Abstract
Human African trypanosomiasis (HAT), despite considerable progress in the control, is still occurring in many countries in both west and central African regions. The HAT situation in the Republic of Congo has always been overshadowed by its neighbor the Democratic Republic of Congo where over 60% of all HAT cases occur. In the Republic of Congo, HAT cases have been significantly reduced to about 20 reported cases yearly and the disease is still prevalent in few foci across the country. Although continuous assessment of HAT situation in Congo is been led by the National Control Program for HAT, research on the vector, parasite, and vector control has received little attention. Because there have not been enough reviews summarizing key findings from studies conducted so far, there is still a poor understanding of the global situation of HAT in Congo. In order to achieve sustainable elimination of HAT in Congo a deep appraisal of HAT situation is required. The present study provides a review of studies conducted on HAT in the republic of Congo since the 1950s to date in order to identify gaps in knowledge and help consolidate the gains and progress towards the elimination of sleeping sickness.
Keywords: human African trypanosomiasis, tsetse fly, trypanosome, distribution, Congo
Human African trypanosomiasis (HAT), also known as sleeping sickness, is a parasitic disease transmitted by tsetse flies (Glossina spp) that has a high lethality unless treated (Büscher et al. 2017). The disease is endemic in sub-Saharan Africa and affects over 65 million people in more than 36 countries particularly those living in rural settings (OMS 2018). Trypanosomiasis due to Trypanosoma brucei gambiense is the most widespread and accounts for 98% of reported human cases of sleeping sickness (OMS 2018). At the beginning of the 20th century, HAT cases were highly prevalent and causing high mortality in populations across the continent (Jamot 1933). Control strategies including active detection of cases by mobile teams and treatment of all febrile cases using compounds such as suramin, pentamidine, and the highly toxic organo-arsenical melarsoprol contributed in significantly reducing the burden of the disease such that the disease was reported residual in most countries after independence in the 1960s (Muraz 1943, Richet 1962). However, in the 1970s, with the stoppage of routine diagnosis and control actions, a resurgence of the disease was observed in historical foci in both west and central Africa. (Saliou and Challier 1976, Duvallet and JF 1979, Lankoande and Ouedele 1982, Solano et al. 2003). Over the last decades the reinforcement of control and surveillance activities across Africa permitted to dramatically reduce its prevalence from 25,841 new cases in 2000 to 2,164 cases in 2016 and 977 cases in 2018 (Franco et al. 2020). In 2009, the number of new cases fell below 10,000 for the first time in 50 years (OMS 2019, Franco et al. 2020). Nevertheless, the disease remains prevalent with a heterogeneous distribution across the continent. Over 60% of HAT cases have been reported in the Democratic Republic of Congo which bears the highest burden of the disease (OMS 2019). Whilst in many countries there are now few active foci the potential for outbreaks and disease resurgence is still important due to the circulation of T. b. gambiense in both human and animal species (Büscher et al. 2018), movement of populations in and out endemic settings, and the presence of competent Tsetse fly in most epidemiological settings (Solano and Torr 2013).
During the last decades important progress has been achieved in the management of gambiense HAT cases with the development of new antibody-based rapid diagnostic tests used for mass and passive screening, the introduction of new and simpler treatments including nifurtimox–eflornithine combination therapy (NECT) and the new oral drug fexinidazole to treat both stages of the disease (Bottieau and Clerinx 2019) (https://apps.who.int/iris/bitstream/handle/10665/326178/9789241550567-eng.pdf 2019).
New indicators were also developed to assess the endemic status of countries and to validate HAT elimination as a public health problem at national level (OMS 2019).
As many countries approach the preelimination and elimination phases, thorough understanding of the epidemiological situation of each endemic setting is required in order to avoid resurgence of the disease which could impede progress registered so far. The Republic of Congo is among countries still affected by HAT almost all being gambiense HAT cases. In 2001 and 2002, the country reported its most largest number of new cases since the 1980s with 894 and 1005 cases respectively following the socio-political crisis that affected the country (Franco et al. 2018). In Congo, HAT remains endemic in five of the twelve administrative units (Bouenza, Niari, Pool, Plateau, and Cuvette). In 2019, 19 cases were reported after examining a total of 11,308 people (PNLTHA 2019). Although much research has been conducted on HAT in Congo, there are still not enough studies summarizing previous and recent findings to identify gaps in knowledge or to document recent dynamics of vectors or parasites. This information is not only a key for the management of current control programs but also to improve disease surveillance in order to achieve HAT elimination by 2030 as planned by the World Health Organization (OMS 2019). In this regard, the objective of this review is to gather information from previous studies in order to provide a better understanding of the dynamic of HAT situation in the Republic of Congo.
Methods
Selection and Data Collection Process
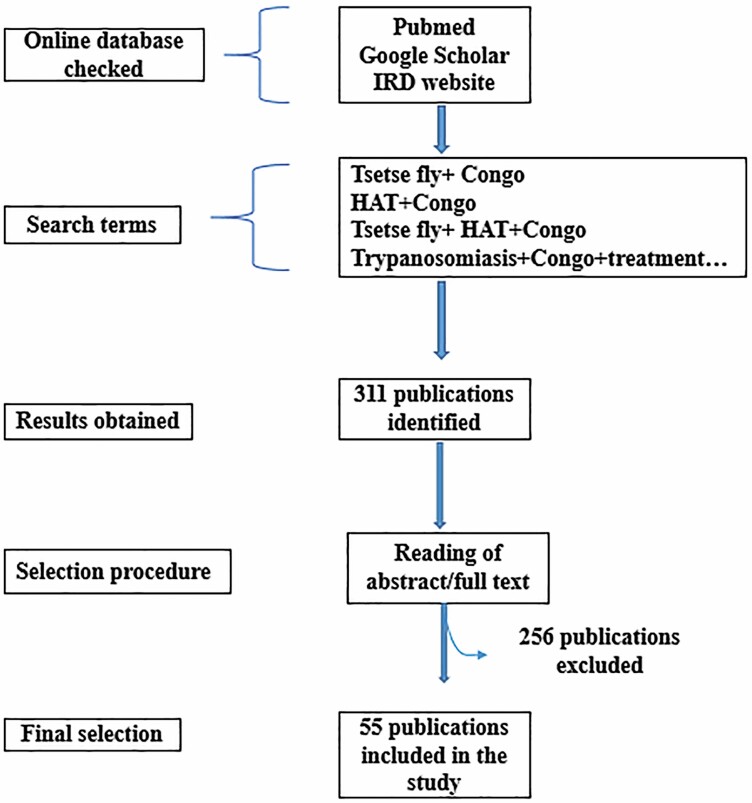
A systematic search of published literature on human trypanosomiasis in the Republic of Congo was done in both English and French using different accessible online bibliographic database such as PubMed, Google Scholar, IRD (Institut de Recherche pour le Developpement) websites with specific/defined keywords such as, ‘Trypanosomiasis and Congo’ or ‘Glossina and Congo’ or ‘Trypanosomiasis and Glossina and Congo’ or ‘maladie du sommeil’ or ‘Trypanosomiase and Congo’ or ‘Trypanosome and glossine and Congo’. Period of literature search included 1950–2020. The search resulted in the identification of 311 publications of which 264 were excluded because of unavailable or nonreporting data on HAT in the Republic of Congo. A Microsoft Excel spreadsheet was used to register information extracted from each selected published study for easy access and data analysis. Information registered included authors names, the year of the study, type of study, and topic covered (main findings). Annual reports from the national sleeping sickness control program of the Republic of Congo were also consulted (Fig. 1). Three researchers were involved in the selection process of papers and they worked independently.
Fig. 1.
Diagram showing the protocol for selection of scientific papers.
Eligibility criteria: Studies included were studies conducted on HAT conducted between 1950 and 2020; were not considered studies on animal trypanosomiasis and experimental studies.
Results
Epidemiology of HAT in Congo
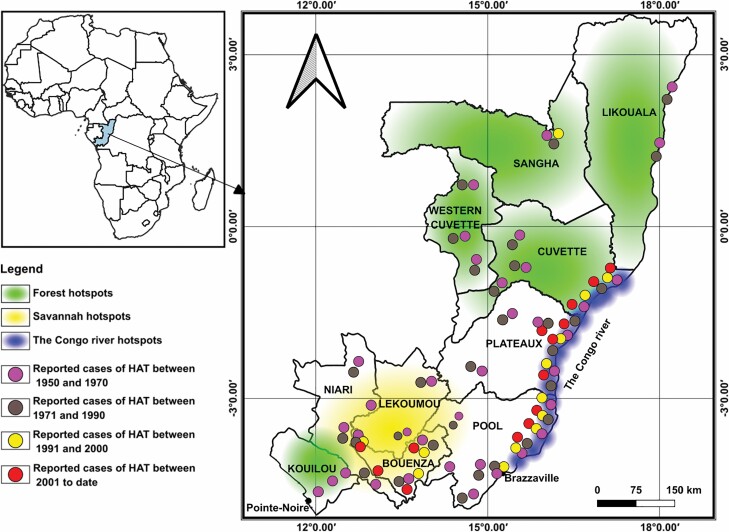
In the 1960s, six main hotspots or active transmission foci of HAT were reported in Congo (Maillot 1962). These include the Congo Atlantic foci extending from Loango in the coast to the south of Brazzaville; the corridor foci extending from the north of Brazzaville to the south of Mpouya; the Nkéni foci covering the district of Gamboma, the Alima foci including the Districts of Mabirou, Fort-Rousset currently called Owando, Ewo, and the north-western of Djambala; the Likouala foci covering the Likouala-Mossaka valley, and the Likouala grassland foci covering the districts of Mossaka-North, Epena, Impfondo, and Dongou. Following active detection and treatment of HAT, the number of cases drastically reduced across the country (Frézil et al. 1975). However studies conducted between 1975 and 1977 reported frequent cases of HAT in three distinct hotspots which were later grouped into three foci with well-defined phytogeographical facies (Frezil et al. 1977) (Table 1):
Table 1.
Number of cases according to year intervals
| Number of cases | Years | References |
|---|---|---|
| 413 | 1950–1970 | Frezil et al. 1975 |
| 2,031 | 1971–1990 | Frezil and Coulm 1977, 1979; Frezil et al. 1975, 1979, 1980, 1981; Gouteux et al. 1987, 1988; Gouteux and Sinda 1990 |
| 2,199 | 2001–to date | Balessegaram et al. 2006, PNLTHA 2015, 2017, 2018, 2019 |
- The savannah hotspot (located between the Kouilou and Pool division)
- The forest hotspot (including the Mbomo ‘Cuvette Congolaise’ and Mayombe forests)
- The Congo River hotspot (comprising two active areas the corridor hotspot situated in the southern part of river Congo and the hotspot of Cuvette situated in the northern part of river Congo).
In the Republic of Congo according to the latest report of the National Program for the Control of human African trypanosomiasis only the foci of savannah and of corridor (Congo river) are still active with regular transmission of HAT; with the localities of Loudima, Ngabe, and Mpouya being at the forefront of the transmission (PNLTHA 2019). In the past, the country recorded fairly high prevalence ranging from 5.5 to 10% (Lancien et al. 1981, Noireau et al. 1991) and most recently, this prevalence has fallen to 0.1% (PNLTHA 2019). It has also been shown that a decrease in the tsetse fly population could lead to a decrease in the prevalence of animal trypanosomiasis (Lancien et al. 1981). Although a significant decrease of HAT transmission has been reported across the country, it is not clear whether these changes are only the results of improved case detection and management and vector control activities conducted so far. Reduced transmission has also been associated to the fragmentation of tsetse fly habitats following extension of human settlements or activities such as agricultural practices, road, or dams constructions which have been significantly expanded during the last decade (Maillot 1952, Taufflieb 1965, Frézil et al. 1972, PNLTHA 2019). The cause of the decrease still warrants further investigation may be clearer (Wamwiri and Changasi 2016). The rapid demographic growth of the population is extending human settlements and pushing more people into tsetse-fly infected zones. In addition the practice of intensive agriculture with the use of large quantities of pesticides could have also considerably affected tsetse flies habitat and leading to an apparent elimination of the disease (Mwanakasale and Songolo 2011). Yet this has not been investigated in Congo and deserves further attention. In eastern and southern Africa, simulation analysis suggested that population growth and land use cause a decline of savannah and forest tsetse fly populations. In these parts of the continent, tsetse fly populations have been confined in discrete habitats around wildlife conservation parks and reserves (Reid et al. 2000, Wamwiri and Changasi 2016).
- HAT situation in the cities of Brazzaville and Pointe Noire
Before and during the colonial period, parasitological positive patients were immediately referred to the reference unit in Brazzaville for treatment and, because of poor health infrastructure in the hinterland, most of the patients diagnosed with HAT nowadays, still travel to Brazzaville for treatment (Frézil and Coulm 1977, PNLTHA 2017). This situation favored the transmission of HAT in Brazzaville due to the presence of local tsetse fly populations in the city. The city of Brazzaville is at the southern limit of the distribution area of Glossina fucipes quanzensis, one of the main vectors of HAT in Congo. Many tsetse flies habitats were described in the ravines and main rivers of the Mʼfoa, Tsiema, Makélékélé, Mfilou, and Djoué districts of Brazzaville (Taufflieb 1965). The city of Brazzaville has been a high hotspot of HAT in Congo until the 1970s (Frézil and Coulm 1977). In 1989 nineteen HAT cases were passively detected in Brazzaville (Jannin et al. 1990). It was not clear whether these were indigenous or imported cases. The practice of brushing and clearing of low vegetation up to 2 m and extension of the city limits (Gouteux et al. 1986b), permitted to reduce the disease burden. Although no tsetse fly has been reported in Brazzaville since the 1990s few HAT cases are regularly reported through passive detection in Brazzaville. In 2015 the national control program for sleeping sleekness reported eight cases through passive detection (PNLTHA 2015). Brazzaville is also vulnerable due to its close proximity (less than 3 km) with the city of Kinshasa where urban cases of HAT are regularly reported (Ebeja et al. 2003, Sumbu et al. 2009).
In the city of Pointe Noire there have been so far not many investigations conducted in the area and just few cases have been reported from the city (Maillot 1962). The city is situated close to Cabinda Angola which still has active HAT foci. With the increase of commercial exchanges with the Cabinda region there could be a possible corridor for HAT transmission. In this regard, there is a need to maintain surveillance of the area through active detection of HAT cases to avoid any resurgence of the disease from this area (Fig. 2).
Fig. 2.
Graphical representation of reported HAT cases.
Vectors (Tsetse Flies)
Of the 33 tsetse fly species described in the Glossinidae family, 11 have been reported in Congo. Species reported so far, belong to the genus Glossina and Nemorhina and include Glossina frezili, Glossina fuscipes quanzensis Pires, 1948. Glossina fuscipes fuscipes Newst., 1910, Glossina palpalis palpalis Rob. Desv., 1830, Glossina (Nemorhina) caligenea, G tabaniformis, G. fusca congolensis, G. nashi, G. schwetzi; G. palicera newsteadi, G. hamingtoni (Maillot and Ceccaldi 1956, Gouteux et al. 1986b, Gouteux 1987, Gouteux et al. 1987, Brunhes et al. 1994) http://www.fao.org/3/p5178f/P5178F06.htm; (Duteurtre and Gouteux 1986). These species have been reported in various sites across the country (Maillot 1952, Taufflieb 1965, Frézil et al. 1972).
Glossina (N.) caligenea and G. frezili, are found in the costal rainforest of Pointe noire (Gouteux and Noireau 1986, Gouteux 1987). Four species including G. f. quanzensis, G. f. fuscipes, G. p. palpalis, G. p. newsteadi, are widely distributed across the country (Maillot 1953, 1960; Maillot 1961). These species are also present in neighboring countries such as Cameroon, Central Africa Republic, Gabon, and DR Congo (Brunhes et al. 1994). Only a single study reported infection of tsetse flies in Congo by T. b. gambiense these flies were of the species G. p. palpalis, G. f. fuscipes, and G. f. quanzensis (Maillot 1956). These species are opportunistic, feed on both human and animal and always display a low infectivity (Frézil et al. 1972). Studies conducted so far indicated the absence of tsetse flies in the city of Brazzaville (Tongue et al. 2009). It is rather possible that tsetse flies distribution may have reduced during the last decades following increase deforestation taking place in most parts of the country.
Significant changes have been recorded in the composition of the Glossina fauna in Congo with many species which have become scarce or have totally disappeared with increasing deforestation and transformation of their natural habitat. G. tabaniformis which was frequent in collections conducted between 1947 and 1953 in Brazzaville (Maillot 1948) is no more present in Brazzaville (Tongue et al. 2009). It is possible that the transformation of it habitats following rapid urbanization of the city of Brazzaville deeply affected its bionomic. This species was also reported from the Plateau Bateke (Lefini foci) (Gouteux 1991).
Glossina schwetzi is a species reported for the first time in the country in 1947 (Taufflieb 1964). This species was also found in collections done in 1961 (Maillot 1961). It was mainly distributed in forest gallery and savannah of the Bouenza. Since 1964 this species has no more been reported (Taufflieb 1964).
Glossina nashi is a species reported in Mayombe and neighboring villages (Maillot 1963, Eouzan 1982). However, it seems like the species is no more present in Congo since repeated samplings conducted in its type locality did not yielded any sample (Bourzat and Gouteux 1990, Gouteux 1991).
Parasites Species
Trypanosoma brucei gambiense, the only pathogen of human sleeping sickness in Congo, was recorded in both human and glossina and was frequent in most sites. Four other Trypanosoma species have been reported in Congo. These include T. congolense, T. vivax, T. simiae, and T. grayi, however they do not cause disease in humans, rather animal African trypanosomiasis (Frézil et al. 1972, Scott et al. 1983, Noireau et al. 1991) (Table 2).
Table 2.
Summary of studies reporting the presence of Glossina species and Trypanosoma species in Congo since the 1950s
| Foci | Sites | Vector species | Year | Species of trypanosoma | Hostsa | References |
|---|---|---|---|---|---|---|
| Corridor hotspot | Brazzaville | G. fuscipes quanzensis | 1953 | T. gambiense | Tsetse flies | (Maillot 1953) |
| Brazzaville | G. fuscipes quanzensis | 1956 | T. brucei gambiense | Tsetse flies | (Maillot and Ceccaldi 1956) | |
| Brazzaville and surroundings | G. fuscipes quanzensis | 1972 |
T.brucei gambiense,
T. grayi, T.simiae |
Tsetse flies, pig | (Frézil et al. 1972) | |
| Kaba Ngomba, Kounzoulou Lipilli and Edouani | G. fuscipes quanzensis | 1981 |
T. congolense,
T. brucei, T. brucei gambiense |
Domestic animals, human | (Lancien et al. 1981) | |
| corridor | — | 1983 | T. brucei gambiense | Sheep | (Scott et al. 1983) | |
| Corridor and Pool | G. fuscipes quanzensis | 1989 | T. brucei gambiense | Sheep, pig | (Noireau et al. 1989) | |
| Brazzaville and Kounzoulou | — | 1991 | T. brucei gambiense | Pig | (Truc et al. 1991) | |
| Savannah hotspot | Loudima | G. palpalis palpalis | 1969 |
T. brucei gambiense,
T. congolense |
Tsetse flies, human | (Adam and Challier 1969) |
| Loudima | G. palpalis palpalis | 1971 |
T. brucei gambiense,
T. vivax, T. grayi |
Tsetse flies | (Adam and Le Pont 1971) | |
| Kingouala-Nsouadi, Kinzaba and Makondo Mabengue | G. palpalis palpalis | 1981 |
T. congolense,
T. brucei, T. brucei gambiense |
Domestic animals, human | (Lancien et al. 1981) | |
| Niari | 1983 | T. brucei gambiense | Sheep | (Scott et al. 1983) | ||
| Bouenza | G. palpalis palpalis | 1989 | T. brucei gambiense | Sheep, pig | (Noireau et al. 1989) | |
| Kimbedi, Kitoundou | G. palpalis palpalis | 1990 | T. congolense | Tsetse flies, sheep, pig, goat | (Noireau et al. 1990) | |
| Savannah hotspot | Kimbedi | — | 1991 | T. congolense | Domestic animals | (Noireau et al. 1991) |
| Kinzaba, Comba and Mbinda | — | 1991 | T. brucei gambiense | Pig | (Truc et al. 1991) | |
| Forest hotspot | Mbomo | G.tabaniformis, G. fuscipes fuscipes | 1981 |
T. congolense,
T. brucei, T. vivax, T. grayi |
Tsetse flies | (Frezil et al. 1981) |
| Makoua | — | 1983 | T. brucei gambiense | Sheep | (Scott et al. 1983) | |
| Mandigo-Kayes | G. caliginea | 1986 | — | — | (Gouteux et al. 1987) | |
| Mandigo-Kayes |
G.tabaniformis,
G. fusca congolensis, G. frezili |
1987 | — | — | (Gouteux 1987) | |
| Sangha | G. fuscipes fuscipes | 1989 | T. brucei gambiense | Sheep, pig | (Noireau et al. 1989) | |
| Mayombe | G. palpalis palpalis | 1990 |
T. congolense (forest), T. grayi, T. vivax and indeterminate species |
Tsetse flies | (Bourzat and Gouteux 1990) | |
| Makoua | — | 1991 | T. brucei gambiense | Pig | (Truc et al. 1991) |
a Refer to the host on which the Trypanosoma was isolated.
Disease Diagnostics
Active screening of the population is recommended by WHO for the control of HAT (WHO Expert Committee on the Control 1998). Several screening techniques are used for trypanosomes detection in different biological fluids (e.g., blood, cerebrospinal fluid, saliva). In past campaigns diagnosis of trypanosomiasis relied on the examination of lymph node or blood (fresh and/or stained) using a microscope. From the 1970s ‘the search for immunoglobulin M (IgM)’ was added to the diagnostic of HAT in the Republic of Congo (Mattern et al. 1961). This technique was tested in 1972 in Loudima, Mpouya, Kinzaba, and Kimongo localities, but showed margin error of at least 10% and was rapidly abandoned in favor of indirect immunofluorescence (IFI) (Frezil 1983). IFI was used for several years for HAT detection (Frézil and Coulm 1977, Gouteux et al. 1988). Despite the fact that it has a high sensitivity, it was found difficult to apply on the field for mass screening because it required qualified personnel (Frezil 1983). Following the development of the CATT (Card Agglutination Test for Trypanosomiasis) by Magnus et al. (Magnus et al. 1978) this tool became the main diagnostic tool used for mass screening of HAT across Africa. The Republic of Congo adopted this technique in the 1980s and it is still used to date in active screening campaigns (Noireau et al. 1991b, PNLTHA 2019). Combination of IFI-CATT was also tested on the field and gave good results. During the last decades several novel and improved techniques were developed for active detection of HAT cases this includes techniques such as light emitting diode fluorescence microscopy (Njiru et al. 2008). The Loop Mediated Isothermal Amplification (LAMP) technique and individual rapid diagnostic tests (RDTs) (Njiru et al. 2008, Büscher et al. 2013, Mitashi et al. 2015). The national sleeping sickness program of Congo started using Rapid Diagnostic Test (HAT sero-K-set test) for the diagnosis of HAT in 2015 (PNLTHA 2015). Yet this tool has not replaced CATT but both are used on the field because of their high sensitivity and specificity (Boelaert et al. 2018). RDT tests particularly HAT sero-K-set test were found to present a sensitivity of 98.5% and a specificity of 98.6% for T. b. gambiense antibody detection (Büscher et al. 2014). The use of RDT is also limited by frequent power supply shortages in most rural settings affecting the conservation of RDT. Although CATT could display a sensitivity of up to 98% for the detection of primary infection cases, it is not reliable to monitor treatment outcome (Lejon et al. 2010). The LAMP (loop mediated isothermal amplification) technique has also been tested in some field sites (PNLTHA 2017).
Management of HAT Cases
Over years, the treatment of sleeping sickness has evolved considerably. Up to the 2000s, patients with sleeping sickness were treated with pentamidine (for stage I patients) and melarsoprol (for stage II patients). Unfortunately, melarsoprol was highly toxic, causing several deaths (Ginoux et al. 1984). In 2000 Congo adopted the use of eflornithine a new compound, more effective and less toxic to replace melarsoprol, (Eozenou et al. 1989, Balasegaram et al. 2006, Balasegaram et al. 2009). Eflornithine was used for stage I and II patients but the nonrespect of treatment protocol by medical personnel particularly for both old and new cases led to an increase in relapse and adverse events (Pépin et al. 2000, Priotto et al. 2007, Priotto et al. 2009, Jansson-Löfmark et al. 2015). The recent introduction of NECT (Nifurtimox–eflornithine combination therapy) in 2007 significantly improved sleeping sickness treatment in Congo. NECT was found to be more efficient for the treatment of patients with stage II trypanosomiasis and was found to limit the emergence of resistant strains (Priotto et al. 2007, Priotto et al. 2009, PNLTHA 2015, 2017).
Vector Control
Vector control is considered as a key component of sleeping sickness control. In several epidemiological foci across Africa it was proven that the implementation of vector control measures alongside medical interventions (diagnostic and treatment) contributed significantly to the reduction of the incidence of HAT cases (Solano and Torr 2013, Courtin et al. 2015, Tirados et al. 2015, Mahamat et al. 2017). Due to the fact that tsetse females produce a limited number of progeny, targets of tsetse flies have been practiced in most epidemiological foci to reduce population densities (Solano and Torr 2013). In Congo, the spraying of sites with 5% DDT was tried in Loudima between 1969 and 1971, unfortunately it was a failure due to the rapid recolonisation of the sites (Adam and Challier 1969, Adam et al. 1969, Adam and Le Pont 1971). Yet with the development of traps there were several trials conducted in some endemic gHAT foci to target and control Tsetse flies. First attempts started with the biconical trap impregnated with insecticide (DDT) (Challier et al. 1977), used during the seventies and eighties to control tsetse flies (Lancien et al. 1981, Gouteux and Noireau 1986). However, these traps were found less efficient for targeting tsetse flies and were replaced (Gouteux et al. 1986a). From 1986 the pyramidal trap was introduced and was found to be more effective in targeting tsetse flies compare to biconical trap (Gouteux et al. 1986b). Although there have been considerable advances with the development of new cost-effective tool such as the tiny target which is more effective in the control of riverine tsetse flies particularly G. fuscipes and G. palpalis species (Esterhuizen et al. 2011, Rayaisse et al. 2011), there have been so far no assessment of it suitability for controlling tsetse flies in Congo. In addition to the fact that the traps are very small easily transportable on the field they also cost much less. The adoption of these traps by the national sleeping sleekness program of Congo might improve disease control in hotspot areas as it was the case in other epidemiological setting with HAT burden (Shaw et al. 2015, Tirados et al. 2015, Lehane et al. 2016, Mahamat et al. 2017).
Conclusion
The study provided an overview of studies conducted so far in the Republic of Congo on vector and trypanosome species distribution, case management, disease prevalence, and control measures. Although surveillance activities conducted by the national sleeping sickness programme suggested significant reduction of HAT cases in Congo, much still need to be done to achieve the elimination of HAT as a public health problem (Tirados 2015, #171). There have been since the 2000 not many research and surveillance activities on the vectors and trypanosomes as a result, it is not clear whether there are additional active foci inland contributing to HAT transmission and requiring further attention. Surveillance activities conducted by the national sleeping sickness programme are conducted once yearly and in a limited number of villages (PNLTHA 2019, #64). All these could limit the accuracy of findings resulting from these surveillance activities.
The limited number of research work conducted by Congolese teams on HAT point the need for strengthening capacities of human resources involved in the fight against HAT in Congo. The fact that Congo is situated close to the Democratic Republic of Congo and the Central Africa Republic where HAT is still highly prevalent (Büscher 2017, #26) could increase the importation of new cases from these affected areas. The following highlights the need for strict surveillance activities along the borders and also in inland settings (Bottieau 2019, #35).
Furthermore, due to the political instability in the central Africa sub-region which is always exposed to civil wars or political or social crises (Franco et al. 2018), it is possible that massive population migration resulting from these crises could influence the dynamic of HAT in Congo and in the subregion as it was the case, in 2015, for the Nola foci in the Central African Republic and a deep attention need to be put on these risk factors which could hinder elimination efforts in Congo and the subregion (Büscher 2017, #26). HAT is a neglected tropical disease which now attracts less funding it is important in this regards that more advocacy and communication be undertaken to have an active participation of all stakeholders and also that the available resources are well managed for improved surveillance of the disease (Büscher 2017, #26).
Several challenges affecting both the treatment, diagnosis, operational implementation, and vector control interventions have also been highlighted in the review and deserve further attention in the context of HAT elimination in Congo and Africa (Bottieau 2019, #35). For instance HAT treatment are not readily available in rural health care centres and patient need to travel to main regional health facilities to receive treatments. New compounds such as fexinidazole still await approval for their use in the country (Organization 2019). Although different diagnostic tools such as LAMP, RDT, and CATT are now available for HAT detection and have improved the diagnosis and treatment of the disease in Congo there are not readily available in all rural health care centre. As the number of cases is reducing it will be important in the course of elimination, to also use sensitive techniques such as molecular technique (PCR) to confirm total clearance of parasites in patients under treatment. The supply chain also proved not to be robust and needs to be reinforced. Regular screening of the population living close to endemic foci need also to be considered (Bottieau 2019, #35).
As Congo now approach elimination targets it is becoming urgent that new strategies including active detection, monitoring, reporting, and vector control intervention be implemented to achieve HAT elimination. Particular emphasis has to be put in reducing tsetse flies densities as these flies could infect on wild animals and transmit pathogens to the population (Simo et al. 2015, #172, Bottieau 2019, #35). Surveillance of the animal reservoir and land use changes which could affect tsetse flies distribution and transmission pattern also need careful consideration to ensure sustainable elimination of HAT in Congo (Bottieau 2019, #35).
Supplementary Material
Acknowledgments
This work received financial support from the OCEAC MTN KFW program. The funding body did not have any role in the design, collection of data, analysis and interpretation of data and in drafting of the manuscript.
Data Availability
The datasets supporting the findings of this paper are included in this paper.
References Cited
- Adam, J., and Challier A.. . 1969. Etude de la transmission de la maladie du sommeil dans le foyer resurgent de Loudima. Organisation d’une compagne de lutte contre les glossines (Mai-Aoiit 1969). Rapport ORSTOM Centre de Brazzaville. [Google Scholar]
- Adam, J.-P., Challier A., and Le Pont. F.. 1969. Etude de la transmission de la maladie du sommeil dans le foyer résurgent de Loudima, district de Loudima, région du Pool, en organisation d’une campagne de lutte contre les glossines (mai-août 1969): République du Congo. [Google Scholar]
- Adam, J. P., and Le Pont F.. . 1971. Contrôle entomologique des résultats des campagnes antiglossines à Loudima, pp. 1–12. ORSTOM, Brazzaville. [Google Scholar]
- Balasegaram, M., Harris S., Checchi F., Ghorashian S., Hamel C., and Karunakara U.. . 2006. Melarsoprol versus eflornithine for treating late-stage Gambian trypanosomiasis in the Republic of the Congo.Bull. World Health Org. 84: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasegaram, M., Young H., Chappuis F., Priotto G., Raguenaud M. E., and Checchi F.. . 2009. Effectiveness of melarsoprol and eflornithine as first-line regimens for gambiense sleeping sickness in nine Médecins Sans Frontières programmes. Trans. R. Soc. Trop. Med. Hyg. 103: 280–290. [DOI] [PubMed] [Google Scholar]
- Boelaert, M., Mukendi D., Bottieau E., Kalo Lilo J. R., Verdonck K., Minikulu L., Barbé B., Gillet P., Yansouni C. P., Chappuis F., . et al. 2018. A phase III diagnostic accuracy study of a rapid diagnostic test for diagnosis of second-stage human African trypanosomiasis in the Democratic Republic of the Congo. Ebiomedicine 27: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottieau, E., and Clerinx. J.. 2019. Human African trypanosomiasis: progress and stagnation. Infect. Dis. Clin. 33: 61–77. [DOI] [PubMed] [Google Scholar]
- Bourzat, D., and Gouteux J.-P.. . 1990. Données préliminaires sur le contact glossines-petits ruminants dans le massif forestier du Mayombe. Revue Élev. Méd. vét. Pays Trop. 43: 199–206. [Google Scholar]
- Brunhes, J., Cuisance D., Geoffroy B., Hervy J.-P., and Lebbe J.. . 1994. Logiciel d’identification Glossine Expert. Manuel illustré d’utilisation. Les glossines ou mouches tsé-tsé. ORSTOM, Paris, France. [Google Scholar]
- Büscher, P., Gilleman Q., and Lejon V.. . 2013. Rapid diagnostic test for sleeping sickness. N. Engl. J. Med. 368: 1069–1070. [DOI] [PubMed] [Google Scholar]
- Büscher, P., Mertens P., Leclipteux T., Gilleman Q., Jacquet D., Mumba-Ngoyi D., Pyana P. P., Boelaert M., and Lejon V.. . 2014. Sensitivity and specificity of HAT Sero-K-SeT, a rapid diagnostic test for serodiagnosis of sleeping sickness caused by Trypanosoma brucei gambiense: a case-control study. Lancet. Glob. Health 2: e359–e363. [DOI] [PubMed] [Google Scholar]
- Büscher, P., Cecchi G., Jamonneau V., and Priotto G.. . 2017. Human African trypanosomiasis. Lancet 390: 2397–2409. [DOI] [PubMed] [Google Scholar]
- Büscher, P., Bart J.-M., Boelaert M., Bucheton B., Cecchi G., Chitnis N., Courtin D., Figueiredo L. M., Franco J.-R., and Grébaut P.. . 2018. Do cryptic reservoirs threaten gambiense-sleeping sickness elimination? Trends Parasitol. 34: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier, A., Eyraud M., Lafaye A., and Laveissière C.. . 1977. Amélioration du rendement du piège biconique pour glossines (Diptera, Glossinidae) par l’emploi d’un cône inférieur bleu. [Google Scholar]
- Courtin, F., Camara M., Rayaisse J. B., Kagbadouno M., Dama E., Camara O., Traoré I. S., Rouamba J., Peylhard M., Somda M. B., . et al. 2015. Reducing human-tsetse contact significantly enhances the efficacy of sleeping sickness active screening campaigns: a promising result in the context of elimination. PLoS Negl. Trop. Dis. 9: e0003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duteurtre, J., and Gouteux J.-P.. . 1986. Les stratégies de lutte antisommeilleuse en République populaire du Congo: recherches et perspectives. Médecine Tropicale 46: 375–380. [PubMed] [Google Scholar]
- Duvallet, G., and JF V.. . 1979. Le foyer de trypanosomiase humaine de Vavona (Republique de Cote d’Ivoire): Enquête clinique, parasitologique et seroimmunologique. [PubMed] [Google Scholar]
- Ebeja, A. K., Lutumba P., Molisho D., Kegels G., Miaka mia Bilenge C., & Boelaert M.. . 2003. La maladie de sommeil dans la region Ville de Kinshasa: une analyse retrospective des donnees de surveillance sur la periode 1996–2000. Trop. Med. Int. Health 8: 949–955. [DOI] [PubMed] [Google Scholar]
- Eouzan, J.-P. 1982. Enquête «glossines» à Dimonika. Rapport de tournée. Archives du Centre ORSTOM de Brazzaville, Brazzaville. [Google Scholar]
- Eozenou, P., Jannin J., Ngampo S., Carme B., Tell G., and Schechter P.. . 1989. A trial treatment with eflornithine of trypanosomiasis caused by Trypanosoma brucei gambiense in the Peoples Republic of the Congo. Medecine tropicale: revue du Corps de sante colonial 49: 149–154. [PubMed] [Google Scholar]
- Esterhuizen, J., Rayaisse J. B., Tirados I., Mpiana S., Solano P., Vale G. A., Lehane M. J., and Torr S. J.. . 2011. Improving the cost-effectiveness of visual devices for the control of riverine tsetse flies, the major vectors of human African trypanosomiasis. PLoS Negl. Trop. Dis. 5: e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, J. R., Cecchi G., Priotto G., Paone M., Diarra A., Grout L., Simarro P. P., Zhao W., and Argaw D.. . 2018. Monitoring the elimination of human African trypanosomiasis: update to 2016. PLoS Negl. Trop. Dis. 12: e0006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, J. R., Cecchi G., Priotto G., Paone M., Diarra A., Grout L., Simarro P. P., Zhao W., and Argaw D.. . 2020. Monitoring the elimination of human African trypanosomiasis at continental and country level: update to 2018. PLoS Negl. Trop. Dis. 14: e0008261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezil, J. 1983. Human trypanosomiasis in the Congo. Human trypanosomiasis in the Congo. ORSTOM, Bondy, France. [Google Scholar]
- Frézil, J.-L., and Coulm J.. . 1977. Conception actuelle de la stratégie antisommeilleuse en République Populaire du Congo, pp. 315–322. InAnnales de la Société Belge de Médecine Tropicale . [PubMed] [Google Scholar]
- Frezil, J. L., and Coulm J.. . 1979. Study on human African trypanosomiasis in the new focus of Mantsoumba. Med. Afr. Noire 26: 41–16. [Google Scholar]
- Frézil, J.-L., Adam J.-P., and Le Pont F.. . 1972. Les glossines de l'agglomération brazzavilloise: situation actuelle (1970-1972). ORSTOM, Bondy, France. [Google Scholar]
- Frézil, J.-L., Coulm J., and Molouba R.. . 1975. Evolution et situation actuelle de la maladie du sommeil dans les foyers historiques de la République Populaire du Congo - fdi:07531 – Horizon. ORSTOM, Bondy, France. [Google Scholar]
- Frezil, J. L., Lancien J., and Carnevale P.. . 1977. Quelques aspects de I’epidémiologie de la Trypanosomiase Humaine en République Populaire du Congo. In ISCTRC/OUA-15th Meeting, Banjul. ORSTOM, Brazzaville, Congo.
- Frézil, J. L., Samba F., and Louembet M. T.. . 1979. Etude du comportement de Trypanosoma brucei gambiense sur petits rongeurs et lémuriens du Congo. Cah. ORSTOM Sér. Entomol. Méd. Parasitol. 12: 119–126. [Google Scholar]
- Frezil, J. L., Eouzan J. P., Alary J. C., Malonga J. R. et., and Ginoux P. Y.. . 1980. Épidémiologie de la Trypanosomiase humaine en République Populaire du Congo. II - Le foyer du Niari. Rapp. final 13e Conf. Techn. OCEAC, Yaoundé. 117–146. [Google Scholar]
- Frezil, J.-L., Lancien J., Yebakima A., Éouzan J.-P., Ginoux P.-Y., and Malonga J.-R.. . 1981. Epidemiologie de la trypanosomiase humaine en Republique populaire du Congo. III. le foyer de Mbomo. Cah. ORSTOM., ser. Ent. mèd. et Parasitol. XIX: 187–195. [Google Scholar]
- Gouteux, J.-P. 1987. Une nouvelle glossine du Congo: Glossina (Austenina) frezili sp. nov. (Diptera: Glossinidae). Trop. Med. Parasitol. 38: 97–100. [PubMed] [Google Scholar]
- Gouteux, J.-P. 1991. La raréfaction de tsé-tsé du groupe fusca en Afrique centrale (Diptera, Glossinidae). Bulletin de la Société entomologique de France. 96: 443–449. [Google Scholar]
- Gouteux, J. P., and Noireau F.. . 1986. Un nouvel écran-piège pour la lutte anti-tsétsé: description et essais dans un foyer congolais de trypanosomiase humaine. Entomologia experimentalis et applicata. 41: 291–297. [Google Scholar]
- Gouteux, J. P., and Sinda D.. . 1990. Community participation in the control of tsetse flies. Large scale trials using the pyramid trap in the Congo. Trop. Med. Parasitol. 41: 49–55. [PubMed] [Google Scholar]
- Ginoux, P., Bissadidi N., and Frezil J.. . 1984. Complications observed in the treatment of trypanosomiasis in the Congo. Medecine tropicale: revue du Corps de sante colonial. 44: 351. [PubMed] [Google Scholar]
- Gouteux, J., Lancien J., Noireau F., and Sinda D.. . 1986a. Control of vectors by trapping and its impact on sleeping sickness in a zone of high density of Glossina fuscipes quanzensis (Lefini River, People’s Republic of the Congo). Trop. Med. Parasitol.: Official Organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ). 37: 101–104. [PubMed] [Google Scholar]
- Gouteux, J., Nkouka E., Noireau F., Frézil J.-L., and Sinda D.. . 1986b. Les glossines de l’agglomération brazzavilloise Congo. I. Répartition et importance des gîtes. Revue d’élevage et de médecine vétérinaire des pays tropicaux. 39: 355–362. [PubMed] [Google Scholar]
- Gouteux, J.-P., Frézil J.-L., Louembet M.-T., and Noireau F.. . 1987. Présence en République Populaire du Congo, de Glossina medicorum Austen, 1911 et G. caliginea Austen, 1911 (Diptera: Muscidae), C. R. Acad. SC. 304: 25. [Google Scholar]
- Gouteux, J.-P., Noireau F., Malonga J.-R., and Frézil J.-L.. . 1988. «Effet de case» et «contamination familiale» dans la maladie du sommeil: essai d’interprétation du phénomène-Exemple de trois foyers congolais. Annales de parasitologie humaine et comparée 63: 315–333. [DOI] [PubMed] [Google Scholar]
- Gouteux, J. P., Toudic A., and Sinda D.. . 1988. The use of sentinel animals for the evaluation of the control of vectors of sleeping sickness: preliminary reports at a Congolese site. Acta Trop. 45: 331–338. https://apps.who.int/iris/bitstream/handle/10665/326178/9789241550567-eng.pdf. 2019. [PubMed] [Google Scholar]
- Jamot, E. 1933. Contribution à l’étude de la maladie du sommeil en Afrique Occidentale Française (Ouagadougou). Doc Tech OCCGE; 492. [Google Scholar]
- Jannin, J., Eozenou P., Ngampos C. J., and Beuzit Y.. . 1990. La place de la DFMO dans le traitement de la THA: l’expérience congolaise. Bull. filiais doc OCEAC. 93: 27–30. [Google Scholar]
- Jansson-Löfmark, R., Na-Bangchang K., Björkman S., Doua F., and Ashton M.. . 2015. Enantiospecific reassessment of the pharmacokinetics and pharmacodynamics of oral eflornithine against late-stage Trypanosoma brucei gambiense sleeping sickness. Antimicrob. Agents Chemother. 59: 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancien, J., Eouzan J.-P., Frezil J.-L., and Mouchet J.. . 1981. Elimination des glossines par piégeage dans deux foyers de trypanosomiase en République Populaire du Congo. ORSTOM, Brazzaville, Congo. XIX(4): 239–246. [Google Scholar]
- Lankoande, S., and Ouedele M.. . 1982. La trypanosomiase humaine dans le foyer de la Volta-Noire (Dédougou-Boromo) Haute-Volta. Médecine d’Afrique noire. 29: 157–161. [Google Scholar]
- Lehane, M., Alfaroukh I., Bucheton B., Camara M., Harris A., Kaba D., Lumbala C., Peka M., Rayaisse J. B., Waiswa C., . et al. 2016. Tsetse control and the elimination of Gambian sleeping sickness. PLoS Negl. Trop. Dis. 10: e0004437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejon, V., Ngoyi D. M., Boelaert M., and Büscher P.. . 2010. A CATT negative result after treatment for human African trypanosomiasis is no indication for cure. PLoS Negl. Trop. Dis. 4: e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus, E., Vervoort T., and Van Meirvenne N.. . 1978. A card-agglutination test with stained trypanosomes (CATT) for the serological diagnosis of T. b. gambiense tripanosomiasis, pp. 169–176. InAnnales de la Société belge de Médecine Tropicale. Societe Belge de Medecine Tropicale, Belgium. [PubMed] [Google Scholar]
- Mahamat, M. H., Peka M., Rayaisse J. B., Rock K. S., Toko M. A., Darnas J., Brahim G. M., Alkatib A. B., Yoni W., Tirados I., . et al. 2017. Adding tsetse control to medical activities contributes to decreasing transmission of sleeping sickness in the Mandoul focus (Chad). PLoS Negl. Trop. Dis. 11: e0005792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillot, l. 1948. Enquêtes entomologiques relatives aux glossines de l'AEF pendant l'année 1948. ORSTOM, Bondy, France. [Google Scholar]
- Maillot, L. 1952. Présence de glossines au Ravin de la Glacière. pp. 3. [Google Scholar]
- Maillot, L. 1953. Les variétés de Glossina palpalis en Afrique Equatoriale Française. Bulletin de la Société de Pathologie Exotique. 46: 1066–1080. [PubMed] [Google Scholar]
- Maillot, L. 1956. Carte de répartition des glossines dans les Etats de l'ancienne fédération d'Afrique Equatoriale Française ORSTOM, Bondy, France. [Google Scholar]
- Maillot, L. 1960. Répartition des glossines dans les Etats de l’ancienne fédération d’Afrique Equatoriale Française. ORSTOM, Bondy, France. [Google Scholar]
- Maillot, L. 1961. Glossines d’Afrique Centrale. 2. Espèces rares ou peu répandues, mais pouvant jouer un rôle comme vecteur. Revue d’élevage et de médecine vétérinaire des pays tropicaux. 14: 439–443. [Google Scholar]
- Maillot, L. 1962. Notice pour la carte chronologique des principaux foyers de la maladie du sommeil dans les etats de l’ancienne federation d’Afrique equatoriale française. Bulletin de l’Institut de Recherches Scientifiques au Congo. 1: 45–54. [Google Scholar]
- Maillot, L. 1963. Glossines d’Afrique Centrale. IV Groupe Fusca: espèces rares (fin). Revue d’élevage et de médecine vétérinaire des pays tropicaux. 16: 419–425. [Google Scholar]
- Maillot, L., and Ceccaldi J.. . 1956. Enquête sur les glossines dans la vallée du M’Filou au niveau de Gamaba à proximité de Brazzaville (janvier-avril 1956). Bulletin-Institut d’Etudes Centrafricaines. 201–208. [Google Scholar]
- Mattern, P., Masseyeff R., Michel R., and Peretti P.. . 1961. Immunochemical studies on the B2-macroglobuIins in sera of patients suffering from Trypanosoma gambiense infections. Ann. Inst. Pasteur. 101: 382–388. [Google Scholar]
- Mitashi, P., Hasker E., Mbo F., Van Geertruyden J. P., Kaswa M., Lumbala C., Boelaert M., and Lutumba P.. . 2015. Integration of diagnosis and treatment of sleeping sickness in primary healthcare facilities in the Democratic Republic of the Congo. Trop. Med. Int. Health. 20: 98–105. [DOI] [PubMed] [Google Scholar]
- Muraz, G. 1943. Lutte contre la maladie du sommeil en AOF et au Togo. Académie des Sciences Coloniales. 8: 593–622. [Google Scholar]
- Mwanakasale, V., and Songolo P.. . 2011. Disappearance of some human African trypanosomiasis transmission foci in Zambia in the absence of a tsetse fly and trypanosomiasis control program over a period of forty years. Trans. R. Soc. Trop. Med. Hyg. 105: 167–172. [DOI] [PubMed] [Google Scholar]
- Njiru, Z. K., Mikosza A. S., Armstrong T., Enyaru J. C., Ndung’u J. M., and Thompson A. R.. . 2008. Loop-mediated isothermal amplification (LAMP) method for rapid detection of Trypanosoma brucei rhodesiense. PLoS Negl. Trop. Dis. 2: e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noireau, F., Osseke F. O., and Gouteux J. P.. . 1990. Impact immediat d'une lutte antivectorielle par piégeage sur l'enzootie de trypanosomose au Sud-Congo. Revue d’élevage et de médecine vétérinaire des pays tropicaux 43: 93–96. [Google Scholar]
- Noireau, F., Lemesre J.-L., and Vervoort T.. . 1991. Absence of serological markers of infection with Trypanosoma brucei gambiense in domestic animals in a sleeping sickness focus in South Congo. Trop. Med. Parasitol. 42: 196–196. [PubMed] [Google Scholar]
- Noireau, F., Force-Barge P., and Cattand P.. . 1991b. Evaluation of Testryp CATT applied to samples of dried blood for the diagnosis of sleeping sickness. Bull. World Health Org. 69: 603. [PMC free article] [PubMed] [Google Scholar]
- Noireau, F., Paindavoine P., Lemesre J. L., Toudic A., Pays E., Gouteux J. P., ... and Frézil J. L.. . 1989. The epidemiological importance of the animal reservoir of Trypanosoma brucei gambiense in the Congo. 2. Characterization of the Trypanosoma brucei complex. Tropical Medicine and Parasitology: Official Organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ), 40: 9–11. [PubMed] [Google Scholar]
- OMS . 2018. Trypanosomiase humaine africaine (maladie du sommeil). https://www.who.int [Google Scholar]
- OMS . 2019. Trypanosomiase humaine africaine (maladie du sommeil). https://www.who.int [Google Scholar]
- Organization, W. H. 2019. WHO interim guidelines for the treatment of gambiense human African trypanosomiasis. World Health Organization, Geneve, Switzerland. [PubMed] [Google Scholar]
- Pépin, J., Khonde N., Maiso F., Doua F., Jaffar S., Ngampo S., Mpia B., Mbulamberi D., and Kuzoe F.. . 2000. Short-course eflornithine in Gambian trypanosomiasis: a multicentre randomized controlled trial. Bull. World Health Organ. 78: 1284–1295. [PMC free article] [PubMed] [Google Scholar]
- PNLTHA. 2015. Rapport annuel d’activités. Programme National de Lutte contre la Trypanosomiase Humaine Africaine. Minsanté, Brazzaville, Congo. [Google Scholar]
- PNLTHA. 2017. Rapport annuel sur la maladie du sommeil. Programme National de Lutte contre la Trypanosomiase Humaine Africaine. Minsanté, Brazzaville, Congo. [Google Scholar]
- PNLTHA. 2019. Rapport annuel sur la maladie du sommeil. Programme National de Lutte contre la Trypanosomiase Humaine Africaine. Minsanté, Brazzaville, Congo. [Google Scholar]
- Priotto, G., Kasparian S., Ngouama D., Ghorashian S., Arnold U., Ghabri S., and Karunakara U.. . 2007. Nifurtimox-eflornithine combination therapy for second-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Congo. Clin. Infect. Dis. 45: 1435–1442. [DOI] [PubMed] [Google Scholar]
- Priotto, G., Kasparian S., Mutombo W., Ngouama D., Ghorashian S., Arnold U., Ghabri S., Baudin E., Buard V., Kazadi-Kyanza S., . et al. 2009. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet. 374: 56–64. [DOI] [PubMed] [Google Scholar]
- Rayaisse, J. B., Esterhuizen J., Tirados I., Kaba D., Salou E., Diarrassouba A., Vale G. A., Lehane M. J., Torr S. J., and Solano P.. . 2011. Towards an optimal design of target for tsetse control: comparisons of novel targets for the control of Palpalis group tsetse in West Africa. PLoS Negl. Trop. Dis. 5: e1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, R. S., Kruska R. L., Deichmann U., Thornton P. K., and Leak. S. G.. 2000. Human population growth and the extinction of the tsetse fly. Agric. Ecosyst. Environ. 77: 227–236. [Google Scholar]
- Richet, P. 1962. La trypanosomiase résiduelle. OMS, comité des experts en trypanosomiase, Genève. [Google Scholar]
- Saliou, P., and Challier A.. . 1976. Compte-rendu de mission dans le foyer de maladie du sommeil de Bouafle (Côte d'Ivoire): étude de la situation de l'endémie et propositions d'un programme de lutte. ORSTOM, Bondy, France. [Google Scholar]
- Scott, C. M., Frézil J. L., Toudic A., and Godfrey D. G.. . 1983. The sheep as a potential reservoir of human trypanosomiasis in the Republic of the Congo. Trans. R. Soc. Trop. Med. Hyg. 77: 397–401. [DOI] [PubMed] [Google Scholar]
- Shaw, A. P., Tirados I., Mangwiro C. T., Esterhuizen J., Lehane M. J., Torr S. J., and Kovacic V.. . 2015. Costs of using “tiny targets” to control Glossina fuscipes fuscipes, a vector of gambiense sleeping sickness in Arua District of Uganda. PLoS Negl. Trop. Dis. 9: e0003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo, G., Fongho P., Farikou O., Ndjeuto-Tchouli P. I. N., Tchouomene-Labou J., Njiokou F., and Asonganyi T.. . 2015. Trypanosome infection rates in tsetse flies in the “silent” sleeping sickness focus of Bafia in the Centre Region in Cameroon. Parasit. Vectors 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano, P., Kone A., Garcia A., Sane B., Michel V., Michel J., Coulibaly B., Jamonneau V., Kaba D., and Dupont S.. . 2003. Representation spatiale des deplacements des malades dans un foyer de trypanosomose humaine africaine de Cote d’Ivoire. Médecine tropicale 63: 577–581. [PubMed] [Google Scholar]
- Solano, P., Torr S. J., and Lehane M. J.. . 2013. Is vector control needed to eliminate gambiense human African trypanosomiasis? Front. Cell. Infect. Microbiol. 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbu, J., De Deken R., Deckers N., Mpiana S., Kabambi P., Tshilenge G., and Boelaert. M.. 2009. Variation spatiale du risque pour les porcs de contracter la trypanosomose dans la zone périurbaine de Kinshasa. Parasite. 16: 153–159. [DOI] [PubMed] [Google Scholar]
- Taufflieb, R. 1964. Glossines et élevage dans la région du Niari. ORSTOM, Bondy, France. [Google Scholar]
- Taufflieb, R. 1965. Les glossines de l’agglomération Brazzavilloise. ORSTOM, Bondy, France. [Google Scholar]
- Tirados, I., Esterhuizen J., Kovacic V., Mangwiro T. N., Vale G. A., Hastings I., Solano P., Lehane M. J., and Torr S. J.. . 2015. Tsetse control and Gambian sleeping sickness; implications for control strategy. PLoS Negl. Trop. Dis. 9: e0003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongué, L. K., Diabakana P. M., Bitsindou P., and Louis F. J.. . 2009. Have tsetse flies disappeared from Brazzaville town? Pan Afr. Med. J. 3: 3. [PMC free article] [PubMed] [Google Scholar]
- Truc, P., Mathieu-Daudé F., and Tibayrenc M.. . 1991. Multilocus isozyme identification of Trypanosoma brucei stocks isolated in Central Africa: evidence for an animal reservoir of sleeping sickness in Congo. Acta Trop. 49: 127–135. [DOI] [PubMed] [Google Scholar]
- Wamwiri, F. N., and Changasi R. E.. . 2016. Tsetse flies (Glossina) as vectors of human African Trypanosomiasis: a review. Biomed Res. Int. 2016: 6201350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Expert Committee on the Control, S. o. A. T., World Health Organization . 1998. Control and surveillance of African Trypanosomiasis: report of a WHO Expert Committee. World Health Organization, Geneve, Switzerland. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the findings of this paper are included in this paper.