Abstract
The authors examined the proportion of US adults that would have their high blood pressure (BP) status changed if systolic BP (SBP) and diastolic BP (DBP) were measured with systematic bias and/or random error versus following a standardized protocol. Data from the 2017–2018 National Health and Nutrition Examination Survey (NHANES; n = 5176) were analyzed. BP was measured up to three times using a mercury sphygmomanometer by a trained physician following a standardized protocol and averaged. High BP was defined as SBP ≥130 mm Hg or DBP ≥80 mm Hg. Among US adults not taking antihypertensive medication, 32.0% (95%CI: 29.6%,34.4%) had high BP. If SBP and DBP were measured with systematic bias, 5 mm Hg for SBP and 3.5 mm Hg for DBP higher and lower than in NHANES, the proportion with high BP was estimated to be 44.4% (95%CI: 42.6%,46.2%) and 21.9% (95%CI 19.5%,24.4%). Among US adults taking antihypertensive medication, 60.6% (95%CI: 57.2%,63.9%) had high BP. If SBP and DBP were measured 5 and 3.5 mm Hg higher and lower than in NHANES, the proportion with high BP was estimated to be 71.8% (95%CI: 68.3%,75.0%) and 48.4% (95%CI: 44.6%,52.2%), respectively. If BP was measured with random error, with standard deviations of 15 mm Hg for SBP and 7 mm Hg for DBP, 21.4% (95%CI: 19.8%,23.0%) of US adults not taking antihypertensive medication and 20.5% (95%CI: 17.7%,23.3%) taking antihypertensive medication had their high BP status re‐categorized. In conclusions, measuring BP with systematic or random errors may result in the misclassification of high BP for a substantial proportion of US adults.
Keywords: blood pressure, measurement error, misclassification, random error
1. INTRODUCTION
The importance of accurately measuring blood pressure (BP) has been emphasized for more than 80 years. 1 The American Heart Association (AHA) has issued multiple scientific statements to inform clinicians and researchers of the proper technique for measuring BP. 2 , 3 Also, the 2017 American College of Cardiology (ACC)/AHA BP guideline provides instructions for obtaining standardized BP measurements. 4 However, recent studies show that BP levels in routine clinical practice are typically overestimated as compared to when measurements are obtained following a standardized protocol. 5 , 6 , 7 , 8
The 2017 ACC/AHA BP guideline lowered the systolic BP (SBP) and diastolic BP (DBP) levels used to define hypertension and it has been estimated that 115 million US adults have elevated BP or hypertension when BP is measured following a standardized protocol. 9 Also, the United States population is aging and the prevalence of obesity has increased over the past several decades. 10 , 11 Obtaining accurate BP measurements is more challenging in older versus younger adults and individuals with progressively larger arm circumferences. 12 , 13 Inaccuracy in BP measurement may lead to the over‐use of antihypertensive medication in some people and under‐use in others. 14
Given the high prevalence of elevated BP and hypertension in the United States and data that BP is not being accurately measured in routine settings, it is important to ascertain the impact of potential errors in BP measurement on the prevalence of high BP among US adults. We used data from the National Health and Nutrition Examination Survey (NHANES) 2017–2018 to estimate the proportion and number of US adults whose high BP status may be over‐ or under‐estimated when BP is measured with a systematic bias or random error.
2. METHODS
The NHANES was designed to assess the health and nutritional status of the non‐institutionalized US population and is conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). 15 For the current analysis, data from the 2017–2018 NHANES cycle were analyzed. Participants were identified using a stratified, multistage, probability sampling method and the current analysis was restricted to adults aged 18 years or older who completed the NHANES interview and examination (Figure S1; n = 5533). Participants who self‐reported being pregnant (n = 55) and those who did not have at least one valid SBP and DBP measurement (n = 292) or were missing information on antihypertensive medication use (n = 10) were excluded. A total of 5176 participants were included in the analyses. The protocol for NHANES 2017–2018 was approved by the NCHS of the CDC Institutional Review Board. Written informed consent was obtained from each participant. The analysis of NHANES data was considered exempt research by the University of Alabama at Birmingham Institutional Review Board.
2.1. Data collection
Data were collected during an in‐home interview and a study examination. Standardized questionnaires were used to assess demographic characteristics that included age, sex and race/ethnicity. Height and weight were measured during the study examination and obesity was defined as a body mass index ≥ 30 kg/m2. Blood and urine samples were collected during the examination. Serum glucose, glycated hemoglobin and serum creatinine were measured using standardized methods. Diabetes was defined as a fasting glucose ≥ 126 mg/dl (≥ 200 mg/dl for those who were not fasting), glycated hemoglobin ≥ 6.5%, or a self‐reported diagnosis of diabetes with the use of insulin or oral glucose lowering medication. Estimated glomerular filtration rate (eGFR) was calculated using the CKD Epidemiology Collaboration equation. 16 Urinary albumin and creatinine levels were measured and used to calculate the albumin‐to‐creatinine ratio. CKD was defined by an eGFR < 60 ml/min/1.73m2 or an albumin‐to‐creatinine ratio ≥ 30 mg/g.
2.2. Blood pressure measurements
Trained physicians measured SBP and DBP up to three times using a mercury sphygmomanometer and an appropriately sized BP cuff following a standardized protocol. The first measurement was performed after participants had been seated quietly with their feet flat on the floor and arm and back supported for 5 min. Subsequent measurements were obtained at 30‐s intervals. The mean of all available measurements was used to define SBP and DBP. Among participants included in the current analysis, 36 (0.6%), 73 (1.3%), and 5067 (98.1%) had one, two, and three SBP measurements, respectively, and 48 (0.8%), 102 (1.8%), and 5026 (97.4%) had one, two or three DBP measurements, respectively. Quality control included re‐certification of physicians every 3 months with retraining if needed. All physicians participated in annual retraining. Additional information on the NHANES BP measurement protocol can be found online. 17 High BP was defined as SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg. 4
2.3. Statistical analysis
All analyses were conducted stratified by antihypertensive medication use. Characteristics of US adults were summarized as percentages for categorical variables and mean with 95% confidence interval (CI) for continuous variables. The proportion and number of US adults with high BP were estimated using the measurements from NHANES and then after assuming BP was measured with a fixed bias. First, we assumed that SBP and DBP was measured 5 and 3.5 mm Hg higher, respectively, and 10 and 7 mm Hg higher, respectively, when compared to following a standardized protocol and we added these amounts to the measured SBP and DBP values from NHANES. Second, we assumed that SBP and DBP was measured 5 and 3.5 mm Hg lower, respectively, and 10 and 7 mm Hg lower, respectively, compared to following a standardized protocol and we subtracted these amounts from the measured SBP and DBP values in NHANES. The magnitudes of bias of 5 mm Hg for SBP and 3.5 mm Hg for DBP was based on the acceptable limit for error in SBP when validating a BP device and comparison of within‐person standard deviations of SBP and DBP resulting in approximately 5 mm Hg difference in SBP being analogous to 3.5 mm Hg difference in DBP. 7 , 18 , 19 The magnitudes of 10 and 7 mm Hg for SBP and DBP, respectively, were selected based on prior studies of the differences in SBP and DBP between routine care and research‐grade measurements. 5 , 6 , 7 , 8 This analysis was conducted for the overall population and in subgroups defined by age (< 65 and ≥ 65 years) and obesity (obese and non‐obese).
Next, we simulated SBP and DBP being measured with random error using a bivariate normal distribution with the correlation between SBP and DBP errors as observed in the NHANES data (ρ = 0.46). First, we assumed measurements were obtained without bias but with random error that had a standard deviation of 15 mm Hg for SBP and 7 mm Hg for DBP. These standard deviations were selected based on data from prior publications on the differences in SBP and DBP values in clinical practice versus research studies. 5 , 6 , 7 , 8 We estimated the proportion with 95% CI of US adults whose high BP status was different when measured in NHANES versus with random error using 10 000 Monte Carlo simulations. Second, as BP measured in a routine clinical practice is often over‐estimated, we did an analysis simulating BP measured with random error and bias. 5 , 6 , 7 , 8 For this analysis, in addition to simulating random error with standard deviation of 15 mm Hg and 7 mm Hg for SBP and DBP, respectively, we simulated a bias of SBP and DBP higher by 5 and 3.5 mm Hg, respectively, and 10 and 7 mm Hg, respectively. In a secondary analysis, the proportion and number of US adults with SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg were estimated using the measurements from NHANES and then after assuming only SBP was measured with fixed bias and random error, separately, and after assuming only DBP was measured with fixed bias and random error, separately. The above analyses were repeated defining high BP as SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg. 20 , 21
All calculations were weighted to obtain nationally representative estimates for the non‐institutionalized US population. These weights were recalibrated based on the proportion of participants missing data by age, sex, and race‐ethnicity, assuming that data within these strata were missing at random. Data analyses were conducted using STATA V16 (Stata Corporation, College Station, TX, USA) and R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Data to replicate this analysis are available on the NHANES website. 15
3. RESULTS
Overall, 23.0% of US adults were taking antihypertensive medication. US adults taking versus not taking antihypertensive medication were more likely to be older and non‐Hispanic black, and have obesity, diabetes, and CKD (Table 1). The distributions of SBP and DBP for US adults not taking and taking antihypertensive medication are presented in Figure S2.
TABLE 1.
Characteristics of US adults taking and not taking antihypertensive medication in 2017–2018
| Taking antihypertensive medication | ||
|---|---|---|
| No (N = 3688) | Yes (N = 1488) | |
| Age group in years, % | ||
| 18–34 | 38.2 | 2.7 |
| 35–44 | 18.8 | 6.0 |
| 45–54 | 15.2 | 16.5 |
| 55–64 | 16.1 | 28.3 |
| 65–74 | 7.5 | 25.4 |
| ≥75 | 4.1 | 21.0 |
| Female sex, % | 51.7 | 52.2 |
| Race/ethnicity, % | ||
| Non‐Hispanic white | 61.4 | 64.0 |
| Non‐Hispanic black | 10.3 | 14.9 |
| Hispanic | 17.7 | 10.6 |
| Non‐Hispanic Asian | 5.8 | 5.4 |
| Other | 4.8 | 5.0 |
| Obese, % | 37.5 | 56.7 |
| Diabetes, % | 6.5 | 33.3 |
| Chronic kidney disease, % | 9.2 | 35.5 |
| Systolic blood pressure, mm Hg | 120 (119 ‐ 121) | 134 (132 ‐ 135) |
| Diastolic blood pressure, mm Hg | 72 (71 ‐ 73) | 73 (72‐75) |
Numbers in the table are column percentage except for systolic blood pressure and diastolic blood pressure which are mean (95% confidence interval).
3.1. Prevalence of high BP
Among US adults not taking antihypertensive medication, the prevalence of high BP was 32.0% (95%CI: 29.6%, 34.4%) (Figure 1, left panel and Table 2, left panel). If SBP and DBP were measured to be 5 and 3.5 mm Hg higher, respectively, than the levels estimated as in NHANES, 44.4% (95%CI: 42.6%, 46.2%) of US adults not taking antihypertensive medication would be estimated to have high BP, representing an increase of 12.4% (95%CI: 10.6%, 14.5%) or 23.6 (95%CI: 19.5, 27.6) million US adults. Assuming SBP and DBP were measured 5 and 3.5 mm Hg lower, respectively, than the levels estimated in NHANES, 21.9% (95%CI: 19.5%, 24.4%) of US adults not taking antihypertensive medication would have high BP, representing a decrease of 10.1% (95%CI: 8.9%, 11.4%) or 19.2 (95%CI: 16.7, 21.7) million fewer US adults. If SBP and DBP measurements were 10 and 7 mm Hg, respectively, higher and lower than the NHANES estimates, the prevalence of high BP would be 29.0% (95%CI: 26.7%, 31.4%) higher and 16.6% (95%CI: 14.6%, 18.7%) lower than when estimated by NHANES.
FIGURE 1.
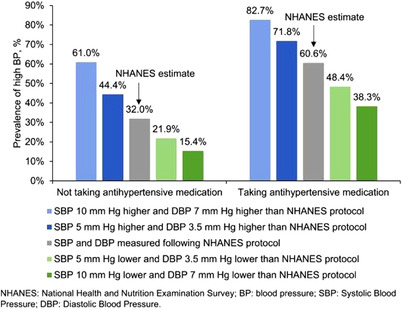
Estimated proportion of US adults with systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 80 mm Hg when blood pressure is measured following a standardized protocol and when it is measured with bias
TABLE 2.
Estimated prevalence and number of US adults with systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg when blood pressure was measured following the NHANES protocol and when it is measured with bias
| US adults not taking antihypertensive medication | US adults taking antihypertensive medication | |||||
|---|---|---|---|---|---|---|
| BP measured following protocol | BP measured following protocol | |||||
| Yes | No – BP measured with bias | Difference (95% CI) | Yes | No – BP measured with bias | Difference (95% CI) | |
| Bias | 5 mm Hg in SBP and 3.5 mm Hg in DBP | 5 mm Hg in SBP and 3.5 mm Hg in DBP | ||||
| NHANES estimate | Higher BP | NHANES estimate | Higher BP | |||
|
Prevalence (95% CI), % |
32.0 (29.6, 34.4) | 44.4 (42.6, 46.2) | 12.4 (10.6, 14.5) | 60.6 (57.2, 63.9) | 71.8 (68.3, 75.0) | 11.2 (9.0, 13.8) |
| N (95% CI), millions | 60.8 (54.6, 61.7) | 84.4 (77.7, 91.1) | 23.6 (19.5, 27.6) | 34.5 (29.8, 39.2) | 40.9 (35.5, 46.3) | 6.4 (4.8, 7.9) |
| NHANES estimate | Lower BP | NHANES estimate | Lower BP | |||
|
Prevalence (95% CI), % |
32.0 (29.6, 34.4) | 21.9 (19.5, 24.4) | ‐10.1 (‐11.4, ‐8.9) | 60.6 (57.2, 63.9) | 48.4 (44.6, 52.2) | ‐12.2 (‐15.1, ‐9.8) |
| N (95% CI), millions | 60.8 (54.6, 61.7) | 41.6 (35.9, 47.3) | ‐19.2 (‐21.7, ‐16.7) | 34.5 (29.8, 39.2) | 27.6 (23.5, 31.6) | ‐6.9 (‐8.7, ‐5.2) |
| Bias | 10 mm Hg in SBP and 7 mm Hg in DBP | 10 mm Hg in SBP and 7 mm Hg in DBP | ||||
| NHANES estimate | Higher BP | NHANES estimate | Higher BP | |||
|
Prevalence (95% CI), % |
32.0 (29.6, 34.4) | 61.0 (59.0, 62.9) | 29.0 (26.7, 31.4) | 60.6 (57.2, 63.9) | 82.7 (79.4, 85.6) | 22.1 (19.3, 25.2) |
| N (95% CI), millions | 60.8 (54.6, 61.7) | 116.0 (105.5, 126.5) | 55.2 (48.2, 62.2) | 34.5 (29.8, 39.2) | 47.1 (40.6, 53.6) | 12.6 (10.0, 15.2) |
| NHANES estimate | Lower BP | NHANES estimate | Lower BP | |||
|
Prevalence (95% CI), % |
32.0 (29.6, 34.4) | 15.4 (13.4, 17.6) | ‐16.6 (‐18.7, ‐14.6) | 60.6 (57.2, 63.9) | 38.3 (35.2, 41.5) | ‐22.3 (‐24.5, ‐20.2) |
| N (95% CI), millions | 60.8 (54.6, 61.7) | 29.3 (25.1, 33.5) | ‐31.5 (‐36.3, ‐26.7) | 34.5 (29.8, 39.2) | 21.8 (18.7, 24.9) | ‐12.7 (‐14.8, ‐10.6) |
Abbreviations: CI, Confidence interval; BP, Blood pressure; NHANES, National Health and Nutrition Examination Survey; SBP, Systolic blood pressure; DBP, Diastolic blood pressure.
The prevalence of high BP among US adults taking antihypertensive medications was 60.6% (95%CI: 57.2%, 63.9%) (Figure 1 right panel and Table 2, right panel). If SBP and DBP were measured to be 5 and 3.5 mm Hg higher, respectively, than the values estimated in NHANES, 71.8% (95%CI: 68.3%, 75.0%) of US adults taking antihypertensive medications would have high BP, representing an increase of 11.2% (95%CI: 9.0%, 13.8%) or 6.4 (95%CI: 4.8, 7.9) million US adults. If SBP and DBP were measured to be 5 and 3.5 mm Hg lower, respectively, 48.4% (95%CI: 44.6%, 52.2%) of US adults would be estimated to have high BP, representing a decrease of 12.2% (95%CI: 9.8%, 15.1%) or 6.9 (95%CI: 5.2, 8.7) million fewer US adults. If SBP and DBP were estimated to be 10 and 7 mm Hg, respectively, higher or lower than the NHANES estimates, the prevalence of high BP among US adults taking antihypertensive medications would be 22.1% (95%CI: 19.3%, 25.2%) higher or 22.3% (95%CI: 20.2%, 24.5%) lower than when estimated by NHANES.
Estimated differences in the prevalence of high BP among US adults < 65 years of age and ≥ 65 years of age when SBP and DBP were measured with bias versus as estimated in NHANES are presented in Table S1 and estimated differences for those without and with obesity are presented in Table S2. Estimated differences in the prevalence of high BP when only SBP and only DBP, were measured with bias versus as estimated in NHANES are presented in Table S3.
3.2. BP measured with random error
Among US adults not taking antihypertensive medication, 21.4% (95% CI 19.8%, 23.0%), including 15.4% (95%CI: 14.0%, 16.7%) without high BP and 6.0% (95%CI: 5.1%, 6.9%) with high BP when BP was measured without random error, had their high BP status re‐categorized when BP was measured with random error (Figure 2). Among US adults taking antihypertensive medication, 20.5% (95%CI: 17.7%, 23.3%), including 11.4% (95%CI: 9.4%, 13.4%) without high BP and 9.1% (95%CI: 7.2%, 11.0%) with high BP when BP was measured without random error, had their high BP status re‐categorized when BP was measured with random error. If BP was measured with random error, the prevalence of high BP among US adults not taking and taking antihypertensive medication increased by 9.4% (95%CI: 5.8%, 12.9%) and 2.2% (95%CI: ‐3.0%, 7.3%), respectively (Table S4). The proportion and number of US adults whose high BP status would be re‐categorized when BP is measured with random error and bias are presented in Figure S3 with the difference in the prevalence of high BP presented in Table S5. The prevalence of high BP among US adults not taking and taking antihypertensive medication increased by 7.6% (95%CI: 4.0%, 11.2%) and 3.0% (95%CI: ‐2.2%, 8.3%), respectively, when only SBP was measured with random error, and by 4.7% (95%CI: 1.2%, 8.2%) and 1.8% (95%CI: ‐3.1%, 6.7%), respectively, when only DBP was measured with random error (Table S6). The proportion and number of US adults whose high BP status would be re‐categorized when only SBP and only DBP were measured with random error are presented in Figure S4.
FIGURE 2.
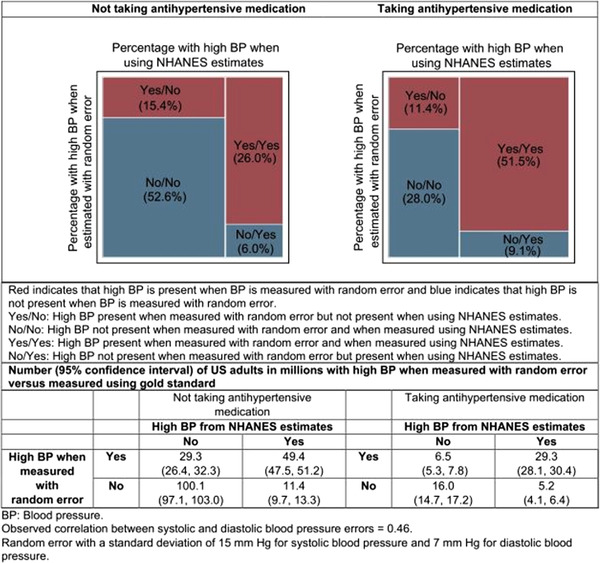
Estimated proportion of US adults with systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 80 mm Hg when blood pressure is measured following a standardized protocol cross‐classified by measurements obtained with random error
3.3. Prevalence of high BP when defined by SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg
Among US adults not taking antihypertensive medication, when SBP and DBP were measured 5 and 3.5 mm Hg higher and lower than the NHANES estimates, the prevalence of high BP was 5.6% (95%CI: 4.3%, 7.2%) higher and 4.5% (95%CI: 3.5%, 5.9%) lower than the NHANES estimate, respectively (Table S7). Among US adults taking antihypertensive medication, the prevalence of high BP when SBP and DBP were measured 5 and 3.5 mm Hg higher and lower than the NHANES estimates, was 9.5% (95%CI: 7.2%, 12.5%) higher and 9.7% (95%CI: 7.7%, 12.4%) lower, respectively. When BP was estimated to be measured with random error with standard deviations of 15 mm Hg and 7 mm Hg for SBP and DBP, 13.2% (95%CI: 11.8, 14.6) and 19.9% (95%CI: 17.3, 22.5) of US adults not taking and taking antihypertensive medication, respectively, had their high BP status re‐categorized (Figure S5). The prevalence of high BP was 7.4% (95%CI: 4.3%, 10.5%) and 4.9% (95%CI: ‐0.2%, 10.0%) higher for those not taking and taking antihypertensive medication, respectively, compared with the NHANES estimates (Table S8).
4. DISCUSSION
In the current study, it was estimated that the prevalence of high BP may be over‐ or under‐estimated substantially among US adults if BP were measured with modest upward or downward systematic bias as compared to when BP is measured following a standardized protocol. In addition to the impact of systematic bias, it was estimated that random error in BP measurement could also result in the misclassification of high BP for a high proportion of US adults. It was estimated that tens of millions of US adults could potentially have their high BP status affected due to systematic bias or random error in the measurement of BP.
A number of studies have reported that BP measured in routine clinical practice is higher than when measurements are obtained following a standardized protocol. 5 , 6 , 7 One study compared BP measurements from 275 patients in outpatient practices with subsequent measurements obtained on the same day following the BP measurement protocol used in the Systolic Blood Pressure Intervention Trial (SPRINT). The mean SBP and DBP measured in clinical practice was 12.7 (95%CI: 10.7, 14.7) mm Hg and 12.0 (95% 10.7, 13.4) mm Hg higher, respectively, than estimates obtained following the SPRINT protocol. 7 Also, among 802 Multi‐Ethnic Study of Atherosclerosis participants, SBP and DBP were 6.0 (95%CI: 4.2, 7.8) mm Hg and 3.5 (95%CI: 2.5, 4.4) mm Hg higher, respectively, during a routine outpatient visit versus the closest study visit, where a protocol was followed to measure BP. 6 As estimated in the current study, the differences in SBP and DBP when measurements are obtained without following a standardized protocol may result in the misclassification of high BP status for a large proportion of US adults.
In the current study, the impact of bias or random error in BP measurement was larger around the SBP/DBP threshold of 130/80 mm Hg, as compared to 140/90 mm Hg, due to more US adults having a BP around this threshold. This is consistent with a study from rural China that reported when SBP and DBP were estimated to be 2 and 1 mm Hg higher than their measured values, respectively, the prevalence of hypertension defined by the SBP/DBP threshold of 130/80 mm Hg and/or taking antihypertensive medication increased from 56.2% to 61.9% (Δ = 5.7%) and when using the threshold of 140/90 mm Hg, the prevalence of hypertension increased from 33.4% to 37.3% (Δ = 3.9%). 22 , 23 A prior study using data from the US NHANES II reported reduction in the prevalence of diastolic hypertension (ie, DBP ≥ 90 mm Hg) by 17% when DBP was modelled to be 2 mm Hg lower as compared to when measured following the NHANES protocol. 24 In addition to providing more contemporary data, evaluating the effect of bias and random error in SBP and DBP, the current study quantified the population level impact of small to moderate biases and random errors in measurement of BP on the prevalence of high BP among US adults by age and obesity status.
There are a number of patient, technician and device‐related factors that may result in BP readings being systematically or randomly different than the true value. 25 , 26 Over‐estimation of BP may result from not following the recommended steps for measuring BP, 25 or using a single BP measurement as compared to the average of two or three measurements over multiple visits as recommended by clinical practice guidelines. 4 , 27 Under‐estimation of BP may be due to use of an oversized cuff, a fast cuff deflation rate, and hearing deficits of the observer if using a manual device. 25 Random error may result from not following the same protocol consistently, or terminal digit preference. 28 , 29 Following a standardized protocol, having trained individuals measure BP, 30 use of validated devices 18 and taking the average of multiple BP measurements 3 , 4 can help minimize both systematic bias and random errors and provide a more precise BP measurement.
Prior studies suggest a widespread lack of proficiency in following a standardized protocol for measuring BP by medical students, clinicians and medical staff. 31 , 32 A survey of 2302 medical professionals conducted by the American Medical Association and AHA reported more than half reported never receiving re‐training after learning to measure BP in school. 33 Measuring BP correctly involves properly preparing a patient by seating them with their back and arm supported, feet flat on floor and legs uncrossed followed by selecting an appropriate size cuff, placing it correctly over patient's bare arm, having a rest period prior to obtaining an initial BP measurement and between measurements and instructing the patient to relax and not talk when their BP is being measured. 4 To ensure BP is measured correctly, re‐training of clinicians and medical staff should occur on a regular basis. 4 , 34 , 35
It is often difficult to measure BP accurately in obese and older individuals. According to the current study, not measuring BP accurately may result in the misclassification of high BP for a large proportion of obese and non‐obese individuals and younger and older adults. Overestimation of BP among obese individuals due to use of under‐sized cuffs, may lead to the over‐use of antihypertensive medication. 12 A study of 305 participants with BP measured three times using an oscillometric device and a mercury device in random order showed that the discrepancy in BP measurements from the two devices was larger in participants aged 75–86 years versus 48–64 years. 36 Accurately measuring BP is important to prevent adverse outcomes of over‐ or undertreatment in these populations.
Education programs have been ineffective in improving the accuracy in BP measurements. 37 The development and implementation of quality control metrics by regulatory and accreditation committees in partnership with policy makers, insurance providers and healthcare systems may result in more accurate BP measurements being obtained as part of routine clinical care. 38
The current study has several strengths. NHANES provides nationally representative estimates for the non‐institutionalized US population. BP was measured following a standardized protocol. Rigorous training and re‐training of physicians on the BP measurement protocol was performed. The results of this study should be interpreted in the context of known and potential limitations. BP was measured at a single visit in NHANES. Clinical practice guidelines recommend the diagnosis of hypertension be based on the mean BP over two or more visits. 3 , 4 BP was measured using a mercury sphygmomanometer, an approach not used in most clinical practice settings. However, a recent comparison study between manual and oscillometric BP measurement devices in NHANES 2017–2018 found a small difference in SBP and DBP (< 2 mm Hg) when a standardized protocol is followed. 39 Finally, bias and random error were assumed to be uniform across all levels of mean BP. However, it is possible that these may be larger at higher BP levels.
The current study estimated that tens of millions of US adults may have their high BP status misclassified when BP is measured with a systematic error. Also, the high BP status for tens of millions of US adults could be affected even when BP is measured without bias but with random error. These data emphasize the need for healthcare providers to use validated BP measurement devices, receive ongoing training and re‐training in the measurement of BP and to follow a standardized protocol to ensure the accurate assessment of BP.
FUNDING
Dr. Hardy receives support through R01HL139716 from the National Heart Lung and Blood Institute (NHLBI). Dr. Jaeger receives support through R01HL144773 from the NHLBI and 15SFRN2390002 from the American Heart Association. Dr. Shimbo receives support through R01HL139716 and K24HL125704 from the NHLBI. Dr. Bress is supported by R01AG065805, K01HL133468, and R01HL139837 from the NHLBI. The funders had no role in the design and conduct of the study; collection, management, analysis, interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICT OF INTEREST
Dr. Bress receives research funding to their institution from Amarin Corporation and Amgen, Inc. unrelated to the current manuscript. No other disclosures were reported.
AUTHOR CONTRIBUTION
Concept and design: Sakhuja, Muntner. Acquisition, analysis, or interpretation of data: Sakhuja. Drafting of the manuscript: Sakhuja. Critical revision of the manuscript for important intellectual content: All authors.Statistical analysis: Sakhuja, Jaeger. Administrative, technical, or material support: Muntner. Supervision: Muntner
Supporting information
Supporting Information
ACKNOWLEDGMENTS
None.
Sakhuja S, Jaeger BC, Akinyelure OP, et al. Potential impact of systematic and random errors in blood pressure measurement on the prevalence of high office blood pressure in the United States. J Clin Hypertens. 2022;24:263–270. 10.1111/jch.14418
REFERENCES
- 1. Wright IS, Schneider RF, Ungerleider HE. Factors of error in blood pressure readings: a survey of methods of teaching and interpretation. Am Heart J. 1938;16(4):469‐476. [Google Scholar]
- 2. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Hypertension. 2005;45(1):142‐161. [DOI] [PubMed] [Google Scholar]
- 3. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35‐e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13‐e115. [DOI] [PubMed] [Google Scholar]
- 5. Drawz PE, Agarwal A, Dwyer JP, et al. Concordance between blood pressure in the systolic blood pressure intervention trial and in routine clinical practice. JAMA Intern Med. 2020;180(12):1655‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmad FS, Chan C, Rosenman MB, et al. Validity of cardiovascular data from electronic sources: the multi‐ethnic study of atherosclerosis and HealthLNK. Circulation. 2017;136(13):1207‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agarwal R. Implications of blood pressure measurement technique for implementation of Systolic Blood Pressure Intervention Trial (SPRINT). J Am Heart Assoc. 2017;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang O, Juraschek SP, Appel LJ, et al. Comparison of automated clinical and research blood pressure measurements: implications for clinical practice and trial design. J Clin Hypertens (Greenwich, Conn). 2018;20(12):1676‐1682. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muntner P, Carey RM, Gidding S, et al. Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol. 2018;71(2):109‐118. Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson LA, Goodman RA, Holtzman D, Posner SF, Northridge ME. Aging in the United States: opportunities and challenges for public health. Am J Public Health. 2012;102(3):393‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state‐level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440‐2450. [DOI] [PubMed] [Google Scholar]
- 12. Fonseca‐Reyes S, de Alba‐García JG, Parra‐Carrillo JZ, Paczka‐Zapata JA. Effect of standard cuff on blood pressure readings in patients with obese arms. How frequent are arms of a ‘large circumference’?. Blood Press Monit. 2003;8(3):101‐106. [DOI] [PubMed] [Google Scholar]
- 13. Reddy AK, Jogendra MR, Rosendorff C. Blood pressure measurement in the geriatric population. Blood Press Monit. 2014;19(2):59‐63. [DOI] [PubMed] [Google Scholar]
- 14. National High Blood Pressure Education Program (NHBPEP)/National Heart, Lung, And Blood Institute (NHLBI) and American Heart Association (AHA) working meeting on blood pressure measurement . Summary Report. National Institutes of Health (NIH). https://www.nhlbi.nih.gov/files/docs/resources/heart/bpmeasu.pdf. Accessed: February 02, 2021
- 15. National Health And Nutrition Examination Survey NHANES 2017‐2018. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017. Accessed: February 11, 2021.
- 16. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blood pressure section of the physician examination NHANES 2017–2018. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017. Accessed: February 11, 2021.
- 18. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018;36(3):472‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen TW, Thijs L, Li Y, et al. Prognostic value of reading‐to‐reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55(4):1049‐1057. [DOI] [PubMed] [Google Scholar]
- 20. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 21. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 22. Fan WG, Xie F, Wan YR, Campbell NRC, Su H. The impact of changes in population blood pressure on hypertension prevalence and control in China. J Clin Hypertens (Greenwich, Conn). 2020;22(2):150‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campbell NRC, Padwal R, Picone DS, Su H, Sharman JE. The impact of small to moderate inaccuracies in assessing blood pressure on hypertension prevalence and control rates. J Clin Hypertens (Greenwich, Conn). 2020;22(6):939‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155(7):701‐709. [PubMed] [Google Scholar]
- 25. Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients' resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017;35(3):421‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Houweling S, Kleefstra N, Lutgers H, Groenier K, BM‐d Jong, Bilo H. Pitfalls in blood pressure measurement in daily practice. Fam Pract. 2005;23(1):20‐27. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz JE, Muntner P, Kronish IM, et al. Reliability of office, home, and ambulatory blood pressure measurements and correlation with left ventricular mass. J Am Coll Cardiol. 2020;76(25):2911‐2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whitlock G, Clark T, Vander Hoorn S, et al. Random errors in the measurement of 10 cardiovascular risk factors. Eur J Epidemiol. 2001;17(10):907‐909. [DOI] [PubMed] [Google Scholar]
- 29. Niyonsenga T, Vanasse A, Courteau J, Cloutier L. Impact of terminal digit preference by family physicians and sphygmomanometer calibration errors on blood pressure value: implication for hypertension screening. J Clin Hypertens (Greenwich, Conn). 2008;10(5):341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rabbia F, Testa E, Rabbia S, et al. Effectiveness of blood pressure educational and evaluation program for the improvement of measurement accuracy among nurses. High Blood Pressure Cardiovasc Prevent. 2013;20(2):77‐80. [DOI] [PubMed] [Google Scholar]
- 31. Rakotz MK, Townsend RR, Yang J, et al. Medical students and measuring blood pressure: results from the American Medical Association Blood Pressure Check Challenge. J Clin Hypertens (Greenwich, Conn). 2017;19(6):614‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drevenhorn E, Hakansson A, Petersson K. Blood pressure measurement–an observational study of 21 public health nurses. J Clin Nurs. 2001;10(2):189‐194. [DOI] [PubMed] [Google Scholar]
- 33. Blood Pressure Measurement Training Research Report. American Medical Association and American Heart Association. 2019. https://www.ama‐assn.org/system/files/2019‐11/market‐research‐survey‐bp‐measurement.pdf. Accessed: March 2, 2021.
- 34. Campbell NRC, Khalsa T, Ordunez P, et al. Brief online certification course for measuring blood pressure with an automated blood pressure device. A free new resource to support World Hypertension Day Oct 17, 2020. J Clin Hypertens. (Greenwich, Conn). 2020;22(10):1754‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pan American Health Organization/World Health Organization . Virtual Course on accurate automated blood pressure measurement (2020). https://www.campusvirtualsp.org/en/node/29166. Accessed November 04, 2021.
- 36. Ni H, Wu C, Prineas R, et al. Comparison of Dinamap PRO‐100 and mercury sphygmomanometer blood pressure measurements in a population‐based study. Am J Hypertens. 2006;19(4):353‐360. [DOI] [PubMed] [Google Scholar]
- 37. Steinman MA, Fischer MA, Shlipak MG, et al. Clinician awareness of adherence to hypertension guidelines. Am J Med. 2004;117(10):747‐754. [DOI] [PubMed] [Google Scholar]
- 38. Improving the measurement of blood pressure: is it time for regulated standards? Ann Intern Med. 2011;154(12):838‐839 [DOI] [PubMed] [Google Scholar]
- 39. Ostchega Y, Nwankwo T, Chiappa M, Wolz M, Graber J, Nguyen DT, Comparing blood pressure values obtained by two different protocols: National Health and Nutrition Examination Survey, 2017–2018. Journal Issue. 01/2021 Vital and health statistics. Series 2, Data evaluation and methods research; no. 187. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


