Abstract
Objective:
To (1) document the prevalence and patterns of hypodontia (permanent tooth agenesis) in Down syndrome (DS) and (2) explore whether maxillary or mandibular hypodontia or simultaneous agenesis of all third molars was associated with differential alterations of the craniofacial morphology.
Materials and Methods:
Longitudinal panoramic radiographs of 25 white patients with DS (12 male, 13 female; mean age, 15.1 years) treated at The Hospital for Sick Children, Toronto, Ontario, Canada, were evaluated to document hypodontia. Cephalometric measurements of subjects with maxillary or mandibular hypodontia or agenesis of all third molars were compared with those of subjects without hypodontia in these regions using analysis of covariance adjusted for age, gender, and proportion of other missing teeth in the total number of missing teeth.
Results:
Hypodontia was seen in 92% of the sample when third molars were considered and in 56% when third molars were not considered. Hypodontia was more prevalent and severe in females. The most frequently agenetic teeth were maxillary and mandibular third molars > maxillary lateral incisors > mandibular second premolars > mandibular incisors > maxillary second premolars > maxillary second molars. Simultaneous agenesis of all third molars was seen in 52% of the sample. Maxillary hypodontia was not associated with significant regional craniofacial differences, while mandibular hypodontia was associated with decreased mandibular length and increased ramus∶body ratio. Agenesis of all third molars was not associated with significant craniofacial differences.
Conclusions:
Hypodontia is widely prevalent in DS. The effect of the syndrome appears to be stronger than that of regional hypodontia in differentially altering the craniofacial morphology.
Keywords: Down syndrome, Hypodontia, Dental agenesis, Missing teeth, Craniofacial morphology, Cephalometrics
INTRODUCTION
Down syndrome (DS) is the most well known and common chromosomal disorder in humans.1 Recent birth statistics in the United States show an increasing prevalence, currently observed at 11.8 per 10,000 births.2 Cephalometric analyses of subjects with DS have described platybasia, reduced maxillary and mandibular alveolar heights, short teeth, reduced maxillary and mandibular dimensions, and forward rotation of the maxillary and mandibular planes, which collectively lead to overclosure and relative mandibular prognathism.3–12 Hypodontia in DS is well known,12–22 with prevalence documented at about 90%12,19,20 if the third molars are considered and between 30%13 and 60%22 when the third molars are not considered in the analysis. A very high prevalence of total agenesis of all third molars has also been described.19
Numerous investigator groups around the world have explored associations between hypodontia and significant alterations in craniofacial morphology in nonsyndromic samples23–31 and craniofacial anomalies such as ectodermal dysplasia32,33 and Pierre Robin sequence.34 Whether an association exists between the location of hypodontia and differential alterations in the regional craniofacial morphology in DS is unknown. This investigation aimed (1) to document the prevalence and patterns of hypodontia (permanent tooth agenesis) in DS, (2) to explore whether maxillary and mandibular hypodontia are associated with greater alteration of the regional craniofacial morphology within DS, and (3) to explore whether agenesis of all third molars is associated with greater alteration of craniofacial morphology within the syndrome.
MATERIALS AND METHODS
This retrospective investigation was conducted using longitudinal panoramic and pretreatment cephalometric radiographs of white patients with DS treated at The Hospital for Sick Children, Toronto, Ontario, Canada, whose pretreatment radiographic records had been acquired before July 2009. The hospital's Research Ethics Board (REB) approved the study.
Radiographic and clinical records of 25 white subjects with DS (12 males, 13 females; mean age, 15.1 years; range, 11.5–18.3 years) were available. Birth, gender, dentition, and treatment details were recorded. Hypodontia was defined and verified as the absence of one or more permanent teeth due to agenesis. The subjects' preorthodontic treatment cephalograms were traced by a single experienced digitizer and analyzed using Dentofacial Planner cephalometric software (Dentofacial Software, Toronto, Ontario, Canada). Other radiographs and clinical charts were reviewed longitudinally to confirm the occurrence and location of the permanent tooth agenesis. Cephalometric measurements specific to the craniofacial regions of interest were made according to the definitions in Tables 1 and 2 (Figure 1). An intraclass correlation coefficient (ICC) analysis of measurements from repeated tracings of one-third of the cephalograms revealed a high level of repeatability (the average ICC was .99).
Table 1.
Landmarks and Definitions Used in Cephalometric Analysis
Table 2.
Linear and Angular Measurements Made in Cephalometric Analysis
Figure 1.
Cephalometric (a) landmarks and (b) measurements.
The null hypotheses framed were that maxillary and mandibular hypodontia were not associated with significant differences in the cephalometric measurements of these regions and that agenesis of all third molars in DS was not associated with significant differences in craniofacial measurements. To test these null hypotheses, the sample was first categorized into maxillary hypodontia and mandibular hypodontia subgroups based on the location of permanent tooth agenesis. Between-group comparisons of measurements representative of the region of interest (maxilla or mandible) were analyzed by a two-way analysis of covariance (ANCOVA) or Friedman's nonparametric ANCOVA, adjusted for age and gender. Similarly, measurements of subjects exhibiting absence of all third molars were compared with those without this presentation. Since dental agenesis is frequently observed in both jaws in DS,12–22 ANCOVA tests were additionally adjusted for the proportion of other missing teeth (vs in the regions of interest) in the total number of missing teeth. Statistical tests were conducted using SAS (version 9.1; SAS Institute Inc, Cary, NC). Differences were considered significant when P < .05.
RESULTS
Hypodontia was prevalent in 92% of our sample with DS when third molars were considered (Table 3). Almost all patients were missing one or more permanent teeth (Figure 2). The average number of missing permanent teeth per affected subject was 4.74. Two subjects did not show hypodontia (Figure 3). Hypodontia was more prevalent among females (100%) than males (83.3%), and the severity in females was also greater. The average number of missing teeth was 5.38 in affected females and 4.10 in males. Among females, 23.1% were missing all quadrant analogues of at least two tooth types. This severe pattern was seen less often in males (16.7%). Among subjects affected by hypodontia, bilateral absence of the same tooth type was also generally more prevalent in females (Table 4).
Table 3.
Prevalence and Patterns of Permanent Tooth Agenesis Observed
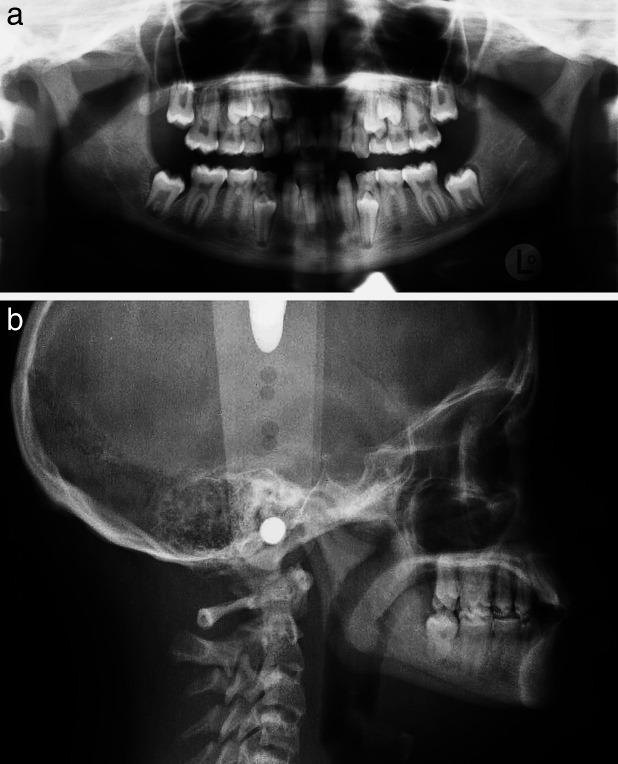
Figure 2.
Radiographs of a subject with Down syndrome with a large number of missing permanent teeth.
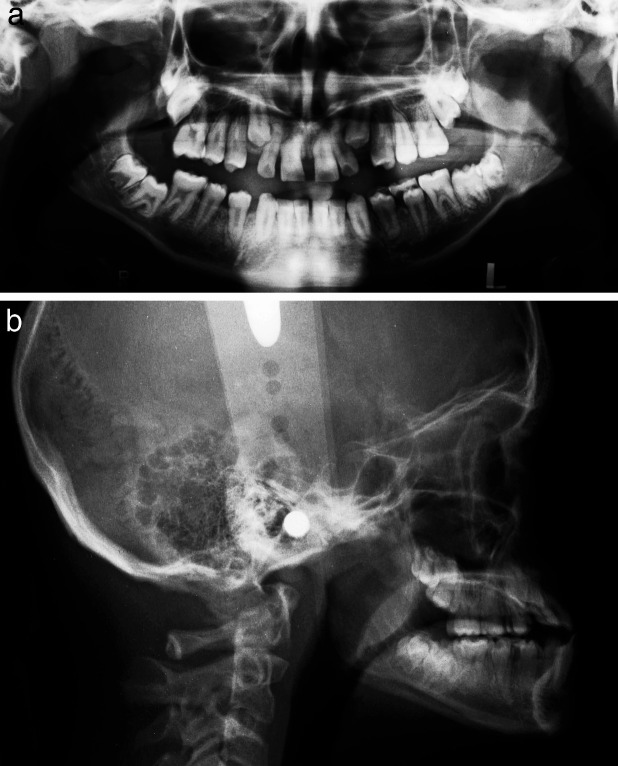
Figure 3.
Radiographs of a subject with Down syndrome without any permanent tooth agenesis.
Table 4.
Prevalence of Bilateral Permanent Tooth Agenesis Observed Within Subjects Having Specific Types of Permanent Tooth Agenesis
Mandibular hypodontia was slightly more prevalent (80%) than maxillary hypodontia (76%) and was much more frequent than maxillary hypodontia in males, whereas the opposite was seen in females (Table 3). In subjects with hypodontia, the average number of missing teeth in the mandible was 2.39 and 2.35 in the maxilla. Although slightly greater numbers of missing teeth were recorded on the right (52.3%) than on the left (47.7%), maxillary and mandibular hypodontia for specific tooth types, when unilateral, was relatively more frequent on the left (72.7%).
When frequencies of hypodontia of individual teeth were examined, the most frequently agenetic teeth were (in decreasing order) maxillary and mandibular third molars > maxillary lateral incisors > mandibular second premolars > mandibular incisors > maxillary second premolars > maxillary second molars. Maxillary lateral incisors and mandibular incisors were missing much more frequently in females (Table 3).
When agenesis of permanent teeth other than third molars was considered, a prevalence of 56% was noted, with greater prevalence in the maxilla (40%) than in the mandible (32%). The average number of teeth missing per affected subject was 2.78 (maxillary, 1.36; mandibular, 1.43). Bilateral absence of a tooth type other than the third molars was seen to be associated with concurrent absence of all third molars in 90% of instances. Simultaneous agenesis of all third molars was seen in 52% of the sample, with greater prevalence in females (61.5%) than males (41.7%).
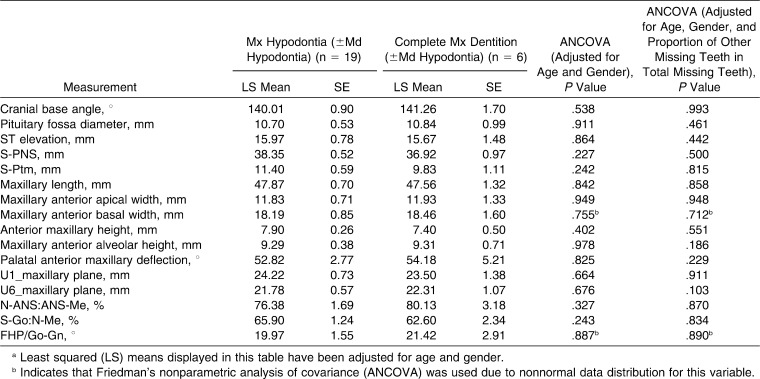
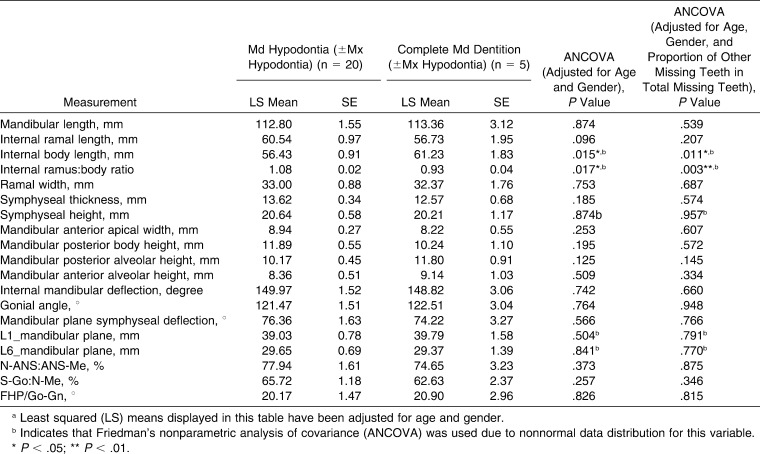
All subjects with maxillary hypodontia exhibited agenesis of at least one maxillary third molar, and 84.2% were missing both maxillary third molars. Comparison of the maxillary measurements of subjects having maxillary hypodontia with those without maxillary hypodontia revealed no significant differences in any of the measurements, both with and without the additional adjusting for the proportion of other (ie, mandibular) missing teeth in the total number of missing teeth (Table 5).
Table 5.
Comparison of Regional Cephalometric Measurements of Subjects With Down Syndrome Having Maxillary Hypodontia With Those Not Having Maxillary Hypodontiaa
On the other hand, all subjects with mandibular hypodontia were missing at least one mandibular third molar, and 80% had agenesis of both mandibular third molars. Comparison of mandibular cephalometric measurements of subjects having mandibular hypodontia with those without mandibular hypodontia (Table 6) revealed a significantly smaller body length (by 4.8 mm; P = .015) and a greater ramus∶body ratio (by 15%; P = .017). These differences remained significant even when additional adjusting was applied for the proportion of other (ie, maxillary) missing teeth in the total number of missing teeth. Other cephalometric measurements were not significantly different.
Table 6.
Comparison of Regional Cephalometric Measurements of Subjects With Down Syndrome Having Mandibular Hypodontia With Those Not Having Mandibular Hypodontiaa
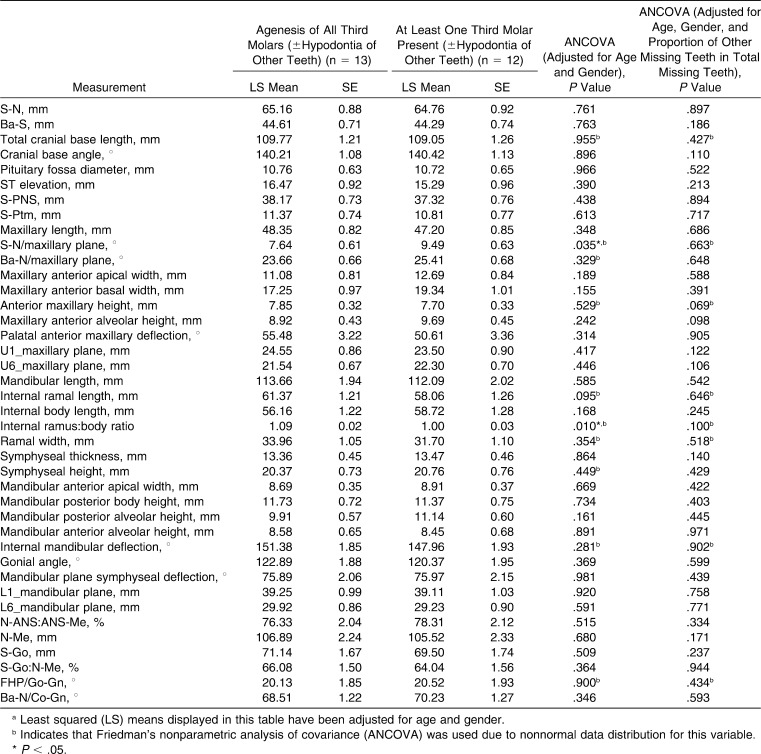
Cephalometric comparison of subjects having agenesis of all third molars and those without this severe presentation (Table 7) showed reduced divergence between the maxillary plane and S-N (1.85°; P = .035), and their ramus∶body ratio was larger (by 9%; P = .010). However, when the comparison was additionally adjusted for the proportion of other missing teeth in the total number of missing teeth, these differences were not significant. No other cephalometric differences were statistically significant.
Table 7.
Comparison of Cephalometric Measurements of Subjects With Down Syndrome Having Agenesis of All Third Molars With Those Having at Least One Third Molar Presenta
DISCUSSION
The prevalence of hypodontia varies in different ethnic groups and regions of the world.35 While the third molars are the teeth most frequently affected by agenesis, when third molars are not considered, the prevalence of hypodontia reported from North America ranges from 3.5%36 to 7.4%,37 with slightly greater prevalence in females. Most studies report that mandibular second premolars, maxillary lateral incisors, maxillary second premolars, and mandibular central incisors are the teeth most often affected (in decreasing order).35 In DS, the prevalence of hypodontia is much greater and more severe12–22 and is recognized as a characteristic phenotypical feature of the syndrome.
In the nonsyndromic population, hypodontia may have multiple causes.38 In DS, it has been hypothesized that altered peripheral nervous system growth and abnormal development of localized chondral elements16 may contribute as potential mechanisms responsible for the greater occurrence of dental agenesis. A recent report described that trigeminal nerve fiber growth and patterning are integrated with tooth morphogenesis, and the report hypothesized that mesenchymal dental follicles fail to form as a result of inadequate local epithelial-mesenchymal interactions due to thyroid deficiency, causing delayed proliferation of nerve cells and decreased rate of neuron production in DS.21 Another reason described earlier implicates poor terminal vascularization of developing tooth buds, causing complete or partial odontoblast degeneration.18
Prevalence rates of agenesis of one or more third molar in the nonsyndromic population have been reported to be between 9%39 and 30.8%40 in North America, while the prevalence of simultaneous agenesis of all third molars is much lower, ranging from 0.50%39 to 2.3%.40 The reported prevalence of third molar agenesis in DS is much higher. It was noted in 88% of our sample, similar to the 84.4% reported by Lomholt et al.20 from Denmark but greater than the 74% reported by Shapira et al.19 from Israel. Agenesis of all third molars was seen in 52% of our sample, similar to the 55% reported by Shapira et al.19
Because of the frequent agenesis of all third molars in our sample, we explored whether this occurrence was associated with any specific craniofacial features. Russel and Kjaer41 hypothesized that an association in DS between the sella turcica structure and the innervations determined occurrence of tooth agenesis due to the proximity of sella to the trigeminal ganglion. In our cephalometric analysis, specific measurements (pituitary fossa diameter, sella turcica elevation from FHP, posterior maxillary vertical height, and the vertical distance of sella from the pterygomaxillary fissure) were included to explore whether the sella and vertical growth of the infrasellar region were differentially affected in individuals with DS having maxillary hypodontia and agenesis of all third molars, respectively.
In the regional cephalometric comparison of subjects having maxillary hypodontia with those without maxillary hypodontia, none of the measurement differences, including those specific for the vertical growth of the sella region, were statistically significant (Table 5). The null hypothesis could not be rejected. On the other hand, in the comparison of regional measurements of subjects having mandibular hypodontia with those without mandibular hypodontia, a significant decrease in body length, with a consequent increase in the ramus∶body ratio, was found (Table 6). A decrease in body length can teleologically be argued to result from the shorter dental lamina (all subjects with mandibular hypodontia had unilateral mandibular third molar agenesis, and 80% had bilateral agenesis). However, this argument was not supported by results of tests for maxillary hypodontia or agenesis of all third molars. The differences in most of the measurements, including mandibular plane angle, face∶height ratio, and Jarabak's ratio, were not significant. These results did not allow for fully rejecting the null hypothesis for the mandibular hypodontia analysis.
Finally, subjects with agenesis of all third molars, when compared with those having at least one third molar, were seen to have relatively less divergence between the anterior cranial base and the palatal plane and an increase in the ramus∶body ratio, but none of the other differences, nor those specific to the sella region, were statistically significant (Table 7). Even these two differences were not statistically significant when the analysis was additionally adjusted for the proportion of other missing teeth. The paucity of significant differences did not allow rejecting the null hypothesis for agenesis of all third molars.
The strengths and limitations of this study should be considered. Confirming hypodontia from longitudinal radiographs ensured that hypodontia patterns were accurately recorded without false-positives. Only preorthodontic treatment cephalograms were analyzed to avoid patient reinclusions and confounding cephalometric data by treatment effects. Only white subjects were included to avoid confounding effects of ethnicity on hypodontia patterns and cephalometric measurements. These considerations, however, limited the number of subjects included. Because of the large prevalence (92%) of hypodontia in the sample, the number of subjects without any hypodontia was too small to allow cephalometric comparison of the regional hypodontia subgroups with a subgroup having DS but without any missing permanent teeth.
Except for the association of mandibular hypodontia with significant differences in body length and ramus∶body ratio, when the statistical analyses were additionally adjusted for the relative proportion of other missing teeth (vs those in the region of cephalometric interest) removed from statistical significance the few other measurements that were significantly different fell out of the range of statistical significance. This indicated that the high overall frequency of missing teeth possibly compounded any differential effect of regional hypodontia on the craniofacial morphology. Keeping these considerations in perspective, and recognizing the known severe craniofacial features of DS, it can be cautiously interpreted that widely prevalent hypodontia in the sample may have contributed to altered craniofacial morphology, but the effect of the syndrome and its characteristic short, under erupted teeth on craniofacial and dentoalveolar dimensions appeared to be stronger than any differential effect regional hypodontia may have had on altering regional craniofacial morphology. Similarly, agenesis of third molars was not associated with further differential alterations in their craniofacial characteristics or with diminished vertical growth proximal to the sella.
CONCLUSIONS
The prevalence of hypodontia noted in the sample with DS was 92%, with the average number of missing teeth per affected subject being 4.74 when third molars were considered, and when third molars were not considered, the prevalence was 56%, with the average number of missing teeth per affected subject being 2.78.
Hypodontia was more prevalent and severe in females.
The most frequently agenetic teeth were (in decreasing order) maxillary and mandibular third molars > maxillary lateral incisors > mandibular second premolars > mandibular incisors > maxillary second premolars > maxillary second molars.
Maxillary hypodontia was not associated with significant differences in maxillary morphology.
Mandibular hypodontia was associated with smaller body length and increased ramus∶body ratio, but other dimensions were not significantly different.
Agenesis of all third molars was associated with a small decrease in maxillary plane to cranial base divergence and increase in ramus∶body ratio, but these differences were not significant when the analysis was additionally adjusted for the proportion of other missing teeth in the total number of missing teeth.
Acknowledgments
The authors would like to acknowledge the contribution of Lynn Cornfoot in tracing and digitizing the cephalograms.
REFERENCES
- 1.Hannekam R. C. M, Krantz I. D, Allanson J. E. Gorlin's Syndromes of the Head and Neck 5th ed. New York, NY: Oxford University Press; 2010. [Google Scholar]
- 2.Shin M, Besser L. M, Kucik J. E, Lu C, Siffel C, Correa A, et al. Prevalence of Down syndrome among children and adolescents in 10 regions of the United States. Pediatrics. 2009;124:1565–1571. doi: 10.1542/peds.2009-0745. [DOI] [PubMed] [Google Scholar]
- 3.Kisling E. Cranial Morphology in Down's Syndrome A Comparative Roentgencephalometric Study in Adult Males. Copenhagen, Denmark: Munksgaard; 1966. [Google Scholar]
- 4.Baer P. N, Coccaro P. J, Baer M. J, Kilham L. Craniofacial manifestation of virus-induced Mongolism in the hamster and Down's syndrome in man. Am J Orthod. 1971;60:221–234. doi: 10.1016/0002-9416(71)90131-x. [DOI] [PubMed] [Google Scholar]
- 5.Frostad W. A, Clea U. J. F, Melosky L. C. Craniofacial complex in the Trisomy 21 syndrome (Down's syndrome) Arch Oral Biol. 1971;16:707–722. doi: 10.1016/0003-9969(71)90116-6. [DOI] [PubMed] [Google Scholar]
- 6.Fink G. B, Madaus W. K, Walker G. F. A quantitative study of the face in Down's syndrome. Am J Orthod. 1975;67:540–553. doi: 10.1016/0002-9416(75)90299-7. [DOI] [PubMed] [Google Scholar]
- 7.Fischer-Brandies H, Schmid R. G, Fischer-Brandies E. Craniofacial development in patients with Down's syndrome from birth to 14 years of age. Eur J Orthod. 1986;8:35–42. doi: 10.1093/ejo/8.1.35. [DOI] [PubMed] [Google Scholar]
- 8.Fischer-Brandies H. Cephalometric comparison between children with and without Down's syndrome. Eur J Orthod. 1988;10:255–263. doi: 10.1093/ejo/10.3.255. [DOI] [PubMed] [Google Scholar]
- 9.Quintanilla J. S, Biedma B. M, Rodriguez M. Q, Mora M. T, Cunqueiro M. M, Pazos M. A. Cephalometrics in children with Down's syndrome. Pediatr Radiol. 2002;32:635–643. doi: 10.1007/s00247-002-0703-x. [DOI] [PubMed] [Google Scholar]
- 10.Gosman S. D, Vineland N. J. Facial development in mongolism. Am J Orthod. 1951;37:332–349. doi: 10.1016/0002-9416(51)90111-x. [DOI] [PubMed] [Google Scholar]
- 11.Alio J. J, Lorenzo J, Iglesias C. Cranial base growth in patients with Down syndrome: a longitudinal study. Am J Orthod Dentofacial Orthop. 2008;133:729–737. doi: 10.1016/j.ajodo.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Suri S, Tompson B, Cornfoot L. Cranial base, maxillary, and mandibular morphology in Down syndrome. Angle Orthod. 2010;80:861–869. doi: 10.2319/111709-650.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen M. M, Blitzer F. J, Arvystas M. G, Bonneau R. H. Abnormalities of the permanent dentition in trisomy G. J Dent Res. 1970;49(6 suppl):1386–1393. doi: 10.1177/00220345700490063901. [DOI] [PubMed] [Google Scholar]
- 14.Orner G. Congenitally absent permanent teeth among mongols and their sibs. J Ment Defic Res. 1971;15:292–302. doi: 10.1111/j.1365-2788.1971.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen G. M, Cleall J. F, Yip A. S. Dentoalveolar morphology and developmental changes in Down's syndrome (trisomy 21) Am J Orthod. 1973;64:607–618. doi: 10.1016/0002-9416(73)90291-1. [DOI] [PubMed] [Google Scholar]
- 16.Russell B. G, Kjaer I. Tooth agenesis in Down syndrome. Am J Med Genet. 1995;55:466–471. doi: 10.1002/ajmg.1320550415. [DOI] [PubMed] [Google Scholar]
- 17.Kumasaka S, Miyagi A, Sakai N, Shindo J, Kashima I. Oligodontia: a radiographic comparison of subjects with Down syndrome and normal subjects. Spec Care Dent. 1997;17:137–141. doi: 10.1111/j.1754-4505.1997.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 18.Mestrovic S. R, Rajic Z, Papic J. S. Hypodontia in patients with Down's syndrome. Coll Antropol. 1998;22(suppl):69–72. [PubMed] [Google Scholar]
- 19.Shapira J, Chaushu S, Becker A. Prevalence of tooth transposition, third molar agenesis, and maxillary canine impaction in individuals with Down syndrome. Angle Orthod. 2000;70:290–296. doi: 10.1043/0003-3219(2000)070<0290:POTTTM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Lomholt J. F, Russell B. G, Stoltze K, Kjær I. Third molar agenesis in Down syndrome. Acta Odontol Scand. 2002;60:151–154. doi: 10.1080/000163502753740160. [DOI] [PubMed] [Google Scholar]
- 21.Reuland-Bosma W, Reuland M. C, Bronkhorst E, Phoa K. H. Patterns of tooth agenesis in patients with Down syndrome in relation to hypothyroidism and congenital heart disease: an aid for treatment planning. Am J Orthod Dentofacial Orthop. 2010;137:584.e1–584.e9. doi: 10.1016/j.ajodo.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Acerbi A. G, de Freitas C, de Magalhães M. H. Prevalence of numeric anomalies in the permanent dentition of patients with Down syndrome. Spec Care Dent. 2001;21:75–78. doi: 10.1111/j.1754-4505.2001.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 23.Wisth P. J, Thunold K, Boe O. E. The craniofacial morphology of individuals with hypodontia. Acta Odontol Scand. 1974;32:281–290. doi: 10.3109/00016357409026344. [DOI] [PubMed] [Google Scholar]
- 24.Nodal M, Kjaer I, Solow B. Craniofacial morphology in patients with multiple congenitally missing permanent teeth. Eur J Orthod. 1994;16:104–109. doi: 10.1093/ejo/16.2.104. [DOI] [PubMed] [Google Scholar]
- 25.Ogaard B, Krogstad O. Craniofacial structure and soft tissue profile in patients with severe hypodontia. Am J Orthod Dentofacial Orthop. 1995;108:472–477. doi: 10.1016/s0889-5406(95)70047-1. [DOI] [PubMed] [Google Scholar]
- 26.Yuksel S, Ucem T. The effect of tooth agenesis on dentofacial structures. Eur J Orthod. 1997;19:71–78. doi: 10.1093/ejo/19.1.71. [DOI] [PubMed] [Google Scholar]
- 27.Endo T, Ozoe R, Yoshino S, Shimooka S. Hypodontia patterns and variations in craniofacial morphology in Japanese orthodontic patients. Angle Orthod. 2006;76:996–1003. doi: 10.2319/082905-303. [DOI] [PubMed] [Google Scholar]
- 28.Bauer N, Heckmann K, Sand A, Lisson J. A. Craniofacial growth patterns in patients with congenitally missing permanent teeth. J Orofac Orthop. 2009;70:139–151. doi: 10.1007/s00056-009-0744-y. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Bassat Y, Brin I. Skeletal and dental patterns in patients with severe congenital absence of teeth. Am J Orthod Dentofacial Orthop. 2009;135:349–356. doi: 10.1016/j.ajodo.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez M. J, Vincente A, Bravo L. A. Third molar agenesis and craniofacial morphology. Angle Orthod. 2009;79:473–478. doi: 10.2319/052008-276.1. [DOI] [PubMed] [Google Scholar]
- 31.Acharya P. N, Jones S. P, Moles D, Gill D, Hunt N. P. A cephalometric study to investigate the skeletal relationships in patients with increasing severity of hypodontia. Angle Orthod. 2010;80:511–518. doi: 10.2319/072309-411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bondarets N, McDonald F. Analysis of vertical facial form in patients with severe hypodontia. Am J Phys Anthropol. 2000;111:177–184. doi: 10.1002/(SICI)1096-8644(200002)111:2<177::AID-AJPA4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Johnson E. L, Roberts M. W, Guckes A. D, Bailey L. J, Phillips C. L, Wright J. T. Analysis of craniofacial development in children with hypohidrotic ectodermal dysplasia. Am J Med Genet. 2002;112:327–334. doi: 10.1002/ajmg.10654. [DOI] [PubMed] [Google Scholar]
- 34.Suri S, Ross R. B, Tompson B. Mandibular morphology and growth with and without hypodontia in Pierre Robin sequence. Am J Orthod Dentofacial Orthop. 2006;130:37–46. doi: 10.1016/j.ajodo.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 35.Polder B. J, Van't Hof M. A, Van der Linden F. P, Kuijpers-Jagtman A. M. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epidemiol. 2004;32:217–226. doi: 10.1111/j.1600-0528.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 36.Glenn F. B. Incidence of congenital missing permanent teeth in a private pedodontic practice. J Dent Child. 1961;28:317–320. [Google Scholar]
- 37.Thompson G. W, Popovich F. Probability of congenitally missing teeth: results in 1191 children in the Burlington Growth Centre in Toronto. Community Dent Oral Epidemiol. 1974;2:26–32. doi: 10.1111/j.1600-0528.1974.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 38.Vastardis H. The genetics of human tooth agenesis: new discoveries for understanding dental anomalies. Am J Orthod Dentofacial Orthop. 2000;117:650–656. [PubMed] [Google Scholar]
- 39.Nanda R. S. Agenesis of third molar in man. Am J Orthod. 1954;40:698–706. [Google Scholar]
- 40.Hellman M. Our third molar teeth, their eruption, presence and absence. Dental Cosmos. 1936;78:750–762. [Google Scholar]
- 41.Russell B. G, Kjaer I. Postnatal structure of the sella turcica in Down syndrome. Am J Med Genet. 1999;87:183–188. doi: 10.1002/(sici)1096-8628(19991119)87:2<183::aid-ajmg11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]