node metastasis in previous studies (i.e., pathologically proven vs clinically defined). In the multivariate analysis, the Ki-67 index was significantly associated with regional lymph node metastasis. Among patients with tumors with Ki-67 index >3%, 68.4% had regional lymph node metastasis, whereas 31.8% of patients with tumors with Ki-67 <3% showed regional lymph node metastasis. In particular, all patients with tumors with Ki-67 >20% showed lymph node metastasis although the tumor size was small (6, 12, and 15 mm for each of the three patients). Tumor size was associated with lymph node metastasis in univariate analysis. Other characteristics such as age, sex, depth of invasion, and tumor location did not show a significant relationship with lymph node metastasis.
Considering both the low rate of clinical recurrence during 3 to 5 years follow-up period in the previous reports and high rate of histopathologically confirmed lymph node metastases in this study, we assume that immediate radical resection is not mandatory even if the locally resected small sized rectal NET showed lymphovascular invasion. However, surgical approach should be discussed with the patients considering significant rate of lymph node metastasis in this setting. If the wait-and-see approach was chosen, sufficient long-term follow-up (more than 10 years) should be recommended for possible delayed recurrence. The cost-effectiveness of surgery versus prolonged surveillance also needs to be clarified by future investigations.
This study had several limitations. First, because this was a retrospective study, the presence of a selection bias cannot be ruled out. Nevertheless, patients with lymphovascular invasion were consistently recommended to undergo radical resection regardless of other tumor characteristics. Therefore, tumor characteristics such as size, depth of invasion, and grade were not significantly different between patients who underwent radical resection and those who did not (data not shown). Second, although this study included the largest number of patients with histopathologically proven lymph node status, a sample of 75 patients is still small for the analysis of oncologic outcomes. Third, long-term clinical follow-up was not possible for most of the patients, and the clinical implication of regional lymph node metastasis in this population remains uncertain. The clinically significant morbidity associated with rectal NETs or the long-term survival rate in operated patients compared with non-operated patients needs to be determined. Fourth, the mitotic count which is one of the indexes of tumor proliferation was not analyzed in this study because a significant proportion of study subjects lacked this data, so the tumor grades were determined based on Ki-67 index only.
In conclusion, this study clearly showed that a high proportion of patients with small rectal NETs (<15 mm) with lymphovascular invasion had locoregional lymph node metastasis. In addition, Ki-67 expression was significantly associated with lymph node metastasis. A prudent attitude needs to be maintained with respect to recommending a wait and see approach, until the long-term safety of local resection and the cost-effectiveness of long-term surveillance are confirmed in this group of patients.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl20364.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: S.J.N., B.C.K. Data acquisition: B.C.K., H.J.C., H.H.J. Data analysis and interpretation: S.J.N., H.H.J., J.K., S.Y.K. Writing - original draft: S.J.N., H.H.J., S.Y.K. Writing - review and editing: B.C.K., H.J.C., J.K., S.Y.K. Approval of final manuscript: all authors.
REFERENCES
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sackstein PE, O'Neil DS, Neugut AI, Chabot J, Fojo T. Epidemiologic trends in neuroendocrine tumors: an examination of incidence rates and survival of specific patient subgroups over the past 20 years. Semin Oncol. 2018;45:249–258. doi: 10.1053/j.seminoncol.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Cho MY, Kim JM, et al. Gastrointestinal Pathology Study Group of Korean Society of Pathologists, author. Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000-2009: multicenter study. Cancer Res Treat. 2012;44:157–165. doi: 10.4143/crt.2012.44.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 5.Bertani E, Ravizza D, Milione M, et al. Neuroendocrine neoplasms of rectum: a management update. Cancer Treat Rev. 2018;66:45–55. doi: 10.1016/j.ctrv.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura K, Osada M, Goto A, et al. Short- and long-term outcomes of endoscopic resection of rectal neuroendocrine tumours: analyses according to the WHO 2010 classification. Scand J Gastroenterol. 2016;51:448–455. doi: 10.3109/00365521.2015.1107752. [DOI] [PubMed] [Google Scholar]
- 7.Ramage JK, De Herder WW, Delle Fave G, et al. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology. 2016;103:139–143. doi: 10.1159/000443166. [DOI] [PubMed] [Google Scholar]
- 8.Anthony LB, Strosberg JR, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (NETs): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767–774. doi: 10.1097/MPA.0b013e3181ec1261. [DOI] [PubMed] [Google Scholar]
- 9.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network, author. Neuroendocrine and adrenal tumors (version 2.2020) [Internet] National Comprehensive Cancer Network; Plymouth Meeting: c2020. [cited 2020 Oct 27]. Available from: https://www.nccn.org/ [Google Scholar]
- 11.Chablaney S, Zator ZA, Kumta NA. Diagnosis and management of rectal neuroendocrine tumors. Clin Endosc. 2017;50:530–536. doi: 10.5946/ce.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.So H, Yoo SH, Han S, et al. Efficacy of precut endoscopic mucosal resection for treatment of rectal neuroendocrine tumors. Clin Endosc. 2017;50:585–591. doi: 10.5946/ce.2017.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy. 2013;45:1039–1046. doi: 10.1055/s-0033-1344794. [DOI] [PubMed] [Google Scholar]
- 14.Sekiguchi M, Sekine S, Sakamoto T, et al. Excellent prognosis following endoscopic resection of patients with rectal neuroendocrine tumors despite the frequent presence of lymphovascular invasion. J Gastroenterol. 2015;50:1184–1189. doi: 10.1007/s00535-015-1079-7. [DOI] [PubMed] [Google Scholar]
- 15.Kwon MJ, Kang HS, Soh JS, et al. Lymphovascular invasion in more than one-quarter of small rectal neuroendocrine tumors. World J Gastroenterol. 2016;22:9400–9410. doi: 10.3748/wjg.v22.i42.9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HS, Kwon MJ, Kim TH, Han J, Ju YS. Lymphovascular invasion as a prognostic value in small rectal neuroendocrine tumor treated by local excision: a systematic review and meta-analysis. Pathol Res Pract. 2019;215:152642. doi: 10.1016/j.prp.2019.152642. [DOI] [PubMed] [Google Scholar]
- 17.Shigematsu Y, Kanda H, Konishi T, et al. Recurrence 30 years after surgical resection of a localized rectal neuroendocrine tumor. Intern Med. 2017;56:1521–1525. doi: 10.2169/internalmedicine.56.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuhashi N, Takahashi T, Tomita H, et al. Evaluation of treatment for rectal neuroendocrine tumors sized under 20 mm in comparison with the WHO 2010 guidelines. Mol Clin Oncol. 2017;7:476–480. doi: 10.3892/mco.2017.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Kim BC, Chang HJ, et al. Rectal neuroendocrine and L-cell tumors: diagnostic dilemma and therapeutic strategy. Am J Surg Pathol. 2013;37:1044–1052. doi: 10.1097/PAS.0b013e3182819f0f. [DOI] [PubMed] [Google Scholar]
- 20.Yamagishi D, Matsubara N, Noda M, et al. Clinicopathological characteristics of rectal carcinoid patients undergoing surgical resection. Oncol Lett. 2012;4:910–914. doi: 10.3892/ol.2012.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimoto Y, Oya M, Kuroyanagi H, et al. Lymph-node metastases in rectal carcinoids. Langenbecks Arch Surg. 2010;395:139–142. doi: 10.1007/s00423-008-0438-8. [DOI] [PubMed] [Google Scholar]
- 22.Okubo Y, Kasajima R, Suzuki M, et al. Risk factors associated with the progression and metastases of hindgut neuroendocrine tumors: a retrospective study. BMC Cancer. 2017;17:769. doi: 10.1186/s12885-017-3769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. Springer; New York: 2010. [Google Scholar]
- 24.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. International Agency for Research on Cancer; Lyon: 2010. [Google Scholar]
- 25.Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455–461. doi: 10.1055/s-2007-1010367. [DOI] [PubMed] [Google Scholar]
- 26.Naunheim KS, Zeitels J, Kaplan EL, et al. Rectal carcinoid tumors: treatment and prognosis. Surgery. 1983;94:670–676. [PubMed] [Google Scholar]
- 27.Jann H, Roll S, Couvelard A, et al. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332–3341. doi: 10.1002/cncr.25855. [DOI] [PubMed] [Google Scholar]
- 28.Caplin M, Sundin A, Nillson O, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88–97. doi: 10.1159/000335594. [DOI] [PubMed] [Google Scholar]
- 29.Shigeta K, Okabayashi K, Hasegawa H, et al. Long-term outcome of patients with locally resected high- and low-risk rectal carcinoid tumors. J Gastrointest Surg. 2014;18:768–773. doi: 10.1007/s11605-014-2468-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim GH, Ye BD, Byeon JS, et al. Endoscopic resection for rectal carcinoid tumor: efficacy and clinical results of follow-up. Intest Res. 2011;9:217–224. doi: 10.5217/ir.2011.9.3.217. [DOI] [Google Scholar]
- 31.Kwak MS, Chung SJ, Yang JI, et al. Long-term outcome of small, incidentally detected rectal neuroendocrine tumors removed by simple excisional biopsy compared with the advanced endoscopic resection during screening colonoscopy. Dis Colon Rectum. 2018;61:338–346. doi: 10.1097/DCR.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 32.Sung HY, Kim SW, Kang WK, et al. Long-term prognosis of an endoscopically treated rectal neuroendocrine tumor: 10-year experience in a single institution. Eur J Gastroenterol Hepatol. 2012;24:978–983. doi: 10.1097/MEG.0b013e3283551e0b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.