Abstract
Background/Aims
Heme oxygenase-1 (HO-1) plays a central role in cellular defense against inflammatory insults, and its induction in macrophages potentiates their efferocytic activity. In this study, we explored the potential role of macrophage HO-1 in the resolution of experimentally induced colitis.
Methods
To induce colitis, male C57BL/6 mice were treated with 2% dextran sulfate sodium (DSS) in the drinking water for 7 days. To investigate efferocytosis, apoptotic colon epithelial CCD 841 CoN cells were coincubated with bone marrow-derived macrophages (BMDMs).
Results
Administration of the HO-1 inhibitor zinc protoporphyrin IX (ZnPP) blunted the resolution of DSS-induced intestinal inflammation and expression of the proresolving M2 macrophage marker CD206. BMDMs treated with apoptotic colonic epithelial cells showed significantly elevated expression of HO-1 and its regulator Nrf2. Under the same experimental conditions, the proportion of CD206-expressing macrophages was also enhanced. ZnPP treatment abrogated the upregulation of CD206 expression in BMDMs engulfing apoptotic colonic epithelial cells. This result was verified with BMDMs isolated from HO-1-knockout mice. BMDMs, when stimulated with lipopolysaccharide, exhibited increased expression of CD86, a marker of M1 macrophages. Coculture of lipopolysaccharide-stimulated BMDMs with apoptotic colonic epithelial cell debris dampened the expression of CD86 as well as the pro-inflammatory cytokines in an HO-1-dependent manner. Genetic ablation as well as pharmacologic inhibition of HO-1 significantly reduced the proportion of efferocytic BMDMs expressing the scavenger receptor CD36.
Conclusions
HO-1 plays a key role in the resolution of experimentally induced colitis by modulating the polarization of macrophages.
Keywords: Acute colitis, Dextran sulfate sodium, Efferocytosis, Macrophage polarization, Resolution of inflammation
INTRODUCTION
Acute inflammation, a protective immune response provoked by infection- or injury-associated danger signals, should be terminated properly to restore the tissue homeostasis.1-3 Failure of resolution of acute inflammation causes the development of chronic diseases including arthritis, asthma, and atherosclerosis.4 Inflammatory bowel disease (IBD) is one of chronic inflammation-associated disorders influenced by environmental stressors, intestinal bacteria, an abnormal immune reaction, some genetic factors, etc.5-7 In a steady state, intestinal epithelial monolayer serves as a barrier between enteric microbiota and immune cell compartment in lamina propria. The chronic inflammatory conditions impair the entire gastrointestinal tract, rendering the underlying tissues more susceptible to pathogen infection continuously.5,8,9
Macrophages have important roles in preparative processes during the inflammation. Macrophages exert functions in inflamed tissues by converting themselves from a pro-inflammatory (M1) to an anti-inflammatory/proresolving (M2) phenotype. M2 macrophages secrete anti-inflammatory cytokines such as interleukin 10 and growth factors. They also control extracellular matrix turnover and remodeling.3,7,10-15
Apoptotic cells are largely produced in infected and injured tissues.8,16 Phagocytic clearance of the apoptotic cell debris, termed “efferocytosis,” is essential for preventing additive inflammatory reactions and autoimmunity.17-19 Notably, uptake of apoptotic cells promotes reprogramming of phagocytic macrophages toward the proresolving phenotype.20-23 Macrophage polarization during efferocytosis involves non-canonical mitochondrial response associated with fatty acid metabolism20 and autophagy.21
Heme oxygenase-1 (HO-1) is an inducible anti-oxidant and anti-inflammatory enzyme, and its expression is mainly regulated by nuclear factor-E2-related factor 2 (Nrf2), a master regulator of stress-responsive gene expression.24 HO-1 has been reported to regulate inflammation by modulating production of anti-/pro-inflammatory cytokines in immune cells.25-29 Furthermore, HO-1 induction in macrophages promotes their phagocytic activity,30 and conversion of macrophages to an anti-inflammatory/proresolving phenotype.25,27,31-33 In this study, we explored the role of HO-1 expressed in macrophages engulfing apoptotic colonic epithelial cells in the resolution of experimentally induced murine colitis.
MATERIALS AND METHODS
1. Animals
Male C57BL/6 mice (6-week-old) were purchased from Raon Bio (Seoul, Korea) and maintained according to the institutional animal care guidelines. Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University (IACUC number: SNU-170725-7) and the University of Ulsan Animal Care and Use Committee (reference number: HTC-14–030).
2. Reagents
Dulbecco’s Modified Eagle’s Medium, Minimum Essential Media, and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, NY, USA). Zinc protoporphyrin IX (ZnPP) and a primary antibody against HO-1 were the products of Enzo Life Sciences (Farmingdale, NY, USA). Primary antibodies for detecting Nrf2 (ab137550) and CD11b (ab8878) were obtained from Abcam (Cambridge, MA, USA). Anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies were provided by Thermo Fisher Scientific (Eugene, OR, USA). Antibodies for TruStain FcX (anti-mouse CD16/32), CD45, CD11b, F4/80, Ly6C, Ly6G, CD86, CD36, and CD206 for flow cytometric analysis were purchased from Biolegend (San Diego, CA, USA). 4’,6-Diamidino-2-phenylindole and phenylmethylsulfonyl fluoride were purchased from Thermo Fisher Scientific and Sigma-Aldrich (St. Louis, MO, USA), respectively.
3. Induction of acute dextran sulfate sodium (DSS)-induced colitis
Seven-week-old male C57BL/6 mice were treated with 2% DSS (36–50 kDa; MP Biomedicals; Santa Ana, CA, USA) dissolved in drinking water for 7 days. Mice were euthanized by CO2 asphyxiation at various time intervals. When necessary, mice were daily injected intraperitoneally with vehicle or ZnPP (25 mg/kg) for 2 weeks after DSS administration.
4. Histologic analysis
Colons were removed from mice and rinsed with cold phosphate buffered saline (PBS) to remove luminal content. Tissue sections were fixed in 10% buffered formalin and embedded in paraffin. The sections were stained with hematoxylin and eosin. The histological scores were determined based on inflammatory cell infiltrates and integrity of the intestinal architecture according to the guideline described elsewhere.34
5. Immunofluorescence
Immunofluorescence was performed on murine colon tissue and isolated leukocytes. Colon-infiltrating myeloid cells were detected using rat monoclonal CD11b antibody (Abcam). Colonic epithelial cells were detected using rat Alexa FluorⓇ 594 anti-mouse CD326 (EpCAM) antibody (Biolegend). Immunofluorescence signals were visualized with secondary antibodies: Alexa FluorTM 488 goat anti-rat immunoglobulin G (IgG) (Thermo Fisher Scientific).
6. Isolation of leukocytes from lamina propria
To isolate lamina propria leukocytes, colons were flushed of their luminal content and opened longitudinally and cut into 1-cm pieces. Epithelial cells were removed by 30-minute incubation with PBS containing 5% FBS (Gibco), 2 mmol/L EDTA at 37℃ under shaking at 275 rpm. Subsequent processes were performed as described previously.35
7. Preparation of bone marrow-derived macrophages (BMDMs)
Bone marrow was harvested from femur and tibia of 8- to 12-week old mice by flushing marrow out with cold PBS. The cells from bone marrow were then filtered through a 70 μm nylon cell strainer. Red blood cells were then removed by red blood cell lysis buffer (iNtRON Biotechnology; Seongnam, Korea). The remaining cells were resuspended in Dulbecco’s Modified Eagle’s Medium (Gibco) containing 10% FBS and 20 ng/mL macrophage colony-stimulating factor (Biolegend), seeded in petri dishes and incubated at 37℃ for 7 days in a humidified incubator containing 5% CO2. Medium containing macrophage colony-stimulating factor (10 ng/mL) was changed once after 3 days of incubation.
8. Measurement of CD206, CD86, and CD36 in BMDMs cocultured with apoptotic colon epithelial cells
Apoptosis was induced in CCD 841 CoN colon epithelial cells by exposure to 180 mJ ultraviolet radiation 4 times and kept overnight at 37℃ and 5% CO2 for 24 hours. Cells were centrifuged at 1,200 rpm for 2 minutes, and pellets were washed 3 times with PBS. The apoptotic colon epithelial cells were added to the BMDMs (5:1 ratio) for 48 hours at 37℃. To inhibit HO-1 activity, BMDMs were pre-incubated with ZnPP (10 μM) for 1 hour before the efferocytosis assay. After incubation, the mixtures were washed with PBS to remove free apoptotic cells and subjected to flow cytometry for the measurement of CD206 and CD36. After generating M1-like macrophages incubated with 100 ng/mL of bacterial lipopolysaccharide (LPS) for 24 hours, the expression of CD86 was measured.
9. Quantitative RT-PCR (qPCR) analysis
Total RNA was isolated from cells with Trizol Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol, and 1 μg of RNA was reverse transcribed using the Moloney murine leukemia virus reverse transcriptase (Promega; Madison, WI, USA). Real-time quantitative PCR was performed on a 7300 Real-Time PCR instrument (Thermo Fisher Scientific) using the RealHelix Premier Quantitative PCR Kit (NanoHelix Co., Ltd.; Daejeon, Korea). The primers used for each RT-PCR reactions are as follows (forward and reverse, respectively): Tnf-α, CCCTCACACTCAGATCATCTTCT and GCTACGACGTGGGCTACAG; Il12b, TGGTTTGCCATCGTTTTGCTG and ACAGGTGAGGTTCACTGTTTG. Gapdh gene was amplified as internal control. Data was analyzed using the comparative Cτ method.
10. Flow cytometry
BMDMs and single cells isolated from colon tissue were incubated with TruStain FcX (anti-mouse CD16/32) (Biolegend) in staining buffer containing 1% FBS and 0.1% NaN3 in PBS to block nonspecific binding to Fc receptor. Specific antibodies for membrane markers (anti-mouse CD45 APC, CD11b Alexa FluorTM 700, F4/80 PerCP/Cy5, Ly6C PE/Cy7, Ly6G APC/Cy7 and CD206 FITC) (Biolegend) were added to samples in the presence of CD16/32 antibody and incubated for 1 hour at 4℃. 4’,6-Diamidino-2-phenylindole was added to the samples about 10 minutes before flow cytometry analysis to gate out dead cells. Gating strategies to identify intestinal macrophages are illustrated in Supplementary Fig. 1. Sequential gating was used to identify specific cell populations. The dead cells were excluded using the viability dye, 4’,6-diamidino-2-phenylindole. The leukocytes were identified and gated by the expression of CD45. The myeloid cells were then further identified and gated by the expression of CD11b. The F4/80 positive macrophages were gated into Ly6G negative and Ly6C negative population to exclude granulocytes and undifferentiated monocytes respectively.
To measure the efferocytic activity of macrophages isolated from colon tissue, EpCAM was used as a marker of colonic epithelial cell debris. Cells were fixed with 4% formaldehyde in PBS for 30 minutes at room temperature and stained with the macrophage marker, F4/80. After permeabilization with fixation/permeabilization buffer set (Thermo Fisher Scientific; Waltham, MA, USA), the cells were stained with anti-mouse EpCAM FITC (Biolegend).
To evaluate the expression of HO-1 in colonic macrophages, cells were fixed and permeabilized as described above. Cells were then incubated with HO-1 primary antibody and goat anti-mouse IgG Fc Cross-Adsorbed secondary antibody (Invitrogen; Rockford, IL, USA). Cells were analyzed by FACS Calibur, LSR Fortessa X-20 and FACS Aria III (BD; Franklin Lakes, NJ, USA) machines. FlowJo software (Ashland, OR, USA) was used to analyze the data.
11. Western blot analysis
Mouse colon tissues were homogenized in ice-cold 1X lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mg/mL leupeptin) supplemented with a protease inhibitor and 0.1 mM phenylmethylsulfonyl fluoride. After periodical vortex mixing for 2 hours at 4℃, the lysates were centrifuged for 15 minutes at 12,000 g.
BMDMs were collected and treated with the same cell lysis buffer containing 0.1 mM phenylmethylsulfonyl fluoride and protease inhibitor for overnight at 4℃, followed by centrifugation for 15 minutes at 12,000 g. The cell lysates were subjected to SDS-polyacrylamide gel electrophoresis according to the standard protocol.
12. Statistical analysis
All data are presented as mean±standard error of the mean. Statistical significance was performed using the Student t-test or one-way analysis of variance with Tukey’s multiple-comparisons post hoc test. All experiments were repeated at least 3 times unless specified. Differences were considered statistically significant at p<0.05.
RESULTS
1. Mice recover from DSS-induced acute colitis
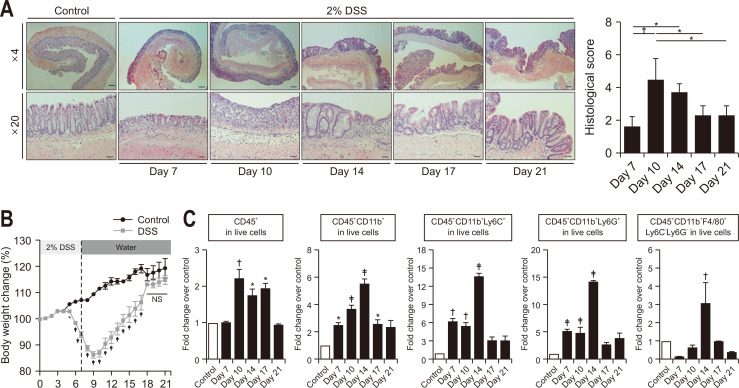
Murine colitis was induced according to the schedule as described in Materials and Methods. DSS-induced intestinal inflammation damages epithelial monolayer lining of colonic wall.36,37 The histological assessment of hematoxylin and eosin-stained tissue sections revealed massive disruption of the epithelial crypt shape which was gradually restored from day 10 (Fig. 1A). DSS-induced acute colitis was almost completely resolved by day 21. DSS-treated mice lost body weight from day 5 to 9, which was recovered gradually thereafter (Fig. 1B). Body weight loss was also associated with shortening of the colon (Supplementary Fig. 2).
Fig. 1.
The resolution of dextran sulfate sodium (DSS)-induced colitis. (A) Representative hematoxylin and eosin staining of colonic tissue sections. Scale bars correspond to 200 μm (upper) and 40 μm (lower). (B) Body weight changes. (C) Relative ratio of infiltrated leukocytes in colonic tissue from the indicated group of mice. Data are expressed as the mean±SEM (n=3).
NS, no significance; SEM, standard error of the mean. *p<0.05, †p<0.01, ‡p<0.001.
2. DSS-induced acute colitis and its resolution are accompanied by differential profiles of leukocyte recruitment
Immune cells in lamina propria were isolated from colon tissue of mice after DSS administration. The proportion of leukocyte subsets in live cells was analyzed by flow cytometry. The leukocyte gating strategy is schematically presented in Supplementary Fig. 1. The increased proportion of CD45+ total leukocytes was sustained from days 10 to 17. The percentage of CD45+CD11b+ myeloid lineage cells gradually increased from day 7 to 14. The proportion of CD45+CD11b+Ly6C+ infiltrated monocytes and CD45+CD11b+Ly6G+ granulocytes escalated from day 7 and peaked on day 14, while that of macrophages peaked on day 14 and then decreased (Fig. 1C).
3. HO-1 is involved in resolution of DSS-induced colitis
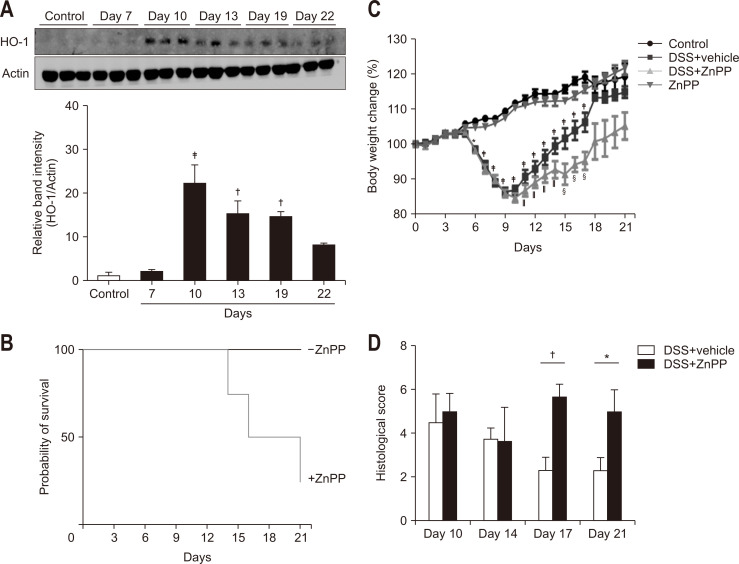
HO-1 catalyzes the reaction that degrades the heme group present in various proteins including hemoglobin. HO-1 has been shown to ameliorate the damaging immune response in several models of intestinal inflammation.38 DSS administration resulted in transient upregulation of HO-1 expression in the colonic mucosa of mice (Fig. 2A) as part of host adaptive survival response to colitis. To examine the role of HO-1 during the resolution of colitis, we utilized the HO-1 inhibitor, ZnPP. After exposure to DSS for 7 days, ZnPP was intraperitoneally injected daily for 2 weeks. As shown in Fig. 2B, the survival rate was dramatically decreased in ZnPP-treated mice while none of mice administered DSS alone died. The mice in the ZnPP-treated group restored their body weight, which had been lost by the DSS administration, to a much lesser extent than did those in the vehicle-treated group (Fig. 2C). Pharmacologic inhibition of HO-1 also delayed the recovery from inflammatory injuries in colonic mucosa as assessed by histologic examination (Fig. 2D). The elevated infiltration of leukocytes caused by DSS gradually returned to the control level in vehicle-treated mice, but ZnPP-treated mice showed a higher level of infiltrated total leukocytes and cells of myeloid lineages. Among the myeloid lineage cells, macrophages were mainly accumulated in the colon tissue of ZnPP-treated mice during the resolution phase (Supplementary Fig. 3).
Fig. 2.
The inhibition of heme oxygenase-1 (HO-1) activity delays the resolution of dextran sulfate sodium (DSS)-induced colitis. Mice were provided drinking water containing 2% DSS for 7 days, followed by regular water for another 14 days. Mice were daily injected with vehicle or 25 mg/kg bodyweight of zinc protoporphyrin IX (ZnPP) after termination of DSS administration. (A) Western blot analysis was performed to detect the expression of HO-1 in whole colon tissue. (B) Survival rates. (C) Body weight changes. (D) Histologic scores. Data are expressed as the mean±SEM (n=3).
SEM, standard error of the mean. Significantly different from the control (*p<0.05, †p<0.01, ‡p<0.001); Significantly different from the DSS + vehicle group (§p<0.05, ‖p<0.001).
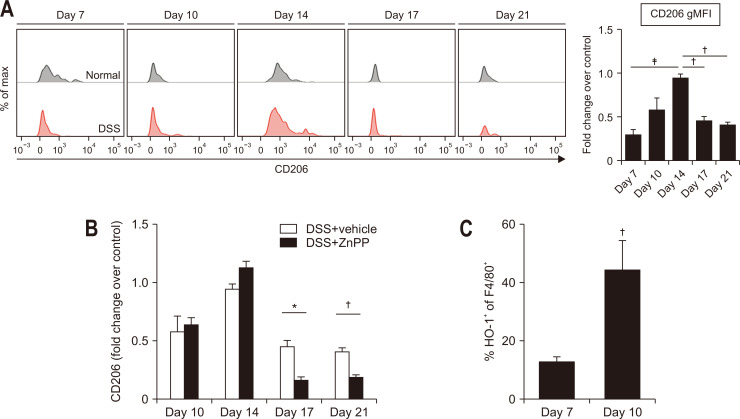
Macrophages orchestrate restoration of the tissue structure and homeostasis by changing their phenotypes from a pro-inflammatory (M1) to an anti-inflammatory/proresolving (M2) state. Macrophage reprogramming for tissue repair is a dynamic and plastic process and can be promoted by phagocytosing dead cells.3,16,20-22,39 In DSS-induced colitis, the expression of an M2 marker (CD206) in colonic macrophages gradually increased until day 14 and then decreased (Fig. 3A). As shown in Fig. 3B, expression of macrophage CD206 was significantly repressed in the colon of ZnPP-treated mice.
Fig. 3.
The enhanced expression of CD206 and heme oxygenase-1 (HO-1) in macrophages of dextran sulfate sodium (DSS)-treated mouse colons. (A) Flow cytometric analysis of CD206 expression in macrophages from the colonic tissue of mice with or without DSS administration. (B) Effects of zinc protoporphyrin IX (ZnPP) on the expression of CD206 in colonic tissue macrophages of DSS-treated mice. Mice were injected intraperitoneally daily with vehicle or ZnPP (25 mg/kg) for 2 weeks after DSS administration. (C) Flow cytometric analysis of HO-1 expression in F4/80+ macrophages. Data are expressed as the mean±SEM (n=3).
gMFI, geometric mean fluorescence intensity; SEM, standard error of the mean. *p<0.05, †p<0.01, ‡p<0.001.
4. The expression of HO-1 is upregulated in macrophages engulfing apoptotic colonic epithelial cells
Our previous studies have demonstrated that HO-1 plays a vital role in efferocytic40 and phagocytic41 activities of macrophages. It has been reported that colonic mucosal CX3CR1+ macrophages are the main producer of HO-1 in intestinal inflammation.29 In line with this notion, the proportion of colonic macrophages expressing HO-1 was also escalated concomitantly with beginning of resolution (Fig. 3C).
5. M2 macrophage polarization is controlled by HO-1 during the engulfment of apoptotic colonic epithelial cells
In the resolution phase, phagocytic removal of apoptotic cells through efferocytosis prevents secondary necrosis of apoptotic cells which triggers an additive inflammatory reaction.1,3,7,42 Immunofluorescence staining performed on fixed leukocytes isolated from murine colitis showed that myeloid cells from both normal and inflammatory colonic tissue similarly participated in engulfing epithelial cells (Fig. 4A 2nd and 4th panels). However, the number of myeloid cells was found to be higher in inflamed colonic tissue compared with that of normal colonic tissue on day 10 (Fig. 1C 2nd panel, and Fig. 4A 1st and 3rd panels). There was a concomitant increase in the proportion of macrophages phagocytosing epithelial cells at the beginning of the resolution phase (Fig. 4B).
Fig. 4.
Engulfment of apoptotic epithelial cells upregulates heme oxygenase-1 (HO-1) expression in macrophages. (A) Immunofluorescence images of fixed colonic tissue sections (1st and 3rd panels) and fixed single myeloid cells (2nd and 4th panels). Myeloid cells isolated from colitis tissues of dextran sulfate sodium (DSS)-treated mice or normal colon tissues were cytospun onto glass slides and subjected to immunofluorescence analysis for detection of colon epithelial cell debris (EpCAM+, red) engulfed by myeloid cells (CD11b+, green). Arrows indicate myeloid cells engulfing colonic epithelial cells. (B) Flow cytometric analysis of macrophages engulfing colonic epithelial cells. (C) Verification of apoptotic death in colonic epithelial cells by Western blot analysis of cleaved PARP. (D) Bone marrow-derived macrophages (BMDMs) were cocultured with colonic epithelial cell debris killed by ultraviolet (UV) radiation and then subjected to Western blot analysis to measure the expression of HO-1 and its transcription factor nuclear factor-E2-related factor 2 (Nrf2). Data are expressed as the mean±SEM (n=3).
PARP, poly (ADP-ribose) polymerase; SEM, standard error of the mean. *p<0.05.
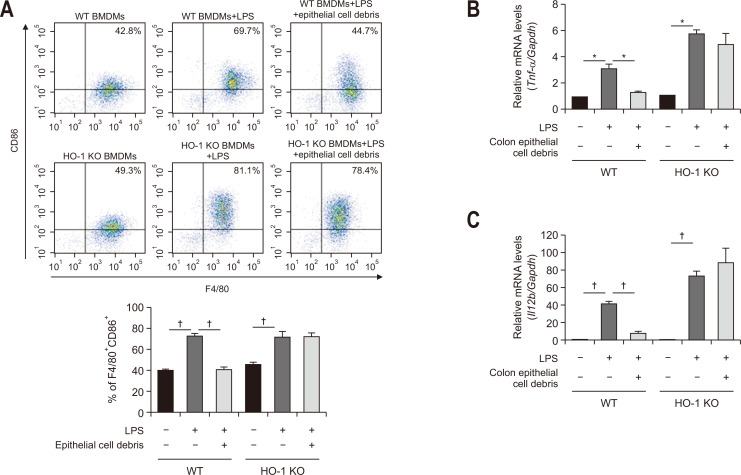
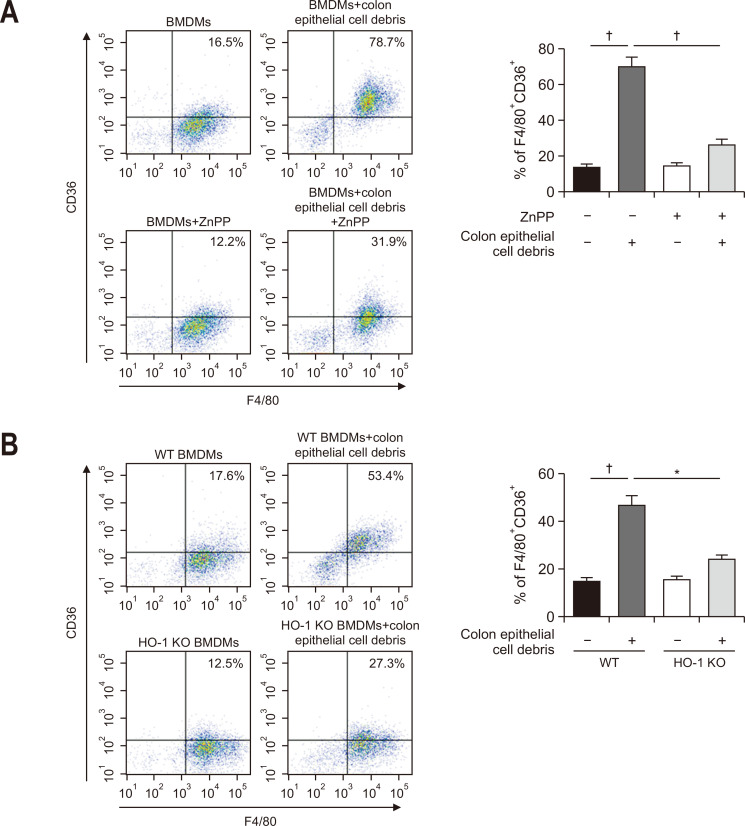
To confirm the direct association between efferocytosis and HO-1 induction in macrophages, BMDMs isolated from mouse femurs were treated with apoptotic colonic epithelial cells generated by ultraviolet irradiation. Ultraviolet-induced cell death was confirmed by the poly (ADP-ribose) polymerase cleavage (Fig. 4C). As a result, the expression of HO-1 and its regulator, Nrf2 was significantly upregulated (Fig. 4D). Coculture of BMDMs with apoptotic colonic epithelial cell debris enhanced expression of the M2 macrophage marker, CD206 (Fig. 5). However, BMDMs engulfing apoptotic colonic epithelial cells attained phenotypic characteristics of M2 macrophages to a lesser extent in the presence of ZnPP (Fig. 5A). The mechanistic dependency of macrophages engulfing dead epithelial cells on HO-1 in their polarization was verified by using BMDMs isolated from HO-1 knockout (KO) mice (Fig. 5B). In contrast, the expression of an M1 macrophage marker CD86 was reduced in LPS-stimulated BMDMs cocultured with apoptotic colonic epithelial cells, which was abrogated in HO-1 deficient mice (Fig. 6A).
Fig. 5.
Role of heme oxygenase-1 (HO-1) in M2-polarized bone marrow-derived macrophages (BMDMs) engulfing apoptotic colonic epithelial cells. CD206 expression in BMDMs mixed with apoptotic epithelial cells in two ways to examine the role of HO-1 in vitro. (A) Murine BMDMs were treated with a pharmacological HO-1 inhibitor, zinc protoporphyrin IX (ZnPP) (10 mM), 1 hour prior to the addition of apoptotic epithelial cells. After incubation for 48 hours, the proportion of BMDMs expressing CD206 was measured by flow cytometry. (B) BMDMs from HO-1 knockout (KO) mice were treated with apoptotic epithelial cells and subjected to flow cytometry. Data are expressed as the mean±SEM (n=3).
WT, wild type; SEM, standard error of the mean. *p<0.01, †p<0.001.
Fig. 6.
Role of heme oxygenase-1 (HO-1) in M1-polarized bone marrow-derived macrophages (BMDMs) phagocytosing colonic epithelial cell debris. (A) BMDMs from wild type (WT) or HO-1 knockout (KO) mice were cocultured with or without CCD 841 CoN colon epithelial cell debris. After 48 hours of incubation, the proportion of macrophages expressing CD86 was assessed by flow cytometry. (B, C) BMDMs derived from WT or HO-1 KO mice were treated with lipopolysaccharide (LPS) (100 ng/mL) for 24 hours. The messenger RNA expression levels of Tnf-α (B) and Il12b (C) in the same macrophages cocultured with or without apoptotic epithelial cell debris for 48 hours were measured by quantitative reverse transcription polymerase chain reaction. *p<0.01, †p<0.001.
M1 macrophages secrete tumor necrosis factor α and interleukin-12, two major macrophage-derived mediators of inflammatory responses in mammals.25 After coculture of LPS-stimulated wild type murine BMDMs with apoptotic CCD 841 CoN cell debris, there was a marked decrease in the expression of messenger RNA transcripts of pro-inflammatory M1 markers, Tnf-α (Fig. 6B) and Il12b (Fig. 6C), but this was attenuated in the BMDMs from HO-1 knockout mice.
CD36 is a prototypic scavenger receptor that is involved in the recognition of apoptotic cells by macrophages. CD36 present on the surface of macrophages mediates efferocytosis following tissue injury and thereby prevents excessive inflammation that could compromise tissue repair.43 The role of CD36 in the pathophysiology of IBD has been suggested.44 CD36 deficiency aggravated DSS-induced colitis. Interestingly, we found that CD36 expression is upregulated in wild type BMDMs stimulated by the apoptotic epithelial cell debris, and this was blunted in BMDMs treated with the HO-1 inhibitor, ZnPP and also in those from HO-1 knockout mice (Fig. 7).
Fig. 7.
Role of heme oxygenase-1 (HO-1) in the expression of CD36 in bone marrow-derived macrophages (BMDMs) engulfing apoptotic colonic epithelial cells. CD36 expression in BMDMs mixed with apoptotic epithelial cells in two ways to examine the role of HO-1 in vitro. (A) Murine BMDMs were treated with a pharmacological HO-1 inhibitor, zinc protoporphyrin IX (ZnPP) (10 mM), 1 hour prior to the addition of apoptotic epithelial cells. After incubation for 48 hours, the proportion of BMDMs expressing CD36 was measured by flow cytometry. (B) BMDMs from HO-1 knockout (KO) mice were treated with apoptotic epithelial cell debris and subjected to flow cytometry. Data are expressed as the mean±SEM (n=3).
WT, wild type; SEM, standard error of the mean. *p<0.01, †p<0.001.
DISCUSSION
In order to investigate the pathophysiological features and therapeutic strategies for the treatment of IBD, several animal models have been developed.45 DSS is one of the most widely used chemical colitogens. This high molecular weight sulfated polysaccharide, when administered to mice as a dissolved form in drinking water, causes disruption of intestinal epithelial monolayer. The resulting pathological features mimic the superficial inflammation seen in ulcerative colitis (UC).35,36,40
The recruitment as well as origin of intestinal macrophages is highly dynamic.46 In other organs, tissue resident macrophages are not derived from monocytes, but rather from embryonic progenitors arising from the yolk sac and/or fetal liver. They have been known to have a key function in homeostasis and inflammation.46-48 However, intestinal macrophages are largely replaced by Ly6Chigh monocytes-derived macrophages in adulthood,49 implying that embryonic macrophages and BMDMs are present together in the gut wall.46 Especially in an inflammatory environment including DSS-induced colitis, colonic tissues show intense accumulation of monocytes and immature macrophages.46,50,51 Consistent with these observations, leukocyte dynamics in the present study also show the accumulation of infiltrated cells of myeloid lineage, including monocytes, granulocytes and macrophages during resolution of DSS-induced colitis.
Macrophage infiltration and recruitment are common features of the early phase of inflammatory insult responsible for tissue injury, but these also lead to resolution of inflammation and tissue repair.52 Depending on the inflammatory milieu, macrophages undergo polarization to one of two phenotypes, classical or pro-inflammatory M1-like and alternatively activated or anti-inflammatory/proresolving M2-like macrophages. Elevated M1 and concomitantly decreased M2 macrophages are commonly observed in the colonic tissues of UC patients.53 Likewise, macrophages have opposing roles in DSS-induced murine colitis that mimics UC. Thus, M1 macrophages contribute to the pathogenesis of colitis whilst M2 macrophages elicit protective functions by promoting tissue repair and by driving epithelial cell regeneration and proliferation.54 When macrophages fail to switch from M1 to M2 polarization, severe colitis arises in mice.55
Our present study demonstrated the accumulation of phagocytic M2 macrophages (F4/80+CD206+) in the mouse colon during the resolution of DSS-induced intestinal inflammation and also polarization of isolated BMDMs engulfing colonic epithelial cell debris toward the M2 phenotype, which appeared to be associated with the macrophage HO-1 activity. BMDMs, when stimulated with LPS, exhibited the increased expression of CD86, one of the typical M1 markers. Coculture of LPS-stimulated BMDMs with apoptotic colonic epithelial cell debris dampened the expression of CD86 as well as the genes encoding pro-inflammatory cytokines (tumor necrosis factor α and IL-12p40) in an HO-1-dependent way.
HO-1 induction occurs in cells and tissues where hemoglobin is degraded by macrophages.38 Bacterial endotoxins or invasive pathogens can also induce HO-1 expression.32,56,57 We found that engulfment of inflammatory apoptotic epithelial cells stimulated HO-1 expression in macrophages. HO-1 induction in macrophages phagocytosing dead cells is considered to compromise the oxidative stress which often accompanies during acute inflammation.21 Notably, pharmacological inhibition of HO-1 activity delayed the resolution of DSS-induced colitis by hampering the M2 macrophage polarization.
In agreement with our observations, administration of an HO-1 inducing agent, hemin ameliorated intestinal inflammation and remedied intestinal mucosal barrier damage by correcting abnormal intestinal macrophage polarization. In addition, hemin inhibited the M1 polarization while enhancing M2 polarization in BMDMs, and these effects were abrogated by silencing HO-1.58 The above findings suggest the therapeutic potential of HO-1 inducers capable of modulating the macrophage functions in the management of IBD. Based on the known contribution of macrophages to intestinal homeostasis, macrophage therapy has been proposed as a means of treating IBD. The transferring purified bone marrow-derived M2 macrophages to mice subjected to DSS-induced colitis was shown to attenuate disease severity.59 Under the same principle, effectively regulating macrophage polarization in vivo may become a potential therapeutic strategy for treating UC.60
Whilst DSS administration induced HO-1 expression in both entire colonic mucosa and tissue macrophages, their kinetics are apparently different. HO-1 is a stress-responsive enzyme, and its expression in the colonic mucosa is induced relatively rapidly upon acute inflammation caused by DSS as adaptive response. In contrast, HO-1 in colonic macrophages is likely to be induced after they engulf the apoptotic epithelial cells. This may account for the delay in the onset of HO-1 expression in macrophages compared with that in the entire colon. Therefore, the epithelial HO-1 induction does not necessarily represent the macrophage-specific HO-1 increased in a recovery phase, but might rather be a result of inflammatory reaction.
In summary, engulfment of apoptotic colonic epithelial cells significantly induces HO-1 expression in macrophages. The HO-1 induction accelerates the resolution of DSS-induced colitis by inducing polarization of macrophages phagocytosing apoptotic colonic epithelial cells. According to Lee et al.,61 an elevated accumulation of apoptotic cells resulting from impaired efferocytosis contributes to the excessive and prolonged colonic inflammation. Of interest, restoration of efferocytosis by adoptive transfer of BMDMs to the inflamed colonic tissue promotes resolution of DSS-induced colitis. In this regard, transfer of HO-1 overexpressing macrophages would be a new therapeutic strategy in the management of IBD, which requires clinical validation.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl210058.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program grant (number: 2021R1A2C2014186 to Y.J.S.), the Priority Research Centres Programme (number: 2014R1A6A1030318 to H.T.C.) and the BK21 FOUR Program (number: 5120200513755) from the National Research Foundation, Republic of Korea.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: S.Y.G., S.H.K., H.N.L. Methodology: S.J.K., J.P., S.H.K., Y.J., H.N.L., W.K., I.A.M. Formal analysis: S.Y.G., S.J.K., J.P., S.H.K., Y.J. Funding acquisition: Y.J.S. Project administration: Y.J.S. Writing - original draft: S.Y.G. Writing - review and editing: Y.J.S. Resources: Y.J., H.T.C., H.K.N. Approval of final manuscript: all authors.
REFERENCES
- 1.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 2.Sansbury BE, Spite M. Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ Res. 2016;119:113–130. doi: 10.1161/CIRCRESAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun. 2018;9:3261. doi: 10.1038/s41467-018-05800-6.3d8d61b400f549ababfbde634195754d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- 7.Farache J, Zigmond E, Shakhar G, Jung S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol Cell Biol. 2013;91:232–239. doi: 10.1038/icb.2012.79. [DOI] [PubMed] [Google Scholar]
- 8.Nunes T, Bernardazzi C, de Souza HS. Cell death and inflammatory bowel diseases: apoptosis, necrosis, and autophagy in the intestinal epithelium. Biomed Res Int. 2014;2014:218493. doi: 10.1155/2014/218493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coskun M. Intestinal epithelium in inflammatory bowel disease. Front Med (Lausanne) 2014;1:24. doi: 10.3389/fmed.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eissa N, Hussein H, Kermarrec L, et al. Chromofungin ameliorates the progression of colitis by regulating alternatively activated macrophages. Front Immunol. 2017;8:1131. doi: 10.3389/fimmu.2017.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas A, Shouval DS, Griffith A, et al. WASP-mediated regulation of anti-inflammatory macrophages is IL-10 dependent and is critical for intestinal homeostasis. Nat Commun. 2018;9:1779. doi: 10.1038/s41467-018-03670-6.d6407c36dbee4770a2372e3380f5d795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi S, Hamada T, Zinsou DGA, et al. PI3K p85α subunit-deficient macrophages protect mice from acute colitis due to the enhancement of IL-10 production. Sci Rep. 2017;7:6187. doi: 10.1038/s41598-017-06464-w.f00f74519225490b8c7048e8656b5c28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayama H, Kohyama M, Okuzaki D, et al. Heme ameliorates dextran sodium sulfate-induced colitis through providing intestinal macrophages with noninflammatory profiles. Proc Natl Acad Sci U S A. 2018;115:8418–8423. doi: 10.1073/pnas.1808426115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 16.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 17.Kawano M, Nagata S. Efferocytosis and autoimmune disease. Int Immunol. 2018;30:551–558. doi: 10.1093/intimm/dxy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das KM, Biancone L. Is IBD an autoimmune disorder? Inflamm Bowel Dis. 2008;14 Suppl 2:S97–S101. doi: 10.1002/ibd.20723. [DOI] [PubMed] [Google Scholar]
- 19.Scott RS, McMahon EJ, Pop SM, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Weinberg S, DeBerge M, et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019;29:443–456. doi: 10.1016/j.cmet.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunha LD, Yang M, Carter R, et al. LC3-associated phagocytosis in myeloid cells promotes tumor immune tolerance. Cell. 2018;175:429–441. doi: 10.1016/j.cell.2018.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. 2017;198:1387–1394. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang N, Shinohara M, Dalli J, et al. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013;190:6378–6388. doi: 10.4049/jimmunol.1202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 25.Vijayan V, Wagener FADTG, Immenschuh S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem Pharmacol. 2018;153:159–167. doi: 10.1016/j.bcp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh SZ, Hegazi RA, Kobayashi T, et al. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. 2011;186:5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med. 2009;206:1167–1179. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marelli G, Erreni M, Anselmo A, et al. Heme-oxygenase-1 production by intestinal CX3CR1+ macrophages helps to resolve inflammation and prevents carcinogenesis. Cancer Res. 2017;77:4472–4485. doi: 10.1158/0008-5472.CAN-16-2501. [DOI] [PubMed] [Google Scholar]
- 30.Onyiah JC, Sheikh SZ, Maharshak N, Otterbein LE, Plevy SE. Heme oxygenase-1 and carbon monoxide regulate intestinal homeostasis and mucosal immune responses to the enteric microbiota. Gut Microbes. 2014;5:220–224. doi: 10.4161/gmic.27290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Nakamura K, Kageyama S, et al. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight. 2018;3:e120596. doi: 10.1172/jci.insight.120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gobert AP, Verriere T, Asim M, et al. Heme oxygenase-1 dysregulates macrophage polarization and the immune response to Helicobacter pylori. J Immunol. 2014;193:3013–3022. doi: 10.4049/jimmunol.1401075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Erben U, Loddenkemper C, Doerfel K, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7:4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 35.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 36.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15.25.1–15.25.14. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirtz S, Popp V, Kindermann M, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–1309. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 38.Sebastián VP, Salazar GA, Coronado-Arrázola I, et al. Heme oxygenase-1 as a modulator of intestinal inflammation development and progression. Front Immunol. 2018;9:1956. doi: 10.3389/fimmu.2018.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yakoub AM, Schulz R, Seiffert M, Sadek M. Autoantigen-harboring apoptotic cells hijack the coinhibitory pathway of T cell activation. Sci Rep. 2018;8:10533. doi: 10.1038/s41598-018-28901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim W, Kim HU, Lee HN, et al. Taurine chloramine stimulates efferocytosis through upregulation of Nrf2-mediated heme oxygenase-1 expression in murine macrophages: possible involvement of carbon monoxide. Antioxid Redox Signal. 2015;23:163–177. doi: 10.1089/ars.2013.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SH, Zhong X, Kim W, et al. Taurine chloramine potentiates phagocytic activity of peritoneal macrophages through up-regulation of dectin-1 mediated by heme oxygenase-1-derived carbon monoxide. FASEB J. 2018;32:2246–2257. doi: 10.1096/fj.201700817R. [DOI] [PubMed] [Google Scholar]
- 42.Lee CS, Penberthy KK, Wheeler KM, et al. Boosting apoptotic cell clearance by colonic epithelial cells attenuates inflammation in vivo. Immunity. 2016;44:807–820. doi: 10.1016/j.immuni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parks BW, Black LL, Zimmerman KA, et al. CD36, but not G2A, modulates efferocytosis, inflammation, and fibrosis following bleomycin-induced lung injury. J Lipid Res. 2013;54:1114–1123. doi: 10.1194/jlr.M035352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oz HS, Zhong J, de Villiers WJ. Pattern recognition scavenger receptors, SR-A and CD36, have an additive role in the development of colitis in mice. Dig Dis Sci. 2009;54:2561–2567. doi: 10.1007/s10620-008-0673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-H. [DOI] [PubMed] [Google Scholar]
- 46.Bain CC, Schridde A. Origin, differentiation, and function of intestinal macrophages. Front Immunol. 2018;9:2733. doi: 10.3389/fimmu.2018.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 50.Bain CC, Scott CL, Uronen-Hansson H, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rugtveit J, Nilsen EM, Bakka A, Carlsen H, Brandtzaeg P, Scott H. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997;112:1493–1505. doi: 10.1016/S0016-5085(97)70030-1. [DOI] [PubMed] [Google Scholar]
- 52.Vinuesa E, Hotter G, Jung M, Herrero-Fresneda I, Torras J, Sola A. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J Pathol. 2008;214:104–113. doi: 10.1002/path.2259. [DOI] [PubMed] [Google Scholar]
- 53.Zhu W, Yu J, Nie Y, et al. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol Invest. 2014;43:638–652. doi: 10.3109/08820139.2014.909456. [DOI] [PubMed] [Google Scholar]
- 54.Horuluoglu BH, Kayraklioglu N, Tross D, Klinman D. PAM3 protects against DSS-induced colitis by altering the M2:M1 ratio. Sci Rep. 2020;10:6078. doi: 10.1038/s41598-020-63143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim E, Kim Y, Lee J, et al. Leucrose, a natural sucrose isomer, suppresses dextran sulfate sodium (DSS)-induced colitis in mice by regulating macrophage polarization via JAK1/STAT6 signaling. J Funct Foods. 2020;74:104156. doi: 10.1016/j.jff.2020.104156. [DOI] [Google Scholar]
- 56.Camhi SL, Alam J, Otterbein L, Sylvester SL, Choi AM. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. Am J Respir Cell Mol Biol. 1995;13:387–398. doi: 10.1165/ajrcmb.13.4.7546768. [DOI] [PubMed] [Google Scholar]
- 57.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y, Wu B, Zhang Z, et al. Heme protects intestinal mucosal barrier in DSS-induced colitis through regulating macrophage polarization in both HO-1-dependent and HO-1-independent way. FASEB J. 2020;34:8028–8043. doi: 10.1096/fj.202000313RR. [DOI] [PubMed] [Google Scholar]
- 59.Hunter MM, Wang A, Parhar KS, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 60.Sun T, Kwong CHT, Gao C, et al. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics. 2020;10:10106–10119. doi: 10.7150/thno.48448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HN, Tian L, Bouladoux N, et al. Dendritic cells expressing immunoreceptor CD300f are critical for controlling chronic gut inflammation. J Clin Invest. 2017;127:1905–1917. doi: 10.1172/JCI89531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.