Abstract
Background
We nested a seroprevalence survey within the TREATS (Tuberculosis Reduction through Expanded Antiretroviral Treatment and Screening) project. We aimed to measure the seroprevalence of SARS-CoV-2 infection and investigate associated risk factors in one community (population ∼27,000) with high prevalence of TB/HIV in Zambia.
Methods
The study design was cross-sectional. A random sample of 3592 individuals aged ≥15 years enrolled in the TREATS TB-prevalence survey were selected for antibody testing. Randomly selected blocks of residence were visited between October 2020 and March 2021. Antibodies against SARS-CoV-2 were detected using Abbott- ARCHITECT SARS-CoV-2 IgG assay.
Results
A total of 3035/3526 (86.1%) individuals had a blood sample taken. Antibody testing results were available for 2917/3035 (96.1%) participants. Overall, 401/2977 (13.5%) individuals tested positive for IgG antibodies. Seroprevalence was similar by sex (12.7% men vs 14.0% women) and was lowest in the youngest age group 15–19 years (9.7%) and similar in ages 20 years and older (∼15%). We found no evidence of an association between seroprevalence and HIV-status or TB. There was strong evidence (p <0.001) of variation by time of enrollment, with prevalence varying from 2.8% (95% CI 0.8–4.9) among those recruited in December 2020 to 33.7% (95% CI 27.7–39.7) among those recruited in mid-February 2021.
Conclusion
Seroprevalence was 13.5% but there was substantial variation over time, with a sharp increase to approximately 35% toward the end of the second epidemic wave.
Keywords: SARS-CoV-2, COVID-19, Zambia, Seroprevalence, Population-based survey, TB/HIV
Introduction
COVID-19 is a rapidly spreading infectious disease caused by the novel coronavirus SARS-CoV-2, which has established a global pandemic. Currently, over 200 million COVID‐19 cases and over 4.8 million deaths have been reported globally, representing a case fatality rate of 2.2% (WHO, 2021). In Africa, there are now more than 8 million COVID-19 reported cases across 47 countries, with 213,000 deaths as of October 7th, 2021 (Africanews, 2021). Although the pandemic initially seemed to have stabilized due to naturally acquired population immunity and vaccine rollout, the disease has been characterized by new waves of infection and the development of more transmissible variants, such as the delta variant.
As of October 11th, 2021, the Zambia National Public Health Institute reported 209,353 COVID-19 cases and 3654 deaths. Many people in Zambia are potentially at risk of developing severe COVID-19 owing to coexisting underlying conditions and a high TB/HIV coinfection rate. With an HIV prevalence of 12.1% among persons aged 15–49 years (DHS, 2018), Zambia is among the 10 countries with the highest burden of HIV (Zambia Statistics Agency, Ministry of Health (MOH) Zambia, and ICF, 2019). Although people living with HIV (PLHIV) may not be at higher risk of contracting SARS-CoV-2 infection, one of the highest risks for developing severe and even fatal COVID-19 disease is among people with poorly controlled or treated HIV (Boulle et al., 2020; Sentongo et al., 2021). In a recent study conducted in Zambia among 443 hospitalized patients with COVID-19 of whom 28% were HIV-positive, PLHIV with severe HIV disease were more likely to develop severe COVID-19 or die from COVID-19 (Chanda et al., 2021). Additionally, in a recent systematic review, TB was a risk factor for COVID-19 in terms of both severity and mortality irrespective of HIV-status (Tamuzi et al., 2020).
Serological assays identify SARS-CoV-2 antibodies, indicating previous infection in unvaccinated persons (Cheng et al., 2020). Population-based serological testing provides estimates of the cumulative incidence of infection and complements diagnostic testing of acute infection in helping to inform the public health response to COVID-19. As the world moves through the vaccine and new variant era, synthesizing seroepidemiology findings is increasingly important to track the spread of infection, identify disproportionately affected groups, and measure progress toward herd immunity (Bobrovitz et al., 2021; Chen et al., 2021).
Our understanding of community-level transmission patterns, seroprevalence, and its correlates remains limited (Mulenga et al., 2021). To our knowledge, only 2 seroprevalence studies have been conducted in Zambia (Lai, Wang, & Hsueh, 2020; Mulenga et al., 2021). We report on results from a SARS-CoV-2 seroprevalence survey (serosurvey) conducted between October 2020 and March 2021. Our aim was to determine the seroprevalence of SARS-CoV-2 infection in a population with high prevalence of TB/HIV coinfection in Zambia, as a measure of the cumulative proportion of the general population who have been infected. We further aimed to determine risk factors for SARS-CoV-2 infection in this population.
Methods
Study Design and Population
The TREATS-COVID study aimed to measure the prevalence and spread of SARS-CoV-2 in Zambia, collecting data from one periurban community and extrapolating to the wider population using mathematical modelling. It is an observational epidemiological study with 3 linked substudies, 1 of which was a serosurvey (Appendix 1). To rapidly gain evidence, we nested the serosurvey within the TREATS TB Prevalence survey (TBPS), a cross-sectional random sample of the community. Participants aged ≥15 years selected for the TBPS were also asked to take part in the serosurvey. Details of the TREATS Project are provided elsewhere (Zambart, 2021).
The study was conducted in a periurban community in Kabwe district, Zambia. This middle-to-high density study community has previously been characterized as part of the HPTN 071 (PopART) and TREATS studies, with a mixed economy that is typical of other Zambian periurban communities (Hayes et al., 2019). HIV prevalence in the community is approximately 15% and TB prevalence is estimated to be in the range of 0.5%–1%. The total population was estimated to be 28,000 individuals, living in approximately 5,300 households (average household size around 5.3), of whom around 17,000 (60%) were aged ≥15 years. More than one-third of the population of Kabwe live in lead-contaminated areas (Watch, 2019) (Appendix 2).
Sample size and sampling strategy
The sampling strategy for the TBPS was used for random selection of serosurvey participants. Sampling was structured according to geographically defined blocks of residence of approximately 150–200 households. A total of 20 blocks covered the whole community. Blocks were randomly ordered from 1–20 and visited sequentially. Every household in the block was visited (at least 3 attempts were made) with the whole block being covered in 2–3 weeks. Closed households in 4 blocks visited in November–December 2020 were revisited at a later stage of the survey (January/February 2021).
Household members aged ≥15 years were invited to participate until the TBPS target sample size of 3500 individuals was reached. The sample size of 3500 individuals was chosen to include all participants of the TBPS in the serosurvey. At the time of the survey, there was no knowledge available on SARS-CoV-2 IgG antibody seroprevalence. Sample size calculations showed that a target of 3500 individuals with 15% HIV-prevalence would give us 84% power to detect a difference in seroprevalence of 6% in participants who were HIV-negative versus 3% in those who were HIV-positive.
Procedures
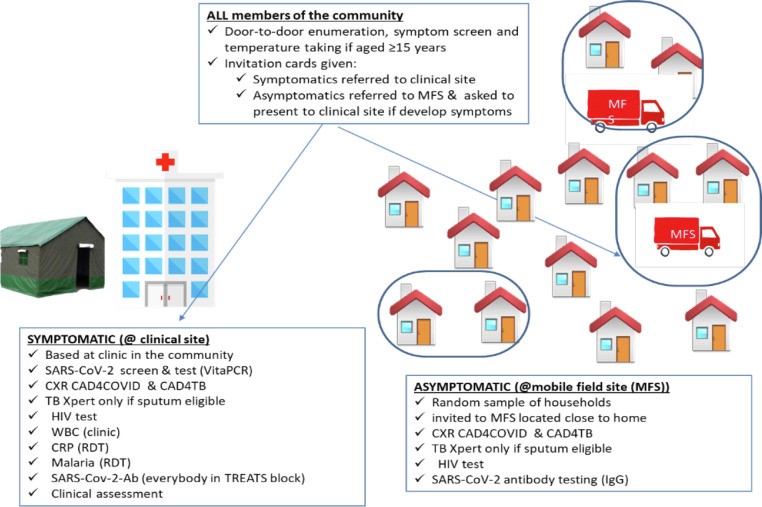
After sensitizing the community, research assistants (RAs) with appropriate personal protective equipment went door-to-door, enumerating the entire population in the selected blocks. During the household visit, written informed consent was obtained for those aged 18 years and older, whereas those aged 15–17 years were asked for verbal assent and their parents/guardians for written informed consent. RAs conducted symptomatic screening—screened participants for COVID-19 symptoms (fever, cough, new shortness of breath, new loss of taste or smell, and fatigue) or for being a (known) household contact of a confirmed COVID-19 case. Individuals screened positive for COVID-19 were invited to go to a clinical site for clinical assessment, whereas those screened negative were invited to attend the TBPS mobile field site (MFS). Absent household members were given invitation cards and asked to attend the clinical site if they had COVID-19 symptoms or MFS if they did not have symptoms. Questionnaire data on seroprevalence risk factors were collected.
At the clinical site, PCR testing (Cepheid Xpert Xpress SARS-CoV-2 assay or VitaPCR RT-PCR assay [Credo-Diagnostics Biomedical, Singapore], depending on availability) using oropharyngeal/nasal specimens was conducted and participants were also all asked for a venous blood sample for SARS-CoV-2 antibody testing. Positive PCR results were communicated to the district for contact tracing. At the MFS, venous blood was collected for antibody testing and TB and HIV procedures were conducted as part of the TBPS (Figure 1 ).
Figure 1.
Summary of study procedures
SARS-CoV-2 IgG immunoassay testing
All blood specimens were transported in cooler boxes with cold packs to a local laboratory in Kabwe on the same day, within 4 hours of collection. Blood was collected in BD Vacutainer Plastic K2EDTA tubes with Lavender BD Hemogard Closure. The blood specimens were registered, centrifuged, and 2 mL plasma aliquots were stored at –80°C. Weekly, the frozen aliquots were transported frozen in freezers installed in a truck to the Zambart Central Laboratory in Lusaka.
Plasma samples were then tested for the presence of SARS-CoV-2 IgG antibodies against the nucleocapsid protein on the Abbott Architect i2000SR automated analyzer using the Abbott SARS-CoV-2 IgG assay (Abbott Park, USA) according to the manufacturer's instructions (Appendix 3). Assay results higher than or equal to the cut-off index value of 1.4 were interpreted as positive for SARS-CoV-2 antibodies.
Data collection and statistical analysis
Tablets were used for data capture. Data from the local server were synchronized daily to a central server at Zambart (Stata version 16.1, STATA Corp., USA) and were used for statistical analysis. The primary outcome—detectable level of SARS-CoV-2 antibodies in plasma—was defined as a binary variable (positive/negative). Seroprevalence of past SARS-CoV-2 infection overall and in subgroups was calculated as the number of individuals testing positive for SARS-CoV-2 antibodies divided by the number of individuals tested and having a valid result.
A population-average logistic regression model, with household as the panel variable to adjust for household clustering and week of participation as a fixed effect, was fitted to the data. Point-prevalence (with 95% CI) for each week of participation was calculated using postestimation of this model with week as the only independent variable. The same regression model was used to obtain adjusted odds ratios (aOR) for the associations between socioeconomic, clinical, and behavioral factors with the prevalence of SARS-CoV-2 infection, adjusted a priori for week of participation, age, and sex.
Simple projections were made to estimate the number of past infections per 1000 population, aged 15 years and older on the basis of our survey. This was compared with number of COVID-19 notifications aged 15 years and older per 1000 population, on the basis of the confirmed notified COVID-19 cases in Kabwe district as reported by the local health authorities and the population size of Kabwe.
Results
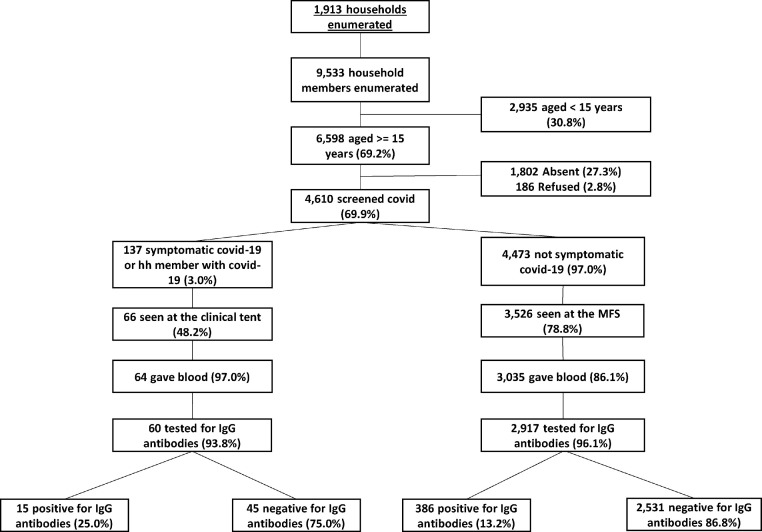
We enumerated 1913 households with 9533 individuals, 69.2% (6598/9533) were aged 15 years or older and 69.9% (4610/6598) agreed to be part of the study and were screened for COVID-19 symptoms. Among those screened, 3.0% (137/4610) self-reported at least one COVID-19 symptom or lived in a household where at least one person had been diagnosed with COVID-19 (figure 2 ). Individuals with a negative symptom screen or who did not report living in a household where at least one person had been diagnosed with COVID-19 and those not seen at the household were referred to the MFS (97.0% [4473/4610]).
Figure 2.
Participants enrolled in the study in Kabwe district, Zambia 2020/2021
A total of 3526 individuals (78.8% of 4473) were seen at the MFS; 3035 (86.1%) provided a blood sample with IgG antibody results available for 2917 (96.1%). Of the 137 who were referred to the clinical site, 66 (48.2%) attended the clinical site and of these, 64 (97.0%) gave a blood sample, and of these 60 (93.8%) were tested for IgG antibodies.
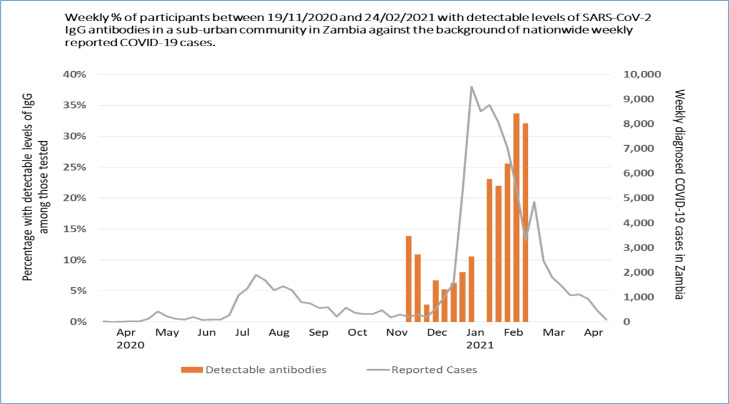
Overall, 401/2977 (13.5%) individuals tested positive for IgG antibodies. There was strong evidence (p <0.001) of variation by time of enrollment, with prevalence varying from 2.8% (95% CI 0.8–4.9) among those recruited early December 2020 to 33.7% (95% CI 27.7–39.7) among those recruited mid-February 2021 (figure 3 ).
Figure 3.
Variation of SARS-CoV-2 Seroprevalence by time of enrolment
A seroprevalence of between 13% and 35% is equivalent to 135–350 per 1000 population having a passed infection of SARS-CoV-2. In May 2021, the health authorities from Kabwe district (population ∼150,000 of 15 years and older) reported ∼500 cumulative confirmed cases of COVID-19 aged 15 years and older since the start of the pandemic, which is equivalent to 3 per 1,000 population.
Risk factors
Seroprevalence was similar in men and women (12.7% vs 14.0%, respectively). Young people (YP) (15–19 years) had the lowest seroprevalence (9.7%); whereas, older age groups had higher prevalence of detectable IgG antibodies varying from 14.3% those aged 20–29 (aOR 1.43; 95% CI 1.03–1.99) to 16.1% in those aged 40–49 (aOR 1.85, 95% CI 1.24–2.74, p = 0.028) (table 1 ).
Table 1.
Associations of key risk factors with seroprevalence of SARS-CoV-2 IgG (Zambia, 2020/2021)
| Number of participants | IgG detected | % | Adjusted OR * | 95%CI | P value | |
|---|---|---|---|---|---|---|
| All | 2,977 | 401 | 13.5% | |||
| Gender | 0.321 | |||||
| Men | 1,246 | 158 | 12.7% | 1.00 | ||
| Women | 1,731 | 243 | 14.0% | 1.12 | (0.89-1.41) | |
| Age | 0.028 | |||||
| 15-19 | 761 | 74 | 9.7% | 1.00 | ||
| 20-29 | 960 | 137 | 14.3% | 1.43 | (1.03-1.99) | |
| 30-39 | 510 | 73 | 14.3% | 1.47 | (1.03-2.09) | |
| 40-49 | 311 | 50 | 16.1% | 1.85 | (1.24-2.74 | |
| 50+ | 435 | 67 | 15.4% | 1.52 | (1.05-2.19) | |
| Household size | 0.999 | |||||
| 6 or more household members | 1,601 | 215 | 13.4% | 1.00 | ||
| 4/5 household members | 833 | 109 | 13.1% | 1.02 | (0.77-1.36) | |
| 3 members | 332 | 47 | 14.2% | 1.00 | (0.69-1.46) | |
| 2 members | 170 | 25 | 14.7% | 1.04 | (0.65-1.68) | |
| 1 member | 41 | 5 | 12.2% | 1.00 | (0.36-2.80) | |
| COVID-19 Diagnosed | 0.704 | |||||
| No | 2969 | 399 | 13.4% | 1.00 | ||
| Yes | 8 | 2 | 25.0% | 1.37 | (0.27-6.92) | |
| Symptoms COVID-19 | 0.467 | |||||
| No | 2925 | 388 | 13.3% | 1.00 | ||
| Yes | 52 | 13 | 25.0% | 1.27 | (0.67-2.42) | |
| Duration of symptoms COVID-19 | 0.025 | |||||
| 0 days (No symptoms) | 2925 | 387 | 13.2% | 1.00 | ||
| 1-3 days | 24 | 1 | 4.2% | 0.16 | (0.01-1.84) | |
| 4-6 days | 8 | 3 | 37.5% | 2.06 | (0.46-9.20) | |
| 7 days or more | 20 | 9 | 45.0% | 3.01 | (1.26-7.24) | |
| Reports a current or past case of COVID-19 in household | 0.984 | |||||
| No | 2966 | 398 | 13.4% | 1.00 | ||
| Yes | 11 | 3 | 27.3% | 0.99 | (0.22-4.34) | |
| HIV status | 0.540 | |||||
| Tested HIV-negative | 1934 | 270 | 14.0% | 1.00 | ||
| Tested HIV-positive | 37 | 3 | 8.1% | 0.45 | (0.12-1.67) | |
| Self-reported HIV-positive | 442 | 62 | 14.0% | 0.85 | (0.61-1.19) | |
| Not tested/tested indeterminate | 564 | 66 | 11.7% | 0.94 | (0.67-1.32) | |
| TB | 0.918 | |||||
| No history of TB | 2851 | 384 | 13.5% | 1.00 | ||
| Self-reported currently on TB-X | 8 | 0 | 0.0% | NA | ||
| History of TB | 110 | 16 | 14.5% | 1.13 | (0.64-1.97) | |
| Bacteriologically confirmed TB | 8 | 1 | 12.5% | 0.99 | (0.17-5.67) | |
| Health Care Worker | 0.261 | |||||
| No | 2945 | 399 | 13.5% | 1.00 | ||
| Yes | 32 | 2 | 6.3% | 0.37 | (0.07-2.09) | |
| Use public transport past 2 weeks | 0.021 | |||||
| No | 898 | 110 | 12.2% | 1.00 | ||
| Yes | 2079 | 291 | 14.0% | 1.32 | (1.04-1.69) | |
| Currently smoking | 0.060 | |||||
| No | 2705 | 370 | 13.7% | 1.00 | ||
| Sometimes | 135 | 20 | 14.8% | 1.09 | (0.64-1.86) | |
| Daily | 137 | 11 | 8.0% | 0.47 | (0.25-0.90) |
Seroprevalence was higher among those with symptoms (15/60, 25.0%) than those without symptoms (386/2917, 13.2%). However, after adjusting for time of enrollment, we found no evidence for higher seroprevalence among those with symptoms (aOR 1.27, 95% CI 0.67–2.42).
Higher seroprevalence was seen in individuals with COVID-19 symptoms for more than 7 days (45.0%; aOR 3.01, 95% CI 1.26–7.24, p = 0.025) compared to asymptomatics. There was no evidence of an association between seroprevalence and underlying conditions including TB and HIV or with self-reported past COVID-19 infection (table 1/Appendix 4b). Participants who reported to have used public transport in the past 2 weeks were more likely to be seropositive than those who did not (aOR 1.32, 95% CI 1.04–1.69, p = 0.021). We found some evidence (p = 0.060) that daily smokers were less likely to have detectable antibodies than nonsmokers (aOR 0.47, 95% CI 0.25–0.90).
Appendix 4a–4c shows the seroprevalence and aOR's for all the socioeconomic, clinical, and behavioral factors we have explored. We found no association between seropositivity and education, marital status, or employment status.
Discussion
We found an overall seroprevalence of SARS-CoV-2 antibodies of 13.5% in an African periurban community, covering the period just before and during the second wave of the COVID-19 epidemic in Zambia (December 2020–March 2021). The seroprevalence from our population-based random sample is much higher than previous estimates from Zambia, which ranged between 2.1%–8.2% (Fwoloshi et al., 2021; Hines JZ, Fwoloshi S, & Kampamba D, 2021; Laban et al., 2021; Mulenga et al., 2021). Our study highlights the widespread exposure to SARS-CoV-2 across this community at a time when the vaccination program had not begun in Zambia.
Several systematic reviews and meta-analyses of SARS-CoV-2 seroprevalence have been conducted worldwide; however, Africa has been under‐represented (Bobrovitz et al., 2021; Chen et al., 2021; Lai et al., 2020; Rostami et al., 2021). Seroprevalence has varied markedly among geographic regions and populations. One review included serological data for more than 5 million study participants from 404 serosurveys done worldwide, with only 8 studies from Africa (Chen et al., 2021). Seroprevalence ranged between 4.2%–18.0% among the different populations (Chen et al., 2021).
In a recent review across 968 studies with 9.3 million participants in 74 countries, seroprevalence was low (median 4.5%, IQR 2.4–8.4%) but varied widely by population and region, from 0.6% in Southeast Asia, East Asia, and Oceania to 19.5% in sub-Saharan Africa (SSA) (Bobrovitz et al., 2021). Six countries from SSA with a median-corrected seroprevalence of 19.5% (IQR 9.0–26.0%) were included in the review. Another review included 47 studies involving 399,265 people from 23 countries with only 1 SSA country (Kenya) (Rostami et al., 2021). Seroprevalence in Kenya varied from 0.37%–22.1%, with a pooled seroprevalence of 3.38% (95% CI 3.05–3.72%) (Rostami et al., 2021).
In another review focused on Africa, 23 studies involving 27,735 individuals were included (Chisale et al., 2021). The pooled seroprevalence of SARS‐CoV‐2 antibodies in Africa was 22% (95% CI 14–31) with very high heterogeneity (Chisale et al., 2021). Seroprevalence was highest in studies conducted in SSA compared to other regions in Africa (Chisale et al., 2021). The high seroprevalence in SSA could be explained by the new variants of the virus spreading across this region before and during the second wave (December 2020) (Mulenga et al., 2021).
Zambia reported the first 2 cases of COVID-19 on March 18th, 2020 (Kanduza, 2020). The first wave of the COVID-19 pandemic occurred between May–August 2020 (peaked July 2020); whereas, the second wave was from December 2020–April 2021 (peaked January 2021). We found a strong time trend of seroprevalence parallel with the second wave of national COVID-19 diagnosed cases. The seroprevalence was in the order of 5%–15% across weeks from November 2020–December 2020. From January 2021–mid-February 2021, the seroprevalence was substantially higher (20%–35%).
The strong time trend in levels of seropositivity we observed could have been due to various reasons. Spatial variation cannot be entirely excluded because geographic blocks were visited at different time-points. Blocks 5–15 were completed within 3 weeks before starting the next one. However, in the first 4 blocks visited, the activities continued for 12 weeks, and seroprevalence in these blocks showed the same time trend as the overall survey. Similarly, in blocks that were adjacent but sampled in different weeks, the prevalence was higher in the blocks sampled at a later time suggesting that the increase in prevalence with time is likely explained by the increased transmission with time and not by spatial variation.
We showed a decline in seroprevalence during the first 5 weeks of the study (November 19th –December 22nd, 2020). This could be explained by spatial variation, random variation, or waning of antibody levels of those infected during the first wave 4–5 months earlier in the year. The increase in weekly seroprevalence from December 23rd, 2020 until around February 20th, 2021 seem to follow the increase in national notified cases during the second wave with a 4–5-week lag time. We show trends by week with seroprevalence in the last weeks reaching approximately 35%, suggesting a high cumulative level of community transmission.
Our survey suggests that the cumulative number of cases (150–350 cases per 1,000) population is much higher than the reported number of confirmed COVID-19 cases as reported by the district health authorities (3 per 1,000) population. Currently, the actual number of people who have been infected with SARS-CoV-2 in Zambia is unknown. Relying on reported COVID-19 cases risks underestimating the true number of infections, given the inadequate testing capacity; the high proportion of asymptomatic individuals and challenges in data and surveillance systems (Chisale et al., 2021; Y-Ling Chi, Clementine Fu, Tom Drake, Hiral Anil Shah, & Javier Guzman, 2021). A testing strategy which focuses purely on testing symptomatic cases is likely to miss a large proportion of SARS-CoV-2 infections (Shaw et al., 2021).
A large population-based serosurvey in Zambia reported that official data on the number of laboratory-confirmed cases were largely underestimating the extent of community transmission (Mulenga et al., 2021). One laboratory-confirmed case was reported for every 92 SARS-CoV-2 infections that occurred in the community (Mulenga et al., 2021). Additionally, a global review reported seroprevalences from national studies that were a median 18.1 times (IQR 5.9–38.7) higher than the corresponding SARS-CoV-2 cumulative incidence (Bobrovitz et al., 2021).
In our study population, ∼13.5% seroprevalence in a population of around 14,850 (≥ 15 years) implies ∼2,004 community members would have prior SARS-CoV-2 infection. However, this is a minimum estimate as the final prevalence of about 35% at the end of the survey may be more reflective of the cumulative infection rate rather than the overall prevalence of about 13.5%, which probably corresponds to an estimate midway through the study. Using a final prevalence of about 35%, measured at the end of February 2021, we estimate that 5197 community members may have been infected. Our study shows that the proportion of individuals who were infected with COVID-19 by the end of the second wave was of the order of one-third in this community that shares features with many others in Zambia. Therefore, it is plausible that many other communities also experienced moderately high levels of infection.
Associated risk factors
Although there is overwhelming evidence on the risk factors for diagnosed SARS-COV-2 infection and disease, literature is scanty on serology (Lalwani et al., 2021). Although it is true that male sex, older age, and comorbidities are associated with higher mortality following infection, their role in acquiring the infection is less clear (Giannouchos, Sussman, Mier Odriozola, Poulas, & Farsalinos, 2020). This study found no difference by sex, consistent with other findings (Bobrovitz et al., 2021; Hallal et al., 2020). However, other studies found that male sex was associated with higher seropositivity owing to various reasons including sex-based immunological differences (Chisale et al., 2021; Galanis, Vraka, Fragkou, Bilali, & Kaitelidou, 2021).
The seroprevalence was lower in YP (∼ 10% among 15–19-year-olds) compared with older age groups (∼15%). Similarly, in a review with 32 studies comprising 41,640 YP and 268,945 adults, YP less than 20 years had 44% lower odds of SARS-CoV-2 infection compared with adults 20 years and older (Viner et al., 2021).
Several studies have shown that individuals with comorbidities are at increased risk for severe COVID-19 disease and death (Dustan, 1990; Edler et al., 2020; Mucheleng'anga et al., 2021; Mwananyanda et al., 2021). In other studies, risk of infection in PLHIV or in patients who had TB was not more than 1.5–2 times higher than for patients negative for HIV or patients without TB (Chanda D et al.; Mulenga et al., 2021). PLHIVmight be immunocompromised and, hence, may have a lower or delayed antibody response after infection. Among participants testing HIV-positive in our survey who were previously unaware of their status and not (yet) on ART, we see lower odds of being seroprevalent than HIV-negative participants (aOR 0.45, 95% CI 0.12–1.67); however, statistical evidence was insufficient for a true difference in the population.
In this study, daily smokers had 50% lower odds of having past infection, similar to previous findings (Paleiron et al., 2021, Miyara et al., 2022). However, the role of smoking in acquiring infection is unclear (Miyara et al., 2022.). We found no associations between seroprevalence and several sociodemographic and socioeconomic risk factors. The small sample size in some subgroups could have affected the ability to detect significant differences or the finding may reflect limited heterogeneity in the living condition of this population. Other studies have shown a strong association between income levels and human development indices (Rostami et al., 2021; Shaw et al., 2021).
We found no evidence that having previous COVID-19 or having a diagnosed case of COVID-19 in the household was associated with seropositivity, probably owing to limited numbers of diagnosed COVID-19 cases. In Brazil, presence of a COVID-19 case (PR 1.39, 95% CI 1.24–1.57) or death (PR 2.14, 95% CI 1.74–2.62) in a household considerably increased the risk of other household members acquiring infection (Lalwani et al., 2021).
We found strong evidence that seropositivity was associated with the duration of COVID-19 symptoms. Individuals with a longer duration of COVID-19 symptoms of 7 days or more were 3 times more likely to be seropositive than those with no symptoms. This finding is not surprising because the sensitivity of antibody tests is low in the first week since symptom onset; 90% of cases are seropositive by 14 days (Deeks et al., 2020).
Study Limitations
Our community was purposefully selected and is not representative of the population of Zambia, although its features are shared by many other peri-urban communities in the country. We might have experienced selection bias in individuals not enrolled in the study. Because they were absent from home they could be potentially at higher risk of SARS-CoV-2 infection due to social mixing.
Seroprevalence findings need to be interpreted with caution as data can underestimate the true number of previously infected individuals (Ward et al., 2020). IgG antibodies against SARS-CoV-2 decline over time, with faster waning of antinucleocapsid antibodies than antispike antibodies (Ward et al., 2020). Some participants could potentially have tested seronegative despite having been infected before being surveyed. Some blood samples collected during the last weeks of the survey could not be tested as a result of shortage of available test kits. Because seroprevalence was higher toward the end the survey, the presented overall seroprevalence could underestimate the actual overall seroprevalence.
Study strengths
Our study was a random sample of the population and used a serological assay recommended in diagnostic algorithms and public health interventions (Andrew Bryan et al., 2020; A. Bryan et al., 2020; Meschi et al., 2020). Additionally, because the study took place over a 6-month period we were able to measure how seroprevalence evolved over time. We have subsequently followed our study participants 6–10 months after the first measurement to get a later-in-time snapshot measure of the prevalence of past infection in the same population and learn also about seroincidence between the second and subsequent epidemic waves. These seroincidence results will be reported in a separate manuscript.
Conclusion
The overall seroprevalence from October 2020–March 2021 was 13.5% but a sharp increase in prevalence was seen (up to 35%) by the end of the second wave in mid-February 2021. There was a strong time trend in levels of seropositivity parallel with the epidemic curve. We provide useful seroepidemiological data needed for public health responses.
Acknowledgments
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Author contributions
KS and HA conceived the study with input from AS, SF, CM, PD, BK, MR, and RH. Sample collection was led by JB with assistance from MC. The laboratory set-up and sample processing were coordinated by JB, CM PD, and BK. BK, PD. MC performed laboratory testing, collected data, and approved the test results.. Data were cleaned and prepared by AS. Statistical analyses was performed by AS. KS and AS contributed equally in writing the paper with input from all authors and revised it critically for intellectual content.
Author agreement
All authors have seen and approved the final version of the manuscript being submitted. The article is the authors’ original work, has not received prior publication, and is not under consideration for publication elsewhere.
Funding source
This project is part of the EDCTP2 program supported by the European Union (grant number RIA2016S-1632-TREATS and RIA2020EF-3004-TREATS-COVID).
Ethical approval
Written informed consent was obtained for adults (aged ≥18 years), whereas those aged 15–17 years were asked for verbal assent and their parents/guardians for written informed consent. The study was approved by the Zambia Biomedical Research and Ethics Committee, the Zambia National Health Research Authority, and the London School of Hygiene and Tropical Medicine Ethics Committee.
Acknowledgment
Authors would like to express their sincere appreciation and gratitude to all members of the TREATS-COVID study team in Zambia, and to the study participants and their communities, and to the Zambart laboratory staff for their contributions to the research. The content herein is solely the responsibility of the authors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.03.021.
Appendix. Supplementary materials
REFERENCES
- Africanews. (2021). Africa COVID-19 statistics. Retrieved 16th April, 2021, from https://www.africanews.com/2020/07/29/coronavirus-in-africa-breakdown-of-infected-virus-free-countries//)
- Bobrovitz N., Arora R.K., Cao C., Boucher E., Liu M., Donnici C.…Cheng M.P. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis. PLOS ONE. 2021;16(6) doi: 10.1371/journal.pone.0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle A., Davies M.-A., Hussey H., Ismail M., Morden E., Vundle Z.…Tamuhla T. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020:ciaa1198. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan A., Fink S.L., Gattuso M.A., Pepper G., Chaudhary A., Wener M.H.…Greninger A.L. Anti-SARS-CoV-2 IgG antibodies are associated with reduced viral load. medRxiv. 2020 doi: 10.1101/2020.05.22.20110551. 20202005.2022.20110551. [DOI] [Google Scholar]
- Bryan A., Pepper G., Wener M.H., Fink S.L., Morishima C., Chaudhary A.…Greninger A.L. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;(8):58. doi: 10.1128/jcm.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda D, Minchella PA, Kampamba D, et al. COVID-19 Severity and COVID-19–Associated Deaths Among Hospitalized Patients with HIV Infection — Zambia, March–December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:807–810. doi: 10.15585/mmwr.mm7022a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chen Z., Azman A.S., Deng X., Sun R., Zhao Z.…Yu H. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. The Lancet Global Health. 2021 doi: 10.1016/S2214-109X(21)00026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.P., Yansouni C.P., Basta N.E., Desjardins M., Kanjilal S., Paquette K.…Papenburg J. Serodiagnostics for Severe Acute Respiratory Syndrome–Related Coronavirus 2. Annals of Internal Medicine. 2020;173(6):450–460. doi: 10.7326/M20-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisale M.R.O., Ramazanu S., Mwale S.E., Kumwenda P., Chipeta M., Kaminga A.C.…Mbakaya B.C. Seroprevalence of anti-SARS-CoV-2 antibodies in Africa: A systematic review and meta-analysis. Rev Med Virol. 2021:e2271. doi: 10.1002/rmv.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S.…Van den Bruel A. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6(6) doi: 10.1002/14651858.cd013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustan H.P. Obesity and hypertension in blacks. Cardiovasc Drugs Ther. 1990;4 Suppl 2:395–402. doi: 10.1007/bf02603183. [DOI] [PubMed] [Google Scholar]
- Edler C., Schröder A.S., Aepfelbacher M., Fitzek A., Heinemann A., Heinrich F.…Sperhake J.-P. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. International Journal of Legal Medicine. 2020;134(4):1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fwoloshi S., Hines J.Z., Barradas D.T., Yingst S., Siwingwa M., Chirwa L.…Agolory S. Prevalence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) among Health Care Workers—Zambia. Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciab273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. Journal of Hospital Infection. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannouchos T.V., Sussman R.A., Mier Odriozola J.M., Poulas K., Farsalinos K. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory–confirmed COVID-19 cases. medRxiv. 2020 doi: 10.1101/2020.06.04.20122481. 2006.2004.20122481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallal P.C., Hartwig F.P., Horta B.L., Silveira M.F., Struchiner C.J., Vidaletti L.P.…Victora C.G. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. The Lancet Global Health. 2020;8(11):e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R.J., Donnell D., Floyd S., Mandla N., Bwalya J., Sabapathy K.…Fidler S. Effect of Universal Testing and Treatment on HIV Incidence — HPTN 071 (PopART) New England Journal of Medicine. 2019;381(3):207–218. doi: 10.1056/NEJMoa1814556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JZ, Fwoloshi S, Kampamba D. SARS-CoV-2 Prevalence among Outpatients during Community Transmission, Zambia, July 2020. Emerging Infectious Diseases. Emerging Infectious Diseases. 2021;27(8):2166–2168. doi: 10.3201/eid2708.210502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanduza A.M. In the moment of making History: The case of COVID-19 in Zambia. Yesterday and Today. 2020:257–273. [Google Scholar]
- Laban N., Bosomprah S., Musukuma-Chifulo K., Simuyandi M., Iyer S., Ng'ombe H.…Chilengi R. Comparable exposure to SARS-CoV-2 in young children and healthcare workers in Zambia [version 1; peer review: 1 approved with reservations] Wellcome Open Research. 2021;6(97) doi: 10.12688/wellcomeopenres.16759.1. [DOI] [Google Scholar]
- Lai C.-C., Wang J.-H., Hsueh P.-R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. International Journal of Infectious Diseases. 2020;101:314–322. doi: 10.1016/j.ijid.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalwani P., Salgado B.B., Filho I.V.P., da Silva D.S.S., de Morais T.B.d.N., Jordão M.F.…Lalwani J.D.B. SARS-CoV-2 seroprevalence and associated factors in Manaus, Brazil: baseline results from the DETECTCoV-19 cohort study. International Journal of Infectious Diseases. 2021;110:141–150. doi: 10.1016/j.ijid.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschi S., Colavita F., Bordi L., Matusali G., Lapa D., Amendola A.…Castilletti C. Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara, M., Tubach, F., Pourcher, V., Morelot-Panzini, C., Pernet, J., Haroche, J., . . . Amoura, Z. Low incidence of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios. doi: 10.32388/WPP19W.3 [DOI]
- Mucheleng'anga L.A., Telendiy V., Hamukale A., Shibemba A.L., Zumla A., Himwaze C.M. COVID-19 and Sudden Unexpected Community Deaths in Lusaka, Zambia, Africa - A Medico-Legal Whole-Body Autopsy Case Series. International Journal of Infectious Diseases. 2021;109:160–167. doi: 10.1016/j.ijid.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga L.B., Hines J.Z., Fwoloshi S., Chirwa L., Siwingwa M., Yingst S.…Malama K. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: a cross-sectional cluster sample survey. The Lancet Global Health. 2021 doi: 10.1016/S2214-109X(21)00053-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwananyanda L., Gill C.J., MacLeod W., Kwenda G., Pieciak R., Mupila Z.…Thea D. Covid-19 deaths in Africa: prospective systematic postmortem surveillance study. BMJ. 2021;372:n334. doi: 10.1136/bmj.n334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleiron N., Mayet A., Marbac V., Perisse A., Barazzutti H., Brocq F.-X.…Bylicki O. Impact of Tobacco Smoking on the Risk of COVID-19: A Large Scale Retrospective Cohort Study. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2021;23(8):1398–1404. doi: 10.1093/ntr/ntab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A., Sepidarkish M., Leeflang M.M.G., Riahi S.M., Nourollahpour Shiadeh M., Esfandyari S.…Gasser R.B. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(3):331–340. doi: 10.1016/j.cmi.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J.A., Meiring M., Cummins T., Chegou N.N., Claassen C., Du Plessis N.…Walzl G. Higher SARS-CoV-2 seroprevalence in workers with lower socioeconomic status in Cape Town, South Africa. PLOS ONE. 2021;16(2) doi: 10.1371/journal.pone.0247852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssentongo P., Heilbrunn E.S., Ssentongo A.E., Advani S., Chinchilli V.M., Nunez J.J., Du P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Scientific Reports. 2021;11(1):6283. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamuzi J.L., Ayele B.T., Shumba C.S., Adetokunboh O.O., Uwimana-Nicol J., Haile Z.T.…Nyasulu P.S. Implications of COVID-19 in high burden countries for HIV/TB: A systematic review of evidence. BMC Infectious Diseases. 2020;20(1):744. doi: 10.1186/s12879-020-05450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L.…Eggo R.M. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared With Adults: A Systematic Review and Meta-analysis. JAMA Pediatrics. 2021;175(2):143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H., Cooke G., Atchison C., Whitaker M., Elliott J., Moshe M.…Elliott P. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365,000 adults. medRxiv. 2020 doi: 10.1101/2020.10.26.20219725. 2010.2026.20219725. [DOI] [Google Scholar]
- WHO. (2021). Coronavirus disease (COVID-19) pandemic. Retrieved 14th April, 2021, from https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Y-Ling Chi, Clementine Fu, Tom Drake, Hiral Anil Shah, & Javier Guzman. (2021). Can Serology Tests and Seroprevalence Surveys Inform a More Effective Vaccine Roll Out? Retrieved 08/06/2021, 2021
- Zambart. (2021). TREATS Project. Retrieved 28th September 2021, from https://treatsproject.org/about-treats/
- Zambia Statistics Agency, Ministry of Health (MOH) Zambia, & and ICF . Zambia Statistics Agency, Ministry of Health, and ICF; Lusaka, Zambia, and Rockville, Maryland, USA: 2019. Zambia Demographic and Health Survey 2018. [Google Scholar]
- Watch, Human Rights. 2019. 'We Have to Be Worried”. The Impact of Lead Contamination on Children's Rights in Kabwe, Zambia ', Accessed 10th February https://www.hrw.org/report/2019/08/23/we-have-be-worried/impact-lead-contamination-childrens-rights-kabwe-zambia.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.