Abstract
Hypertension is the most common comorbidity in patients with coronavirus disease 2019 (COVID‐19) and increases in‐hospital mortality. Day‐by‐day blood pressure (BP) variability (BPV) is associated with clinical outcomes in hypertensive patients. However, little information is available on the association of BPV with the outcomes of COVID‐19 patients with hypertension. This study aimed to demonstrate whether day‐by‐day in‐hospital BPV had prognostic significance in these patients. The authors included 702 COVID‐19 patients with hypertension from Huoshenshan Hospital (Wuhan, China), who underwent valid in‐hospital BP measurements on at least seven consecutive days. Day‐by‐day BPV was assessed by standard deviation (SD), coefficient of variation (CV), and variation independent of mean (VIM). Overall, patients with severe COVID‐19 and non‐survivors had higher BPV than moderate cases and survivors, respectively. Additionally, higher BPV was correlated with greater age and higher levels of C‐reactive protein, procalcitonin, high‐sensitive cardiac troponin I, and B‐type natriuretic peptide. In multivariable Cox regression, SD of systolic BP (SBP) was predictive of mortality [hazard ratio (HR) 1.17, 95% confidence interval (CI) 1.05–1.30] as well as acute respiratory distress syndrome (ARDS) (HR 1.09, 95% CI 1.01–1.16). Similar trends were observed for CV and VIM of SBP, but not indices of diastolic BP variability. The authors demonstrated that day‐by‐day in‐hospital SBP variability can independently predict mortality and ARDS in COVID‐19 patients with hypertension. And high BPV might be correlated with severe inflammation and myocardial injury. Further studies are needed to clarify whether early reduction of BPV will improve the prognosis of these patients.
Keywords: blood pressure variability, COVID‐19, hypertension, mortality, prognosis
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has quickly developed into a global pandemic since December 2019. With high transmission and lack of specific treatment, more than 200 million COVID‐19 cases have been confirmed worldwide, and four million patients had progressed to death as of September 2021, which poses a great threat to global health. SARS‐CoV‐2 mainly attacks the respiratory system, with some patients progressing to acute respiratory distress syndrome (ARDS). 1 As reported, patients with comorbidities are more likely to develop severe pneumonia and adverse clinical outcomes. 2 , 3 Hypertension has been reported to be the most common comorbidity in COVID‐19 patients and it is associated with pronounced increase in disease severity and the risk of mortality. 4 , 5 Hence, it should be treated to prevent adverse outcomes.
Blood pressure (BP) monitoring in clinical settings or out of office has generally been used for clinical management of hypertension. Recently, blood pressure variability (BPV), which represents the fluctuation of BP in a period of time, has been identified as an accurate assessment of BP that helps to avoid oscillations of mean BP. 6 Increasing evidence suggests that BPV is associated with higher risk of incident cardiovascular diseases and all‐cause mortality, which is independent of mean BP. 7 , 8 , 9 Day‐by‐day BP measurement is commonly used to assess mid‐term BPV, which provides incremental value of risk assessment over BP level. 6
However, it remains to be determined whether BPV correlates with adverse outcomes in COVID‐19 patients with hypertension. Therefore, in this study, we investigated 702 COVID‐19 patients with hypertension who were consecutively admitted to Huoshenshan Hospital in Wuhan, China. The aim of the study was to investigate the relationships between BPV and background factors, and further demonstrate whether day‐by‐day in‐hospital BPV had prognostic significance in these patients.
2. METHODS
2.1. Study design and patients
This was a retrospective cohort study of consecutive patients at Huoshenshan Hospital (Wuhan, China) from February 4, 2020 to April 11, 2020. The Huoshenshan Hospital was established by the government as a designated center to provide medical care for patients with COVID‐19. The inclusion criteria for this study were patients (1) aged at least 18 years, (2) diagnosed with confirmed COVID‐19, and (3) having a history of hypertension prior to admission. The exclusion criteria were patients with valid BP measurements on less than seven consecutive days during hospitalization.
The diagnosis and severity categorization of COVID‐19 in the study patients were based on the World Health Organization interim guidance, 10 and the diagnosis and treatment protocol for novel coronavirus pneumonia provided by the Chinese National Health Commission. 11 Patients with suspected COVID‐19 were admitted to the hospital to confirm SARS‐CoV‐2 infection. Throat swabs and plasma samples were collected to detect new coronavirus nucleic acids (ORF1ab gene and the N gene) by reverse transcriptase‐polymerase chain reaction, and specific IgM and IgG by the immune colloidal gold technique.
Hypertensive patients were defined as patients diagnosed with hypertension before admission to our hospital for management of COVID‐19. 12 History of hypertension was collected from electronic medical records. The study was approved by the Human Ethics Committee of Huoshenshan Hospital (No. HSSLL023) and complied with the Declaration of Helsinki. Oral informed consent was obtained from each patient at enrollment. Written consent was waived by the ethics committee in view of urgency of data collection amid outbreaks of the infectious disease.
2.2. Data collection
Patients’ demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records. All data were checked independently by two physicians. The laboratory parameters included leukocyte count, neutrophil count, lymphocyte count, D‐dimer, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, lactate dehydrogenase (LDH), high‐sensitive cardiac troponin I (hs‐TnI), B‐type natriuretic peptide (BNP), high‐sensitive C‐reactive protein (hs‐CRP), and procalcitonin (PCT). Reference values for the laboratory parameters were based on the results that are seen in 95% of the healthy population. The choice of antihypertensive treatment during hospitalization was at the physician's discretion, and anti‐hypertensive drugs included renin‐angiotensin‐aldosterone system inhibitors (angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker), β‐blocker, calcium channel blocker, and diuretic. Antiviral therapy included arbidol, oseltamivir, and ribavirin.
2.3. BP measurement and variability
Day‐by‐day BP measurements were collected from the electronic nursing records. Trained nurses measured the BP of patients daily between 6:00 a.m. and 9:00 a.m. (before breakfast and intake of anti‐hypertensive drugs) using an automatic cuff sphygmomanometer (Omron HEM‐7122, Omron Healthcare, Kyoto, Japan). 13 , 14 Two BP measurements were taken at 15‐s intervals and the average BP was recorded in the medical documents. BP measurements of the first 14 days were included in the BPV analyses. BP measurements with systolic BP < 70 or > 250 mm Hg, or diastolic BP < 40 or > 140 mm Hg were discarded to retain physiologically meaningful measurements in the analysis and to ensure the reliability of BPV indexes. 15 Day‐by‐day BPV was evaluated using three indices including the standard deviation (SD) of BP, coefficient of variation (CV) of BP, and variation independent of mean (VIM). 16 CV was calculated using the formula: CV = SD/mean×100. VIM was calculated as follows: VIM = Mx×SD/meanx, where M is the average of mean systolic BP (SBP) or diastolic BP (DBP) among the study patients, and x was derived from the fitting curve (SD = k×meanx). 17
2.4. Clinical outcomes
For this study, all patients were hospitalized and had definite outcomes. The criteria for discharge were absence of fever for more than 3 days, obvious remission of respiratory symptoms, obvious resolution of inflammation on pulmonary imaging, and two consecutive respiratory tract swab samples negative for nuclei acid and obtained at least 24 h apart. 11 The primary endpoint was in‐hospital mortality. The secondary endpoint was ARDS, which was diagnosed based on the Berlin definition. 18 Onset date was defined as the day when COVID‐19 symptoms were first noticed. The final follow‐up date was April 11, 2020.
2.5. Statistical analysis
Continuous variables with normal distribution were presented as mean ± SD, and those with non‐normal distribution were presented as median (interquartile range, IQR). Categorical variables were presented as counts (percentages). Two continuous variables were compared with independent Student's t‐test or Mann‐Whitney U‐test, as appropriate. Categorical variables were compared with χ2 test or Fisher's exact test. Correlation analyses between BPV and background data were calculated using Spearman's rank correlation. The mortality between groups stratified by BPV median was compared using Kaplan–Meier plots and log‐rank tests. 19 Hazard ratios (HRs) with 95% confidence intervals (CIs) of BPV were calculated using univariable and multivariable Cox proportional hazard models. Previous studies have demonstrated the risk factors for critical or fatal cases in COVID‐19. 4 , 5 , 20 , 21 These confounders were included in the univariable Cox regression analysis, and variables with p < .1 were entered to the stepwise multivariable Cox regression. p value < .05 was considered statistically significant. All statistical analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0 (Inc., La Jolla, USA).
3. RESULTS
A total of 2864 hospitalized adult patients were diagnosed with COVID‐19 in the study center from February 4, 2020 to April 11, 2020. Among these patients, 867 had preexisting hypertension. After excluding 165 patients without blood pressure measurement on seven consecutive days, 702 patients were included in the final analysis (Figure 1). The median age of the patients was 66.0 (58.0, 73.0) years, and 367 (52.2%) patients were males. The median time from illness onset to discharge was 44.0 (32.0, 55.3) days. The median number of days of BP measurement in the hospital was 13.0 (9.0, 14.0) days.
FIGURE 1.
Study flow chart
3.1. Comparison between patients stratified by disease severity and survival
Among the study patients, 418 (59.5%) were diagnosed with moderate COVID‐19, whereas 284 (40.5%) were severe or critical cases (Table 1). Compared with moderate cases, patients with severe and critical cases were older [69.5 (62.0, 76.0) vs. 65.0 (57.0, 71.0) years, p < .001], had higher proportions of comorbidities, including diabetes, asthma, COPD; and were more likely to receive diuretic for the antihypertensive treatment. For laboratory findings, severe and critical cases had higher leukocyte count, hs‐CRP, and PCT levels, indicating enhanced inflammatory responses. In addition, LDH, hs‐TnI, and BNP levels were much higher in severe and critical cases, as a result of cardiac involvement during SARS‐CoV‐2 infection. For BP measurement, compared with moderate cases, severe and critical cases had lower mean DBP, but higher BPV, as follows: SD SBP, CV SBP, VIM SBP, and CV DBP. However, no significant difference in mean SBP was observed between the two cohorts.
TABLE 1.
Demographics and clinical characteristics of hypertensive patients stratified by severity and survival of COVID‐19
| Variable | Total (n = 702) | Moderate (n = 418) | Severe and critical (n = 284) | p‐value | Survivor (n = 680) | Non‐survivor (n = 22) | p‐value |
|---|---|---|---|---|---|---|---|
| Age (years) | 66.0 (58.0, 73.0) | 65.0 (57.0, 71.0) | 69.5 (62.0, 76.0) | <.001 | 66.0 (58.0, 73.0) | 71.5 (65.0, 78.8) | .012 |
| Male, n (%) | 367 (52.2) | 205 (49.0) | 162 (57.0) | .037 | 352 (51.8) | 15 (68.2) | .129 |
| Smoking history, n (%) | 44 (6.3) | 24 (5.7) | 20 (7.0) | .485 | 43 (6.3) | 1 (4.5) | 1.000 |
| Symptoms, n (%) | |||||||
| Fever | 490 (69.8) | 293 (70.1) | 197 (69.4) | .836 | 476 (70.0) | 14 (63.6) | .522 |
| Cough | 471 (67.1) | 272 (65.1) | 199 (70.1) | .167 | 457 (67.2) | 14 (63.6) | .726 |
| Dyspnea | 274 (39.0) | 158 (37.8) | 116 (40.8) | .417 | 267 (39.3) | 7 (31.8) | .481 |
| Muscle ache | 178 (25.4) | 104 (24.9) | 74 (26.1) | .725 | 172 (25.3) | 6 (27.3) | .834 |
| Diarrhea | 30 (4.3) | 18 (4.3) | 12 (4.2) | .959 | 29 (4.3) | 1 (4.5) | 1.000 |
| Comorbidities, n (%) | |||||||
| Diabetes | 177 (25.2) | 93 (22.2) | 84 (29.6) | .028 | 173 (25.4) | 4 (18.2) | .440 |
| Coronary heart disease | 82 (11.7) | 44 (10.5) | 38 (13.4) | .248 | 76 (11.2) | 6 (27.3) | .048 |
| Asthma | 37 (5.3) | 16 (3.8) | 21 (7.4) | .038 | 34 (5.0) | 3 (13.6) | .194 |
| COPD | 32 (4.6) | 13 (3.1) | 19 (6.7) | .026 | 28 (4.1) | 4 (18.2) | .010 |
| Chronic kidney disease | 19 (2.7) | 8 (1.9) | 11 (3.9) | .116 | 16 (2.4) | 3 (13.6) | .019 |
| Tumor | 18 (2.6) | 11 (2.6) | 7 (2.5) | .891 | 17 (2.5) | 1 (4.5) | .440 |
| Laboratory findings | |||||||
| Leukocyte count (10 9 /L) | 5.96 (4.90, 7.38) | 5.70 (4.70, 7.00) | 6.51 (5.00, 8.25) | <.001 | 5.90 (4.85, 7.20) | 9.05 (5.49, 13.5) | <.001 |
| Neutrophil count (10 9 /L) | 3.73 (2.81, 5.02) | 3.49 (2.73, 4.58) | 4.24 (3.04, 6.10) | <.001 | 3.67 (2.78, 4.95) | 7.84 (4.39, 12.2) | <.001 |
| Lymphocyte count (10 9 /L) | 1.44 (1.02, 1.79) | 1.54 (1.15, 1.93) | 1.27 (0.82, 1.64) | <.001 | 1.46 (1.05, 1.82) | 0.75 (0.44, 1.01) | <.001 |
| D‐dimer (mg/L) | 0.53 (0.30, 1.17) | 0.41 (0.23, 0.77) | 0.86 (0.45, 1.83) | <.001 | 0.51 (0.29, 1.10) | 3.66 (2.41, 7.24) | <.001 |
| ALT (u/L) | 21.9 (14.6, 36.8) | 21.4 (14.2, 35.3) | 22.6 (15.2, 38.7) | .121 | 21.5 (14.4, 35.9) | 38.2 (20.1, 67.3) | <.001 |
| AST (u/L) | 19.7 (15.5, 26.7) | 18.6 (15.1, 23.8) | 21.5 (16.6, 32.1) | <.001 | 19.5 (15.4, 25.9) | 45.5 (32.1, 56.4) | <.001 |
| Creatinine (μmol/L) | 65.5 (55.8, 78.9) | 65.5 (56.3, 78.1) | 65.5 (54.3, 79.5) | .950 | 65.4 (55.8, 78.4) | 79.5 (54.2, 140.5) | .026 |
| LDH (u/L) | 190.1 (160.0, 230.4) | 175.3 (155.2, 206.4) | 207.9 (174.5, 264.5) | <.001 | 188.6 (159.2, 224.7) | 413.1 (280.2, 523.8) | <.001 |
| hs‐TnI (ng/ml) | 0.01 (0.01, 0.02) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.03) | <.001 | 0.01 (0.01, 0.01) | 0.06 (0.02, 0.33) | <.001 |
| BNP (pg/ml) | 15.6 (0.01, 60.7) | 0.01 (0.01, 36.5) | 41.9 (0.01, 127.7) | <.001 | 14.0 (0.01, 55.8) | 103.8 (43.2, 321.9) | <.001 |
| hs‐CRP (mg/L) | 3.37 (1.17, 14.6) | 2.13 (0.92, 6.95) | 7.87 (2.14, 34.4) | <.001 | 3.06 (1.15, 12.78) | 121.1 (51.7, 135.9) | <.001 |
| PCT (ng/ml) | 0.06 (0.03, 0.16) | 0.05 (0.03, 0.12) | 0.07 (0.04, 0.17) | .002 | 0.05 (0.03, 0.14) | 0.38 (0.14, 1.10) | <.001 |
| Treatment, n (%) | |||||||
| ACEI/ARB | 78 (11.1) | 42 (10.0) | 36 (12.7) | .277 | 77 (11.3) | 1 (4.5) | .515 |
| β blocker | 104 (14.8) | 54 (12.9) | 50 (17.6) | .086 | 102 (15.0) | 2 (9.1) | .643 |
| Calcium channel blocker | 406 (57.8) | 237 (56.7) | 169 (59.5) | .460 | 398 (58.5) | 8 (36.4) | .038 |
| Diuretic | 42 (6.0) | 14 (3.3) | 28 (9.9) | <.001 | 40 (5.9) | 2 (9.1) | .867 |
| Antiviral therapy | 305 (43.4) | 167 (40.0) | 138 (48.6) | .023 | 292 (42.9) | 13 (59.1) | .133 |
| Glucocorticoid | 80 (11.4) | 28 (6.7) | 52 (18.3) | <.001 | 71 (10.4) | 9 (40.9) | <.001 |
| Blood pressure measurements | |||||||
| mean SBP (mm Hg) | 130.0 (123.4, 138.0) | 130.1 (123.4, 137.1) | 129.9 (123.4, 138.7) | .867 | 130.0 (123.4, 137.9) | 130.9 (125.5, 140.2) | .815 |
| SD SBP (mm Hg) | 11.6 (9.11, 14.2) | 11.2 (8.80, 13.6) | 11.7 (9.47, 14.8) | .017 | 11.4 (9.07, 14.0) | 16.3 (12.5, 23.4) | <.001 |
| CV SBP (%) | 8.87 (7.01, 10.7) | 8.76 (6.75, 10.4) | 9.05 (7.46, 11.0) | .017 | 8.82 (6.99, 10.6) | 12.4 (9.72, 18.3) | <.001 |
| VIM SBP (units) | 11.6 (9.18, 14.0) | 11.5 (8.82, 13.6) | 11.9 (9.76, 14.5) | .018 | 11.5 (9.13, 13.8) | 16.3 (12.7, 23.9) | <.001 |
| mean DBP (mm Hg) | 78.1 (74.1, 82.9) | 78.9 (74.7, 83.9) | 77.3 (73.2, 81.4) | <.001 | 78.2 (74.2, 82.9) | 74.0 (69.8, 76.5) | .001 |
| SD DBP (mm Hg) | 7.77 (6.49, 9.96) | 7.75 (6.45, 9.72) | 7.90 (6.57, 10.2) | .151 | 7.75 (6.45, 9.84) | 11.2 (8.87, 13.1) | <.001 |
| CV DBP (%) | 9.95 (8.32, 12.4) | 9.77 (8.24, 12.1) | 10.4 (8.45, 13.0) | .013 | 9.88 (8.28, 12.2) | 15.6 (12.0, 17.3) | <.001 |
| VIM DBP (units) | 7.85 (6.55, 9.87) | 7.75 (6.51, 9.64) | 8.03 (6.63, 10.2) | .059 | 7.76 (6.52, 9.70) | 11.4 (9.40, 13.5) | <.001 |
Abbreviations: COVID‐19, coronavirus disease 2019; COPD, Chronic obstructive lung disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; hs‐TnI, high‐sensitive cardiac troponin I; BNP, B‐type natriuretic peptide; hs‐CRP, high‐sensitive C‐reactive protein; PCT, procalcitonin; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; CV, coefficient of variation; VIM, variation independent of mean.
Among the study patients, 680 (96.9%) patients were discharged and 22 (3.1%) died. Compared with survivors, non‐survivors were also significantly older [(72.0 (65.0, 81.0) vs. 66.0 (58.0, 73.0) years, p = .005], and had higher proportions of comorbidities, including coronary heart disease, COPD, and chronic kidney disease. In addition, non‐survivors were less likely to receive calcium channel blockers for treatment of hypertension. For laboratory findings, non‐survivors had more severe inflammatory responses (indicated by higher leukocyte count, hs‐CRP, and PCT levels) and organ damage than survivors. For example, more severe coagulation function dysfunction (elevated D‐dimer), liver dysfunction (elevated ALT and AST), renal injury (elevated creatinine), myocardial injury (elevated LDH and hs‐TnI), and cardiac dysfunction (elevated BNP) were observed in non‐survivors. Consistently, compared with survivors, all BPV indices were higher in non‐survivors. However, there were no significant differences in symptoms between groups stratified by severity and survival of COVID‐19.
3.2. Correlations of clinical data with BPV
Correlation coefficients between BPV and background factors were illustrated in Table 2. BPV was correlated with various background factors. For instance, SD SBP showed significant positive relationships with age, D‐dimer, LDH, hs‐TnI, BNP, hs‐CRP, and PCT. In addition, SD DBP showed significant positive relationships with hs‐TnI and BNP. Furthermore, Kaplan–Meier survival analysis was used to compare the mortality between groups stratified by BPV median. As shown in Figure 2A, two (0.9%) died among patients with lower SD SBP (SD SBP < 11.6 mm Hg), while 20 (5.7%) died among patients with higher SD SBP (SD SBP ≥11.6 mm Hg), that is, patients with higher SD SBP had significantly higher rate of mortality than those with lower SD SBP. Besides, higher SD DBP was also correlated with higher mortality (Figure 2B).
TABLE 2.
Correlation analyses of clinical data with BPV in COVID‐19 patients with hypertension
| Variable | SD SBP | CV SBP | VIM SBP | SD DBP | CV DBP | VIM DBP |
|---|---|---|---|---|---|---|
| Age | 0.15* | 0.12* | 0.12* | −0.03 | 0.06 | 0.01 |
| Sex | 0.02 | 0.03 | 0.04 | 0.01 | −0.02 | 0.00 |
| Smoking history | 0.07 | 0.09 | 0.09 | 0.00 | 0.02 | 0.01 |
| Coronary heart disease | 0.05 | 0.05 | 0.05 | −0.05 | −0.01 | −0.03 |
| ACEI/ARB | 0.03 | −0.01 | −0.01 | −0.02 | −0.05 | −0.03 |
| β blocker | 0.03 | 0.03 | 0.03 | 0.04 | 0.02 | 0.03 |
| Calcium channel blocker | 0.08 | 0.03 | 0.03 | 0.03 | 0.00 | 0.02 |
| Diuretic | 0.07 | 0.06 | 0.06 | 0.05 | 0.06 | 0.05 |
| Antiviral therapy | 0.04 | 0.04 | 0.04 | 0.00 | 0.01 | 0.00 |
| Glucocorticoid | 0.08 | 0.09 | 0.09 | 0.05 | 0.07 | 0.06 |
| Leukocyte count | 0.09 | 0.09 | 0.09 | 0.07 | 0.07 | 0.07 |
| D‐dimer | 0.11* | 0.11* | 0.11* | 0.04 | 0.11* | 0.07 |
| ALT | −0.06 | −0.04 | −0.04 | −0.04 | −0.03 | −0.04 |
| AST | 0.01 | 0.02 | 0.03 | 0.01 | 0.04 | 0.02 |
| Creatinine | 0.06 | 0.07 | 0.07 | −0.03 | −0.01 | −0.02 |
| LDH | 0.11* | 0.11* | 0.10* | 0.04 | 0.09 | 0.06 |
| hs‐TnI | 0.22* | 0.19* | 0.19* | 0.14* | 0.18* | 0.16* |
| BNP | 0.24* | 0.22* | 0.21* | 0.11* | 0.17* | 0.14* |
| hs‐CRP | 0.19* | 0.18* | 0.18* | 0.09 | 0.14* | 0.11* |
| PCT | 0.15* | 0.16* | 0.16* | 0.02 | 0.09 | 0.05 |
Abbreviations: BPV, blood pressure variability; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; hs‐TnI, high‐sensitive cardiac troponin I; BNP, B‐type natriuretic peptide; hs‐CRP, high‐sensitive C‐reactive protein; PCT, procalcitonin; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker..
p < 0.05.
FIGURE 2.
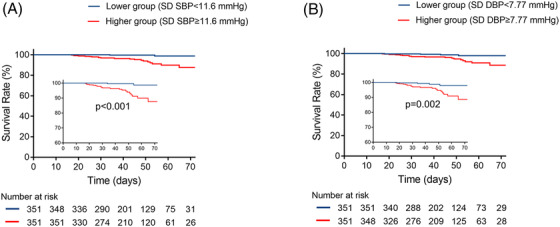
Kaplan–Meier survival curves for mortality in patients with higher and lower BPV. Patients with higher SD SBP (≥11.6 mm Hg) exhibited significantly higher incidence of mortality (A). In addition, patients with higher SD DBP (≥7.77 mm Hg) exhibited significantly higher incidence of mortality (B). BPV, blood pressure variability; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure
3.3. Prognostic value of BPV for adverse clinical outcomes
Of all patients, 64 (9.1%) developed ARDS. Table 3 shows univariable Cox regression analyses for the mortality and ARDS in COVID‐19 patients with hypertension. There was no significant association between mean SBP and adverse outcomes in univariable Cox regression. In univariate Cox regression model, SBP variability parameters, including SD (HR 1.29, 95% CI 1.20–1.40), CV (HR 1.38, 95% CI 1.27–1.51) and VIM (HR 1.28, 95% CI 1.20–1.37) of SBP, were predictive of mortality. Additionally, variables with p < .1 in univariable regression were entered to multivariable Cox model, included age, comorbidities (coronary heart disease and COPD), medication (calcium channel blocker, antiviral therapy, and glucocorticoid), laboratory parameters (D‐dimer > 0.55 mg/L, AST > 40 u/L, creatinine > 133 μmol/L, LDH > 250 u/L, hs‐TnI > 0.04 ng/ml, BNP > 100 pg/ml, hs‐CRP > 4 mg/L, PCT > 0.05 ng/ml) and mean DBP. In the multivariate Cox regression model, the SD SBP (HR 1.17, 95% CI 1.05–1.30) was consistently predictive of mortality (Table 4). The predictive value of SBP variability for ARDS was also observed in the univariable and multivariate models. For DBP indices, mean DBP and all of DBP variability could significantly predict mortality and ARDS in univariable Cox model, but not in multivariable model.
TABLE 3.
Univariable Cox regression analyses for the mortality and ARDS in COVID‐19 patients with hypertension
| Mortality(n = 22) | ARDS (n = 64) | |||
|---|---|---|---|---|
| Variable | HR (95%CI) | p‐value | HR (95%CI) | p‐value |
| Age | 1.05 (1.01–1.10) | .018 | 1.05 (1.02–1.07) | <.001 |
| Sex | 2.07 (0.85–5.09) | .111 | 1.22 (0.75–2.00) | .429 |
| Smoking history | 0.67 (0.09–4.99) | .696 | 0.68 (0.21–2.19) | .522 |
| Diabetes | 0.60 (0.20–1.77) | .351 | 0.79 (0.44–1.43) | .439 |
| Coronary heart disease | 3.29 (1.29–8.42) | .013 | 1.38 (0.68–2.79) | .373 |
| COPD | 4.06 (1.37–12.0) | .011 | 4.27 (2.17–8.41) | <.001 |
| Tumor | 2.23 (0.30–16.64) | .434 | 3.29 (1.19–9.11) | .022 |
| ACEI/ARB | 0.41 (0.05–3.05) | .383 | 0.87 (0.38–2.02) | .748 |
| β blocker | 0.45 (0.10–1.93) | .281 | 1.77 (1.00–3.13) | .049 |
| Calcium channel blocker | 0.39 (0.16–0.92) | .032 | 1.00 (0.61–1.65) | .988 |
| Diuretic | 1.68 (0.39–7.21) | .482 | 4.19 (2.23–7.87) | <.001 |
| Antiviral therapy | 2.27 (0.97–5.34) | .059 | 1.25 (0.76–2.06) | .372 |
| Glucocorticoid | 4.65 (1.98–10.91) | <.001 | 5.29 (3.21–8.72) | <.001 |
| Leukocyte count < 4×10 9 /L | 1.14 (0.15–8.50) | .896 | 0.05 (0.00–13.05) | .287 |
| D‐dimer > 0.55 mg/L | 8.79 (2.03–38.07) | .004 | 14.69 (5.31–40.63) | <.001 |
| AST > 40 u/L | 16.73 (7.01–39.93) | <.001 | 4.62 (2.69–7.91) | <.001 |
| Creatinine > 133 μmol/L | 9.76 (3.78–25.19) | <.001 | 4.32 (2.13–8.76) | <.001 |
| LDH > 250 u/L | 42.06 (9.83–180.01) | <.001 | 12.20 (7.11–20.91) | <.001 |
| hs‐TnI > 0.04 ng/ml | 17.73 (6.84–45.95) | <.001 | 8.37 (94.64–15.09) | <.001 |
| BNP > 100 pg/ml | 6.06 (2.46–14.92) | <.001 | 4.81 (2.76–8.38) | <.001 |
| hs‐CRP > 4 mg/L | 76.81 (2.26–2613.39) | .016 | 26.25 (8.23–83.72) | <.001 |
| PCT > 0.05 ng/ml | 17.44 (2.31–131.57) | .006 | 6.61 (3.11–14.08) | <.001 |
| mean SBP | 1.00 (0.96–1.04) | .950 | 0.99 (0.97–1.02) | .567 |
| mean DBP | 0.88 (0.83–0.94) | <.001 | 0.90 (0.87–0.94) | <.001 |
| Assessments of BPV | ||||
| SD SBP | 1.29 (1.20–1.40) | <.001 | 1.19 (1.13–1.25) | <.001 |
| CV SBP | 1.38 (1.27–1.51) | <.001 | 1.27 (1.19–1.35) | <.001 |
| VIM SBP | 1.28 (1.20–1.37) | <.001 | 1.20 (1.14–1.25) | <.001 |
| SD DBP | 1.34 (1.21–1.48) | <.001 | 1.18 (1.10–1.27) | <.001 |
| CV DBP | 1.26 (1.18–1.34) | <.001 | 1.16 (1.11–1.22) | <.001 |
| VIM DBP | 1.35 (1.23–1.48) | <.001 | 1.20 (1.13–1.29) | <.001 |
ARDS, acute respiratory distress syndrome; HR, hazard ratio; CI, confidence interval; COPD, Chronic obstructive lung disease; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; hs‐TnI, high‐sensitive cardiac troponin I; BNP, B‐type natriuretic peptide; hs‐CRP, high‐sensitive C‐reactive protein; SBP, systolic blood pressure; DBP, diastolic blood pressure; PCT, procalcitonin.
TABLE 4.
Multivariable Cox regression analyses for the mortality and ARDS with BPV in COVID‐19 patients with hypertension
| Mortality (n = 22) | ARDS (n = 64) | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p‐value | HR (95% CI) | p‐value |
| SBP variability | ||||
| SD SBP (mm Hg) | 1.17 (1.05–1.30) | .004 | 1.09 (1.01–1.16) | .021 |
| CV SBP (%) | 1.13 (0.99–1.29) | .076 | 1.10 (1.01–1.19) | .027 |
| VIM SBP (units) | 1.10 (0.99–1.22) | .076 | 1.07 (1.01–1.14) | .028 |
| DBP variability | ||||
| SD DBP (mm Hg) | 0.77 (0.48–1.24) | .283 | 1.11 (0.96–1.28) | .149 |
| CV DBP (%) | 0.97 (0.70–1.34) | .854 | 1.07 (0.97–1.09) | .192 |
| VIM DBP (units) | 0.98 (0.65–1.49) | .929 | 1.10 (0.96–1.27) | .166 |
Hazard ratios of BPV in multivariable Cox regression were adjusted for the variables with p < .1 in univariable Cox regression.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
4. DISCUSSION
Based on in‐hospital BP measurement, this study demonstrated the prognostic significance of day‐by‐day BPV for adverse outcomes in COVID‐19 patients with hypertension. The main finding of the study was that SBP variability rather than DBP variability predicted mortality and ARDS in COVID‐19 patients with hypertension, independent of other validated risk factors. In addition, BPV was correlated with inflammation and organ damage markers, such as hs‐CRP, PCT, hs‐TnI, and BNP.
Previous large‐scale studies in China reported that the prevalence of hypertension in COVID‐19 patients ranged from 15% to over 30%. 4 , 8 , 21 , 22 , 23 In the present study, 30.3% of patients with COVID‐19 had hypertension. A meta‐analysis assessing the effect of cardiovascular comorbidities on COVID‐19 patients, reported that hypertension was associated with 3.67‐fold (95% CI 2.31–5.83) increased risk for mortality in the unadjusted model. 24 Therefore, it is of great value to investigate the risk factors for adverse outcomes in COVID‐19 patients with hypertension, and this can guide physicians in early medical management of these patients. In line with previous studies, our data suggested that non‐survivors were more likely to have excessive inflammations and severe organ damage than survivors. 21 , 25 Additionally, compared with moderate cases, patients with severe and critical COVID‐19 had higher BPV, as assessed by SD, CV, and VIM of SBP and DBP. Similarly, non‐survivors had higher BPV than survivors, which implied the associations between BPV and adverse outcomes.
Undoubtedly, hypertensive patients can benefit from reduction in BP levels in clinical practice. Moreover, more studies have identified additional benefits of reducing BPV in the prevention of cardiovascular events and adverse outcomes. 6 The Finn‐Home Study suggested that higher variability parameters of morning BP, but not evening BP, were independent predictors of cardiovascular events and mortality, probably resulting from the activation of the sympathetic nervous system and an increase in platelet aggregability. 26 Consistently, the estimation of BPV in our study was derived from in‐hospital BP measured in the morning. In fact, BPV has been identified to be predictive of adverse outcomes in several diseases, such as, hypertension, transient ischemic attack, heart failure, diabetes, under hemodialysis, and even in the general population. 15 , 19 , 27 , 28 , 29 , 30 Intriguingly, in the present study, higher SD SBP was associated with in‐hospital mortality and ARDS in COVID‐19 patients with hypertension. Several studies have investigated the risk factors for mortality among COVID‐19 patients and demonstrated that older males with comorbidities, including hypertension, diabetes, coronary heart disease, COPD, tumor, and severe organ damage had greater risk of mortality. 4 , 5 , 20 , 21 After we adjusted for these confounders, in this study, the variability of SBP, but not DBP, could consistently predict adverse outcomes among COVID‐19 patients with hypertension. Although Li eand coworkers 31 , 32 conducted similar studies, this study included larger sample size and excluded cases without sufficient BP readings to reduce its confounding impact. Our data implied that stable level of blood pressure has better prognosis than fluctuating level in COVID‐19 patients with hypertension.
The mechanisms involved in the association between increased day‐by‐day BPV and adverse outcomes in COVID‐19 patients with hypertension are unknown. BPV is a complex phenomenon that is affected by multiple intrinsic and extrinsic factors. Aging and hypertension‐induced arterial stiffness can cause BP fluctuation and increased variability. 27 Similarly, the results in this study have demonstrated the relationship between age and BPV. In addition, inflammation markers have been reported to be associated with BPV in hypertensive patients, the same as this study identified. 33 Indeed, SARS‐CoV‐2 infection has been considered a cytokine storm syndrome, and COVID‐19 patients with ARDS showed 10‐ to 60‐ fold higher levels of interleukin‐1β and interleukin‐6 than patients with moderate cases. 34 , 35 , 36 Excessive inflammatory responses may therefore partly explain the association between high BPV and adverse outcomes in COVID‐19 patients with hypertension. We also found the correlations of BNP and hs‐TnI with BPV, perhaps implying the detrimental role of cardiac dysfunction and myocardial injury on BP stability. Previous study has reported the relationship between visit‐to‐visit SBP variability and the rate of myocardial infarction. 37 Additionally, Diaz and coworkers 38 , 39 suggested that high BPV was associated with endothelial dysfunction, which could account for the link between BPV and vascular injury diseases. Recently, Varga and coworkers 40 found evidence that SARS‐CoV‐2 directly infected endothelial cell via the protein angiotensin‐converting enzyme 2, and induced endotheliitis in several organs. Viral infection increases the risk of adverse outcomes for hypertensive patients with pre‐existing endothelial dysfunction, which may provide another underlying mechanism linking high BPV and adverse outcomes in COVID‐19 patients with hypertension. Further studies are required to confirm the mechanism underlying this effect and to clarify whether reducing BPV improves the prognosis of COVID‐19 patients with hypertension.
5. LIMITATIONS
This study has several limitations. First, due to its retrospective design, some cases patients incomplete medical records, and laboratory tests were not performed in all patients, which may have caused bias in our analyses. Second, history of antihypertensive medication prior to admission could not be ascertained, and this prevented us from excluding the confounding effect of this on the outcomes of the patients. Third, this study included only Chinese hypertensive patients with COVID‐19, and further studies are needed to clarify these results in other populations with COVID‐19.
6. CONCLUSIONS
To the best of our knowledge, this study for the first time demonstrates that higher day‐by‐day in‐hospital SBP variability can independently predict mortality and ARDS in COVID‐19 patients with hypertension. In addition, high BPV may be correlated with severe inflammation and myocardial injury. Early reduction of BPV may improve the prognosis of these patients. The underlying mechanisms that link higher BPV and adverse outcomes need to be clarified in further studies.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
Lan Huang, Chunyan He, Chuan Liu, and Jie Yang contributed to the conception or design of the study. Hu Tan and Ping Li contributed to the data collection. Yang Shen and Limin Zhang checked the data. Chunyan He, Hedong Xiang, Jingbin Ke, Fangzhengyuan Yuan, Renzheng Chen, Ran Cheng, and Hailin Lv performed the statistical analysis. Chunyan He, Chuan Liu, Jie Yang, Hu Tan, and Yuanqi Yang drafted the manuscript. Lan Huang, Xiaohan Ding, and Xubin Gao reviewed the manuscript. Lan Huang performed supervision. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We appreciated all the patients enrolled and all the stuffs involved in this study. This work was supported by Scientific Research Project of Huoshenshan Hospital.
He C, Liu C, Yang J, et al. Prognostic significance of day‐by‐day in‐hospital blood pressure variability in COVID‐19 patients with hypertension. J Clin Hypertens. 2022;24:224–233. 10.1111/jch.14437
Chunyan He, Chuan Liu, and Jie Yang contributed equally to this work.
REFERENCES
- 1. Li X, Ma X. Acute respiratory failure in COVID‐19: is it “typical” ARDS?. Crit Care. 2020;24(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐19: evidence from meta‐analysis. Aging (Albany N Y). 2020;12(7):6049‐6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81(2):e16‐e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoshide S, Yano Y, Mizuno H, Kanegae H, Kario K. Day‐by‐day variability of home blood pressure and incident cardiovascular disease in clinical practice: the J‐HOP Study (Japan morning surge‐home blood pressure). Hypertension. 2018;71(1):177‐184. [DOI] [PubMed] [Google Scholar]
- 8. Smith TO, Sillito JA, Goh CH, et al. Association between different methods of assessing blood pressure variability and incident cardiovascular disease, cardiovascular mortality and all‐cause mortality: a systematic review. Age Ageing. 2020;49(2):184‐192. [DOI] [PubMed] [Google Scholar]
- 9. Wang J, Shi X, Ma C, et al. Visit‐to‐visit blood pressure variability is a risk factor for all‐cause mortality and cardiovascular disease: a systematic review and meta‐analysis. J Hypertens. 2017;35(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: Interim guidance. https://apps.who.int/iris/handle/10665/330893
- 11. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Chin. Med. J. (Engl.). 2020;133(9):1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 13. Japanese Society of Hypertension . Blood pressure monitor test results. https://www.jpnsh.jp/data/ketsuatsukei2020/forhome2020‐3omron.pdf
- 14. Takahashi H, Yoshika M, Yokoi T. Validation of three automatic devices for the self‐measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010: the Omron HEM‐7130, HEM‐7320F, and HEM‐7500F. Blood Press Monit. 2015;20(2):92‐97. [DOI] [PubMed] [Google Scholar]
- 15. Juhanoja EP, Niiranen TJ, Johansson JK, et al. Outcome‐driven thresholds for increased home blood pressure variability. Hypertension. 2017;69(4):599‐607. [DOI] [PubMed] [Google Scholar]
- 16. Dolan E, O'Brien E. Blood pressure variability: clarity for clinical practice. Hypertension. 2010;56(2):179‐181. [DOI] [PubMed] [Google Scholar]
- 17. Fukuda K, Kai H, Kamouchi M, et al. Day‐by‐day blood pressure variability and functional outcome after acute ischemic stroke: Fukuoka stroke registry. Stroke. 2015;46(7):1832‐1839. [DOI] [PubMed] [Google Scholar]
- 18. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 19. Amari Y, Morimoto S, Iida T, et al. Characteristics of visit‐to‐visit blood pressure variability in hemodialysis patients. Hypertens Res. 2019;42(7):1036‐1048. [DOI] [PubMed] [Google Scholar]
- 20. Yu C, Lei Q, Li W, et al. Clinical characteristics, associated factors, and predicting COVID‐19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med. 2020;59(2):168‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang S, Wang J, Liu F, et al. COVID‐19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43(8):824‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Guan B, Su T, et al. Impact of cardiovascular disease and cardiac injury on in‐hospital mortality in patients with COVID‐19: a systematic review and meta‐analysis. Heart. 2020;106(15):1142‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo X, Zhou W, Yan X, et al. Prognostic value of C‐reactive protein in patients with Coronavirus 2019. Clin Infect Dis. 2020;71(16):2174‐2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home‐measured blood pressure and heart rate: the Finn‐Home study. Hypertension. 2012;59(2):212‐218. [DOI] [PubMed] [Google Scholar]
- 27. Kikuya M, Ohkubo T, Metoki H, et al. Day‐by‐day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52(6):1045‐1050. [DOI] [PubMed] [Google Scholar]
- 28. Rossignol P, Girerd N, Gregory D, Massaro J, Konstam MA, Zannad F. Increased visit‐to‐visit blood pressure variability is associated with worse cardiovascular outcomes in low ejection fraction heart failure patients: insights from the HEAAL study. Int J Cardiol. 2015;187:183‐189. [DOI] [PubMed] [Google Scholar]
- 29. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895‐905. [DOI] [PubMed] [Google Scholar]
- 30. Ushigome E, Matsumoto S, Oyabu C, et al. Prognostic significance of day‐by‐day variability of home blood pressure on progression to macroalbuminuria in patients with diabetes. J Hypertens. 2018;36(5):1068‐1075. [DOI] [PubMed] [Google Scholar]
- 31. Li FK, An DW, Guo QH, et al. Day‐by‐day blood pressure variability in hospitalized patients with COVID‐19. J Clin Hypertens (Greenwich). 2021;23(9):1675‐1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nam JH, Park JI, Kim BJ, et al. Clinical impact of blood pressure variability in patients with COVID‐19 and hypertension. Blood Press Monit. 2021;26(5):348‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim KI, Lee JH, Chang HJ, et al. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J. 2008;72(2):293‐298. [DOI] [PubMed] [Google Scholar]
- 34. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with Coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID‐19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9(5):1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suchy‐Dicey AM, Wallace ER, Mitchell SV, et al. Blood pressure variability and the risk of all‐cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens. 2013;26(10):1210‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diaz KM, Veerabhadrappa P, Kashem MA, et al. Relationship of visit‐to‐visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 2012;35(1):55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakano C, Morimoto S, Nakahigashi M, et al. The relationships between visit‐to‐visit blood pressure variability and renal and endothelial function in chronic kidney disease. Hypertens Res. 2015;38(3):193‐198. [DOI] [PubMed] [Google Scholar]
- 40. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]


