Abstract
Objective:
To test the null hypothesis that there is no significant difference between the chitosan-containing and conventional nonfluoridated dentifrices in inhibition of enamel demineralization around orthodontic brackets.
Materials and Methods:
Sixteen orthodontic patients who were scheduled to have extraction of four first premolars for orthodontic reasons were divided into two groups after the power of the study was estimated. Patients in the experimental group were instructed to use chitosan-containing dentifrice (AloeDent), and patients in the control group were instructed to use nonfluoridated dentifrice (Sensodyne Mint). After 60 days, the teeth were extracted and longitudinally sectioned. The demineralization was assessed by cross-sectional microhardness. The determinations were made at the bracket edge cementing limits and at occlusal and cervical points, 100 µm and 200 µm away from the edge. In all these positions, indentations were made at depths of 10, 20, 30, 50, 70, and 90 µm from the enamel surface. Analysis of variance (ANOVA) and Tukey test were used for statistical evaluation at P < .05 level.
Results:
ANOVA showed statistically significant differences for the factors of dentifrice type, position, and depth (P = .000). Statistically significant differences for microhardness values between two tested dentifrices were observed up to 20 µm of depth from the enamel surface (P < .05). Lower microhardness values were found for nonfluoridated dentifrice. Significant microhardness differences were also determined between materials at occlusal and cervical 0 µm positions (P < .05). At these positions, chitosan-containing dentifrice showed lower demineralization than the control.
Conclusion:
Chitosan-containing dentifrice may reduce the enamel decalcification found in patients with poor oral hygiene. The null hypothesis is rejected.
Keywords: Chitosan, Tooth demineralization, Dental caries, Orthodontic brackets
INTRODUCTION
Despite the advances in orthodontic materials and techniques, the development of cavitations around the brackets during treatment continues to be a problem.1 Fixed appliances make it difficult for young patients to maintain adequate oral hygiene during orthodontic treatment.2 These appliances are linked to a high risk of developing white-spot lesions.3 The prevalence of new decalcifications among orthodontic patients with fixed appliances is reported to range from 13% to 75%.3,4 Patients with orthodontic brackets have an elevated risk of caries, and enamel lesions can occur within a month, irrespective of mechanical plaque control and whether fluoridated dentifrice is used.3,5,6
Several methods have been used to prevent or reduce enamel demineralization during orthodontic treatment, including fluoride application in various forms, enamel sealants, rigorous oral-hygiene regimens using glass-ionomer cement for bonding bracket and modified appliance designs.7–9
Chitosan and modified chitosans are interesting candidates in this respect. Chitosan, a natural linear biopolyaminosaccharide is obtained by alkaline deacetylation of chitin.10–12 Chitin is chemically a polymeric N-acetyl-D-glucosamine and is mainly contained in the shells of crabs and shrimps.13 Particular properties of chitosan are especially important. It has a pH of 6.3, which is suitable to buffer the oral pH value high enough to prevent the deleterious action of organic acids on the tooth surface.14 This material is also biocompatible and biodegradable. It is positively charged and combines with the bacterial cell wall and membrane with bacteriostatic and bactericidal results.15,16 Muzzarelli et al.17 demonstrated that chitosan exhibit bactericidal action against several pathogens, including Streptococcus mutans. This is especially important since S. mutans is known to be the principal etiological factor of dental caries.18
Water-soluble, reduced chitosan was used as a mouth-rinse agent, and it displayed an antibacterial and plaque-reducing action.19 Furthermore, recent studies have demonstrated that chewing chitosan-containing gum effectively inhibited the growth of cariogenic bacteria in saliva.20
It is well-known that sub-minimal inhibitory concentrations of several antibacterial compounds impair expression or production of bacterial adhesions, thus interfering with adherence to and colonization of host tissues.21 Therefore, considering that, prevention from bacterial adhesion can easily be achieved in the oral cavity by the use of dentifrices containing these agents.
Studies in dental literature have demonstrated the positive effects of chitosan-containing materials on the pH changes in bacterial dental plaque14 and also showed good antimicrobial effectiveness of these materials.15–21
In a different perspective, amorphous calcium-phosphate–containing orthodontic adhesive,22–24 antibacterial monomer-containing adhesive,25 and different topical agents26 have been investigated in reducing enamel demineralization around brackets. However, no in vivo studies have been performed to investigate the efficiency of a dentifrice on enamel demineralization around orthodontic brackets.
Therefore, the aim of this study was to evaluate the in vivo effects of a chitosan-containing dentifrice in reducing enamel demineralization around orthodontic brackets and to compare the chitosan-containing dentifrice with the conventional nonfluoridated dentifrice. For these purposes, the null hypothesis assumed that there is no significant difference between the chitosan-containing and nonfluoridated dentifrices in inhibition of enamel demineralization around orthodontic brackets.
MATERIALS AND METHODS
This study was approved by the Ethical Committee on Research of the Gülhane Military Medical Academy, Ankara, Turkey. A power analysis was completed by G*Power Ver 3.0.10. (Franz Faul, Universität Kiel, Germany) software. Based on 1∶1 ratio between groups, a sample size of eight patients in each group would give more than 80% power to detect significant differences with 0.40 effect size and at a significance level of α = .05.
Inclusion Criteria
The inclusion criteria were as follows:
Extraction of first premolars due to orthodontic reasons;
No active caries lesions, no staining, no enamel defect, or no initial caries lesion on premolars that were planned to be investigated;
Normal salivary flow rate (>1.0 mL/min);
Normal buffer capacity (final pH: 6.5–7.2);
No allergic reaction to any of the ingredients in dentifrices; and
Regular tooth brushing habit.
Exclusion Criteria
The exclusion criteria were as follows:
Poor oral hygiene,
Occurrence of allergic reaction or mucosal irritation due to any of the ingredients in dentifrices, and
Unexpected side effects.
Sixteen orthodontic patients who were scheduled to have four first premolar teeth extracted for orthodontic reasons were invited to participate in the study according to selection criteria, and all patients and their families provided informed consent.
All patients received a full-mouth cleaning to remove plaque in preparation for bonding. For evaluating the baseline demineralization values of all selected teeth, a portable battery-powered laser fluorescence device, DIAGNOdent Pen (KaVo, Germany), was used.22 The scores of two groups were less than 13, and this indicates that there was no demineralization; both were equivalent for caries risk.
Patients were divided into two equal groups: one experimental and one control. After oral hygiene training, patients were given one of the two dentifrices randomly:
Group 1 (experimental) was given chitosan-containing dentifrice (AloeDent, Optima-Health Nutrition Ltd, Cardiff, Wales, UK), and
Group 2 (control) was given nonfluoridated dentifrice (Sensodyne Mint, GlaxoSmithKline Consumer Healthcare, Brentford, UK).
Orthodontic brackets were bonded with Transbond XT (3M Unitek, Monrovia, Calif), a resin-based composite. A 37% phosphoric acid gel (3M Espe, St Paul, Minn) was used for 15 seconds. The teeth were rinsed with water for 30 seconds and dried with an oil-free source for 20 seconds. Transbond XT (3M Unitek) primer was applied to the etched surface in a thin film and not cured. Adhesive paste was applied to the bracket base (Dyna-Lok series, 3M Unitek), and the bracket was positioned on the tooth and pressed firmly into place. The excess adhesive was removed from around the bracket with a scaler, and the adhesive was light cured from the mesial and distal for 10 seconds each (total time 20 seconds). A light-emitting diode unit (Elipar Freelight 2, 3M-Espe) was used for curing the specimens for 20 seconds. All patients were instructed to brush their teeth twice a day with the given dentifrice and were advised against using any additional preparations.
For the testing procedure, 32 brackets were bonded for each group (16 upper and 16 lower first premolars in both groups). After 60 days, the teeth were extracted and stored in a refrigerator in flasks containing gauze dampened with 2% formaldehyde, pH 7.0, until the analysis. Demineralization was evaluated by cross-sectional microhardness method according to the literature.6,22–28
Cross-sectional Microhardness Analysis
One operator who was blinded from the group allocation carried out the microhardness analysis (Dr Ozcan). The roots of the teeth were removed with a water-cooled diamond disk. The crowns were hemisectioned vertically into mesial and distal halves with a large 15 HC wafering blade in an Isomet low-speed saw (Buehler, Lake Bluff, Ill). The hemisections were cut into a cervical portion and an occlusal portion. Both portions were embedded in self-curing epoxy resin (Epo-Kwick, Buehler), leaving the cut face exposed. The half crown sections were polished with abrasive paper discs (320, 600, and 1200 grit) and polished with a 1-µm diamond spray and a cloth polishing disc (Buehler). A microhardness tester (HMV-700, Shimadzu, Kyoto, Japan) under a 2N load for 15 seconds was used for the microhardness analysis.
Forty-eight indentations were made in each half crown from eight positions and six depths according to the definitions of Pascotto et al.6 On the buccal surface, indentations were made under the bracket. In the occlusal and cervical regions, indentations were made at the edge (0) of the bracket and at 100 µm and 200 µm away from it. Indentations were also made in the middle third of the lingual surface of each half crown, as another control. In all these positions, six indentations were made at 10, 20, 30, 50, 70, and 90 µm from the external surface of the enamel. The values of Vickers hardness number (VHN) found in the two half crowns were averaged.
Statistical Analysis
Data analyses were performed by using Statistical Package for Social Sciences (SPSS, Ver 13.0, SPSS Inc, Chicago, Ill). The Shapiro-Wilks normality test and the Levene variance homogeneity test were applied to the microhardness data. The data showed normal distribution, and there was homogeneity of variances between the groups.
Analysis of variance (ANOVA) was used to evaluate the effect of materials, depths from the enamel surface, positions, and their interactions. For multiple comparisons, the Tukey honestly significant difference (HSD) test was used. The statistically significant level was set at P < .05 level.
For evaluating the intra- and interobserver agreement, the microhardness measurements were done by two investigators using the same instrument at two separate times, and Cohen's kappa scores were determined.
RESULTS
The kappa scores for the assessment of intra- and interobserver agreement were higher than 0.80, which implies substantial agreement between the observers.
ANOVA showed statistically significant differences for the factors of dentifrice type, position, and depth (P = .000). The interactions (dentifrice type/position, dentifrice type/depth, position/depth and dentifrice type/position/depth) were also statistically significant (P = .000) (Table 1).
Table 1.
Statistical Comparisons and the Results of ANOVA
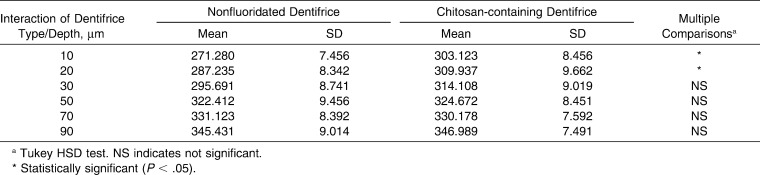
Descriptive statistics and multiple comparisons of microhardness for chitosan-containing and conventional nonfluoridated dentifrice at different depths from the enamel surface are shown in Table 2. The interaction between dentifrice type and depth had significant differences at the depths of 10 µm and 20 µm from the enamel surface. Less demineralization was found on enamel around the brackets for the experimental group compared with the control group.
Table 2.
Descriptive Statistics and Multiple Comparisons of Microhardness (VHN) for Chitosan-containing and Conventional Dentifrice Systems at Different Depths From the Enamel Surface
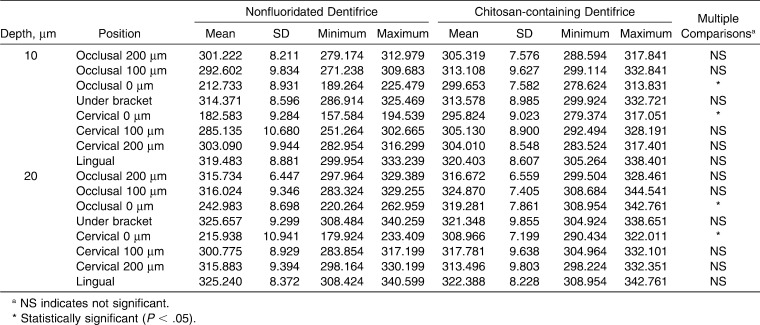
All descriptive statistics and multiple comparisons of microhardness of two dentifrice systems at different observation positions are shown in Table 3. Statistically significant microhardness differences were determined for the two tested groups (P < .05). Chitosan-containing dentifrice showed higher microhardness (occlusal 0 µm: 319.356 VHN; cervical 0 µm: 320.156 VHN) than the conventional dentifrice (occlusal 0 µm: 279.214 VHN; cervical 0 µm: 281.451 VHN).
Table 3.
Descriptive Statistics and Multiple Comparisons of Microhardness (VHN) of Two Dentifrice Systems at Different Observation Positions
All descriptive statistics and multiple comparisons of microhardness of two dentifrice systems and positions at depths of 10 µm and 20 µm are shown in Table 4. The highest demineralization values were determined at occlusal and cervical 0 µm positions for the control group at 10 µm and 20 µm depth.
Table 4.
Descriptive Statistics and Multiple Comparisons of Microhardness (VHN) of Two Dentifrice Systems and Positions at Depth of 10 and 20 µm
According to these results, the null hypothesis of the present study is rejected.
DISCUSSION
Present findings showed that the use of chitosan-containing dentifrice during 60 days of orthodontic treatment was able to prevent demineralization of enamel, and its use can be suggested in prevention of white-spot lesions. To our knowledge, this study is the first to compare the use of a specific dentifrice with a conventional nonfluoridated dentifrice by evaluating condition with microhardness testing enamel demineralization around orthodontic brackets in an in vivo condition, by microhardness testing.
In the past, to use fewer patients and for ethical considerations, preventive effects of various products such as fluoride-releasing materials against demineralization were investigated by using a split-mouth study design.27 A split-mouth design was unsuitable for this investigation. Moreover oral hygiene characteristics are not always homogeneously distributed over the within-patient experimental units, and this heterogeneity can reduce the efficiency of split-mouth designs. As suggested by Pascotto et al.,6 the current experimental design was chosen instead of the split-mouth technique to avoid the carry-across effect. Therefore, subjects were divided into two groups. The baseline clinical, radiologic, salivary, and laser fluorescence assessments were done for standardization. It was determined that all patients in both groups were equivalent with regard to caries risk or demineralization activity.
Instead of in vitro studies with extracted teeth, our model had several advantages27: the development of the caries lesions was studied in vital teeth; minimal patient cooperation was required; no special diet was required; and because the protected enamel surface allowed the accumulation of thick plaque, no other site was at risk of caries with this procedure.
The demineralization values of enamel under two internal controls (under the bracket and at the lingual surface) bonded to the two types of teeth were used to evaluate the effect of acid etching and enamel demineralization.6
In this study, the mineral loss was assessed by cross-sectional microhardness, an accepted analytic method. This method was preferred to evaluate demineralization and caries because a strong correlation coefficient (r = 0.91) was reported by Featherstone and coworkers28 between enamel microhardness scores and the percentage of mineral loss in the caries lesions.
Pascotto et al.6 observed reduced enamel hardness in the cervical region of the bracket compared with that in the occlusal area. These findings were similar to current findings. In vivo, the explanation for this may be that the greater dental plaque accumulation and patient difficulty in cleaning this area. In vitro, the explanation would be less mineralization and higher carbonate on the cervical surface than in the occlusal region.6 Interestingly in the present study, different from the previous findings,6,27,28 similar mineral loss was observed at the cervical and the occlusal region at 0 µm positions. Statistically significant microhardness differences were determined at these regions between the tested materials. Control group showed lower hardness values that indicate more mineral loss than the tested materials.
While others have reported that the demineralization of the enamel around orthodontic brackets can extend as far as 75 µm below the enamel surface, Gorton and Featherstone1 and Pascotto et al.6 reported that enamel demineralization extended to only 30 µm from the enamel surface in vivo. They permitted their subjects to brush their teeth, which most probably removed some or all of the plaque and microorganisms accountable for demineralization. In this study, for extensive and controlled assessment, the indentations we made were at 10, 20, 30, 50, 70, and 90 µm from the external surface of the enamel to observe mineral changes at the outermost part of the enamel. The results of the current study demonstrate that, up to 20-µm depth from enamel surface, the microhardness values for nonfluoridated dentifrice were lower than for the experimental dentifrice, and the difference was statistically significant.
Different from our findings, de Moura et al.27 found lesion depths up to 70 µm from the enamel surface. Our results showed that demineralization existed only up to 20 µm of depth from the enamel surface. This could be explained by the experimental model used; they allowed more plaque accumulation and impaired its removal by tooth brushing. Multiple comparisons of demineralization for dentifrice type and position at the 10 µm depth from the enamel surface showed statistically significant differences at all positions in both the cervical and occlusal margins evaluated on the buccal surface.
Orthodontic attachments make a patient's dental hygiene more difficult, and the accumulation of plaque is easier around the brackets. Our results confirmed this by showing the lower demineralization values at the occlusal and cervical margins at 0 µm positions (Table 3).
To date, the use of chitosan-containing chewing gum and mouth rinse has been found to be an effective method for preventing demineralization of enamel. Hayashi et al.20 have reported that chitosan-containing chewing gum was more effective than chitosan-containing mouth rinse. Hayashi et al.20 have also reported that chitosan released from the gum base could be preserved at the bacteriostatic level in the saliva. The antimicrobial activity of chitosan is dependent on its molecular weight and degree of deacetylation.29 Fujiwara et al.15 have proved that the highly deacetylated and lower molecular chitosan showed bactericidal activity. Present findings suggested that the supplementation of chitosan to dentifrice is an effective method to control the demineralization of enamel around brackets.
CONCLUSION
Chitosan-containing dentifrice may reduce the enamel decalcification found in patients with poor oral hygiene.
REFERENCES
- 1.Gorton J, Featherstone J. D. B. In vivo inhibition of demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2003;123:10–14. doi: 10.1067/mod.2003.47. [DOI] [PubMed] [Google Scholar]
- 2.Tanna N, Kao E, Gladwin M, Ngan P. W. Effects of sealant and self-etching primer on enamel decalcification. Part I: an in-vitro study. Am J Orthod Dentofacial Orthop. 2009;135:199–205. doi: 10.1016/j.ajodo.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Wenderoth C. J, Weinstein M, Borislow A. I. Effectiveness of a fluoride-releasing sealant in reducing decalcification during orthodontic treatment. Am J Orthod Dentofacial Orthop. 1999;116:629–634. doi: 10.1016/s0889-5406(99)70197-6. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick L, Geiger A. M, Gwinnett A. J. Incidence of white spot formation after bonding and banding. Am J Orthod Dentofacial Orthop. 1982;81:93–98. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 5.Øgaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94:68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 6.Pascotto R. C, Navarro M. F. L, Capelozza Filho L, Cury J. A. In vivo effect of a resin-modified glass ionomer cement on enamel demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2004;125:36–41. doi: 10.1016/s0889-5406(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 7.Ghiz M. A, Ngan P, Kao E, Martin C, Gunel E. Effects of sealant and self-etching primer on enamel decalcification. Part II: an in-vivo study. Am J Orthod Dentofacial Orthop. 2009;135:206–213. doi: 10.1016/j.ajodo.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 8.Chung C. K, Millett D. T, Creanor S. L, Gilmour W. H, Foye R. H. Fluoride release and cariostatic ability of a compomer and a resin-modified glass ionomer cement used for orthodontic bonding. J Dent. 1998;26:533–538. doi: 10.1016/s0300-5712(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 9.Fricker J. P. Bonding and debonding with a light-activated resin-modified glass-ionomer cement. Aust Orthod J. 1996;14:78–80. [PubMed] [Google Scholar]
- 10.Muzzarelli R. A. Chitins and chitosans as immuneadjuvants and non-allergenic drug carriers. Mar Drugs. 2010;21:292–312. doi: 10.3390/md8020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts G. A. F. Chitin Chemistry. Houndmills, UK: MacMillan Press; 1992. [Google Scholar]
- 12.Rinaudo M, Domard A. In: Chitin and Chitosan Sources Chemistry Biochemistry Physical Properties and Applications. Skjak-Brack G, Anthonsen T, Sandford P, editors. London, UK: Elsevier Applied Science; 1989. pp. 1–37. [Google Scholar]
- 13.Petri D. F, Donegá J, Benassi A. M, Bocangel J. A. Preliminary study on chitosan modified glass ionomer restoratives. Dent Mater. 2007;23:1004–1010. doi: 10.1016/j.dental.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Shibasaki K, Sano H, Matsukubo T, Takaesu Y. Effects of low molecular chitosan on pH changes in human dental plaque. Bull Tokyo Dent Coll. 1994;35:33–39. [PubMed] [Google Scholar]
- 15.Fujiwara M, Hayashi Y, Ohara N. Inhibitory effect of water-soluble chitosan on growth of Streptococcus mutans. New Microbiol. 2004;27:83–86. [PubMed] [Google Scholar]
- 16.Vishu Kumar A. B, Varadaraj M. C, Gowda L. R, Tharnathan R. N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem J. 2005;391:167–175. doi: 10.1042/BJ20050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzzarelli R, Tarsi R, Filippini O, Giovanetti E, Biagini G, Varaldo P. E. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob Agents Chemother. 1990;34:2019–2023. doi: 10.1128/aac.34.10.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkinson H. F. Adherence and accumulation of oral streptococci. Trends Microbiol. 1994;2:209–212. doi: 10.1016/0966-842x(94)90114-k. [DOI] [PubMed] [Google Scholar]
- 19.Bae K, Jun E. J, Lee S. M, Paik D. I, Kim J. B. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin Oral Investig. 2006;10:102–107. doi: 10.1007/s00784-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi Y, Ohara N, Ganno T, et al. Chewing chitosan-containing gum effectively inhibits the growth of cariogenic bacteria. Arch Oral Biol. 2007;52:290–294. doi: 10.1016/j.archoralbio.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhanel G. G, Nicolle L. E. Effect of subinhibitory antimicrobial concentrations (sub-MICs) on in-vitro bacterial adherence to uroepithelial cells. J Antimicrob Chemother. 1992;29:617–627. doi: 10.1093/jac/29.6.617. [DOI] [PubMed] [Google Scholar]
- 22.Uysal T, Amasyali M, Koyuturk A. E, Sagdic D. Efficiency of amorphous calcium phosphate-containing orthodontic composite and resin modified glass ionomer on demineralization evaluated by a new laser fluorescence device. Eur J Dent. 2009;3:127–134. [PMC free article] [PubMed] [Google Scholar]
- 23.Uysal T, Amasyali M, Ozcan S, Koyuturk A. E, Akyol M, Sagdic D. In vivo effects of amorphous calcium phosphate–containing orthodontic composite on enamel demineralization around orthodontic brackets. Aust Dent J. 2010;55:285–291. doi: 10.1111/j.1834-7819.2010.01236.x. [DOI] [PubMed] [Google Scholar]
- 24.Uysal T, Amasyali M, Ozcan S, Koyuturk A. E, Sagdic D. Amorphous calcium phosphate-containing orthodontic composites. Do they prevent demineralisation around orthodontic brackets? Aust Orthod J. 2010;26:10–15. [PubMed] [Google Scholar]
- 25.Uysal T, Amasyali M, Ozcan S, Koyuturk A. E, Sagdic D. Effect of antibacterial monomer–containing-adhesive on enamel demineralization around orthodontic brackets. In vivo study. Am J Orthod Dentofacial Orthop. In press doi: 10.1016/j.ajodo.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Uysal T, Amasyali M, Koyuturk A. E, Ozcan S. Effects of different topical agents on enamel demineralization around orthodontic brackets. In vivo and in vitro study. Aust Dent J. 2010;55:268–274. doi: 10.1111/j.1834-7819.2010.01233.x. [DOI] [PubMed] [Google Scholar]
- 27.de Moura M. S, de Melo Simplício A. H, Cury J. A. In-vivo effects of fluoridated antiplaque dentifrice and bonding material on enamel demineralization adjacent to orthodontic appliances. Am J Orthod Dentofacial Orthop. 2006;130:357–363. doi: 10.1016/j.ajodo.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Featherstone J. B. D, ten Cate J. M, Shariati M, Arends J. Comparison of artificial caries-like lesion by quantitative microradiography and microhardness profiles. Caries Res. 1983;17:385–391. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- 29.No H. K, Park N. Y, Kee S. H, Meyers S. P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74:65–72. doi: 10.1016/s0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]