Abstract
Background
Postpartum haemorrhage (PPH) (bleeding from the genital tract after childbirth) is a major cause of maternal mortality and disability, particularly in under‐resourced areas. In these settings, uterotonics are often not accessible. There is a need for simple, inexpensive techniques which can be applied in low‐resourced settings to prevent and treat PPH. Uterine massage is recommended as part of the routine active management of the third stage of labour. However, it is not known whether it is effective. If shown to be effective, uterine massage would represent a simple intervention with the potential to have a major effect on PPH and maternal mortality in under‐resourced settings.
Objectives
To determine the effectiveness of uterine massage after birth and before or after delivery of the placenta, or both, to reduce postpartum blood loss and associated morbidity and mortality.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2013).
Selection criteria
All published, unpublished and ongoing randomised controlled trials comparing uterine massage alone or in addition to uterotonics before or after delivery of the placenta, or both, with non‐massage.
Data collection and analysis
Two researchers independently considered trials for eligibility, assessed risk of bias and extracted the data using the agreed form. Data were checked for accuracy. The effect of uterine massage commenced before or after placental delivery were first assessed separately, and then the combined for an overall result.
Main results
This review included two randomised controlled trials. The first trial included 200 women who were randomised to receive uterine massage or no massage following delivery of the placenta, after active management of the third stage of labour including use of oxytocin. The numbers of women with blood loss more than 500 mL was small, with no statistically significant difference (risk ratio (RR) 0.52, 95% confidence interval (CI) 0.16 to 1.67). There were no cases of retained placenta in either group. The mean blood loss was significantly less in the uterine massage group at 30 minutes (mean difference (MD) ‐41.60 mL, 95% CI ‐75.16 to ‐8.04) and 60 minutes after trial entry (MD ‐77.40 mL, 95% CI ‐118.71 to ‐36.09). The need for additional uterotonics was significantly reduced in the uterine massage group (RR 0.20, 95% CI 0.08 to 0.50).
For use of uterine massage before and after delivery of the placenta, one trial recruited 1964 women in Egypt and South Africa. Women were assigned to receive oxytocin, uterine massage or both after delivery of the baby but before delivery of the placenta. There was no added benefit for uterine massage plus oxytocin over oxytocin alone as regards blood loss greater than or equal to 500 mL (average RR 1.56, 95% CI 0.44, 5.49; random‐effects) or need for additional use of uterotonics (RR 1.02, 95% CI 0.56 to 1.85).
The two trials were combined to examine the effect of uterine massage commenced either before or after delivery of the placenta. There was substantial heterogeneity with respect to the blood loss 500 mL or more after trial entry. The average effect using a random‐effects model found no statistically significant differences between groups (average RR 1.14, 95% CI 0.39 to 3.32; random‐effects).
Authors' conclusions
The results of this review are inconclusive, and should not be interpreted as a reason to change current practice. Due to the limitations of the included trials, more trials with sufficient numbers of women are needed in order to estimate the effects of sustained uterine massage. All the women compared in this review received oxytocin as part of the active management of labour. Recent research suggests that once an oxytocic has been given, there is limited scope for further reduction in postpartum blood loss. Trials of uterine massage in settings where uterotonics are not available, and which measure women's experience of the procedure, are needed.
Keywords: Female; Humans; Pregnancy; Labor Stage, Third; Uterus; Massage; Massage/methods; Oxytocics; Oxytocin; Postpartum Hemorrhage; Postpartum Hemorrhage/prevention & control; Randomized Controlled Trials as Topic; Time Factors; Uterine Contraction
Plain language summary
Uterine massage for preventing postpartum haemorrhage
Bleeding after childbirth (postpartum haemorrhage) is the leading cause of maternal deaths in Sub‐Saharan Africa and Egypt, and yet it is largely preventable. Possible causes of heavy bleeding directly following childbirth or within the first 24 hours are that the uterus fails to contract after delivery (uterine atony), a retained placenta, inverted or ruptured uterus, and cervical, vaginal, or perineal tears.
In well‐resourced settings haemorrhage is reduced by routine active management of delivery of the placenta, called the third stage of labour, using a drug to stimulate contraction of the uterus such as oxytocin. Uterine massage after delivery of the placenta can also promote contraction of the uterus. This involves placing a hand on the woman's lower abdomen and stimulating the uterus by repetitive massaging or squeezing movements.
This review included two controlled trials in which women were randomly assigned to receive uterine massage or no massage with active management of the third stage of labour, including the routine use of oxytocin.
In one trial involving 200 women, uterine massage was given every 10 minutes for 60 minutes after delivery of the placenta effectively reduced blood loss, and the need for additional uterotonics, by some 80%. The numbers of women losing more than 500 mL of blood were too small for meaningful comparison. Two women in the control group and none in the uterine massage group needed blood transfusions.
The second trial involved 1964 women who were assigned to receive oxytocin, uterine massage or both after delivery of the baby and before delivery of the placenta. There was no added benefit for uterine massage when oxytocin was used.
The results of this review are inconclusive. The methodological quality of the two included trials was high but it is possible that there were differences in the procedures used in the study sites. Disadvantages of uterine massage include the use of staff time, and discomfort caused to women. The findings should not change the recommended practice. It is likely that any reduction in blood loss was limited with the use of oxytocin in these trials. Uterine massage may also have increased apparent blood loss by pressing pooled blood out from the uterine cavity. There is a need for more trials, especially in settings where uterotonics are not available. Uterine massage could be a simple inexpensive intervention if proved effective.
Background
Postpartum haemorrhage (PPH) (excessive bleeding from the genital tract after childbirth) is a major cause of maternal mortality and disability, particularly in under‐resourced areas (Fawcus 1995). It is the leading cause of maternal mortality in Sub‐Saharan Africa (Lazarus 2005). The South African National Committee for the Confidential Enquiries into Maternal Deaths analysed 3406 reported maternal deaths for the years 2002 to 2004. Overall, 9.5% were due to PPH (NCCEMD 2006). In Egypt, in spite of the drop in maternal mortality ratio from 174/100,000 live births in 1992 to 1993 to 80/100,000 live births in the year 2000 (MOH 2000), PPH is still the leading cause and responsible for 34% of maternal deaths.
Deaths from PPH remain most common in areas where access to health services is poorest. In these settings, poor nutrition, malaria and anaemia may aggravate the effects of PPH. In well‐resourced settings with healthier populations and adequate health services, deaths from PPH are extremely rare, as effective methods are available to reduce and treat PPH. These include routine active management of the third stage of labour and facilities for resuscitation, blood transfusion and surgical interventions. In the 2002 to 2004 South African National Confidential Enquiry into Maternal Deaths, 83% of the 313 deaths from PPH were found to be 'clearly preventable' (NCCEMD 2006). For these reasons, strategies to reduce deaths from PPH have been a focus of attempts to achieve the Millennium Development Goal of reducing maternal mortality by 75% by 2015. Primary PPH, heavy bleeding directly following childbirth or within 24 hours thereafter, is the most common type of PPH and can be caused by uterine atony, retained placenta, inverted or ruptured uterus, and cervical, vaginal, or perineal lacerations. Uterine atony, when the uterus fails to contract after delivery, is the most important cause of primary PPH (WHO 2000). Methods leading to contraction of the uterus and correction of atony will reduce the amount of bleeding after delivery.
Recommendations for the prevention of PPH such as the joint statement of the International Confederation of Midwives and the International Federation of Gynaecologists and Obstetricians (ICM/FIGO 2004) recommend routine massage of the uterus after delivery of the placenta. Uterine massage involves placing a hand on the woman's lower abdomen and stimulating the uterus by repetitive massaging or squeezing movements. Massage is thought to stimulate uterine contraction, possibly through stimulation of local prostaglandin release and thus to reduce haemorrhage. However, it is not done routinely after delivery in a systematic way. If shown to be effective, it would have important advantages as it is inexpensive and requires no access to medication or other specialised services, and could be used in any location in which women give birth. Disadvantages include the use of staff time, and discomfort caused to women. However, there is very little empirical research to evaluate the effectiveness of this method. There is therefore a need to evaluate systematically the effectiveness of uterine massage for preventing PPH.
Objectives
To determine the effectiveness of uterine massage after birth and before or after delivery of the placenta, or both, to reduce postpartum blood loss and associated morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials comparing uterine massage alone or as part of the active management of labour (including uterotonics) before or after delivery of the placenta, or both, with non‐massage. We planned to exclude from the analyses quasi‐randomised trials (for example, those randomised by date of birth or hospital number). We planned to included studies reported only in abstract form if sufficient information to evaluate the trial was available. If not, they would be included in the 'Studies awaiting classification' category and be included in the analyses when published as full reports.
Types of participants
Women who have given birth vaginally or by caesarean section.
Types of interventions
Uterine massage commencing after birth of the baby, before or after delivery of the placenta, or both, compared with no intervention or a 'dummy' procedure to mask allocation or with alternative methods or alternative forms of uterine massage, with or without other third stage co‐interventions.
Types of outcome measures
Primary outcomes
Blood loss 500 mL or more after trial entry.
Placenta delivered more than 30 minutes after birth.
Secondary outcomes
Blood loss 1000 mL or more after trial entry.
Mean blood loss after trial entry.
Mean time to placental delivery.
Use of additional uterotonics
Use of other procedures for management of postpartum haemorrhage.
Haemoglobin level after 12 to 24 hours less than 8 g/dL or blood transfusion.
Blood transfusion.
Maternal death or severe morbidity (organ failure, intensive care unit admission for more than 24 hours, major surgery).
Women's experience including pain/discomfort.
Caregiver's experience.
Cost.
Only outcomes with available data appear in the analysis table. We planned that outcome data that we did not pre‐specify, but which were reported by the trial authors, would be labelled as such in the analysis but not used for the conclusions.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 April 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
For additional searching performed for the previous version of the review, please seeAppendix 1
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 2.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we would have consulted a third person. In the case of trials involving the review authors, an independent person made the decision regarding inclusion of the study.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we would have consulted a third person. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor. In the case of trials involving the review authors, an independent person assessed for risk of bias.
(1) Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal allocation to interventions prior to assignment and assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We describe for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion. In future updates, if we identify cluster‐randomised trials we will include them in the analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates we will explore the impact of including studies with high levels of missing data (>10%) in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the T² was greater than zero and either an I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there had been 10 or more studies in the meta‐analysis we planned to investigate reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry. For continuous outcomes, we would have used the test proposed by Egger 1997, and for dichotomous outcomes, we would have used the test proposed by Harbord 2006. If asymmetry was detected in any of these tests or was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary is treated as the average range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not have combined trials.
Where we used random‐effects analyses, the results are presented as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, when adequate data are available, we will analyse data in the following subgroups.
Uterine massage commencing before or after delivery of the placenta.
With or without routine uterotonics.
With or without controlled cord traction.
We will include all outcomes in the sub‐group analysis.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2011). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates of this review, as more data become available, we will use sensitivity analysis to explore aspects of the trials that might affect the results. These will include:
type of uterine massage;
type of co‐interventions;
trial quality.
Results
Description of studies
Two trials are included in this review. Abdel‐Aleem 2006 is a small randomised trial conducted in a teaching hospital located in a developing country (Egypt). The other study (Abdel‐Aleem 2010) is a larger trial from the same group conducted in teaching hospitals in Egypt and South Africa. For the purposes of this review, the data for each site for the latter trial are presented separately due to heterogeneity between sites. In our analyses, the data are included as Abdel‐Aleem 2010 Egypt and Abdel‐Aleem 2010 S Africa. In both trials, women were randomly allocated to receive uterine massage or no massage after active management of the third stage of labour, including the routine use of oxytocin 10 units. Uterine massage was commenced before (Abdel‐Aleem 2010 Egypt; Abdel‐Aleem 2010 S Africa) or after delivery of the placenta (Abdel‐Aleem 2006). The first trial (Abdel‐Aleem 2006) recruited 200 women assigned at random to receive uterine massage or no massage after delivery of the placenta, in addition to active management of the third stage of labour. The second trial (Abdel‐Aleem 2010 Egypt; Abdel‐Aleem 2010 S Africa) included 1964 women, assigned randomly to receive one of three interventions after delivery of the baby and before delivery of the placenta. These interventions were oxytocin (10 IU), uterine massage, or both. In this review we include only the oxytocin versus both oxytocin and uterine massage groups (i.e. uterine massage versus no uterine massage with oxytocin as a co‐intervention in both groups).
For further details of the included studies, seeCharacteristics of included studies.
Risk of bias in included studies
See table of Characteristics of included studies, particularly the 'Methods' section. The sequence generation and allocation concealment for both trials was rated as 'low risk of bias' and there was a low proportion of losses to follow‐up (losses to follow‐up occurred only in the South African arm of the second trial (Abdel‐Aleem 2010 S Africa)). There was no blinding of participants and caregivers, which raises the possibility of biased assessment of outcomes. Because the primary outcome assessment was dependent on objective measurement of blood loss, the risk of bias was considered low. The methodological quality of both included trials was high.
Effects of interventions
Analysis 1. Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth
There was substantial heterogeneity with respect to the proportion of women with blood loss greater than or equal to 500 mL (Analysis 1.1). The average effect found no statistically significant difference between groups (average risk ratio (RR) 1.56, 95% confidence interval (CI) 0.44, 5.49; random‐effects). Retained placenta for more than 30 minutes occurred only in the South African cohort (Abdel‐Aleem 2010 S Africa), and was not significantly different between groups (RR 0.79, 95% CI 0.33 to 1.88) (Analysis 1.2). Haemoglobin levels after 12 to 24 hours were measured only in the South African cohort (Abdel‐Aleem 2010 S Africa), and there were no statistically significant differences in haemoglobin less than 8 g/dL (RR 0.63, 95% CI 0.21 to 1.88) (Analysis 1.9) or need for additional use of uterotonics (RR 1.02, 95% CI 0.56 to 1.85) (Analysis 1.3). There were four blood transfusions in each group (too few for statistical analysis) (Analysis 1.4).
1.1. Analysis.
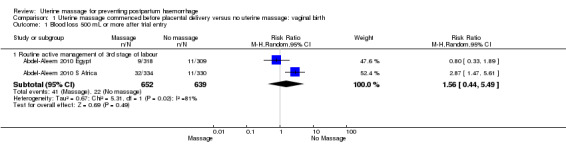
Comparison 1 Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth, Outcome 1 Blood loss 500 mL or more after trial entry.
1.2. Analysis.
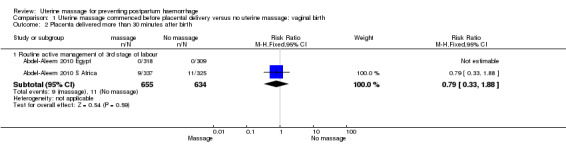
Comparison 1 Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth, Outcome 2 Placenta delivered more than 30 minutes after birth.
1.9. Analysis.
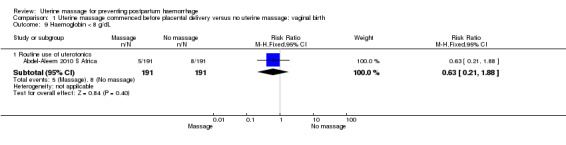
Comparison 1 Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth, Outcome 9 Haemoglobin < 8 g/dL.
1.3. Analysis.
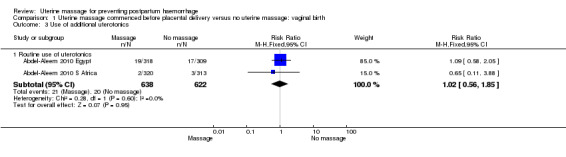
Comparison 1 Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth, Outcome 3 Use of additional uterotonics.
1.4. Analysis.
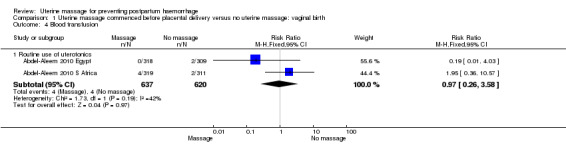
Comparison 1 Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth, Outcome 4 Blood transfusion.
Analysis 2. Uterine massage after placental delivery versus no massage: vaginal birth
In the first trial (Abdel‐Aleem 2006), the mean blood loss was less in the uterine massage group at 30 (mean difference (MD) ‐41.60 mL, 95% CI ‐75.16 to ‐8.04 (Analysis 2.4)) and 60 minutes after trial entry (MD ‐77.40 mL, 95% CI ‐118.71 to ‐36.09 (Analysis 2.5)). The need for additional uterotonics was reduced in the uterine massage group (RR 0.20, 95% CI 0.08 to 0.50 (Analysis 2.6)). Two blood transfusions were administered in the control group (Analysis 2.8).
2.4. Analysis.
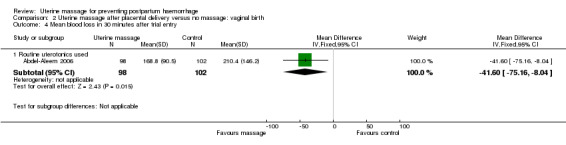
Comparison 2 Uterine massage after placental delivery versus no massage: vaginal birth, Outcome 4 Mean blood loss in 30 minutes after trial entry.
2.5. Analysis.
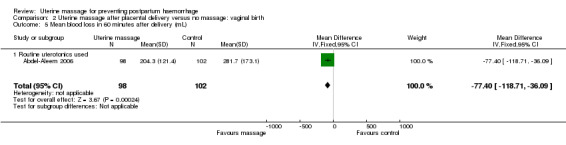
Comparison 2 Uterine massage after placental delivery versus no massage: vaginal birth, Outcome 5 Mean blood loss in 60 minutes after delivery (mL).
2.6. Analysis.
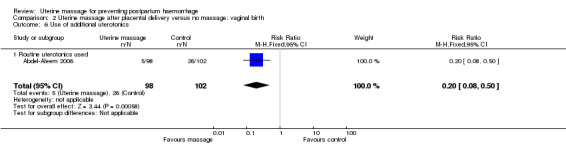
Comparison 2 Uterine massage after placental delivery versus no massage: vaginal birth, Outcome 6 Use of additional uterotonics.
2.8. Analysis.
Comparison 2 Uterine massage after placental delivery versus no massage: vaginal birth, Outcome 8 Blood transfusion.
The numbers of women with blood loss more than 500 mL was small, with no statistically significant difference (RR 0.52, 95% CI 0.16 to 1.67) (Analysis 2.1). There were no cases of retained placenta in either group.
2.1. Analysis.
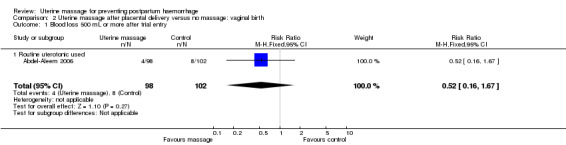
Comparison 2 Uterine massage after placental delivery versus no massage: vaginal birth, Outcome 1 Blood loss 500 mL or more after trial entry.
Analysis 3. Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth
Concerning the effect of uterine massage commenced either before or after delivery of the placenta, we included the results of both included studies (Abdel‐Aleem 2006; Abdel‐Aleem 2010 Egypt; Abdel‐Aleem 2010 S Africa).There was substantial heterogeneity with respect to the blood loss 500 mL or more after trial entry. The average effect using a random‐effects model found no statistically significant differences between groups (average RR 1.14, 95% CI 0.39 to 3.32; random‐effects, I² = 77% (Analysis 3.1)). The use of additional uterotonics was not statistically different between the groups (RR 0.52, 95% CI 0.15, 1.81; random‐effects, I² = 78% (Analysis 3.3)).
3.1. Analysis.
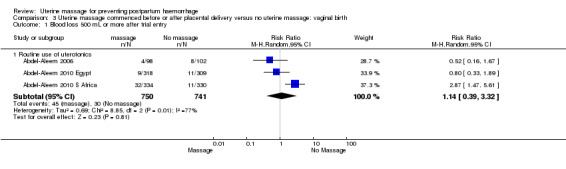
Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 1 Blood loss 500 mL or more after trial entry.
3.3. Analysis.
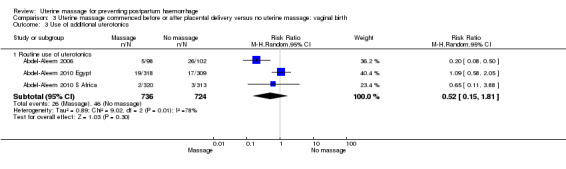
Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 3 Use of additional uterotonics.
Discussion
One small trial found evidence of reduced blood loss with uterine massage after placental delivery. However, overall, the trials do not provide compelling evidence of the effectiveness of uterine massage.
The heterogeneity between trials and sites is cause for cautious interpretation of the results of this review. The heterogeneity raises the possibility that differences in trial procedures between sites may affect the results. For example, it is possible that uterine massage may express blood from the uterine cavity, while in the control group pooled blood in the uterine cavity is not measured, leading to a relative under‐estimation of blood loss. This could mask a beneficial effect of uterine massage. This effect is suggested by the fact that in the South African site (Abdel‐Aleem 2010 S Africa), measured blood loss was significantly more in the uterine massage group, yet there was a trend to higher haemoglobin levels at 12 to 24 hours. In view of these uncertainties, the results of this review should be regarded as inconclusive.
The data reviewed included only women who had received routine uterotonics. In this situation, it is likely that their uteri were optimally contracted, and the potential for further benefit from uterine massage was limited.
These results should therefore not be extrapolated to settings where routine uterotonics are not used or not available.
There were no data on women's experience of discomfort during the procedure.
Authors' conclusions
Implications for practice.
The joint statement of ICM/FIGO 2004 on management of the third stage of labour, advises uterine massage after delivery of the placenta to prevent postpartum haemorrhage. The results of this review are inconclusive, and should not be interpreted as a reason to change current practice.
Implications for research.
It is remarkable that a practice, which has been widespread for decades, has been subjected to so little formal research. Further research to determine the effectiveness of uterine massage should be a high priority, especially as it is an intervention that can be used in any setting, particularly where uterotonics are not available. Such trials should include an assessment of women's views. Due to the limitations of the included trials, trials with sufficient numbers to estimate the effects of sustained uterine massage with greater precision, particularly with respect to objective outcomes such as postpartum anaemia, are needed. In particular, trials of uterine massage in settings where uterotonics are not available are of great importance. Recent research suggests that once an oxytocic has been given, as was the case in the studies included in this review, there is limited scope for further reduction in postpartum blood loss (Widmer 2010). In settings with no access to uterotonics, the potential for uterine massage to reduce blood loss may differ from that when uterotonics are used. If shown to be effective, uterine massage would represent a simple intervention with the potential to have a major effect on postpartum haemorrhage and maternal mortality in under‐resourced settings.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2016 | Amended | Amended analysis 1.2.1 and 3.2.1 to correct an error in the data. The overall result for this outcome (placenta delivered more than 30 minutes after birth) remains unchanged. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 30 June 2011 | New citation required and conclusions have changed | The findings of the earlier version of the review supported the use of uterine massage as part of the management of the third stage of labour. The findings of the current version are inconclusive, with considerable heterogeneity between results from different sites. |
| 4 February 2011 | New search has been performed | Search updated. One new trial identified and included Abdel‐Aleem 2006 but results are included in the analyses as Abdel‐Aleem 2010 Egypt and Abdel‐Aleem 2010 S Africa. This update is now comprised of two included studies. |
| 2 July 2010 | Amended | Contact details edited. |
| 18 March 2008 | Amended | Converted to new review format |
Acknowledgements
We are most grateful to Frances Kellie for assistance with the review, and to Sonja Henderson and the Pregnancy and Childbirth team for technical support.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team) and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Previous searches
In addition, we searched CENTRAL (The Cochrane Library 2011, Issue 2 of 4), PubMed (1966 to June 2011) using the term 'uterine massage', and searched reference lists of key papers.
Appendix 2. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Abdel‐Aleem 2006.
Selection of studies
We assessed for inclusion all potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, consulted an outside person.
Data extraction and management
We designed a form to extract data. The review authors extracted the data using the agreed form. Discrepancies were resolved through discussion. The Review Manager software (RevMan 2008) was used to double enter all the data.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of methodological quality of included studies
We assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Methods used for generation of the randomisation sequence were described for each trial.
(1) Selection bias (randomisation and allocation concealment)
We assigned a quality score for each trial, using the following criteria: (A) adequate concealment of allocation: such as telephone randomisation, consecutively‐numbered, sealed opaque envelopes; (B) unclear whether adequate concealment of allocation: such as list or table used, sealed envelopes, or study does not report any concealment approach; (C) inadequate concealment of allocation: such as open list of random‐number tables, use of case record numbers, dates of birth or days of the week.
We decided to exclude trials with inadequate allocation concealment (C).
(2) Attrition bias (loss of participants, for example, withdrawals, dropouts, protocol deviations)
We assessed completeness to follow up using the following criteria: (A) less than 5% loss of participants; (B) 5% to 9.9% loss of participants; (C) 10% to 19.9% loss of participants; (D) more than 20% loss of participants.
We decided to exclude outcomes with 10% or more missing data.
(3) Performance bias (blinding of participants, researchers and outcome assessment)
We assessed blinding using the following criteria:
blinding of participants (yes/no/unclear);
blinding of caregiver (yes/no/unclear);
blinding of outcome assessment (yes/no/unclear).
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If heterogeneity was found, this was to be explored by sensitivity analysis followed by random‐effects analysis if required.
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but used different methods. If there was evidence of skewness, this was to be reported.
Unit of analysis issues
Cluster‐randomised trials
We decided to include cluster‐randomised trials in the analyses along with individually randomised trials. However, we did not identify any cluster‐randomised trials but if we do in the future, we will use the methods in Appendix 1.
Dealing with missing data
We decided to analyse data on all participants with available data in the group to which they are allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants are not analysed in the group to which they were randomised, and there was sufficient information in the trial report or the information could be obtained from the trial authors, we would attempt to restore them to the correct group.
Assessment of heterogeneity
We decided to apply tests of heterogeneity between trials, if appropriate, using the I² statistic. If we had identified high levels of heterogeneity among the trials (exceeding 50%), we would have explored it by prespecified subgroup analysis and performed sensitivity analysis. A random‐effects meta‐analysis would be used as an overall summary if this was considered appropriate.
Subgroup analyses
We decided to conduct planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001.
The plan was to carry out the following subgroup analyses:
women delivered vaginally or by caesarean section;
comparisons with or without the use of routine oxytocics;
uterine massage commenced before or after placental delivery.
Sensitivity analyses
Sensitivity analyses are planned to explore the effect of trial quality assessed by concealment of allocation by excluding studies with inadequate allocation of concealment (rated B) and to assess the possible effect of publication bias by a sensitivity analysis excluding trials not identified from prospective trial registers.
Appendix 3. Methods to be used for cluster‐randomised controlled trials
Their sample sizes will be adjusted using the methods described in Gates 2005 using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, this will be reported and sensitivity analyses conducted to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
Data and analyses
Comparison 1. Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after trial entry | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Routine active management of 3rd stage of labour | 2 | 1291 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.44, 5.49] |
| 2 Placenta delivered more than 30 minutes after birth | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Routine active management of 3rd stage of labour | 2 | 1289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.33, 1.88] |
| 3 Use of additional uterotonics | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Routine use of uterotonics | 2 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.56, 1.85] |
| 4 Blood transfusion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Routine use of uterotonics | 2 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.26, 3.58] |
| 5 Need for manual removal of placenta | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Routine use of uterotonics | 2 | 1289 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.18, 17.62] |
| 6 Blood loss > 1000 mL | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Routine use of uterotonics | 2 | 1291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.31, 28.35] |
| 7 Mean blood loss at 30 minutes | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Mean blood loss at 60 minutes | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Haemoglobin < 8 g/dL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Routine use of uterotonics | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.88] |
| 10 maternal mortality | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.5. Analysis.
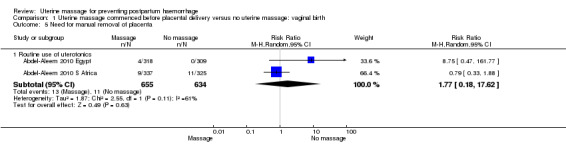
Comparison 1 Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth, Outcome 5 Need for manual removal of placenta.
1.6. Analysis.
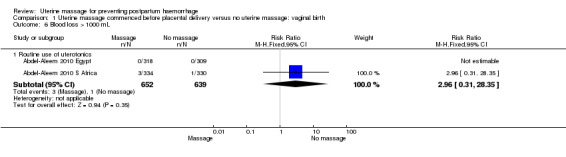
Comparison 1 Uterine massage commenced before placental delivery versus no uterine massage: vaginal birth, Outcome 6 Blood loss > 1000 mL.
Comparison 2. Uterine massage after placental delivery versus no massage: vaginal birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after trial entry | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.67] |
| 1.1 Routine uterotonic used | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.67] |
| 2 Placenta delivered more than 30 minutes after birth | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Routine uterotonic used | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood loss 1000 mL or more after trial entry | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mean blood loss in 30 minutes after trial entry | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Routine uterotonics used | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐41.60 [‐75.16, ‐8.04] |
| 5 Mean blood loss in 60 minutes after delivery (mL) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐77.40 [‐118.71, ‐36.09] |
| 5.1 Routine uterotonics used | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐77.40 [‐118.71, ‐36.09] |
| 6 Use of additional uterotonics | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.08, 0.50] |
| 6.1 Routine uterotonics used | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.08, 0.50] |
| 7 Haemoglobin level after 12 to 24 hours less than 8 g/dL | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood transfusion | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Routine uterotonics used | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal death or severe morbidity | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Routine uterotonics used | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.2. Analysis.
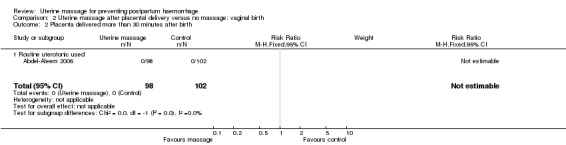
Comparison 2 Uterine massage after placental delivery versus no massage: vaginal birth, Outcome 2 Placenta delivered more than 30 minutes after birth.
2.9. Analysis.
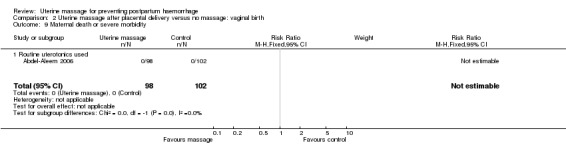
Comparison 2 Uterine massage after placental delivery versus no massage: vaginal birth, Outcome 9 Maternal death or severe morbidity.
Comparison 3. Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss 500 mL or more after trial entry | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Routine use of uterotonics | 3 | 1491 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.39, 3.32] |
| 2 Placenta delivered more than 30 minutes after birth | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Routine use of uterotonics | 3 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.33, 1.88] |
| 3 Use of additional uterotonics | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Routine use of uterotonics | 3 | 1460 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.15, 1.81] |
| 4 Blood transfusion | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Routine use of uterotonics | 3 | 1457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.26, 3.58] |
| 5 Need for manual removal of placenta | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Routine use of uterotonics | 2 | 1289 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.18, 17.62] |
| 6 Blood loss > 1000 mL | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Routine use of uterotonics | 2 | 1291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.31, 28.35] |
| 7 Mean blood loss at 30 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Routine use of uterotonics | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐41.60 [‐75.16, ‐8.04] |
| 8 Mean blood loss at 60 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Routine use of uterotonics | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐77.40 [‐118.71, ‐36.09] |
| 9 Haemoglobin < 8 g/dL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Routine use of uterotonics | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.88] |
| 10 maternal mortality | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Routine use of uterotonics | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.2. Analysis.
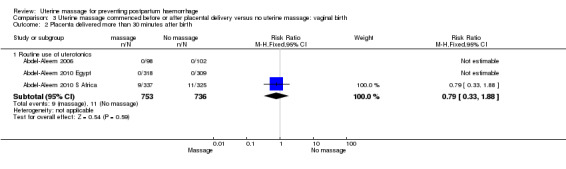
Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 2 Placenta delivered more than 30 minutes after birth.
3.4. Analysis.
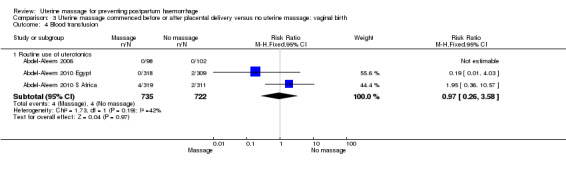
Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 4 Blood transfusion.
3.5. Analysis.
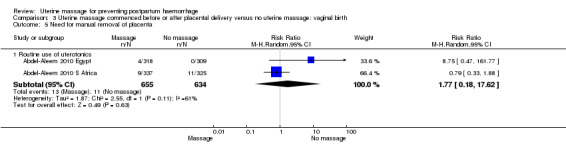
Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 5 Need for manual removal of placenta.
3.6. Analysis.
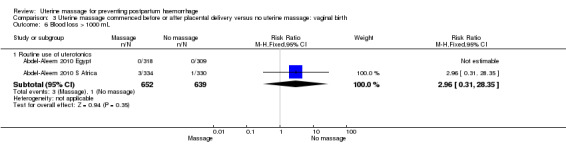
Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 6 Blood loss > 1000 mL.
3.7. Analysis.

Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 7 Mean blood loss at 30 minutes.
3.8. Analysis.

Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 8 Mean blood loss at 60 minutes.
3.9. Analysis.
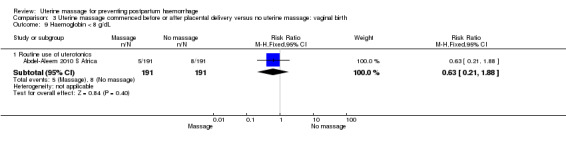
Comparison 3 Uterine massage commenced before or after placental delivery versus no uterine massage: vaginal birth, Outcome 9 Haemoglobin < 8 g/dL.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Aleem 2006.
| Methods | Randomised clinical trial. Allocation in computer‐generated random sequence by numbered, sealed, opaque envelopes. Attrition bias: no. Blinding of participants: no. Blinding of caregiver: no. Blinding of outcome assessment: yes. | |
| Participants | 200 women delivered vaginally. Women were enrolled shortly after birth of the baby, by opening the sealed allocation envelope. Setting: labour ward in a University hospital, Assiut, Egypt. | |
| Interventions | Uterine massage after delivery of the placenta, every 10 minutes for 60 minutes (98 women). Control group: no intervention (102 women). All women received active management of the third stage of labour including oxytocin 10 units. | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque, sequentially‐numbered envelopes containing allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data were analysed. |
| Selective reporting (reporting bias) | Low risk | Nothing to suggest reporting bias. |
| Other bias | Low risk | Nothing to suggest other bias. |
Abdel‐Aleem 2010 Egypt.
| Methods | Randomised by means of computer‐generated random numbers held within sealed, opaque, sequentially numbered envelopes. | |
| Participants | Women 18 years or older with uncomplicated pregnancy and spontaneous birth. | |
| Interventions | Uterine massage from birth of the baby for 30 minutes, versus uterine massage plus oxytocin 10 units IM, vs oxytocin alone. For this review, the latter 2 groups are included. | |
| Outcomes | Primary
Secondary
|
|
| Notes | Assiut, Egypt and East London Hospital Complex, South Africa. The data were presented separately for the 2 sites because of some heterogeneity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque, sequentially‐numbered envelopes containing allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small proportion of missing data SA site only. |
| Selective reporting (reporting bias) | Low risk | Nothing to suggest reporting bias. |
| Other bias | Low risk | Nothing to suggest other bias. |
Abdel‐Aleem 2010 S Africa.
| Methods | Randomised by means of computer‐generated random numbers held within sealed, opaque, sequentially numbered envelopes. | |
| Participants | Women 18 years or older with uncomplicated pregnancy and spontaneous birth. | |
| Interventions | Uterine massage from birth of the baby for 30 minutes, versus uterine massage plus oxytocin 10 units IM, vs oxytocin alone. For this review, the latter 2 groups are included. | |
| Outcomes | Primary
Secondary
|
|
| Notes | Assiut, Egypt and East London Hospital Complex, South Africa. The data were presented separately for the 2 sites because of some heterogeneity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque, sequentially‐numbered envelopes containing allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small proportion of missing data SA site only. |
| Selective reporting (reporting bias) | Low risk | Nothing to suggest reporting bias. |
| Other bias | Low risk | Nothing to suggest other bias. |
Hb: haemoglobin IM: intramuscular vs: versus
Differences between protocol and review
Reported outcomes which were not specified in the protocol have been included in the review and identified as non‐prespecified data.
Contributions of authors
For the first version of the review, H Abdel‐Aleem (HA) wrote the first draft of the protocol; GJ Hofmeyr (GJH) revised the protocol; HA and GJH independently extracted data; HA wrote the first draft of the review; GJH revised the review; M Abdel‐Aleem (MA) developed the data extraction form, assisted with data extraction and approved the review.
For this update, GJH extracted data and wrote the first draft of the review; HA and MA undertook the meta‐analysis, reconstructed the review and revised the manuscript. GJH revised the review in response to peer reviewer and editorial comments.
Sources of support
Internal sources
(GJH) Effective Care Research Unit, University of the Witwatersrand, University of Fort Hare, Eastern Cape Department of Health, South Africa.
Department of Obstetrics and Gynecology, Assiut University Hospital, Assiut, Egypt.
External sources
No sources of support supplied
Declarations of interest
HA and GJH are co‐authors of Abdel‐Aleem 2006. HA, MA and GJH are co‐authors of Abdel‐Aleem 2010 Egypt / Abdel‐Aleem 2010 S Africa. One of the Pregnancy and Childbirth Group editors (AM Gulmezoglu) evaluated the studies for inclusion in the review, including assessment for risk of bias.
Edited (no change to conclusions)
References
References to studies included in this review
Abdel‐Aleem 2006 {published data only}
- Abdel‐Aleem H, Hofmeyr GJ, Shokry M, El‐Sonoosy E. Uterine massage and postpartum blood loss. International Journal of Gynecology & Obstetrics 2006;93(3):238‐9. [DOI] [PubMed] [Google Scholar]
Abdel‐Aleem 2010 Egypt {published data only}
- Abdel‐Aleem H, Singata M, Abdel‐Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics 2010;111(1):32‐6. [DOI] [PubMed] [Google Scholar]
Abdel‐Aleem 2010 S Africa {published data only}
- Abdel‐Aleem H, Singata M, Abdel‐Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics 2010;111(1):32‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Abdel‐Aleem 2010
- Abdel‐Aleem H, Singata M, Abdel‐Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynaecology and Obstetrics 2010;111(1):32‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. London: BMJ Books, 2001. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fawcus 1995
- Fawcus S, Mbizvo MT, Lindmark G, Nystrom L, Maternal mortality study group. Community based investigation of causes of maternal mortality in rural and urban Zimbabwe. Central African Journal of Medicine 1995;41:105‐13. [PubMed] [Google Scholar]
Gates 2005
- Gates S. Methodological Guidelines. In: the Editorial Team. Pregnancy and Childbirth Group. About The Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2005, Issue 2.
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICM/FIGO 2004
- International Confederation of Midwives (ICM), International Federation of Gynaecologists and Obstetricians (FIGO). Joint statement: management of the third stage of labour to prevent post‐partum haemorrhage. Journal of Midwifery and Women's Health 2004;49:76‐7. [DOI] [PubMed] [Google Scholar]
Lazarus 2005
- Lazarus JV, Lalonde V. Reducing postpartum hemorrhage in Africa. International Journal of Gynecology & Obstetrics 2005;88:89‐90. [DOI] [PubMed] [Google Scholar]
MOH 2000
- Anonymous. Egyptian Ministry of Health and Population (MOP). National Maternal Mortality Study. Egyptian Ministry of Health and Population, 2000. [Google Scholar]
NCCEMD 2006
- National Committee for the Confidential Enquiries into Maternal Deaths. Saving Mothers. Third Report on Confidential Enquiries into Maternal Deaths in South Africa 2002‐2004. South Africa: Department of Health, 2006. [MEDLINE: ] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
WHO 2000
- World Health Organization (WHO). Vaginal bleeding after childbirth. Managing Complications in Pregnancy and Childbirth: a Guide for Midwives and Doctors. WHO/RHR/00.7. Geneva: WHO, 2000:S25‐S34. [Google Scholar]
Widmer 2010
- Widmer M, Blum J, Hofmeyr GJ, Carroli G, Abdel‐Aleem H, Lumbiganon P, et al. Misoprostol as an adjunct to standard uterotonics for treatment of post‐partum haemorrhage: a multicentre, double‐blind randomised trial. Lancet 2010;375(9728):1808‐13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hofmeyr 2008
- Hofmeyr GJ, Abdel‐Aleem H, Abdel‐Aleem MA. Uterine massage for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006431.pub2] [DOI] [PubMed] [Google Scholar]


