Abstract
Objective:
To evaluate the color fading in aqueous solutions of the blue dot wear-compliance indicators of the Invisalign Teen® System outside the oral cavity.
Materials and Methods:
The compliance indicators in the Invisalign Teen aligners were tested for color resistance in various aqueous models with no saliva involved.
Results:
Color fading was observed as a function of time, pH, and temperature while compliance indicators were stored in drinking water or sour soft drinks and in conjunction with the use of cleaning tablets and a dishwasher. The findings of color fading were consistent with the color changes observed when the aligners were being worn by patients. Color fading, notably as observed in connection with acidic soft drinks and cleaning techniques, introduces uncertainty into the assessment of actual patient compliance, as reflected by the fading colors of compliance indicators.
Conclusion:
Compliance indicators are not immune to simple intentional or unintentional manipulations. Therefore, they can best show an estimate of wear time but cannot be recommended as objective wear-time indicators.
Keywords: Removable aligners, Invisalign® Teen, Compliance indicator, Encapsulated dye
INTRODUCTION
Compliance is a mandatory for effective treatment with a removable orthodontic appliance.1,2 Studies of self-reported wear times as compared to the results of clinical assessment have shown that reliable information was obtained in only 43% of patients.3 A total of 140 patients with a mean age of 12.7 years were surveyed by questionnaire.4 The majority expressed a desire to wear their appliances only at night, and they did not wish to have wear times prescribed. When patients were informed that their headgear wear times were being recorded, they did tend to wear the headgear more regularly but still fell short of the actual instructions given.1
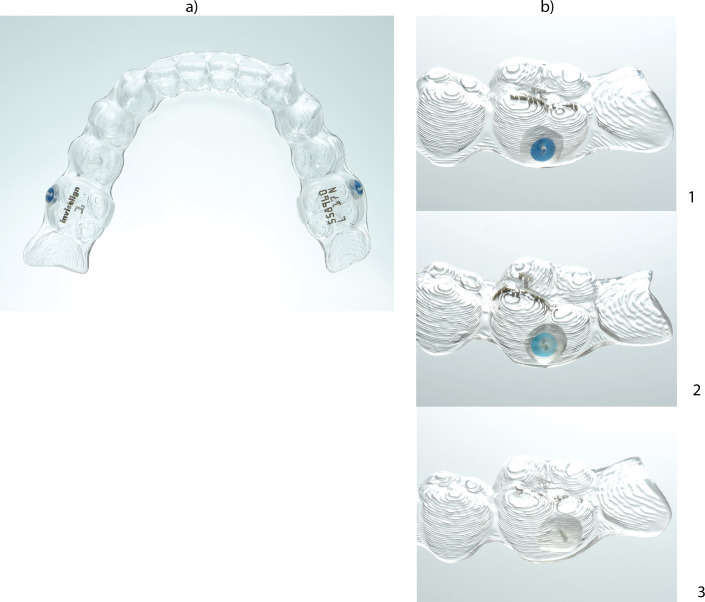
Align Technology recently started distributing a compliance indicator that was designed for use on young patients treated with the Invisalign® Teen system.5 According to the manufacturer, the compliance indicator uses the food dye Erioglaucine disodium salt, which is encapsulated in the clear Invisalign Teen aligner and is released from the polymer in the presence of oral fluid.6 The amount of dye loss will correspond with the amount of time the aligner was worn in the oral cavity. Two different blue dot wear indicators (fast and slow fading) are used to ensure that individual patients' different saliva compositions are appropriately accounted for. The different color fading is based on the different amount of the diffused dye determined by the pore sizes of the polymer. The blue dots are embedded in the vestibular part of the molar segments of the aligners (Figure 1). Wear time is determined by assessing the way in which the compliance indicators change color as the aligner is worn. The clinician is required to evaluate five potential color changes (ranging from dark blue/dark blue to clear/clear) to obtain a graphic representation of the wear time.
Figure 1.
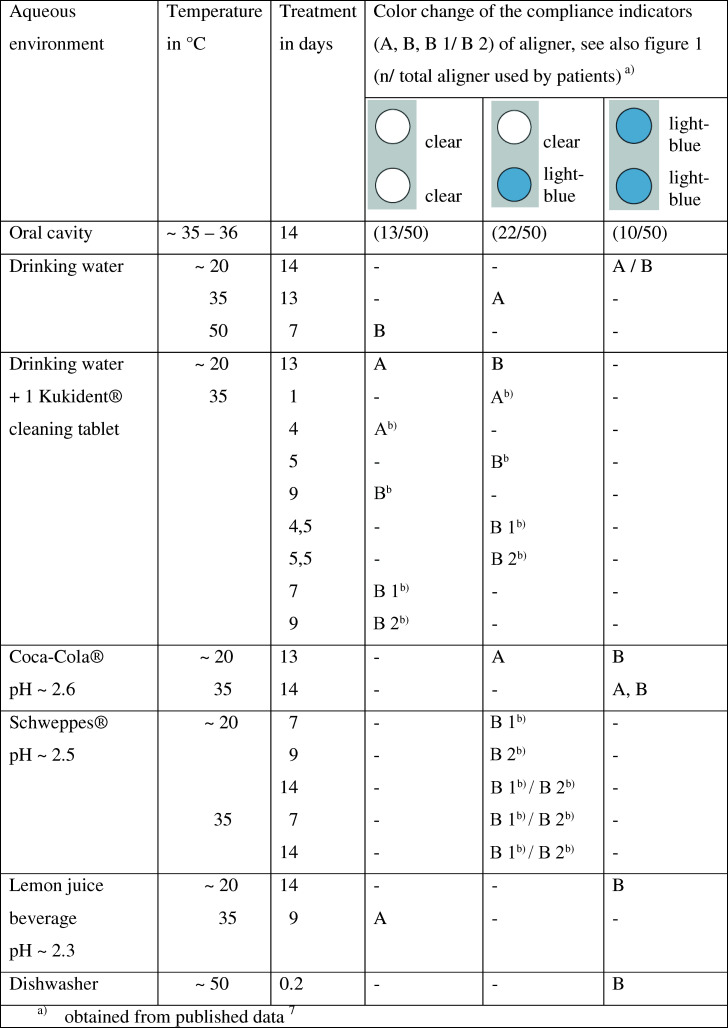
(a) Removable Invisalign Teen® Aligner equipped with two dark blue compliance indicators. (b) Site of aligners with enclosed “fast” compliance indicators. The color intensity of the blue compliance indicator is shown in 1b before treatment (dark blue), in 2b during treatment (light blue), and in 3b after treatment (clear) of the aligner in the aqueous environment (see Table 1).
In a study of 14 patients who were prescribed Invisalign Teen® with clear aligners and embedded color indicators, it was concluded that the service times determined from the compliance indicators showed good agreement with the number of service hours reported by the patients. The positive assessment of compliance based on compliance indicators, however, rested on very different color changes observed in individual patients against a background of similar wearing patterns. Following the initial wear time of 2 weeks, the compliance indicators revealed that, of the nine patient examples listed, four patients showed a clear/clear pattern, four patients showed a clear/blue pattern, and one patient showed a blue/blue pattern, although all patients were expected to have worn the aligners for 20 to 22 hours in a 24-hour period. The theoretical possibility that patients included in the study may have attempted to manipulate the compliance indicator system was consciously ruled out.5
Given the widely diverging color changes, it remains unclear whether the variance in the values obtained may have been caused by different wear times as a result of lack of patient compliance or whether any unknown factors may have exerted an influence on the fading of the embedded dye. These considerations prompted us to investigate factors that might have contributed to the color changes.
MATERIALS AND METHODS
Seven Invisalign Teen® aligners (A) were obtained directly from the manufacturer (Align Technology Inc, Santa Clara, Calif). Fourteen Invisalign Teen® aligners (B) were provided by an orthodontic office that routinely uses the Invisalign® system.
The light-dependent color stability of the food dye Erioglaucine disodium salt (also known as Alphazurine FG, FD&C Blue 1, Brilliant Blue FCF, or Acid Blue 9), which was obtained from Sigma-Aldrich Chemie (Munich, Germany), was proven by irradiation with an ultraviolet lamp (254 nm) for 2 weeks. The color stability of the dissolved dye was tested by dissolving a spatula tip of solid Erioglaucine in 250 mL of drinking water, resulting in a dark blue solution. The pH-, time-, and temperature-dependent color stability of this blue solution was proven in a range of pH 2–9 by adding 1 N HCl or 1 N NaOH and keeping it in these solutions for 2 weeks at 20°C or 35°C.
The oxidation-dependent color fading of the blue dye solution was tested by adding Kukident cleaning tablets (Reckitt Benckiser, Mannheim, Germany) <1?show=[to]?>to the solution and storing it at 20°C or 35°C. Among other chemicals, these tablets contain an oxidant compound in the form of sodium carbonate peroxide.
The color fading of the compliance indicator was investigated by storing the Invisalign Teen® aligners in 250 mL drinking water at 20°C, 35°C, and 50°C as well as in different aqueous solutions at 20°C and 35°C. The first solution studied was created by adding a Kukident cleaning tablet to 250 mL drinking water. Commercially available soft drinks and a fruit juice were used for the remaining solutions: Coca-Cola (Berlin, Germany), Schweppes Original Bitter Lemon (Schweppes, Kreuztal, Germany), and lemon juice (Hitchcock Sportfit Fruchtsaft, Rheinberg, Germany). Aligners were stored in 250 mL of each aqueous medium.
RESULTS
Color Stability of Food Dye Erioglaucine Disodium Salt
The Erioglaucine disodium salt that was used as the compliance indicator was light resistant in its solid and nonembedded form. The pH-dependent color stability of the food dye can be postulated, because a deep blue aqueous solution of the dye in the range of pH 2–9 retained its color throughout the 2 weeks. This finding was also obtained when lemon concentrate (20% by vol) was added, regardless of whether the solution was kept at 20°C or 35°C over the 2-week period. In contrast, the blue aqueous solution lost its color entirely within 24 hours of adding a Kukident cleaning tablet and maintaining the solution at 35°C, while storage at 20°C delayed the process of complete color loss by a few days (Table 1). The loss of color of the dye was presumably a result of oxidative degradation in the wake of peroxide formation triggered by the Kukident tablet dissolving in water.
Table 1.
Color Fading of the Blue Compliance Indicators After Soaking of Unused Aligners (A) in 250 mL of Different Aqueous Media at Different Temperatures, pH, and Time in Comparison to the Color Change of the Compliance Indicators of 50 Aligners After Use by Patients for 17–22/24 Hours Over 14 Days (B)
Color Fading of the Compliance Indicator in Different Aqueous Solutions
The pH-, time-, and temperature-dependent fading patterns of the embedded compliance indicator were determined. To this end, the aligners were stored in drinking water and only in various common highly acidic soft drinks (but not including saliva), as the aligners under study embedded the dye in the form of a stable sodium salt. Acidic soft drinks were therefore more likely to destabilize the dye; in neutral or slightly alkaline beverages, the sodium salt form of the dye was more likely to be preserved. Alcoholic beverages were not included. Testing was carried out for a maximum of 2 weeks, as the aligners included in the Invisalign Teen® system were routinely replaced within 2 or 3 weeks over the course of treatment. The aligners were stored in various liquids at both room temperature (∼20°C) and/or at 35°C. The latter temperature was used to simulate thermal conditions present in the oral cavity: ranges of 33.10°C–37.80°C7 and 35°C–36°C8 have been reported. Temperature-dependent color fading was additionally evaluated by storing the aligners in drinking water at 50°C, since temperatures may reach peaks of 58.8°C in the anterior segment and 54°C in the premolar area (notably when drinking hot beverages).8 A wide variety of cleaning procedures have been used on removable appliances (eg, Kukident cleaning tablets, mechanical cleaning, or application of vinegar).9 Kukident cleaning tablets and cleaning in a dishwasher were included in the present study of color changes in aligners. Aligners obtained from two different sources (A = obtained directly from the manufacturer; B = provided by an orthodontic office) were used to determine whether the dye was encapsulated with sufficient reproducibility during manufacturing to yield consistently reproducible color changes in aligners. In addition, parallel testing was conducted with aligners from the same patients (B1/B2).
Color Fading After Storage in Water
The instructions for use provided by the manufacturer suggested that the embedded dye would fully retain its blue color until exposed to moisture and temperatures equal to or higher than body temperature. However, we observed that the compliance indicators of two aligners belonging to different sources would change their “fast” and “slow” color formulation from dark to light blue when stored in drinking water, even at approximately 20°C, which is considerably below body temperature (see Table 1). Corresponding fading of the compliance indicators was found in patients taking part in the 12-week study after they had worn the aligners for 17.5 to 23 hours in a 24-hour period.
The initial dark blue of the “fast” formulation faded completely when the aligner A was stored in water at 35°C over 13 days, while the dark blue color of the “slow” formulation changed to light blue under the same conditions. The same pattern was observed on graphical analysis of 22 of 50 compliance indicators in patients who had each worn a total of six aligners for 2 weeks each. At 50°C, the dark blue color of both compliance indicators of a used aligner faded to clear after only 7 days.
Color Fading After Storage in Water with One Kukident Tablet
Color fading was fast when a Kukident cleaning tablet was added to the drinking water holding the aligner. In this environment, both color formulations (“fast” and “slow”) included in the compliance indicators of aligner A changed from dark blue to clear within 13 days. Under the same conditions, the color of the other aligner (B) changed from dark to light blue. When the same experiment was conducted at 35°C, the “fast” color formulation in aligner A took 24 hours to fade, and both formulations faded within 4 days. The aligner B showed a clear/light blue pattern of fading after 5 days, which faded to clear/clear after 9 days. When the experiment was repeated with aligners B1 and B2, these changed to light blue/clear within 4.5 and 5.5 days, respectively. Complete fading was noted after 7 days with aligner B1 and after 9 days with aligner B2. Complete fading of both formulations was also observed with the compliance indicators in 13 of 50 aligners of different patients worn for 2 weeks for the prescribed wearing time, while the same pattern was not observed in the remaining 37 aligners.
Color Alterations on Storage in Acidic Soft Drinks
Color changes were also observed when the aligners were stored in Coca-Cola, a very popular drink and one of the most acidic (pH 2.6)10 soft drinks available. At ∼20°C, the dark blue color of the new aligners (A) changed to clear (fast formulation) or light blue (slow formulation) within 13 days, while the worn aligners (B) acquired a light blue/light blue color during the same period of time. Again, the fading pattern obtained with Coca-Cola would have suggested good patient compliance, even though the aligners had never been worn. At 35°C, both formulations resulted in a light blue/light blue pattern within 2 weeks. Presumably, this weak change was caused by more CO2 escaping from the Coca-Cola solution at 35°C than at 20°C, such that the initially low pH value would rise toward a pH of 7 as the reaction proceeded.
Marked changes were also observed when the compliance indicators were stored in Schweppes Original Bitter Lemon (pH 2.5). When the aligners B were stored at roughly 20°C, one of both formulations of the indicator completely lost its color, while the other formulation turned light blue. Strikingly, aligner B1 took 7 days to achieve this color change, while aligner B2 took 9 days. The fading was complete for both compliance indicators by that time and did not progress any further when the aligners were stored in Schweppes for up to 2 weeks. Similar fading patterns were observed at 35°C. Much as with Coca-Cola as the medium, the fact that different temperatures did not cause a difference in fading patterns was presumably a result of CO2 escaping at higher temperatures, raising the pH and reducing the acidic effect of the Schweppes medium. This assumption was supported by the storage of the aligners in lemon juice (pH ∼2.3). At 20°C, the initially dark blue color of both indicators changed to light blue within 2 weeks. At 35°C, by contrast, the compliance indicators completely lost their color in 9 days. This pronounced effect of temperature was not observed with Coca-Cola and Schweppes as media and was presumably a result of the noncarbonated nature of the lemon juice. In other words, the acidic pH of the lemon juice (∼2.3) remained unchanged at 35°C or even decreased slightly, whereas the acid content of acidic soft drinks increased slightly at higher temperatures.11
Color Alterations After Cleaning in a Dishwasher
Color fading was also observed when the aligners were subjected to dishwashing. After two program cycles in the dishwasher (roughly 5 hours), including the use of a commercially available detergent and temperatures of up to 50°C, the compliance indicators changed from dark blue to light blue.
DISCUSSION
This investigation adds to the existing series of reports dealing with the Invisalign® system.12–14 Interindividual differences in color change patterns had, in the past, been attributed to different compositions of saliva. Since the dark blue color remained unchanged when the food dye was stored in nonencapsulated form in aqueous media within the same temperature (20°C/35°C) and pH (2–9) ranges, the observed fading of the compliance indicators was not caused by instability of the dye. The reason for the different fading patterns among aligners was presumably caused by the manufacturing of different encapsulations of the dye, with each pair consisting of a “fast” and a “slow” compliance indicator. The encapsulations of the “fast” and “slow” formulations were located on alternating sides of the aligners. It was not possible to determine the locations of the “fast” vs “slow” formulations on the aligners.
Once the aligner was exposed to aqueous medium, the way in which the blue dye physically diffused from the encapsulation in a time-, pH-, and temperature-dependent fashion would result in a gradual change from dark blue to clear. The addition of Kukident cleaning tablets presumably induced not only diffusion but also chemical reactions that gave rise to colorless metabolites of the encapsulated dye.
Therefore, our experiments indicate that the wide variation of color changes noted between aligners despite similar wear times5 are not caused solely by individual differences in the composition of saliva (as postulated by the manufacturer) but also by effects demonstrated in the present study for the first time. The finding that the compliance indicators of Invisalign Teen taken from the same source and tested in identical experimental setups (eg, drinking water plus Kukident cleaning tablet at 35°C) failed to show identical fading patterns would suffice to account for the large variances in fading that can occur even in patients who strictly adhered to the prescribed wear times. Another possible source of error is in the very technique of determining wear times based on the fading patterns of compliance indicators, as it requires the investigator to rate the color changes on a five-point scale. Because of its inherent subjectivity, this method does not yield objective wear times. Based on experience and on the precision of fit presented by the aligners, the orthodontist can judge their patients' compliance even without the use of compliance indicators.
It would appear that patients could easily manipulate the color indicators, either on purpose or unintentionally. Clinicians should remember this possibility whenever, for instance, an aligner does not fit, even though the color indicators would seem to suggest an adequate level of patient compliance. One should also bear in mind that undesirable color changes may be present because the patient forgot to remove the aligner from the mouth during drinking. Situations in which aligners no longer fit during treatment, requiring extensive new treatment planning despite the fact that the indicators of the aligners have completely or partially faded, will constitute a dilemma. Clinicians will have to assume in this situation that the aligners have been worn by the patient as instructed, and this is also what the patients themselves (or their parents) may think. Based on current thinking, the finding of color indicators having duly faded would effectively rule out that patient compliance was lacking. Consequently, this factor would no longer be suspected in the event of treatment failure, but the focus of suspicion might well shift to the Invisalign Teen system or the clinician's skills.
CONCLUSIONS
The compliance indicator encapsulated in the clear Invisalign Teen aligners was released in different aqueous solutions in the absence of oral fluid outside the oral cavity.
Color changes deviating from the graphically determined baseline throughout the treatment period with removable aligners could be caused by the following reasons: the aligners were left in the mouth during drinking, were kept in water, were cleaned with tablets containing oxidizing agents, or were cleaned in a dishwasher.
The fading pattern of the compliance indicators is not suitable as an objective measure of wear time.
Acknowledgments
We would like to thank Dr T. Drechsler (private practice, Wiesbaden, Germany) and Align Technology Inc for kindly providing the Invisalign Teen aligners used in these experiments. The study was supported by the Fortüne Program (project no. 1850-0-0).
REFERENCES
- 1.Brandao M, Pinho H. S, Urias D. Clinical and quantitative assessment of headgear compliance: a pilot study. Am J Orthod Dentofacial Orthop. 2006;129:239–244. doi: 10.1016/j.ajodo.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Lee S. J, Ahn S. J, Kim T. W. Patient compliance and locus of control in orthodontic treatment: a prospective study. Am J Orthod Dentofacial Orthop. 2008;133:354–358. doi: 10.1016/j.ajodo.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 3.Sahm G, Bartsch A, Witt E. [Orthodontic treatment from the patients' and parents' viewpoint–the results of a practical and clinical questionnaire study (II)] Fortschr Kieferorthop. 1990;51:336–344. doi: 10.1007/BF02167542. [DOI] [PubMed] [Google Scholar]
- 4.Schott T. C, Göz G. Young patients' attitudes toward removable appliance wear times, wear-time instructions and electronic wear-time measurements–results of a questionnaire study. J Orofac Orthop. 2010;71:108–116. doi: 10.1007/s00056-010-9925-y. [DOI] [PubMed] [Google Scholar]
- 5.Tuncay O. C, Bowman S. J, Nicozisis J. L, Amy B. D. Effectiveness of a compliance indicator for clear aligners. J Clin Orthod. 2009;43:263–268. [PubMed] [Google Scholar]
- 6.Abolfathi A, Chen J, Li C, Tricca R, Wu B. inventors; Align Technology, Inc, assignee System and methods for dental appliance compliance indication. June 30, 2009 US patent 7,553,157 B2. [Google Scholar]
- 7.Ovsenik M, Farcnik F, Kosorok T, Zupancic S, Volk J. Mouth temperature during removable orthodontic appliance wear. Eur J Orthod. 2006;28:e245. [Google Scholar]
- 8.Moore R. J, Watts J. T, Hood J. A, Burritt D. J. Intra-oral temperature variation over 24 hours. Eur J Orthod. 1999;21:249–261. doi: 10.1093/ejo/21.3.249. [DOI] [PubMed] [Google Scholar]
- 9.Serbesis-Tsarudis C, Ruf S. Reinigung herausnehmbarer kieferorthopädischer Apparaturen - Eine deutschlandweite Umfrage. J Orofac Orthop. 2009;70:447. [Google Scholar]
- 10.Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Res. 2004;38(suppl 1):34–44. doi: 10.1159/000074360. [DOI] [PubMed] [Google Scholar]
- 11.Barbour M. E, Finke M, Parker D. M, Hughes J. A, Allen G. C, Addy M. The relationship between enamel softening and erosion caused by soft drinks at a range of temperatures. J Dent. 2006;34:207–213. doi: 10.1016/j.jdent.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Kravitz N. D, Kusnoto B, Agran B, Viana G. Influence of attachments and interproximal reduction on the accuracy of canine rotation with Invisalign. A prospective clinical study. Angle Orthod. 2008;78:682–687. doi: 10.2319/0003-3219(2008)078[0682:IOAAIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Kravitz N. D, Kusnoto B, BeGole E, Obrez A, Agran B. How well does Invisalign work? A prospective clinical study evaluating the efficacy of tooth movement with Invisalign. Am J Orthod Dentofacial Orthop. 2009;135:27–35. doi: 10.1016/j.ajodo.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Vicens J, Russo A. Comparative use of Invisalign by orthodontists and general practitioners. Angle Orthod. 2010;80:425–434. doi: 10.2319/052309-292.1. [DOI] [PMC free article] [PubMed] [Google Scholar]