Abstract
Background
Patients with hemophilia have deficiencies in intrinsic coagulation factors and can develop inhibitors that limit the effectiveness of replacement coagulation factors. Marstacimab, a human monoclonal antibody, binds and inhibits the human tissue factor pathway inhibitor. Marstacimab is currently under development as a potential prophylactic treatment to prevent bleeding episodes in patients with hemophilia A and B.
Objective
To assess the effects of marstacimab alone or in combination with the bypassing agent recombinant factor FVIIa (rFVIIa) or activated prothrombin complex concentrate (aPCC) on thrombin generation and bleeding.
Methods
Marstacimab and/or rFVIIa or aPCC were added to hemophilic A or B plasma or nonhemophilic plasma in vitro. Hemostatic activity was measured using the thrombin generation assay. In vivo effects were assessed using a mouse acute bleeding model. Male hemophilia A mice were dosed with marstacimab plus aPCC before tail clip; blood loss was quantified by measuring hemoglobin.
Results
Marstacimab plus rFVIIa or aPCC slightly increased peak thrombin levels compared with either agent alone. This increase was within the reported range for nonhemophilic plasma and did not exceed levels observed in nonhemophilic plasma treated with marstacimab alone. Hemophilia A mice that received 200 U/kg aPCC had significantly reduced bleeding (62%) compared with vehicle‐treated mice (p < 0.05), and marstacimab plus aPCC reduced bleeding by 83.3% compared with vehicle (p= 0.0009).
Conclusions
Marstacimab alone or with bypassing agents increased hemostasis in hemophilia plasma without generating excessive thrombin. The hemostatic activity of marstacimab plus aPCC was confirmed in hemophilia A mice.
Keywords: blood coagulation, hemophilia A, hemophilia B, lipoprotein‐associated coagulation inhibitor, tissue factor pathway inhibitor
Essentials.
Marstacimab is a human monoclonal antibody targeting tissue factor pathway inhibitor (TFPI).
Effects of marstacimab and bypassing agents on thrombin generation were tested in vitro.
Marstacimab plus a bypassing agent increased hemostasis without inducing hypercoagulation.
Using a mouse injury model, marstacimab and a bypassing agent reduced bleeding.
1. INTRODUCTION
Hemophilia A and B are hereditary bleeding disorders characterized by deficiencies in factor VIII (FVIII) or factor IX (FIX), respectively, of the intrinsic coagulation pathway. 1 , 2 Reduced activity of these intrinsic factors leads to inadequate generation of thrombin for the conversion of fibrinogen to fibrin and the development of a stable clot. 3 Hemophilia has been primarily managed by replacing deficient clotting factors using recombinant protein‐ or plasma‐derived products. 1 New nonfactor agents, such as emicizumab, were recently approved for hemophilia A. 1 , 4 However, neutralizing antibodies, or inhibitors, develop in approximately 20%–30% of patients with severe hemophilia A who are receiving replacement clotting factors, with inhibitor development more rare in patients with severe hemophilia B. 2 , 5 , 6 Two commonly used bypassing treatments for the resolution of bleeding in hemophilia patients with inhibitors are recombinant factor VIIa (rFVIIa, eptacog alfa) and activated prothrombin complex concentrate (aPCC). 1
Tissue factor pathway inhibitor (TFPI) is a Kunitz‐type serine protease inhibitor that negatively regulates thrombin generation within the extrinsic coagulation pathway. 7 TFPI is largely found in the microvascular endothelium and is stored in multiple pools, including plasma and platelets. 8 , 9 TFPI inhibits the functions of the Xa (FXa) and FVIIa/tissue factor (TF) complex. 10 , 11 Marstacimab (previously PF‐06741086) is a fully human monoclonal antibody designed to target the extrinsic tissue factor/FVIIa pathway by binding to the Kunitz‐2 domain (3.7 nM) 12 , 13 of human TFPI, thereby neutralizing its inhibitory activity. 14 By inhibiting TFPI, FXa generation is increased, even in the absence of FVIII or FIX, leading to increased thrombin production and clot formation, thus bypassing the deficiencies in the intrinsic coagulation pathway. 7 , 15 Marstacimab, a nonreplacement hemostatic agent, is in development as a potential subcutaneous prophylactic treatment to prevent bleeding episodes in patients with hemophilia A or B, with or without inhibitors. By targeting the extrinsic and common pathway, marstacimab would not be affected by inhibitors. 16 , 17
Thrombotic events have been associated with concomitant use of a bypass agent (an aPCC: FEIBA) for treatment of breakthrough bleeding episodes during prophylaxis of patients with inhibitors with the nonfactor treatment emicizumab. Nonfatal thrombotic events were also observed in two studies with anti‐TFPI monoclonal antibodies in development, resulting in either termination or interruption of the study. 18 Patients with inhibitors receiving treatment with marstacimab may concomitantly receive treatment with rFVIIa or aPCC after a breakthrough bleeding event. 19 Therefore, there is a potential concern for excessive thrombin generation with the use of marstacimab in combination with rFVIIa or aPCC. To investigate this consideration, we studied the effects of marstacimab on thrombin generation in the presence or absence of rFVIIa or aPCC in hemophilic plasma with or without inhibitors. In addition, an acute tail clip injury model in hemophilia A mice was used to evaluate the hemostatic activity of marstacimab with or without aPCC in vivo.
2. METHODS
2.1. Materials and reagents
Marstacimab was produced by Pfizer Inc. rFVIIa (eptacog alfa) was obtained from Novo Nordisk and aPCC (FEIBA) was obtained from Baxalta Inc. 20 and reconstituted according to the package insert. Citrated platelet‐poor severe hemophilia A (FVIII deficient), severe hemophilia A with an inhibitor, severe hemophilia B (FIX deficient), and human normal (nonhemophilic) pooled plasma were obtained from George King Biomedical (Overland Park, KS). Severe hemophilia B inhibitor plasma (FIX immune‐depleted with FIX inhibitory antibody) was obtained from Affinity Biologicals. All hemophilia plasma had less than 1% coagulation factor activity. 21 The thrombin generation reagents, PPP‐Reagent LOW, thrombin calibrator, and Flu‐Ca kit (comprising Fluo‐Buffer and Fluo‐Substrate containing the fluorogenic substrate solubilized in dimethyl sulfoxide) were obtained from Diagnostica Stago. The calibrated automated thrombogram (CAT), including the Fluoroskan Ascent fluorescent plate reader (Thrombinoscope BV, Maastricht, the Netherlands), and Thrombinoscope software (version 5) were also obtained from Diagnostica Stago.
2.2. Thrombin generation assays
The effect of marstacimab alone and in combination with rFVIIa or aPCC was evaluated in citrated, platelet‐poor plasma from individuals with severe hemophilia A or B, with or without inhibitors. Control reactions included (1) vehicle‐treated hemophilic plasma, (2) untreated nonhemophilic plasma, and (3) nonhemophilic plasma dosed with 16 μg/ml of marstacimab. Thrombin generation in platelet‐poor human nonhemophilic plasma was evaluated in each thrombin generation assay (total number of assays = 15). The effect of marstacimab on thrombin generation was also measured in nonhemophilic plasma, which has the full complement of coagulation factors. Marstacimab was added to the plasma samples at a concentration of 16 μg/ml to approximate the maximum plasma concentration following steady state for a 2‐mg/kg subcutaneous dose based on modeling or a single 300‐mg subcutaneous dose. 14 , 22 The thrombin generation assay is a global assay that measures multiple phases of thrombin generation, including the lag time (initiation phase), activation phase, and inactivation phase. 23 Thrombin generation was evaluated using the CAT. Buffers used in the rFVIIa procedure included 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (HEPES) buffer (pH 7.4, including 20 mM HEPES, 150 mM sodium chloride, and 1% bovine serum albumin) and phosphate buffered saline (PBS; containing 137 mM sodium chloride, 2.7 mM potassium chloride, 8.1 mM disodium phosphate, 1.47 mM monopotassium phosphate). The vehicle comprised 50% PBS/50% HEPES buffer, rFVIIa (5 μl in HEPES buffer), or HEPES buffer (5 μl) added to 70 μl of hemophilic plasma samples. A total of 5 µl of marstacimab (diluted in PBS) or PBS (5 μl) were added to the rFVIIa‐dosed hemophilic plasma samples (75 μl) and to the HEPES buffer‐treated hemophilic plasma (75 μl). In addition, 5 μl of marstacimab (at 16 μg/ml) was assayed in HEPES buffer‐treated nonhemophilic plasma (75 μl). Control untreated nonhemophilic plasma (80 μl) was included in the analysis.
Similarly, to evaluate the effect of marstacimab on thrombin generation in combination with aPCC, assays were performed either as individual treatments with increasing concentrations of aPCC (0.063, 0.125, 0.5, and 1 U/ml), marstacimab (0.5, 1, 2, 4, 8, and 16 μg/ml), and vehicle, or as combination studies where the final plasma concentrations of aPCC and marstacimab were 1 U/ml and 16 μg/ml, respectively. A concentration of 1 U/ml aPCC was used to approximate plasma levels achieved with routine aPCC dosing at 100 U/kg. For all assays, the plasma volume was 70 μl. Buffers used in this study included PBS, aPCC diluent (trisodium citrate dihydrate 13.6 mM, sodium chloride 136.9 mM with pH 7.4) and vehicle (50% PBS/50% aPCC diluent). Test reagents were added to the respective plasma as follows: (1) 10 μl of marstacimab alone or aPCC alone (in 50% PBS/50% aPCC diluent); (2) for combination studies: 5 μl of marstacimab (PBS) and 5 μl of aPCC (aPCC diluent); (3) for vehicle‐treated hemophilic control plasma (baseline): 10 μl of vehicle; (4) for control untreated nonhemophilic plasma: 10 μl of vehicle; and (5) for nonhemophilic plasma dosed with 5 μl of marstacimab and 5 μl of aPCC diluent.
For all thrombin generation assays, 20 μl of PPP‐Reagent LOW reagent (final concentration 4 μM and 1 pM TF) was added manually to the plasma and each reaction was run in duplicate. Duplicate reference‐calibrated reactions (20 μl thrombin calibrator with 80 μl of vehicle‐dosed hemophilic or nonhemophilic plasma or 80 μl of untreated nonhemophilic plasma) were run in parallel. Samples were incubated at 37°C for 5 min, and reactions were then initiated by the addition of 20 μl of FluCa buffer, containing calcium chloride and fluorogenic substrate, for a total reaction volume of 120 μl. Fluorescence of plasma reactions was read at 37°C at 20‐s intervals on a Fluoroskan Ascent fluorometer and compared with the reference thrombin calibrator reactions to determine thrombin concentrations. The intensity of the fluorescence signal was continuously monitored at 37°C using the CAT. Data generated by Thrombinoscope software was exported to Microsoft Excel (version 2010). Thrombograms (nM thrombin vs time) were plotted using Excel (rFVIIa only) or GraphPad Prism.
2.3. Acute tail clip injury in hemophilia A mice
Marstacimab binds to and inhibits TFPI activity with broad species cross‐reactivity, including mouse TFPI with a 6‐fold higher affinity than human TFPI. 12 The thrombin generation assays were conducted in platelet‐poor plasma that lacked many of the components necessary for in vivo coagulation, including platelets, which are also a source of TFPI. 17 , 24 A model of acute bleeding in hemophilia A mice (i.e., the acute tail clip injury model) was used to evaluate the combined hemostatic activity of marstacimab and aPCC in vivo. In the mouse, acute injury induces blood loss and is indicative of coagulation function. Hemophilia A mice have been used to assess hemostatic agents in vivo. The tail clip is a severe injury model in mice with hemophilia in which a small portion of the tail (3 mm) is transected, resulting in significant blood loss. Small studies have been performed to investigate the effects of rFVIIa and marstacimab in the mouse bleeding model. Studies investigating these effects remain a challenge given that high levels of human rFVIIa are needed to reduce blood loss following tail injury. 25 An approximate 50% reduction in bleeding events with the combination of marstacimab and rFVIIa has been observed (Figure S1).
Male hemophilia A mice, aged 9–27 weeks old and each weighing 20–50 g, were obtained from Charles River Laboratories (Wilmington, MA) and acclimated for at least 3 days before the experimental procedure. Each mouse received a single intravenous dose of marstacimab (0.5 mg/kg) 30 minutes before tail clip alone or in combination with various concentrations of aPCC (50, 100, and 200 U/kg), which were administered via the tail vein 5 min before tail clip. The suboptimal dose of marstacimab used in this model was based on previous studies of marstacimab in the hemophilia A mouse model, which showed little to no hemostatic effect at doses of marstacimab <1 mg/kg. 26 The doses of aPCC (50, 100, and 200 U/kg) were chosen based on a previously reported study of aPCC, where minimal blood loss was observed at 100 and 200 U/kg and at clinically used doses. 27 Control mice received PBS. Mice were anesthetized with a cocktail of ketamine 100 mg/kg and xylazine 10 mg/kg delivered intraperitoneally and were placed on a heated platform before the tail clip injury. The tails were immersed in 50 ml of prewarmed PBS at 37°C for 2 min. A 3‐mm tail transection was made, and blood was collected into PBS for 10 min. The hemostatic effect was determined by measuring the volume of blood loss as described later.
A quantitative assessment of blood loss was determined by measuring the hemoglobin content of the blood collected in PBS. To collect erythrocytes, sample tubes were centrifuged, and the pelleted erythrocytes were suspended in 10 ml of lysis buffer (8.3 g/L ammonium chloride, 1.0 g/L potassium bicarbonate, and 0.037 g/L EDTA). The absorbance of the sample was measured at 575 nm using a spectrophotometer. The absorbance values were converted to total volume of blood loss (μl) using a standard curve. All experiments were performed within guidelines and were reviewed and approved by the Pfizer Institutional Animal Care and Use Committee.
3. RESULTS
Thrombin generation was evaluated in a total of 15 plasma samples, 13 with hemophilia A and two with hemophilia B. Consistent with the TFPI inhibitory activity of marstacimab, the addition of marstacimab to human nonhemophilic platelet‐poor plasma shortened the lag time and resulted in increased thrombin generation, ranging from 160 to 257 nM (Tables 1 and 2). These peak thrombin levels are within the range reported in an earlier study of nonhemophilic plasmas (287 ± 61 nM). 28 In untreated human nonhemophilic plasma, peak thrombin ranged from 90 to 143 nM (Tables 1 and 2).
TABLE 1.
Peak thrombin levels and lag time parameter from thrombin generation assay for marstacimab and rFVIIa
| Group | Vehicle a | rFVIIa 2 μg/ml | Marstacimab (16 μg/ml) | Marstacimab (16 μg/ml) rFVIIa (2 μg/ml) | Nonhemophilic plasma a | Nonhemophilic plasma marstacimab (16 μg/ml) |
|---|---|---|---|---|---|---|
| Peak thrombin levels, nM | ||||||
| Hemophilia A (n = 6) | 26–76 | 45–113 | 82–172 | 102–178 | 128–135 | 198 (n = 1) b |
| Hemophilia A with inhibitor 3−261 BU (n = 3) | 24–66 | 46–89 | 88–166 | 99–157 | 130–132 | 198–199 |
| Hemophilia B (n = 1) | 81 | 131 | 174 | 167 | 143 | 171 |
| Hemophilia B with inhibitor 14 BU (n = 1) | 32 | 60 | 72 | 117 | 133 | 228 |
| Lag time, min | ||||||
| Hemophilia A (n = 6) | 2.50–4.66 | 0.83–2.88 | 1.67–4.10 | 1.00–2.33 | 3.08–4.33 | 2.33 |
| Hemophilia A with inhibitor 3 −1261 BU (n = 3) | 2.50–3.50 | 1.67–1.83 | 1.67–2.50 | 1.00–1.50 | 3.08–3.25 | 2.00–2.33 |
| Hemophilia B (n = 1) | 2.33 | 0.83 | 2.00 | 0.83 | 2.92 | 2.33 |
| Hemophilia B with inhibitor 14 BU (n = 1) | 3.01 | 1.00 | 2.84 | 1.17 | 4.09 | 2.84 |
Thrombin generation was measured in the presence of 1 pM tissue factor and 4 µM phospholipid. Nonhemophilic plasma was derived from a pool and analyzed with each hemophilia plasma sample. Vehicle = 50% phosphate buffered saline/50% HEPES buffer.
Abbreviation: BU, Bethesda unit; HEPES, 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid; rFVIIa, recombinant factor VIIa.
The ranges of peak thrombin from each plasma type are provided.
N = 1 for the nonhemophilic plasma marstacimab (16 µg/ml).
TABLE 2.
Peak thrombin levels and lag time parameter from thrombin generation assay for marstacimab and aPCC
| Group | Vehicle a | aPCC 1 U/ml | Marstacimab 16 µg/ml | Marstacimab 16 µg/ml aPCC 1 U/ml | Nonhemophilic plasma a | Nonhemophilic plasma marstacimab 16 µg/ml a |
|---|---|---|---|---|---|---|
| Peak thrombin levels, nM | ||||||
| Hemophilia A with inhibitor 88–160 BU (n = 4) | 15–19 | 75–110 | 73–110 | 209–241 | 95–135 | 182–257 |
| Hemophilia B with inhibitor 14 BU (n = 1) | 31 | 163 | 70 | 254 | 90 | 160 |
| Lag time, min | ||||||
| Hemophilia A with inhibitor 88–160 BU (n = 4) | 3.50–5.18 | 2.17–2.51 | 3.67–4.35 | 2.00–2.34 | 4.00–4.85 | 3.00–3.51 |
| Hemophilia B with inhibitor 14 BU (n = 1) | 4.67 | 2.17 | 3.83 | 2.17 | 4.00 | 3.00 |
Thrombin generation was measured in the presence of 1 pM tissue factor and 4 µM phospholipid. Nonhemophilic plasma was derived from a pool and analyzed with each hemophilia plasma sample. The ranges of peak thrombin parameters from each plasma type are provided. Vehicle = 50% phosphate buffered saline/50% aPCC diluent.
Abbreviations: aPCC, activated prothrombin complex concentrate; BU, Bethesda unit; U, unit.
Ranges of controls included with each analysis.
3.1. Effects of marstacimab in combination with rFVIIa on thrombin generation
Hemophilia leads to the inadequate generation of thrombin, and this was markedly reduced in vehicle‐treated hemophilia A and B plasmas with or without inhibitors compared with human nonhemophilic plasma (Tables 1 and 2). The concentration of rFVIIa in the plasma was 2 μg/ml and approximated the plasma levels that could be observed following dosing of rFVIIa 90 and 180 μg/kg body weight. 29
First, in hemophilia A plasma without inhibitors, the addition of rFVIIa 2 μg/ml resulted in an increase in peak thrombin generation (45–113 nM) compared with vehicle‐treated hemophilia A (26–76 nM) (Table 1). In hemophilia B plasma treated with rFVIIa 2 μg/ml, peak thrombin levels reached 131 nM compared with 81 nM in vehicle‐treated hemophilia B plasma. A shortening of the lag time was also observed after the addition of rFVIIa. The addition of marstacimab (16 μg/ml) alone resulted in an increase in peak thrombin in both hemophilia A (82–172 nM) and hemophilia B (174 nM) plasma. Combining marstacimab (16 μg/ml) and rFVIIa (2 μg/ml) resulted in higher peak thrombin concentration (102–178 nM) and shortening of lag time in hemophilia A plasma compared with vehicle‐treated plasma, perhaps suggesting a minimal additive effect. Peak thrombin concentrations after combined treatment with marstacimab and rFVIIa were comparable with those observed in nonhemophilic plasma and did not exceed the level observed in nonhemophilic plasma dosed with marstacimab (228 nM; Table 1).
In hemophilia A plasma samples with inhibitors (3, 102, and 1261 Bethesda units [BU]) or hemophilia B plasma with an inhibitor (14 BU), marstacimab 16 μg/ml alone also increased peak thrombin concentrations (88–166 nM and 72 nM, respectively), compared with vehicle treatment (24–66 and 32 nM, respectively; Figure 1; Table 1). A minimal additive effect in peak thrombin generation may be observed with the combination of marstacimab 16 μg/ml plus rFVIIa 2 μg/ml in severe hemophilia A or B plasma with inhibitors. The midpoint peak thrombin levels achieved with marstacimab 16 μg/ml alone or in combination with rFVIIa were similar to those observed in nonhemophilic plasma and did not exceed the level observed in nonhemophilic plasma dosed with marstacimab (228 nM).
FIGURE 1.
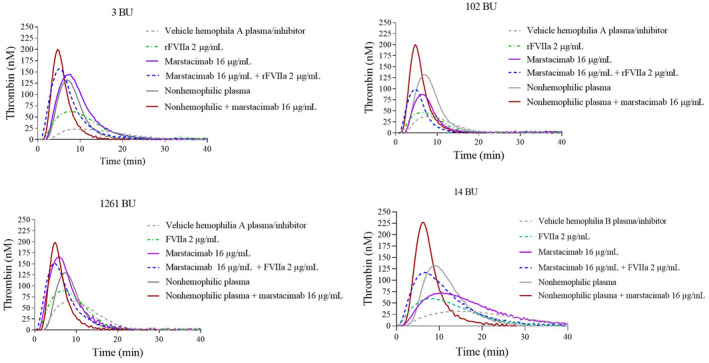
Effect of marstacimab and/or rFVIIa on thrombin generation in nonhemophilic plasma and severe hemophilia A and B human plasma with inhibitors. Thrombin generation was measured in the presence of 1 pM TF and 4 µM phospholipids. Shown are the thrombograms from each donor hemophilia A plasma with an inhibitor (3, 102, and 1261 BU) and hemophilia B with an inhibitor (14 BU). The following were added to each individual hemophilia A plasmas: vehicle (50% PBS/50%HEPES); rFVIIa (2 μg/ml), marstacimab alone (16 μg/ml); and marstacimab (16 μg/ml) in combination with rFVIIa (2 μg/ml). Thrombin generation was also measured in nonhemophilic plasma alone and with marstacimab (16 μg/ml). Abbreviations: BU, Bethesda unit; HEPES, 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid; PBS, phosphate buffered saline; rFVIIa, recombinant factor VIIa; TF, tissue factor
3.2. Effects of marstacimab in combination with aPCC on thrombin generation
Concentration‐dependent increases in peak thrombin levels were observed after the addition of aPCC alone to hemophilia A plasma with inhibitors or to hemophilia B plasma with an inhibitor (Figure 2, Table 2). The lag time decreased at 0.063 U/ml, with no further decrease observed at concentrations >0.063 U/ml. The addition of marstacimab alone to hemophilia plasma increased peak thrombin at >0.5 μg/ml in all hemophilia plasmas tested. An additive effect was observed with the addition of aPCC 1 U/ml to marstacimab at a concentration of 0.5 μg/ml in all hemophilia plasmas, with an increase in the peak thrombin parameter. The peak thrombin reached a plateau at marstacimab concentrations >0.5 μg/ml in combination with aPCC 1 U/ml.
FIGURE 2.
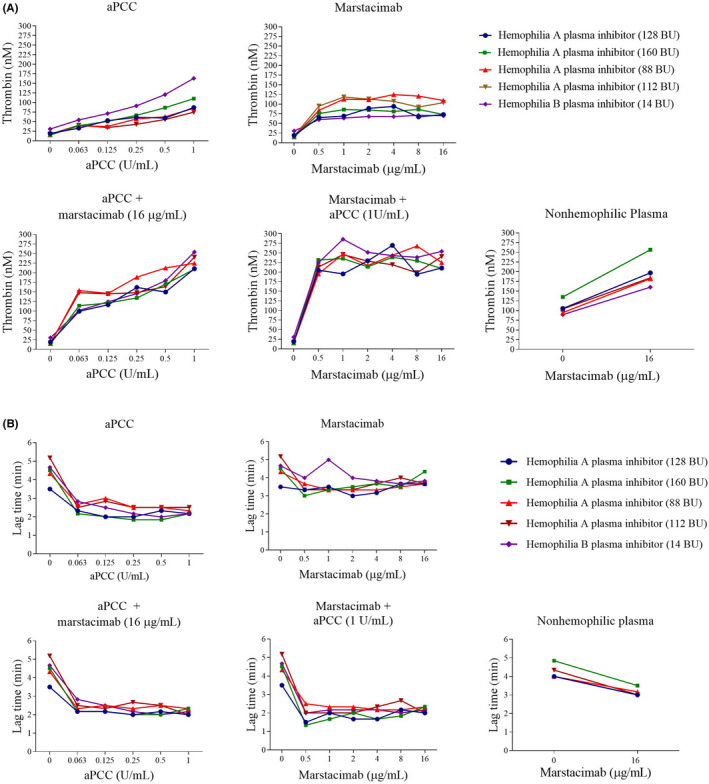
Effect of marstacimab alone or in combination with aPCC on (A) peak thrombin level and (B) thrombin generation assay lag time in human hemophilia A and B plasma with inhibitors. Thrombin generation was measured in the presence of 1 pM TF and 4 µM phospholipids. Marstacimab or aPCC were added to the plasma for assay as indicated. Thrombin generation was also measured in nonhemophilic plasma alone and with marstacimab (16 µg/ml) and was included in each thrombin generation assay. Vehicle = 50% phosphate‐buffered saline (PBS)/50% aPCC diluent. Abbreviations: aPCC, activated prothrombin complex concentrate; BU, Bethesda unit; TF, tissue factor
The addition of aPCC 1 U/ml resulted in an increase in thrombin generation in hemophilia A (75–110 nM) and B (163 nM) inhibitor plasmas compared with vehicle treatment (15–19 nM and 31 nM, respectively) (Table 2). A shortening of the lag time was also observed after treatment with aPCC. The addition of marstacimab 16 μg/ml alone also resulted in an increase in thrombin generation, including higher peak thrombin concentration (73–110 nM in hemophilia A and 70 nM in hemophilia B) and a shortening of lag time compared with vehicle‐treated hemophilic plasma. An additive effect on thrombin generation of 2.1–3.6 times was observed after cotreatment with marstacimab 16 μg/ml and aPCC 1 U/ml compared with either agent alone (Figure 2A). The peak thrombin levels (209–254 nM) observed with this combination were within the reported range for nonhemophilic plasmas (287 ± 61 nM). 28 Although marstacimab 16 μg/ml and aPCC 1 U/ml as individual treatments both decreased the lag time in hemophilia plasma, no additive effect on lag time was observed when marstacimab and aPCC were used in combination (Figure 2B).
3.3. Effects of marstacimab and aPCC in a mouse acute bleeding model
As expected, the hemophilia A mice treated with a suboptimal dose of marstacimab (0.5 mg/kg) exhibited no reduction in blood loss compared with vehicle‐treated control mice (Figure 3). aPCC doses of 50 and 100 U/kg were chosen to correspond to clinical doses of aPCC, 30 and the dose of 200 U/kg was chosen to represent a high dose of aPCC. 30 Concomitant use of low‐dose marstacimab (0.5 mg/kg) and 100 U/kg aPCC showed a slight trend toward improvement in hemostasis: a 49.7% decrease in bleeding compared with vehicle‐treated mice (p > 0.05). Hemophilia A mice that received 200 U/kg aPCC had significantly reduced bleeding (62.0%) compared with vehicle‐treated mice (p < 0.05). An additive effect was observed for the combination of marstacimab 0.5 mg/kg plus high‐dose aPCC 200 U/kg, exhibiting a significant reduction in blood loss (83.3%) compared with vehicle‐treated mice (p = 0.0009) (Figure 3). Variability in the volume of blood loss was observed in the vehicle‐treated mice (Figure 3).
FIGURE 3.
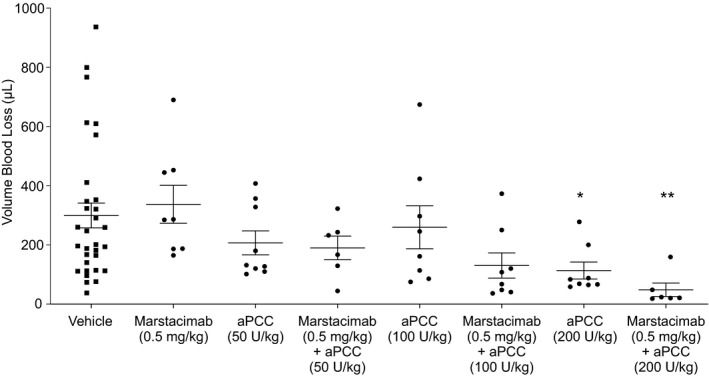
Hemostatic activity of marstacimab in combination with ascending doses of aPCC in hemophilia A mice. Marstacimab and aPCC were administered to hemophilia A mice alone or in combination at the indicated doses before the tail clip injury. All measurements are presented as mean ± SEM. Kruskal‐Wallis test with Dunn's multiple comparison correction used for comparison to vehicle control mice. *p < 0.05; **p, 0.0009; U, Unit. Abbreviations: aPCC, activated prothrombin complex concentrate; SEM, standard error of the mean
4. DISCUSSION
Hemophilia leads to an inadequate generation of thrombin. The TFPI inhibitory activity of marstacimab on thrombin generation alone or in combination with rFVIIa or aPCC was studied in plasma from donors with severe hemophilia as well as nonhemophilic plasma, which contains the full complement of coagulation factors, including FVIII and FIX. Marstacimab improved thrombin generation parameters in all hemophilia plasmas studied. A potential safety concern is excessive thrombin generation with the use of marstacimab in combination with rFVIIa or aPCC. The addition of rFVIIa to marstacimab resulted in a minimal increase in thrombin generation compared with marstacimab alone. The peak thrombin levels achieved with the combination of the anti‐TFPI antibody marstacimab plus rFVIIa in all hemophilic plasma samples did not exceed those achieved in nonhemophilic plasma that was treated with vehicle.
Activated prothrombin complex concentrates, such as FEIBA, also contain both the coagulation zymogens and activated proteins; the cofactors FVIII, factor V; and protein S, and the coagulation inhibitors protein C and TFPI. 19 , 31 In vitro mechanism of action studies with FEIBA showed that the thrombin‐FXa complex was a key component of FEIBA, but other plasma factors contribute to the mechanism. 19 , 31 In addition, the reported levels of total TFPI in 1 U of FEIBA are between 10 and 30 ng/ml. In patients with hemophilia, TFPI levels did not accumulate after 3 months of prophylaxis or on‐demand treatment with FEIBA. 19 , 31
TFPI negatively regulates thrombin generation within the extrinsic pathway of coagulation by rapidly inactivating the protease functions of FXa and the FVIIa/TF (FXa/FVIIa/TF) complex. Mechanistically, marstacimab binds to the TFPI Kunitz‐2 domain and increases FXa and thus hemostasis. In our studies, we added aPCC and marstacimab at concentrations corresponding to plasma levels that could be achieved clinically after dosing. This resulted in increased thrombin generation in hemophilia A and hemophilia B inhibitor plasmas, including higher peak thrombin concentration compared with either agent alone. However, thrombin levels were within the range reported in studies of nonhemophilic plasma 28 and marstacimab in nonhemophilic plasma (this study), alleviating concerns of excessive coagulation with combined treatment. The administration of either marstacimab or aPCC decreased the thrombin generation assay lag time parameter in hemophilia plasma, but no further decrease was observed in the lag time with the combination of marstacimab plus aPCC. To summarize, the combined treatment with marstacimab and rFVIIa or aPCC in vitro increased thrombin generation in hemophilia A and hemophilia B inhibitor plasmas without inducing excessive coagulation. The findings of the current study support evidence from an in vitro study with platelet‐rich and platelet‐poor plasma from patients with hemophilia in which marstacimab, at concentrations ranging from 1 to 100 nM (0.16–16 μg/ml) improved in thrombin generation. 16 In platelet‐rich plasma, the effect of marstacimab on decreasing thrombin generation assay lag time was more pronounced. The differences observed in platelet‐rich plasma could be attributable to the assay conditions with the presence of platelet membrane phospholipids or could potentially reflect the contribution of platelet factor V(a).
In a hemophilia A mouse, severe tail transection model, administration of a suboptimal dose of marstacimab in combination with aPCC 200 U/kg restored hemostasis. Compared with vehicle‐treated mice, aPCC 200 U/kg alone significantly reduced bleeding by 62.0%, whereas the combination of marstacimab plus aPCC reduced bleeding by 83.8%. This is consistent with the additive effects observed with the addition of aPCC plus marstacimab to hemophilia plasma. The hemophilia mouse model provides a tool to study the effect of hemostatic compounds in vivo, though there are limitations to the model. In the mouse, the levels of plasma TFPI are approximately 20 times higher compared with humans. 32 The properties of the human isoforms TFPIα and TFPIβ are similar in the mouse, including platelet TFPIα. However, the predominant form in mouse plasma, TFPIγ, is an isoform not found in humans. 32 The TFPI Kunitz‐2 domain is found in all mouse isoforms and marstacimab, which binds to the Kunitz‐2 domain of TFPI, would bind to all mouse TFPI isoforms. 32 In our assays, the addition of aPCC alone restored hemostasis at 200 U/kg; this effect was enhanced in combination with marstacimab in our mouse model. This in vivo mouse study was designed to look at the effect of concomitant administration of marstacimab and aPCC on hemostasis alone, excluding other hemostatic parameters.
In addition to the plasma pool of TFPIα, which was investigated in these in vitro studies, the TFPIβ isoform is expressed on the surface of endothelial cells. 8 , 9 An important limitation of the in vitro study is that it did not address the TFPI pool associated with endothelial cells, the inhibition of which may potentially contribute to a thrombotic event. Another potential limitation is that this was an in vitro thrombin generation study in which marstacimab and bypassing agents were added to the hemophilia plasma. This may be a different environment from the plasma of patients in vivo, and, therefore, extrapolating this to humans should be done with caution. In a phase 1 clinical trial, following the subcutaneous administration of a single dose of marstacimab at 300 mg, the peak thrombin observed in participants was around 200 nM and the maximum plasma concentration was 16.5 μg/ml. 14 These values are similar to the peak thrombin observed in our study after the addition of marstacimab 16 μg/ml to nonhemophilic plasma, suggesting that in vitro assays are capturing aspects of thrombin generation observed in vivo. 14 The number of donor patient plasmas with inhibitors studied was small but plasmas contained a wide range of inhibitors, from a very low‐titer inhibitor of 3 BU to a high‐titer inhibitor of 1261 BU, and all showed similar thrombin generation responses after the addition of marstacimab and bypassing agents.
The nonclinical studies provide support for the combined use of marstacimab and bypass agents, rFVIIa or aPCC, in patients with inhibitors who may have a breakthrough bleeding event. Although the in vitro thrombin generation assay has some limitations, studies have shown a correlation of the assay with thrombosis. 33 In a study using a sequence‐identical analog of emicizumab with aPCC, the peak thrombin level was increased up to 17‐fold. 34 The in vitro spike in studies of hemophilia plasma treated with marstacimab in combination with bypass agents, rFVIIa or aPCC, showed increased thrombin generation without excessive coagulation. Finally, in nonclinical 10‐day studies, the cumulative and potential additive pharmacodynamic effects of the combined repeat‐dose intravenous administration of marstacimab plus rFVIIa or of subcutaneous marstacimab plus intravenous FEIBA, were evaluated in hemostatically normal male rats. 35 The marstacimab systemic exposures exceeded the predicted exposure at the clinical dose of subcutaneous 300 mg/week. These nonclinical studies in rats with normal levels of FIX and FVIII provide additional rationale for the concomitant use of marstacimab and rFVIIa (90 μg/kg) or aPCC (100 U/kg) for the treatment of breakthrough bleeding events in patients with hemophilia A or B with inhibitors in the phase 3 trial. 35 Marstacimab is currently in clinical development, with an ongoing phase 3 study investigating its prophylactic efficacy in patients with severe hemophilia A or B with or without inhibitors (clinicaltrials.gov identifier NCT03938792).
5. CONCLUSION
Results of in vitro and in vivo assays support increased hemostasis through combined use of the anti‐TFPI antibody marstacimab and the bypassing agents rFVIIa or aPCC, without inducing excessive coagulation.
RELATIONSHIP DISCLOSURE
All authors are employees of Pfizer Inc. and may own stock/options in the company.
AUTHOR CONTRIBUTIONS
Debra Pittman contributed to the study design. Swapnil Rakhe participated in the collection and assembly of data. Debra Pittman participated in manuscript preparation. All authors had full access to the data and contributed to data interpretation. All authors participated in the critical review and revision of the manuscript and granted approval of the final manuscript for submission.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Medical writing and editorial support were provided by Jessica D. Herr, PharmD, of Peloton Advantage, an OPEN Health company, and funded by Pfizer Inc. The authors acknowledge the marstacimab development team.
Pittman DD, Rakhe S, Bowley SR, Jasuja R, Barakat A, Murphy JE. Hemostatic efficacy of marstacimab alone or in combination with bypassing agents in hemophilia plasmas and a mouse bleeding model. Res Pract Thromb Haemost. 2022;6:e12679. doi: 10.1002/rth2.12679
Handling Editor: Dr Henri Spronk
Funding information
This study was sponsored by Pfizer Inc. No author received an honorarium or other form of financial support related to the development of this manuscript.
REFERENCES
- 1. Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1‐e47. [DOI] [PubMed] [Google Scholar]
- 2. Franchini M. The modern treatment of haemophilia: a narrative review. Blood Transfus. 2013;11:178‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brummel‐Ziedins KE, Wolberg AS. Global assays of hemostasis. Curr Opin Hematol. 2014;21:395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809‐818. [DOI] [PubMed] [Google Scholar]
- 5. World Federation of Hemophilia . World Federation of Hemophilia report on the annual global survey 2010. 2011. Available at: http://www1.wfh.org/publications/files/pdf‐1427.pdf Accessed: Apr 30, 2021.
- 6. Gouw SC, van der Bom JG, Ljung R, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368:231‐239. [DOI] [PubMed] [Google Scholar]
- 7. Peterson JA, Maroney SA, Mast AE. Targeting TFPI for hemophilia treatment. Thromb Res. 2016;141(suppl 2):S28‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Broze GJ Jr, Girard TJ. Tissue factor pathway inhibitor: structure‐function. Front Biosci (Landmark Ed). 2012;17:262‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maroney SA, Mast AE. New insights into the biology of tissue factor pathway inhibitor. J Thromb Haemost. 2015;13:S200‐S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Girard TJ, Warren LA, Novotny WF, et al. Functional significance of the Kunitz‐type inhibitory domains of lipoprotein‐associated coagulation inhibitor. Nature. 1989;338:518‐520. [DOI] [PubMed] [Google Scholar]
- 11. Baugh RJ, Broze GJ Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273:4378‐4386. [DOI] [PubMed] [Google Scholar]
- 12. Apgar J, Parng C, Benard S, Juo S, Tam M, Tu C, Rakhe S, Jin M, Svenson K, Murphy JE, Stahl M & Pittman DD X‐ray crystallography and hemostatic activities of a neutralizing anti‐TFPI antibody, marstacimab [poster]. Presented at: Annual Congress of the International Society on Thrombosis and Haemostasis; July 12‐14, 2020.
- 13. Apgar J, Parng C, Benard S, et al. X‐ray crystallography and hemostatic activities of a neutralizing anti‐TFPI antibody, marstacimab [abstract]. Res Pract Thromb Haemost. 2020;4(suppl 1):PB0240. [Google Scholar]
- 14. Cardinal M, Kantaridis C, Zhu T, et al. A first‐in‐human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of PF‐06741086, an anti‐tissue factor pathway inhibitor mAb, in healthy volunteers. J Thromb Haemost. 2018;16:1722‐1731. [DOI] [PubMed] [Google Scholar]
- 15. Hilden I, Lauritzen B, Sørensen BB, et al. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model. Blood. 2012;119:5871‐5878. [DOI] [PubMed] [Google Scholar]
- 16. Patel‐Hett S, Martin EJ, Mohammed BM, et al. Marstacimab, a tissue factor pathway inhibitor neutralizing antibody, improves coagulation parameters of ex vivo dosed haemophilic blood and plasmas. Haemophilia. 2019;25:797‐806. [DOI] [PubMed] [Google Scholar]
- 17. Maroney SA, Haberichter SL, Friese P, et al. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931‐1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahlangu JN. Progress in the development of anti‐tissue factor pathway inhibitors for haemophilia management. Front Med (Lausanne). 2021;8:670526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varadi K, Tangada S, Loeschberger M, et al. Pro‐ and anticoagulant factors facilitate thrombin generation and balance the haemostatic response to FEIBA(®) in prophylactic therapy. Haemophilia. 2016;22:615‐624. [DOI] [PubMed] [Google Scholar]
- 20. FEIBA [package insert]. Lexington, MA: Baxalta US Inc., 2018.
- 21. Rakhe S, Patel Hett S, Murphy JE, Pittman DD. An antibody to tissue factor pathway inhibitor (PF‐06741086) in combination with recombinant factor VIIa increases hemostasis in hemophilia plasma without excessive thrombin generation [abstract]. Blood. 2016;128:2566. [Google Scholar]
- 22. Parng C, Singh P, Pittman DD, et al. Translational pharmacokinetic/pharmacodynamic characterization and target‐mediated drug disposition modeling of an anti‐tissue factor pathway inhibitor antibody, PF‐06741086. J Pharm Sci. 2018;107:1995‐2004. [DOI] [PubMed] [Google Scholar]
- 23. Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4‐15. [DOI] [PubMed] [Google Scholar]
- 24. Novotny WF, Girard TJ, Miletich JP, Broze GJ Jr. Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020‐2025. [PubMed] [Google Scholar]
- 25. Ivanciu L, Toso R, Margaritis P, et al. A zymogen‐like factor Xa variant corrects the coagulation defect in hemophilia. Nat Biotechnol. 2011;29:1028‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jasuja R, Barakat A, Murphy JE, Pittman DD. An antibody to tissue factor pathway inhibitor (TFPI) restores hemostasis after the onset of bleeding in hemophilic a mouse injury models [abstract]. Blood. 2016;128:3761. [Google Scholar]
- 27. Holmberg HL, Lauritzen B, Tranholm M, Ezban M. Faster onset of effect and greater efficacy of NN1731 compared with rFVIIa, aPCC and FVIII in tail bleeding in hemophilic mice. J Thromb Haemost. 2009;7:1517‐1522. [DOI] [PubMed] [Google Scholar]
- 28. Dargaud Y, Beguin S, Lienhart A, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93:475‐480. [DOI] [PubMed] [Google Scholar]
- 29. Scharling B, Nielsen GG, Klitgaard T, et al. Comparison of coagulant activity of factor VII and activated factor VII activity assays when used for determination of recombinant activated factor VII levels in plasma. Blood Coagul Fibrinolysis. 2007;18:677‐684. [DOI] [PubMed] [Google Scholar]
- 30. FEIBA NF [product monograph]. Toronto, ON, Canada: Shire Pharma Canada, 2018.
- 31. Turecek PL, Váradi K, Gritsch H, Schwarz HP. FEIBA: mode of action. Haemophilia. 2004;10(suppl 2):3‐9. [DOI] [PubMed] [Google Scholar]
- 32. Girard TJ, Grunz K, Lasky NM, Malone JP, Broze GJ Jr. Re‐evaluation of Mouse tissue factor pathway inhibitor and comparison of mouse and human tissue factor pathway inhibitor physiology. J Thromb Haemost. 2018;16(11):2246‐2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipets EN, Ataullakhanov FI. Global assays of hemostasis in the diagnostics of hypercoagulation and evaluation of thrombosis risk. Thrombosis J. 2015;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartmann R, Feenstra T, Valentino L, Dockal M, Scheiflinger F. In vitro studies show synergistic effects of a procoagulant bispecific antibody and bypassing agents. J Thromb Haemost. 2018;16:1580‐1591. [DOI] [PubMed] [Google Scholar]
- 35. Peraza MA, Brady JT, Young LJ, et al. Studies of Marstacimab, an anti‐tissue factor pathway (TFPI) inhibitor, in combination with factor replacement or bypassing agents [abstract F‐FP‐LBA‐4.1]. Haemophilia. 2020;26(suppl 4):154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


