Abstract
Objective:
To examine modifications in sleep pattern and in craniofacial morphology of adolescents with mandibular retrognathism.
Materials and Methods:
Sixteen subjects at maximum pubertal growth (12.6 years [±11.5 months]) were selected and treated for 12 months with maxillary expansion and mandibular advancement with a Herbst appliance. Cephalometric radiography and magnetic resonance imaging were obtained prior to and after treatment and were compared using the paired Student's t-test or the nonparametric Wilcoxon rank-sum test. Four polysomnographic recordings were obtained with pressurized nasal cannulae and were analyzed by analysis of variance.
Results:
The length of the mandible was increased, while the antero-posterior position of the maxilla remained stable. The posterior airway space was increased, the length of the tongue was preserved, and the hyoid bone was moved to a more anterior position. After Herbst treatment, sleep efficiency, sleep latency, rapid eye movement (REM) sleep latency, and percentage of REM sleep remained stable. We did observe a reduction (P < .05) in the relative proportions of stage 1 and stage 3–4 (from 4.30 ± 1.99 to 2.61 ± 1.83 for stage 1 and from 25.78 ± 7.00 to 19.17 ± 7.58 for stages 3–4) as well as an increase (P < .01) in the percentage of stage 2 after treatment (49.03 ± 6.25 to 56.90 ± 6.22). There was a reduction (P < .05) in the number of respiratory effort–related arousals (7.06 ± 5.37 to 1.31 ± 1.45 per hour of sleep) due to an increase (P < .01) in airway volume.
Conclusions:
In the short term, the increase in airway space improved nocturnal breathing associated with the correction of mandibular retrognathism.
Keywords: Adolescents, Mandibular retrognathism, Sleep-disordered breathing, Polysomnography, Upper airway volume
INTRODUCTION
Among school-aged children, 8% to 10% snore, and the incidence of obstructive sleep apnea syndrome (OSA) is estimated to be 2%,1 though there are no studies available on the prevalence of upper airway resistance syndrome. In adolescents, it is unknown whether sleep apnea is an extension of the clinical disorder of childhood, with adenotonsillar hypertrophy as a major risk factor,2 or whether it represents an early manifestation of the adult form of sleep apnea, for which obesity and mandibular retrognathism figure as major risk factors.3 Altogether, this evidence indicates that the exact mechanism of OSA is not fully understood.
OSA or upper airway resistance in adolescents is a dynamic process resulting from a combination of structural and neuromotor abnormalities rather than from structural alterations alone. Craniofacial abnormalities commonly occur in OSA patients and may predispose them to apnea through an adverse effect on upper airway dimensions.4 The relationship of the mandible to the cranial base was retrognathic in children and adolescents with a history of upper airway obstruction.2,3,5 Furthermore, skeletal Class II pattern—with a reduction in mandible length, an increased overbite, and a hyoid bone in a more superior position—was also reported5,6 in children with OSA. Nevertheless, maxillary constriction may also play a role in the pathophysiology of OSA7,8 and is often associated with other craniofacial abnormalities, such as sagittal mandibular deficiency and narrowing.
Thus, the primary aim of this investigation was to determine whether adolescents with Class II, division 1 malocclusion, mandibular retrognathism, and mild transverse maxillary deficiency would present with early alterations in sleep pattern and in nocturnal breathing in relation to patterns described in the literature. Since that early intervention with oral appliances (for shifting the mandible anteriorly or expanding the maxilla) promotes remodeling of the jaw in children and adolescents and because this kind of malocclusion during development is immediately indicated for treatment, we also evaluated whether treatment with a Herbst appliance and a rapid maxillary expander would correct the craniofacial architecture and bring these values to normal levels to prevent progressive problems.
MATERIALS AND METHODS
The subjects for this prospective study were screened for orthopedic-functional treatment from a total of 840 patients (9 to 14 years of age) that were evaluated for orthodontic treatment at the department of orthodontics. This group comprised 16 subjects and both genders, with a mean age of 12.6 years ± 11.5 months, which accomplished all inclusion criteria. Average body mass index (BMI) was 18.3 ± 1.8 kg/m2. At the end of the treatment, the mean age was 13.8 years ± 12.1 months, with an average BMI of 18.7 ± 1.8 kg/m2. The cut-offs for overweight and obese individuals were established according to guidelines of the International Obesity Task Force.9
Inclusion criteria include the following: full Class II, division 1 malocclusion; mandibular retrognathism; mild transverse maxillary deficiency; and habitual snoring. All patients were in the midst of the pubertal growth peak (stage 3 or stage 4 of skeletal maturation). Exclusion criteria include the following: conditions that may obstruct the upper airways (acute rhinitis, septum deviation, adenotonsillar hypertrophy, tumors, and presence of polyps), obesity, periodontal disease, and dental caries. A control group was not selected because it is deemed unethical to withhold treatment for adolescents with Class II malocclusion and mandibular retrognathism in pubertal growth peak. This study received the appropriate ethical clearance and was conducted in accordance with Brazilian regulations (USP No. 79/04).
Dental Appliance
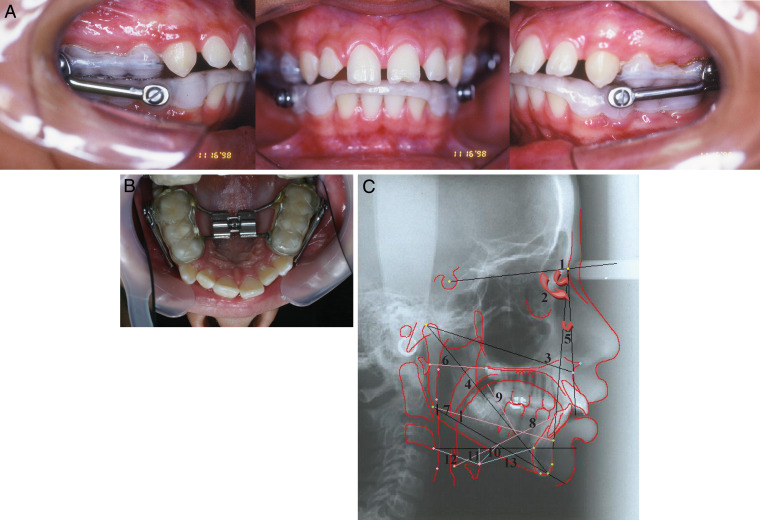
All patients were treated using the acrylic-splint Herbst appliance to the point of mandibular advancement (Figure 1A). The average treatment time was 12 months. The mandible was initially advanced 6.0 mm and was opened 4.0 mm vertically. Subsequent stepwise anterior activations were needed. Along with the mandibular advancement treatment, mild rapid palatal expansion was performed by adapting a rapid maxillary expander at the Herbst appliance (Figure 1B). The average expansion time was 15 days. The mean maxillary expansion achieved was 3.19 mm, and the retention period was completed within the complete treatment period. The Herbst treatment phase was followed by a multibracket treatment phase for final tooth alignment.
Figure 1.
(A) The acrylic-splint Herbst appliance. (B) Rapid maxillary expander adapted to the Herbst appliance. (C) Cephalometric tracing: 1. SNA; 2. SNB; 3. Co-A; 4. Co-Gn; 5. ANB; 6. A1-PNS; 7. posterior airway space; 8. tongue length; 9. tongue height; 10. H-MP (hyoid bone to mandibular plane); 11. H-H1 (hyoid bone to Sinf-C3 plane); 12. H-C3 (linear distance between hyoid bone and C3); and 13. H-Sinf (linear distance between hyoid bone and Sinf).
Cephalometry
Two radiographs in an upright position were taken for each patient: before and 12 months after Herbst treatment. The following cephalometric measurements (Figure 1B) were used:
Craniofacial analysis: SNA, SNB, Co-A, Co-Gn;
Maxillomandibular relation: ANB;
Oropharyngeal analysis: A1-PNS, posterior airway space, tongue length, tongue height, H-MP (vertical position of hyoid bone), H-H1 (vertical position of hyoid bone), H-C3, and H-Sinf; and
Dentoalveolar analysis: upper central incisor. PP, upper central incisor-ANSperp, upper central incisor-PP, upper molar-ANSperp, upper molar-PP, lower central incisor. GoMe, lower central incisor-Sinf, lower central incisor-GoMe, lower molar-Sinf, lower molar-GoMe.
Polysomnography
Polysomnography studies (PSGs) were performed on an Alice Host (Healthdyne/Respironics®) at the Sleep Institute. Nasal airflow was recorded using both a nasal cannula/pressure transducer system and a thermocouple. The criteria adopted for sleep-wake scoring met the accepted international standards. Arousals were defined according to American Sleep Disorders Association criteria, while scoring of respiratory events followed the American Academy of Sleep Medicine and American Thoracic Society criteria.
We performed four PSGs: adaptation night, baseline, after 5 months (before additional sagittal advancement), and 12 months after Herbst treatment. The following sleep parameters were assessed: sleep latency; rapid eye movement (REM) sleep latency; sleep efficiency; all sleep stages (stages 0, 1, 2, 3–4, and REM) calculated as a percentage of total sleep time; respiratory events (obstructive apnea, hypopnea, respiratory effort–related arousal, and respiratory disturbance index score); number of arousals, and mean oxygen saturation.
Magnetic Resonance Imaging
Patients were submitted to neck magnetic resonance imaging (MRI) before and after treatment. The MRIs were performed on a 1.5-Tesla (T) scanner in a Philips®-Intera with anterior neck coil Syn Head/Neck Combination-Philips®. Subjects were placed in a supine position with the head in a neutral anatomic position.
The technical parameters were as follows: repetition time, 337 milliseconds; echo time, 10 milliseconds; field of view, 245 mm; 12 slices of 3 mm thickness with an 0.3-mm gap. The matrix was 256 × 512, 4NEX (number of signal averages), with upper and lower saturation pulses. The following anatomical airway space volumes were determined: nasopharynx, oropharynx, hypopharynx, and total volume.
For practical purposes and for the sake of precision in the execution of this phase, we elected to use the Easyvision software for Workstation-Philips, which is approved by the Food and Drug Administration. All duplicated measurements were obtained for all variables. Measurement error was assessed.
Statistical Analysis
The comparisons between the measurements (cephalometric evaluation and airway space volume) before and after treatment were made using the paired Student's t-test. All variables that were compared with this test had a normal distribution and similar variances.
Whenever a cephalometric variable presented a non-normal distribution of data or differing variances, the comparison was made by the nonparametric Wilcoxon rank-sum test. Analyses of the sleep variables were performed using parametric testing and repeated-measures analysis of variance. The post hoc Tukey test was used when necessary. Results were considered significant when P ≤ .05.
RESULTS
Changes in PSG and MRI
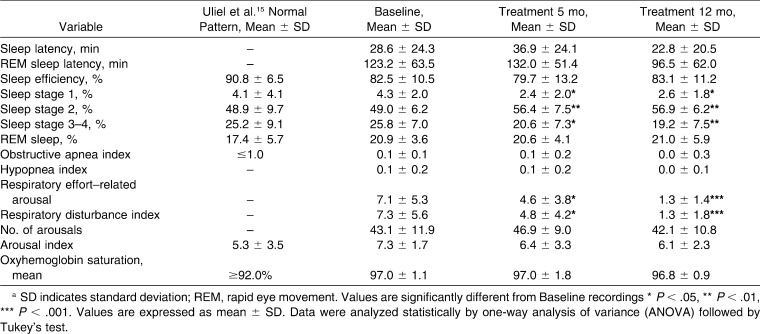
The percentage of sleep stage 1 (Table 1) decreased [F(3,45) = 6.17; P < .01] after 5 and 12 months of treatment (P < .05). The percentage of sleep stage 2 was significantly enhanced [F(3,45) = 6.63; P < .001] after both posttreatment periods (P < .01). Comparison of the percentages of stages 3–4 over different periods highlighted a reduction [F(3,45) = 6.04; P < .01] in the two posttreatment recordings (P < .05 and P < .01, respectively).
Table 1.
Sleep Parametersa
The number of respiratory effort–related arousals (RERAs) and the respiratory disturbance index (RDI) decreased [F(3,45) = 8.66; P < .001] in both posttreatment recordings (P < .05 and P < .001, respectively).
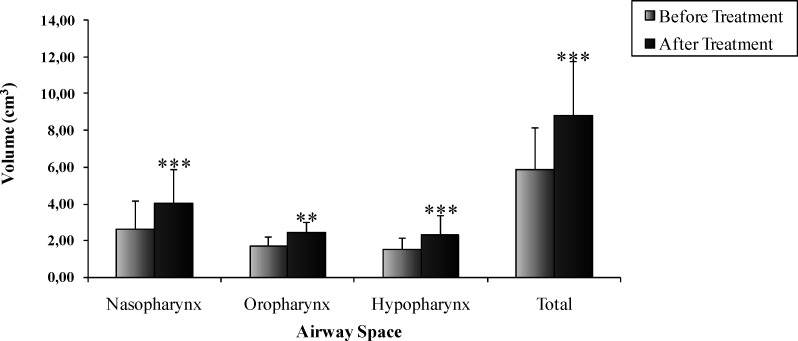
Statistically significant increases were observed for the nasopharynx (P < .001), oropharynx (P < .01), hypopharynx (P < .001), and total (P < .001) volumes evaluated before and after orthopedic functional treatment (Figure 2).
Figure 2.
Volume in each part of the upper airway before and after treatment, obtained by MRI. The values are expressed in mean ± standard deviation (SD) (* P < .05, ** P < .01, *** P < .001). N = 16 patients.
Changes in Cephalometry
The craniofacial analysis (Table 2) demonstrated the SNA angle maintenance and that the effective length of the maxilla (Co-A) was enhanced by 1.1 mm. The effective length of the mandible (Co-Gn) was enhanced by 6.1 mm with functional treatment. The mandibular symphysis moved anteriorly, the SNB angle increased (2.50°), and the ANB angle diminished by 2.6° compared to pretreatment values.
Table 2.
Cephalometric Variablesa
Analysis of oropharyngeal dimensions demonstrated that the posterior airway space was enhanced by 3.2 mm. The tongue height was enhanced by 3.0 mm. The hyoid bone maintained its vertical position and moved 2.0 mm anteriorly.
During treatment, the upper incisors were retroclined about 5.4° and retruded 2.5 mm. It was also verified that distal movement of the maxillary molars (2.3 mm), which maintained their vertical position, occurred. The lower incisors were proclined (8.1°) and protruded (2.6 mm). The Herbst treatment enhanced the mesial movement (2.3 mm) and extrusion (1.5 mm) of the mandibular molars.
DISCUSSION
Our adolescents were treated at the peak of their pubertal growth period, during which marked biologic, cognitive, emotional, and social alterations take place. Before the treatment period, the adolescents presented with a high RDI (7.16 events per hour of sleep) sum of the apnea, hypopnea, and RERA indexes as a result of an increase in the number of events of RERA. We did not observe events of apnea or hypopnea. Normal children or adolescents have less than one apnea episode per hour of sleep, and hypopnea and RERA are probably rare.10 Thus, the polysomnographic features of childhood OSA differ from those of adults. Instead of repetitive discrete apneas, children and adolescents often exhibit a pattern of partial obstructive hypoventilation characterized by snoring, phasic oxyhemoglobin desaturation, hypercapnia, and upper airway resistance.11
Sleep-disordered breathing during childhood and adolescence is associated with an anatomic abnormality of the upper airway. The most common anomalies are hypertrophic tonsils, adenoids, retrognathic mandibles, and transverse maxillary deficiencies. These anomalies decrease the radius of nasal and oral airways, and the outcome is increased upper airway resistance. This increase may not be significant during periods of wakefulness, when the adolescent spends most time upright, but it may become clinically significant at night during sleep, when the youngster is lying in bed in a horizontal position with airway narrowing as a result of a reduction in tonic activity of the pharyngeal dilator muscle.12
After treatment with the Herbst appliance, we observed a reduction in sleep stages 1 and 3–4, along with an increase in stage 2 of non-REM (NREM) sleep. These results are in accordance with previous findings,13 which report that mature adolescents had a reduction in stage 4 and an increase in stage 2 when compared with prepubertal adolescents, and it was suggested that brain maturation may be responsible for these differences. However, the sleep pattern modifications occurred among patients between the ages of 12.6 and 13.8 years, a more specific age range than the ages of 11 and 17 years of patients in the study previously reported.13 The sleep efficiency, percentage of REM sleep, and number of arousals remained stable. Proportion of time spent in the different stages of sleep and sleep efficiency preserved were previously reported14,15 in children with sleep-disordered breathing.
In the craniofacial architecture, the initial SNA measurement was comparable with normal values published in the literature. Thus, the maxilla was well positioned in the craniofacial complex before treatment. This angle remained stable, and the effective length of the maxilla was enhanced by 1.1 mm during treatment with the Herbst appliance and rapid maxillary expander. However, the total expansion effect consists of a downward and forward movement of the maxillary complex, with a resulting increase in the nasal canal and an improvement in nasal airflow.16 We hypothesized that our results (showing stable SNA) were less pronounced because we made a smaller expansion (3.2 mm) than described in the other studies (8.0 mm and 5.0 mm)4,7 and because a headgear-type inhibition effect on maxillary growth was proportioned by the Herbst appliance.
The initial SNB angle in our study was diminished (74.7°) in relation to the normal pattern. After treatment, the sagittal position of the mandibular base changed significantly relative to the initial position. The effective length of the mandible increased (6.1 mm) and the mandibular symphysis moved anteriorly, resulting in an enhancement of the SNB angle (2.5°), similar to results of previous studies.17,18
The retroposition of the mandibular complex was associated with reduced retrobasilingual space, mouth breathing, nasopharyngeal airway obstructions, and relative retrodisplacement of the tongue.19 After treatment, the anterior displacement of the mandible and the hyoid bone caused an anterior traction of the tongue, which increased the posterior airway space (3.2 mm), reduced airway resistance, and facilitated nocturnal breathing. Thus, the orthopedic treatment in our patients exerted a direct beneficial effect through anterior repositioning of the tongue and improvement of swallowing. Proper swallowing favors a return of normal lingual tone, which reduces the hypotonia that causes the tongue to fall back during the hypotonic stage of REM sleep.7 We hypothesized that the reduction in the hypotonia of the tongue was represented by the enhancement in tongue height.
The enlargement of the upper airway was also verified by volumetric assessment performed by MRI. We observed a statistically significant increase in all portions that were under scrutiny. The nasopharynx and oropharynx volumes increased, probably from the associated effects of rapid maxillary expansion and mandibular advancement. In the hypopharynx region, volume increase was observed as a result of the mandibular protrusion occurring after treatment.
These morphologic and functional modifications were clinically significant with regard to nocturnal breathing when upper airway resistance decreased to about 4.63 events of RERA per hour of sleep after 5 months and to 1.31 events after 12 months. The reduction in airway resistance was progressive during the treatment period. Because the maxillary expansion was performed only during the first 2 weeks after initiating the treatment, we therefore speculate that the final improvement was specifically due to the subsequent sagittal advancements of the mandible performed until the overcorrected Class I relation was achieved.
In this study, the treatment was accomplished by posterior maxillary and anterior mandibular tooth movements. The mandibular dental changes are due to anchorage loss as a result of the mesially directed forces exerted on the mandibular teeth by the telescope mechanism. Herbst treatment enhanced an extrusion of the lower molars, while other lower and upper teeth maintained their vertical positions. Similar findings were also previously reported.17,20 Therefore, we can conclude that Class II correction is a result of both skeletal and dental components and that mandibular anchorage loss is a reality with which the orthodontist has to live.
The Herbst appliance has many advantages when compared with removable functional appliances such as the activator, bionator, and Fränkel. The Herbst appliance is partially fixed to the teeth, works continuously (24 hours a day), and does not require compliance for its correct function. Furthermore, the treatment effects of the Herbst exceed those mediated by orthopedic removable appliances in a much shorter period of time.
Our patients received treatment for an anatomic alteration that must be corrected in the growth period in order to normalize the relationship between maxilla and mandible to produce a normal occlusion and a more aesthetically pleasing profile. Class II dentoskeletal disharmony does not exhibit a tendency for self-correction along with growth, in association with a worsening of the deficiency in mandibular dimensions, as recently reported in two clinical studies.21,22 Craniofacial growth in subjects with untreated Class II malocclusion is essentially similar to that in untreated subjects with normal occlusion at all developmental intervals,23 with the exception of a significant deficiency in the size of the mandible at the growth spurt, which was still present at the completion of active craniofacial growth.21
In the long term, this treatment may help to eliminate predisposing factors to OSA, such as mandibular retrognathism, and to decrease the risk of OSA development in adulthood. An unavoidable limitation of our study is that we had a small sample, but this is a prospective study with consecutive patients, none of whom withdrew or were eliminated during treatment. Moreover, this was a difficult study to conduct because of the complexity of the examinations, which go beyond the orthodontic treatment period itself.
CONCLUSIONS
As a result of the treatment of mandibular advancement and maxillary expansion, we obtained a reduction in the number of RERA events, indicating improved respiration and less effort expended in breathing during sleep.
Mouth breathing and persistent snoring, symptoms particularly indicative of OSA, which were reported by parents and verified by means of clinical and polysomnographic evaluations, ceased after treatment.
Acknowledgments
The authors would like to thank Prof. Julio W. Vigorito. The authors are very appreciative of the help from Fernanda Almeida and Monica Andersen, who reviewed the manuscript. This work was supported by grants from Associação Fundo de Incentivo à Psicofarmacologia (AFIP) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (CEPID 98/14303-3 to S.T.).
REFERENCES
- 1.Wildhaber J. H, Moeller A. Sleep and respiration in children: time to wake up! Swiss Med Wkly. 2007;137:689–694. doi: 10.4414/smw.2007.11986. [DOI] [PubMed] [Google Scholar]
- 2.Shintani T, Asakura K, Kataura A. Adenotonsillar hypertrophy and skeletal morphology of children with obstructive sleep apnea syndrome. Acta Otolaryngol Suppl. 1996;523:222–224. [PubMed] [Google Scholar]
- 3.Arens R, Marcus C. L. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 4.Cistulli P. A, Palmisano R. G, Poole M. D. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep. 1998;21:831–835. doi: 10.1093/sleep/21.8.831. [DOI] [PubMed] [Google Scholar]
- 5.Kawashima S, Peltomaki T, Sakata H, Mori K, Happonen R. P, Ronning O. Craniofacial morphology in preschool children with sleep-related breathing disorder and hypertrophy of tonsils. Acta Paediatr. 2002;91:71–77. doi: 10.1080/080352502753457996. [DOI] [PubMed] [Google Scholar]
- 6.Cozza P, Polimeni A, Ballanti F. A modified monobloc for the treatment of obstructive sleep apnoea in paediatric patients. Eur J Orthod. 2004;26:523–530. doi: 10.1093/ejo/26.5.523. [DOI] [PubMed] [Google Scholar]
- 7.Villa M. P, Malagola C, Pagani J, Montesano M, Rizzoli A, Guilleminault C, Ronchetti R. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med. 2007;8:128–134. doi: 10.1016/j.sleep.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Cistulli P. A. Rapid maxillary expansion in obstructive sleep apnea—hope on the horizon? Sleep. 2004;27:606–607. [PubMed] [Google Scholar]
- 9.Cole T. J, Bellizzi M. C, Flegal K. M, Dietz W. H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–878. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 11.Rosen C. L, D'Andrea L, Haddad G. G. Adult criteria for obstructive sleep apnea do not identify children with serious obstruction. Am Rev Respir Dis. 1992;146:1231–1234. doi: 10.1164/ajrccm/146.5_Pt_1.1231. [DOI] [PubMed] [Google Scholar]
- 12.Tangel D. J, Mezzanotte W. S, White D. P. Influence of sleep on tensor palatine EMG and upper airway resistance in normal men. J Appl Physiol. 1991;70:2574–2581. doi: 10.1152/jappl.1991.70.6.2574. [DOI] [PubMed] [Google Scholar]
- 13.Jenni O. G, Achermann P, Carskadon M. A. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 14.Goh D. Y, Galster P, Marcus C. L. Sleep architectures and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:682–686. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 15.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–878. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 16.da Silva Filho O. G, Boas M. C, Capelozza Filho L. Rapid maxillary expansion in the primary and mixed dentitions: a cephalometric evaluation. Am J Orthod Dentofac Orthop. 1991;100:171–179. doi: 10.1016/s0889-5406(05)81524-0. [DOI] [PubMed] [Google Scholar]
- 17.Schütz T. C. B, Vigorito J. W, Rodrigues C. R. M. D, Domínguez-Rodríguez G. C. Cephalometric evaluation of dental modifications during treatment with Herbst appliance in adolescents with Class II, division 1 malocclusion. Ortodontia. 2002;35:22–34. [Google Scholar]
- 18.Schütz T. C. B, Vigorito J. W, Domínguez-Rodríguez G. C. Cephalometric evaluation of skeletal and facial modifications during treatment with Herbst appliance in adolescents with Class II, division 1 malocclusion. Ortodontia. 2003;36:44–61. [Google Scholar]
- 19.Hochban W, Brandenburg U. Morphology of the viscerocranium in obstructive sleep apnoea syndrome—cephalometric evaluation of 400 patients. J Craniomaxillofac Surg. 1994;22:205–213. doi: 10.1016/s1010-5182(05)80559-1. [DOI] [PubMed] [Google Scholar]
- 20.Pancherz H. Vertical dentofacial changes during Herbst appliance treatment. A cephalometric investigation. Swed Dent. 1982;15:189–196. [PubMed] [Google Scholar]
- 21.Franchi L, Baccetti T, Stahl F, McNamara J. A., Jr Thin-plate spline analysis of craniofacial growth in Class I and Class II subjects. Angle Orthod. 2007;77:595–601. doi: 10.2319/070506-275. [DOI] [PubMed] [Google Scholar]
- 22.Stahl F, Baccetti T, Franchi L, McNamara J. A., Jr Longitudinal growth changes in untreated subjects with Class II division 1 malocclusion. Am J Orthod Dentofac Orthop. 2008;134:125–137. doi: 10.1016/j.ajodo.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Carroll J. L, McColley S. A, Marcus C. L, Curtis S, Loughlin G. M. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108:610–618. doi: 10.1378/chest.108.3.610. [DOI] [PubMed] [Google Scholar]