Abstract
Background
The question of whether asthma is causally related to gastrointestinal disorders remained unanswered so far. Thus, this study investigated whether there is such a relation and whether the time of onset of asthma plays a role in the occurrence of the following gastrointestinal disorders: peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD), irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD) including the distinction between Crohn’s disease (CD) and ulcerative colitis (UC).
Methods
Using summary data of genome-wide association studies (GWASs), we ran Mendelian randomization analyses based on up to 456,327 European participants. Outlier assessment, a series of sensitivity analyses and validation of IBD results in a second GWAS were performed to confirm the results.
Results
Presented ORs represent the average change in the outcome per 2.72-fold increase in the prevalence of the exposure. Genetically predicted childhood-onset asthma was positively associated with PUD, GORD, and IBS with similar odds ratios near 1.003 and adjusted P-values from 0.007 (GORD) to 0.047 (PUD). Furthermore, it was inversely related to IBD (OR = 0.992, 95% CI: 0.986, 0.998, adjusted P = 0.023) and suggestively associated with its UC subtype (OR = 0.990, 95% CI: 0.982, 0.998, adjusted P = 0.059). There were no associations between genetically predicted adult-onset asthma and the mentioned gastrointestinal disorders.
Conclusions
This study provides evidence that the presence of asthma onset in childhood increases the risk for GORD, PUD, and IBS but decreases the risk for IBD in adults. The lower risk for IBD may be attributed to a lower risk primarily for UC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02283-7.
Keywords: Asthma, Gastrointestinal disorders, Mendelian randomization, Crohn’s disease, Ulcerative colitis, Peptic ulcer disease, Gastroesophageal reflux disease, Irritable bowel syndrome, Inflammatory bowel disease
Background
Evidence from observational studies suggests a complex interplay between chronic lung disorders (e.g., asthma, chronic obstructive pulmonary disease, cystic fibrosis) and gastrointestinal diseases [1–3]. Furthermore, studies reported the occurrence of intestinal symptoms as part of a viral respiratory infection [4, 5]. This indicates that there is a cross-talk between these organ systems, termed as “gut-lung axis” [6]. A number of exposures in early-life have been linked to both the predisposition towards respiratory diseases and changes in the intestinal microbiota. However, clear mechanisms responsible for the intestinal-pulmonary cross-talk are not yet clear [7].
The most prevalent chronic respiratory disease in children and adults is asthma [8]. Several observational studies for example focused on the relationship between asthma and inflammatory bowel disease (IBD) [9], asthma and irritable bowel syndrome (IBS) [10], and asthma and gastroesophageal reflux disease (GORD) [11]. It was found that people with asthma are three times more likely to have gastroesophageal reflux than healthy people [11]. Additionally, it could be shown that the risk of asthma in subjects with IBS is twice the risk in persons without the disease [10]. However, so far, it is not clear which disease causes the other. This largely unclear relationship could not be clarified even in systematic reviews and meta-analyses [9, 10, 12].
This study aims to close this gap and is therefore dedicated to the causal effects of both childhood- and adult-onset asthma on peptic ulcer disease (PUD), GORD, IBS, and IBD.
Methods
Study design and population
Since observational studies are prone to reverse causation and unmeasured confounding, we performed a Mendelian randomization (MR) analysis to investigate causal effects of asthma and the time of onset on the mentioned gastrointestinal disorders [13]. The MR approach uses genetic variants, which are randomly allocated at meiosis and therefore independent of potential confounders biasing observational studies, as proxies for a risk factor in an instrumental variable analysis. Beyond that, to be considered as a valid instrument, a genetic variant must be strongly associated with the risk factor of interest and not affect the outcome directly, but only through exposure. For each genetic instrument, a causal estimate (known as Wald ratio) is calculated by dividing the variant-outcome by the variant-exposure association [14]. Subsequently, all Wald ratios are combined into an overall estimate using meta-analysis tools.
In a nested 2-sample MR within one study cohort, we considered summary statistics of GWASs including 314,633, 327,253, and 456,327 UK Biobank (UKB) participants with European ancestry for childhood-onset asthma, adult-onset asthma, and gastrointestinal disorders, respectively. In the following, we will refer to this as one-sample MR. Overall 13,962 asthma-diagnosed (main and secondary ICD10) cases between 0 and 19 years of age and 26,582 cases between 20 and 60 years of age were compared to the control group of 300,671 individuals without any allergic disease, such as asthma and hay fever [15] (Table 1). Regarding the gastrointestinal disorders, out of 456,327 subjects, 3.7% were diagnosed with PUD, 12% with GORD, 6.5% with IBS, and 1.5% with IBD [16] (Table 2).
Table 1.
Description of the UKB exposures [15]. In both cases, the control group contains 300,671 individuals without any allergic disease
| Childhood-onset asthma | Adult-onset asthma | |
|---|---|---|
| Considered age range for onset | 0 to 19 | 20 to 60 |
| Sample size | 314,633 | 327,253 |
| Cases, abs (rel) | 13,962 (0.044) | 26,582 (0.081) |
| Associated independent SNPs | 102 | 47 |
| Instrumental SNPs/instrumental SNPs in validation analyses | 89/76 | 42/38 |
Abbreviations: SNPs single nucleotide polymorphisms
Table 2.
Description of the outcomes according to Wu et al. [16]
| PUD | GORD | IBS | IBD | |
|---|---|---|---|---|
| Sample size | 456,327 | 456,327 | 456,327 | 456,327 |
| Cases, abs (rel) | 16,666 (0.037) | 54,854 (0.120) | 29,524 (0.065) | 7045 (0.015) |
Abbreviations: PUD peptic ulcer disease, GORD gastroesophageal reflux disease, IBS irritable bowel syndrome, IBD inflammatory bowel disease
Additionally, a two-sample MR validation analysis was performed with IBD as outcome using GWAS summary data of 86,640 European individuals from the International Inflammatory Bowel Disease Genetics (IIBDG) consortium (Table 3). Beyond that, these data allowed us to investigate the outcomes CD and ulcerative colitis UC as subtypes of IBD with 20,550 and 17,647 cases vs. 41,642 and 47,179 controls, respectively [17, 18].
Table 3.
Description of the outcome IBD and its subtypes Crohn’s disease and ulcerative colitis according to Liu et al. [17] used in validation analyses
| IBD | Crohn’s disease | Ulcerative colitis | |
|---|---|---|---|
| Sample size | 86,640 | 62,192 | 64,826 |
| Cases, abs (rel) | 38,155 (0.44) | 20,550 (0.33) | 17,647 (0.27) |
Abbreviations: IBD inflammatory bowel disease
As possible instruments, we selected single nucleotide polymorphisms (SNPs) with an imputation score ≥0.8 that were strongly associated with asthma based on the genome-wide significance threshold of P = 5 · 10−8. After the linkage disequilibrium clumping with a stringent cut-off r2 = 0.001, we harmonized the respective exposure and outcome datasets using effect allele frequencies, while removing palindromic SNPs with intermediate allele frequencies (i.e., minor allele frequency >0.42).
Thus, 89 childhood-onset and 42 adult-onset asthma associated SNPs remained as possible independent instruments for the one-sample MR analyses (Additional file 1: Tables S1 and S2). In the two-sample setting 76 potential genetic instruments were used for childhood-onset and 38 for adult-onset asthma, respectively (Additional file 1: Tables S3 and S4).
Statistical analyses
Assessing heterogeneity is an important part of a MR study to identify invalid instruments. Wald ratios are assumed to estimate the same causal effect. Thus, heterogeneous SNPs that differ significantly from other Wald ratios indicate instruments that influence the outcome through an additional path (i.e., directly or through a confounder of the exposure-outcome association) rather than only through the exposure (known as horizontal pleiotropy). As a result, the estimates will be biased especially in presence of unbalanced pleiotropy.
In the main analyses, estimates were calculated using the radial inverse-variance weighted (IVW) approach with modified second-order weights in an iterative way [19]. If MR assumptions are met, this method provides the highest statistical power and accounts also for balanced pleiotropy. In each iteration step, pleiotropic SNPs were quantified and detected by calculating and testing the SNP-specific Cochran’s Q-statistics (based on a type I error αQ = 0.01) as well as by graphical evaluation. Moreover, we assessed all identified heterogeneous SNPs responsible for possible unbalanced horizontal pleiotropy using PhenoScanner database [20, 21] (Additional file 1: Tables S5 to S8). To account for different patterns of pleiotropy, sensitivity analyses were applied to the final models that provided consistent point estimates under different assumptions. The weighted median approach allows up to 50% of the selected genetic instruments to be invalid. The weighted mode leads to consistent estimates, even if there are more than 50% invalid instruments. The MR-PRESSO method uses the global and SNP-specific observed residual sum of squares to test for general horizontal pleiotropy and outliers, respectively. Additionally, it provides a distortion test comparing estimates before and after outlier removal. As a final step, we applied a many weak instrument analysis using the MR-RAPS approach with a robust loss function and consideration of overdispersion.
Estimates were presented as odds ratios (ORs) with 95% confidence intervals (CIs) and can be interpreted as the average change in the outcome per 2.72-fold increase in the prevalence of the respective binary exposure. It is important to note that since the estimates have no clear interpretation except for the direction, they were only used to test whether a causal effect exists [22]. The type I error was set to α = 0.05. Due to multiple testing P-values were adjusted by the Benjamini-Hochberg procedure (Additional file 1: Table S9). All analyses were performed using the open-source statistical software R (version: 4.1.0).
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Estimates presented hereafter were obtained by the radial IVW approach with modified second-order weights as the principal analysis. Given P-values (Padj) were adjusted for multiple testing.
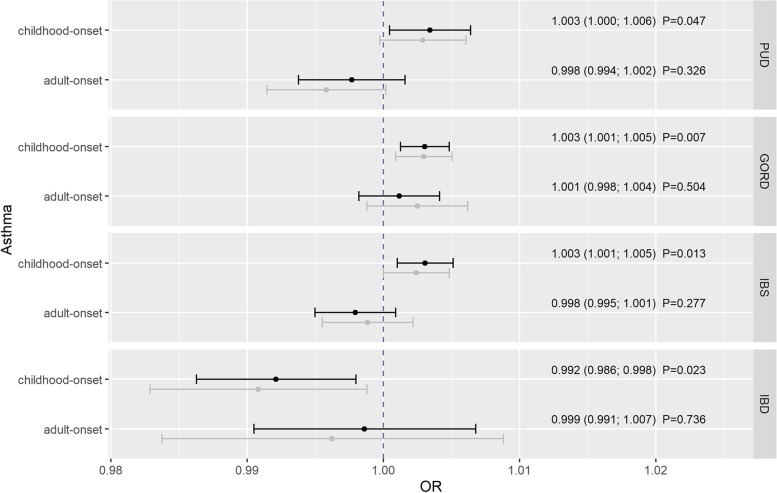
After removal of pleiotropic SNPs according to the Additional file 1: Tables S5 to S8, genetically predicted childhood-onset asthma was positively related to PUD (ORIVW =1.003, 95% CI: 1.000; 1.006, Padj =0.047), GORD (ORIVW =1.003, 95% CI: 1.001; 1.005, Padj =0.007), and IBS (ORIVW =1.003, 95% CI: 1.001; 1.005, Padj =0.013), but inversely to IBD (ORIVW =0.992, 95% CI: 0.986; 0.998, Padj =0.023) (Fig. 1). However, no notable associations could be observed between genetically predicted adult-onset asthma and the mentioned gastrointestinal disorders (Fig. 1).
Fig. 1.
Estimates given as odds ratios (ORs) and 95% confidence intervals for the effect of childhood- and adult-onset asthma on peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD), irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). Estimates were derived by the inverse-variance weighted method with modified second-order weights. Reported P-values were adjusted for multiple testing using the Benjamini-Hochberg procedure. Gray estimates represent the results before and black estimates the results after outlier-removal. PUD, peptic ulcer disease; GORD, gastroesophageal reflux disease; IBS, irritable bowel syndrome; IBD, inflammatory bowel disease
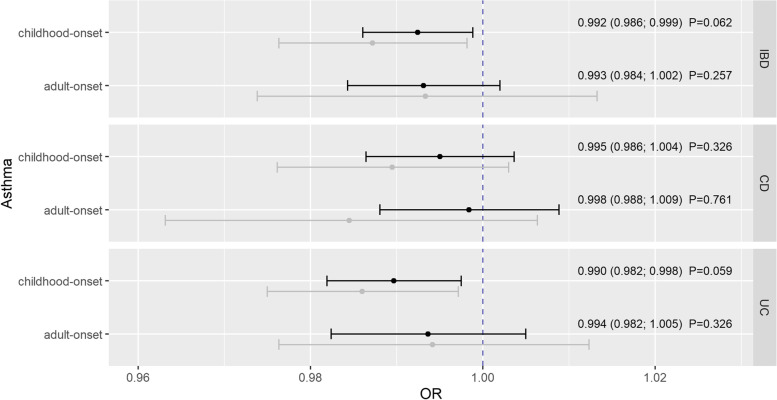
The validation analysis confirmed our findings for the association between childhood-onset asthma and IBD showing similar and consistent effect estimates but with loss of statistical significance after correction for multiple testing (quantified by adjusted P-values in Fig. 2). Thus, genetically predicted childhood-onset asthma was inversely associated with IBD (ORIVW =0.992, 95% CI: 0.986; 0.999, Padj =0.062) (Fig. 2). The same applies to the relationship between childhood-onset asthma and UC (ORIVW =0.990, 95% CI: 0.982; 0.998, Padj =0.059), while no notable association could be observed with CD (ORIVW =0.995, 95% CI: 0.986; 1.004, Padj =0.326). Again, no associations could be observed between adult-onset asthma and IBD, CD, and UC (Fig. 2).
Fig. 2.
Estimates given as odds ratios (ORs) and 95% confidence intervals for the effect of childhood- and adult-onset asthma on peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD), irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). Estimates were derived by the multiplicative random effects inverse-variance weighted method (except the effect of adult-onset asthma on ulcerative colitis that was estimated by the Wald atio approach). Reported P-values were adjusted for multiple testing using the Benjamini-Hochberg procedure. Gray estimates represent the results before and black estimates the results after outlier-removal. IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis
Except the association of genetically predicted childhood-onset asthma and UC, where the weighted mode led to an inconsistent point estimate, and despite of some heterogeneity quantified by the Q-statistics, the results were supported by the pleiotropic-robust approaches within sensitivity analyses (Additional file 1: Figs. S1-S4, Tables S10 and S11). Beyond that, removal of heterogeneous SNPs within the iterative radial approach did not substantially change the effect estimates (Figs. 1 and 2).
Discussion
This is the first study showing evidence that childhood-onset asthma increases the risk for PUD, GORD, and IBS but decreases the risk for IBD (which may mainly be attributed to UC) later in life. The findings were supported by consistent magnitude and direction of robust methods. No conclusions were possible regarding the impact of adult-onset asthma on these gastrointestinal disorders due to weak effect estimates and inconsistencies within sensitivity analyses.
In observational studies, a frequent co-occurrence of asthma with IBD was reported [9, 12, 23]. A population-based Canadian case-control study using health administrative data including 3087 cases with Crohn’s disease, 2377 cases with ulcerative colitis, and 402,800 control subjects found that asthma was associated with Crohn’s disease (adjusted OR 1.45; 95% CI 1.31–1.60) and with early and late-onset ulcerative colitis. There was no association between asthma and ulcerative colitis among subjects aged 17 to 40 years [23]. A systematic review and meta-analysis including 18 matched-control and cohort studies found a positive association of asthma with Crohn’s disease and ulcerative colitis [12]. In that study, the risk of asthma was increased in patients with UC, but in patients with existing asthma, no increased risk of UC was observed. The authors assumed that geographic differences and differences in study design might be responsible for the high degree of heterogeneity between the included studies [12]. While in some countries asthma was associated with a higher risk of Crohn’s disease, in other countries, a negative association between these two diseases was found [12]. The present MR study showed an inverse association between childhood but not adulthood asthma and IBD and thus contributes to the evidence on this topic.
Asthma is also frequently associated with IBS, a disease that manifests with variable and fluctuating symptoms, such as abdominal pain, bloating, headache, and muscle pain [24]. In a retrospective analysis from Taiwan using National Health Insurance data, a bidirectional association between asthma and IBS was reported [25]. The relative risk of IBS was 1.57 [95% CI = 1.47–1.68] in the asthma cohort in comparison to subjects without asthma [25]. A meta-analysis including 8 case-control and 2 cross-sectional studies concluded that the risk of asthma is significantly higher in IBS patients and vice versa [10]. Patients with asthma first had twice the risk of having IBS and patients with IBS first had twice the risk of asthma [10]. Our study confirmed a causal effect of asthma on IBS; however, this only applies to asthma in childhood and the occurrence of IBS later in life. No causal relationship could be found for adulthood onset asthma and IBS.
A number of studies reported on the association of GORD and asthma [11, 26]. Although there seems to be a strong relationship between these two diseases, most prior studies were cross-sectional or case-control studies, thus, the direction of causality remained undetermined [11]. In a systematic review including 28 studies, the sample size weighted prevalence of gastroesophageal symptoms in patients with asthma was 59.2% (in controls 38.1%) and the average prevalence of asthma in subjects with GORD was 4.6% (in controls 3.9%) [11]. The authors concluded that there is a clear paucity of data on the causal direction of the association. The present MR study found a positive causal association between childhood asthma and GORD in adulthood, a result that strengthens the evidence of a causal relationship and thus contributes to clarifying the direction of the relationship.
There are no observational studies on the association between asthma and PUD. The present MR analysis suggested a causal effect of asthma onset in childhood on the development of peptic ulcer later in life. No causal effect of asthma onset in adults on peptic ulcer risk was found.
Prior studies reported that obesity and psychological traits such as depression or anxiety are associated with functional gastrointestinal disorders, e.g., irritable bowel syndrome and gastroesophageal reflux disease GERD [27–30]. Also, previous genome-wide association studies (GWASs) on GERD have found genetic overlaps with established risk factors such as obesity and depression [31, 32]. Thus, in our MR analysis, bias due to pleiotropic effects by other pathways than the investigated cannot be entirely excluded. However, to reduce pleiotropy, we identified pleiotropic genetic variants within an iterative approach and confirmed them using the PhenoScanner database.
In that way, we excluded SNPs strongly associated with possible confounding factors like obesity and medication use and compared the results before and after outlier removal. Furthermore, we performed a number of sensitivity analyses, which failed to find evidence for unbalanced horizontal pleiotropy in the final models. In addition, we used a stringent selection threshold (P < 5 · 10−8) to minimize weak instrument bias.
Nowadays, asthma is recognized as a complex syndrome rather than a single disease and the mechanisms determining childhood asthma might not necessarily lead to asthma in adulthood [8]. A possible explanation for the present findings could be that the asthma phenotype in childhood is more uniform compared to asthma in adulthood, in which various mechanisms might be involved in the development of the disease [8]. These differences might result in more precise data with higher explained variance by genetic risk factors ( 25.6% in childhood-onset vs. 10.6% in adult-onset asthma [15]). Furthermore, regarding the earlier time point of disease manifestation and therefore lower susceptibility to reverse causation [33], the cause-effect-chain for individuals with childhood-onset asthma is more sustainable than in case of a disease onset later in life.
Several underlying mechanisms may explain the link between asthma onset in childhood and the different intestinal diseases. It has been postulated that early life exposures are associated with a predisposition towards respiratory diseases and with changes in the intestinal microbiota [7]. For example, recurrent antibiotic treatment has detrimental effects on the diversity of the microbiota in childhood [34] and is significantly associated with a later onset of asthma [35, 36] and—as shown in murine studies—with a loss of protection against respiratory viruses [37]. Other studies found that cesarean birth is accompanied by an alteration of the gut microbiome in early infancy [38] and a predisposition towards the onset of asthma in childhood [39]. Whether these early-life factors also impact on the airway microbiota has not been elucidated yet. There is no question that there is a cross-talk between the intestinal and airway microbiota compartments [6]. However, so far, the mechanisms how both compartments interact are not fully elucidated.
Our study has several strengths. The MR concept is less susceptible to unobserved confounding and reverse causality that are issues of observational studies. Outlier-assessment and a wide range of sensitivity analyses that accounted for different patterns of pleiotropy increased the robustness of the results and thus strengthened the evidence of our findings.
The study has also notable limitations. One-sample MR is known to have less statistical power compared to the two-sample MR [40]. However, in large biobanks, such as UK Biobank, the results from one- and two-sample MRs are shown to be similar except for the MR-Egger method [41]. Moreover, we were able to validate the results for IBD and investigate its subgroups using a summary statistics from a second GWAS in a two-sample setting. Another limitation is that the magnitude of provided estimates cannot be directly compared to estimates from observational studies. Thus, we were only able to compare the direction of the estimates and strengthen evidence for a causal relationship. Finally, sex-specific and ethnic-stratified analyses were not possible due to the lack of suitable summary level data.
Conclusions
In conclusion, we found that genetically predicted asthma onset in childhood is causally associated with a higher risk of GORD, PUD, and IBS and a lower risk of IBD in adults from the general population. Physicians should pay increased attention to whether gastrointestinal disease develops in patients who had manifest asthma in childhood. The pathophysiologic mechanisms underlying the cross-talk between the gut and the lung have to be explored in further investigations.
Supplementary Information
Additional file 1: Fig. S1 Sensitivity analyses of the effect of childhood-onset asthma on gastrointestinal disorders. Fig. S2 Sensitivity analyses of the effect of adult-onset asthma on gastrointestinal disorders. Fig. S3 Sensitivity analyses of the effect of childhood-onset asthma on IBD including subtypes. Fig. S4 Sensitivity analyses of the effect of adult-onset asthma on IBD including subtypes. Table S1. SNPs used in the analyses of the effect of childhood-onset asthma on gastrointestinal disorders. Table S2. SNPs used in the analyses of the effect of adult-onset asthma on gastrointestinal disorders. Table S3. SNPs used in the analyses of the effect of childhood-onset asthma on IBD including subtypes. Table S4. SNPs used in the analyses of the effect of adult-onset asthma on IBD including subtypes. Table S5. Identified pleiotropic SNPs of the effect of childhood-onset asthma on gastrointestinal disorders. Table S6. Identified pleiotropic SNPs of the effect of adult-onset asthma on gastrointestinal disorders. Table S7. Identified pleiotropic SNPs of the effect of childhood-onset asthma on IBD including subtypes. Table S8. Identified pleiotropic SNPs of the effect of adult-onset asthma on IBD including subtypes. Table S9. Estimates, standard errors, raw P-values, and Benjamini-Hochberg-adjusted P-values from the Mendelian randomization analyses. Table S10. Heterogeneity statistics of the effect of asthma on gastrointestinal disorders. Table S11. Heterogeneity statistics of the effect of asthma on IBD including subtypes.
Acknowledgements
Not applicable.
Abbreviations
- CD
Crohn’s disease
- CI
Confidence interval
- GORD
Gastroesophageal reflux disease
- GWAS
Genome-wide association study
- IBD
Inflammatory bowel disease
- IBS
Irritable bowel syndrome
- MR
Mendelian randomization
- OR
Odds ratio
- PUD
Peptic ulcer disease
- SNP
Single nucleotide polymorphism
- UC
Ulcerative colitis
Authors’ contributions
DF analyzed the data and created the tables and plots. CM did the literature research and contributed together with DF to the data collection. All authors wrote the manuscript and contributed to data interpretation. JL and CM reviewed the final manuscript. All authors read and approved the final version.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors did not receive funding for this study. Funding information of the genome-wide association studies is specified in the cited studies.
Availability of data and materials
The present study is based on summary-level data that have been made publically available. Summary data from genome-wide association studies for childhood-onset and adult-onset asthma are available at https://genepi.qimr.edu.au/staff/manuelF/gwas_results/main.html [42, 43]. Data for gastrointestinal disorders can be obtained from https://cnsgenomics.com/content/data [44–47] and https://www.ibdgenetics.org [48].
Declarations
Ethics approval and consent to participate
Not applicable, since the study is based on summary-level data. In all original studies, ethical approval and consent to participate had been obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roussos A, Koursarakos P, Patsopoulos D, Gerogianni I, Philippou N. Increased prevalence of irritable bowel syndrome in patients with bronchial asthma. Respir Med. 2003;97(1):75–79. doi: 10.1053/rmed.2001.1409. [DOI] [PubMed] [Google Scholar]
- 2.Baral V, Connett G. Acute intestinal obstruction as a presentation of cystic fibrosis in infancy. J Cyst Fibros. 2008;7(4):277–279. doi: 10.1016/j.jcf.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Keely S, Hansbro PM. Lung-gut cross talk: a potential mechanism for intestinal dysfunction in patients with COPD. Chest. 2014;145(2):199–200. doi: 10.1378/chest.13-2077. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211(12):2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dilantika C, Sedyaningsih ER, Kasper MR, Agtini M, Listiyaningsih E, Uyeki TM, et al. Influenza virus infection among pediatric patients reporting diarrhea and influenza-like illness. BMC Infect Dis. 2010;10:3. doi: 10.1186/1471-2334-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12(4):843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 7.Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017;5(3):224–234. doi: 10.1016/S2213-2600(16)30187-4. [DOI] [PubMed] [Google Scholar]
- 9.Zergham AS, Sekhon AK, Mebasher A, Tserenpil G, Malik BH. Inflammatory bowel disease and obstructive pulmonary disease: a two-way association? Cureus. 2020;12(1):e6836. doi: 10.7759/cureus.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshmukh F, Vasudevan A, Mengalie E. Association between irritable bowel syndrome and asthma: a meta-analysis and systematic review. Ann Gastroenterol. 2019;32(6):570–577. doi: 10.20524/aog.2019.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56(12):1654–1664. doi: 10.1136/gut.2007.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuenzig ME, Bishay K, Leigh R, Kaplan GG, Benchimol EI, Crowdscreen SRRT. Co-occurrence of asthma and the inflammatory bowel diseases: a systematic review and meta-analysis. Clin Transl Gastroenterol. 2018;9(9):188. doi: 10.1038/s41424-018-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 14.Teumer A. Common methods for performing mendelian randomization. Front Cardiovasc Med. 2018;5:51. doi: 10.3389/fcvm.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic architectures of childhood- and adult-onset asthma are partly distinct. Am J Hum Genet. 2019;104(4):665–684. doi: 10.1016/j.ajhg.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Murray GK, Byrne EM, Sidorenko J, Visscher PM, Wray NR. GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat Commun. 2021;12(1):1146. doi: 10.1038/s41467-021-21280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyette P, Boucher G, Mallon D, Ellinghaus E, Jostins L, Huang H, et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat Genet. 2015;47(2):172–179. doi: 10.1038/ng.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowden J, Spiller W, Del Greco MF, Sheehan N, Thompson J, Minelli C, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int J Epidemiol. 2018;47(4):1264–1278. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–4853. doi: 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32(20):3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33(10):947–952. doi: 10.1007/s10654-018-0424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuenzig ME, Barnabe C, Seow CH, Eksteen B, Negron ME, Rezaie A, et al. Asthma is associated with subsequent development of inflammatory bowel disease: a population-based case-control study. Clin Gastroenterol Hepatol. 2017;15(9):1405–1412. doi: 10.1016/j.cgh.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 24.Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20(22):6759–6773. doi: 10.3748/wjg.v20.i22.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen TC, Lin CL, Wei CC, Chen CH, Tu CY, Hsia TC, et al. Bidirectional association between asthma and irritable bowel syndrome: two population-based retrospective cohort studies. PLoS One. 2016;11(4):e0153911. doi: 10.1371/journal.pone.0153911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amarasiri LD, Pathmeswaran A, de Silva HJ, Ranasinha CD. Prevalence of gastro-oesophageal reflux disease symptoms and reflux-associated respiratory symptoms in asthma. BMC Pulm Med. 2010;10:49. doi: 10.1186/1471-2466-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106(6):642–649. doi: 10.1016/s0002-9343(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 28.Argyrou A, Legaki E, Koutserimpas C, Gazouli M, Papaconstantinou I, Gkiokas G, et al. Risk factors for gastroesophageal reflux disease and analysis of genetic contributors. World J Clin Cases. 2018;6(8):176–182. doi: 10.12998/wjcc.v6.i8.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SY, Kim HJ, Lim H, Kong IG, Kim M, Choi HG. Bidirectional association between gastroesophageal reflux disease and depression: Two different nested case-control studies using a national sample cohort. Sci Rep. 2018;8(1):11748. doi: 10.1038/s41598-018-29629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang P, Friedenberg F. Obesity and GERD. Gastroenterol Clin N Am. 2014;43(1):161–173. doi: 10.1016/j.gtc.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong JS, An J, Han X, Law MH, Nandakumar P, Me Research T, et al. Multitrait genetic association analysis identifies 50 new risk loci for gastro-oesophageal reflux, seven new loci for Barrett’s oesophagus and provides insights into clinical heterogeneity in reflux diagnosis. Gut. 2021. 10.1136/gutjnl-2020-323906. [DOI] [PMC free article] [PubMed]
- 32.An J, Gharahkhani P, Law MH, Ong JS, Han X, Olsen CM, et al. Author Correction: Gastroesophageal reflux GWAS identifies risk loci that also associate with subsequent severe esophageal diseases. Nat Commun. 2019;10(1):5617. doi: 10.1038/s41467-019-13526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess S, Swanson SA, Labrecque JA. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur J Epidemiol. 2021;36(3):253–257. doi: 10.1007/s10654-021-00726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fouhy F, Guinane CM, Hussey S, Wall R, Ryan CA, Dempsey EM, et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother. 2012;56(11):5811–5820. doi: 10.1128/AAC.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91(441):48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 36.Toivonen L, Schuez-Havupalo L, Karppinen S, Waris M, Hoffman KL, Camargo CA, et al. Antibiotic treatments during infancy, changes in nasal microbiota, and asthma development: population-based cohort study. Clin Infect Dis. 2021;72(9):1546–1554. doi: 10.1093/cid/ciaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 39.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38(4):629–633. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor AE, Davies NM, Ware JJ, VanderWeele T, Smith GD, Munafo MR. Mendelian randomization in health research: using appropriate genetic variants and avoiding biased estimates. Econ Hum Biol. 2014;13:99–106. doi: 10.1016/j.ehb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021. [DOI] [PMC free article] [PubMed]
- 42.Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Adult-onset asthma. QIMR Berghofer Medical Research Institute. 2019. [Google Scholar]
- 43.Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Childhood-onset asthma. QIMR Berghofer Medical Research Institute. 2019. [Google Scholar]
- 44.Wu Y, Murray GK, Byrne EM, Sidorenko J, Visscher PM, Wray NR. Peptic ulcer disease (PUD). Program in Complex Trait Genomics. 2021. [Google Scholar]
- 45.Wu Y, Murray GK, Byrne EM, Sidorenko J, Visscher PM, Wray NR. Gastro-oesophageal reflux disease (GORD). Program in Complex Trait Genomics. 2021. [Google Scholar]
- 46.Wu Y, Murray GK, Byrne EM, Sidorenko J, Visscher PM, Wray NR. Irritable bowel syndrome (IBS). Program in Complex Trait Genomics. 2021. [Google Scholar]
- 47.Wu Y, Murray GK, Byrne EM, Sidorenko J, Visscher PM, Wray NR. Inflammatory bowel diseases (IBD). Program in Complex Trait Genomics. 2021. [Google Scholar]
- 48.Consortium IMSG, Consortium IIG, Liu JZ, Sv S, Huang H, Ng SC, et al. Latest combined GWAS and Immunochip trans-ancestry summary statistics. IBD Genetics. 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1 Sensitivity analyses of the effect of childhood-onset asthma on gastrointestinal disorders. Fig. S2 Sensitivity analyses of the effect of adult-onset asthma on gastrointestinal disorders. Fig. S3 Sensitivity analyses of the effect of childhood-onset asthma on IBD including subtypes. Fig. S4 Sensitivity analyses of the effect of adult-onset asthma on IBD including subtypes. Table S1. SNPs used in the analyses of the effect of childhood-onset asthma on gastrointestinal disorders. Table S2. SNPs used in the analyses of the effect of adult-onset asthma on gastrointestinal disorders. Table S3. SNPs used in the analyses of the effect of childhood-onset asthma on IBD including subtypes. Table S4. SNPs used in the analyses of the effect of adult-onset asthma on IBD including subtypes. Table S5. Identified pleiotropic SNPs of the effect of childhood-onset asthma on gastrointestinal disorders. Table S6. Identified pleiotropic SNPs of the effect of adult-onset asthma on gastrointestinal disorders. Table S7. Identified pleiotropic SNPs of the effect of childhood-onset asthma on IBD including subtypes. Table S8. Identified pleiotropic SNPs of the effect of adult-onset asthma on IBD including subtypes. Table S9. Estimates, standard errors, raw P-values, and Benjamini-Hochberg-adjusted P-values from the Mendelian randomization analyses. Table S10. Heterogeneity statistics of the effect of asthma on gastrointestinal disorders. Table S11. Heterogeneity statistics of the effect of asthma on IBD including subtypes.
Data Availability Statement
The present study is based on summary-level data that have been made publically available. Summary data from genome-wide association studies for childhood-onset and adult-onset asthma are available at https://genepi.qimr.edu.au/staff/manuelF/gwas_results/main.html [42, 43]. Data for gastrointestinal disorders can be obtained from https://cnsgenomics.com/content/data [44–47] and https://www.ibdgenetics.org [48].