Abstract
Objective:
To develop a mouse orthodontic organ culture model and examine early-induced changes in osteoblast differentiation markers within the periodontal ligament (PDL) and alveolar bone.
Methods:
Mandibles from 4- to 12-week-old transgenic mice were dissected and hemisected. A conventional superelastic orthodontic spring (25 grams) was bonded to the incisor and first molar on one side of the mandible; the other side served as a control. Dissected mandibles were cultured for 6 hours and then were histologically analyzed for proliferation (BrdU immunostaining) and fluorescent protein expression. Additionally, an in vivo model using the same methods was applied to 3.6 Col1-GFP transgenic mice.
Results:
In vitro, after 6 hours of orthodontic loading, a significant increase was noted in 3.6Col1-GFP– and BSP-GFP–positive cells within the tension side of the PDL compared with unloaded controls. On the compression side, a significant decrease in positive cells in 3.6Col1-GFP mice was observed in the PDL compared with unloaded controls. In vivo, the same tendencies were found.
Conclusion:
This novel in vitro mandibular tooth movement organ culture model coupled with transgenic mouse technology provides a powerful tool for delineating initial cellular and molecular events of orthodontic tooth movement.
Keywords: Tooth movement, Organ culture, Green fluorescent proteins, Collagen I, Bone sialoprotein
INTRODUCTION
Orthodontically induced bone remodeling is dependent on conversion of orthodontic forces into intracellular signals within mechanosensitive cells. This information must then be communicated to other nonmechanosensitive cells to produce a coordinated response of alveolar bone resorption and new bone formation.1 The alveolar bone and periodontal ligament (PDL) contain a variety of cells at different stages of osteoblast differentiation. During orthodontic tooth movement, osteoblasts on the tension side are generated from osteoprogenitor cells, which reside within the PDL2,3 and differentiate through a number of defined maturational stages. For example, cells expressing the 3.6 kb fragment of rat type I collagen mRNA define the preosteoblastic stage of differentiation. The osteoblastic stage of differentiation is characterized by expression of bone sialoprotein (BSP) and, finally, terminally differentiated osteoblasts and osteocytes express DMP-1 sialoprotein.4–6
In light of transgenic mouse technology, the mouse provides an ideal animal model by which to study orthodontic tooth movement. In an attempt to generate a visual marker that would reflect the stages of osteoblast maturation, we decided to use transgenic mice containing the 3.6 kb fragment of the rat collagen type I promoter fused to a green fluorescent protein7 and the bone sialoprotein promoter fused to topaz fluorescent protein transgenes.8 We chose these markers because (1) they mark preosteoblastic and osteoblastic cells, respectively; (2) they have been used in a variety of bone remodeling models5,6,9,10; and (3) expression of both BSP and Col3.6 has been shown in previous studies to increase during orthodontic tooth movement in a murine model.11–13 In vivo mouse models examining the early effects of orthodontic force–induced changes on osteoblast differentiation are problematic because of the small size of the mouse mandible and the technical difficulty of insertion and achievement of a reproducible orthodontic appliance placement. To overcome these limitations, mandibular organ culture systems have been used.
Recently, an orthodontic tooth movement organ culture model has been reported in the rat.14 Therefore, the purposes of this study were to develop a mouse orthodontic organ culture model and to examine early-induced changes in osteoblast differentiation markers within the PDL and alveolar bone. Greater understanding of how orthodontic forces affect early osteoblast differentiation temporally and spatially within the PDL and alveolar bone is critical for the development of treatment strategies aimed at modulating tooth movement.
MATERIALS AND METHODS
Experimental Animals
All animal experimental procedures were approved by the University of Connecticut Animal Care and Use Committee.
For in vitro studies, five 4- to 6-week-old 3.6Col1-GFP9 and three 4-week-old BSP-GFP transgenic mice8 were used. The mice were constructed at the University of Connecticut Health Center by Dr David Rowe. Mice were euthanized with carbon dioxide (CO2) followed by cervical dislocation. Three hours before sacrifice, the mice were injected intraperitoneally with 0.1 mg bromodeoxyuridine (BrdU) per gram body weight. Mouse mandibles were dissected and hemisected. After orthodontic appliance delivery, experimental and control mandibles were cultured for 6 hours in sterile culture medium.
For in vivo studies, 12-week-old 3.6Col1GFP mice (n = 3) were anesthetized with 87 mg/kg ketamine and 13 mg/kg xylazine, and an orthodontic spring appliance was placed. The mice were euthanized after 6 hours, and their mandibles were dissected and processed for histologic analysis. Three hours before sacrifice, the mice were injected intraperitoneally with 0.1 mg BrdU per gram body weight.
Appliance Delivery
For both in vivo and in vitro studies, only left mandibles were treated with an active spring appliance; the contralateral side served as the control. A conventional 0.010 × 0.030-inch superelastic Sentalloy spring (GAC, Dentsply, Bohemia, NY) was used to apply orthodontic force. Based on the force/deflection curve, a force of 25 grams was delivered upon spring deactivation at a distance of 0.45 mm from the initial length of the spring.
Self-etching primer (Transbond Plus Self-Etching Primer, 3M Unitek, St Paul, Minn) was applied to the occlusal surface of the first molar and incisor. One metal attachment of the spring was bonded to the incisor with light-cured dental adhesive resin cement (Transbond, 3M Unitek) via a light-curing unit (Flashlite 1401, Discus Dental, Los Angeles, Calif). The other end of the spring was bonded to the occlusal surface of the first molar (Figure 1A).
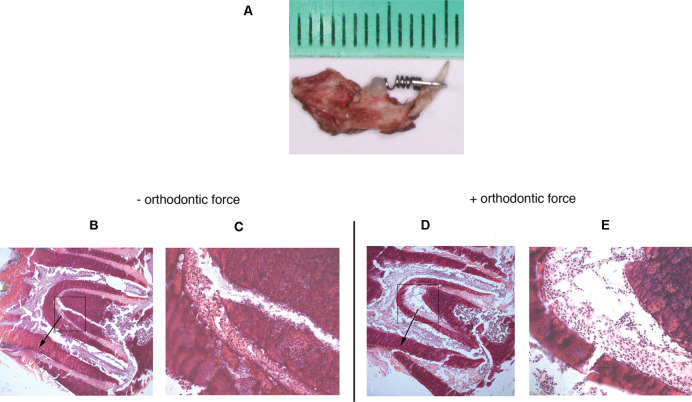
Figure 1.
(A) Spring appliance bonded to hemisected mouse mandibles. (B and C) Hematoxylin/eosin-stained sagittal cross sections of unloaded and loaded molars (D and E) after 6 hours. Arrow shows the direction of the orthodontic force.
Organ Culture
The culture medium consisted of α-MEM containing 100 U/mL penicillin, 100 g/mL streptomycin, 100 mg/mL glutamine, 20% heat-inactivated fetal calf serum, and 0.15 mg/mL ascorbic acid. Each specimen was placed in a multiwell tissue culture dish containing an 8.0 µm pore size insert (PET Track-Etched Membrane, it4ip, Seneffe, Belgium) immersed completely in the culture medium and incubated at 37°C for 6 hours.
Histology
After incubation in culture medium during in vitro experiments, or immediately after euthanasia in in vivo experiments, the mandibles were fixed for 5 days in 10% formalin at 4°C. After fixation, mandibles were washed three times in 1× phosphate-buffered solution and were placed in 30% sucrose for an additional 24 hours. After spring removal, tissues were embedded in tissue-embedding medium (Cryomatrix, Termo Shandon, Pittsburgh, Pa) on dry ice and were immersed in methyl butane for 10–15 minutes. Midsagittal frozen sections (5 µm) were obtained from the mesial and distal roots of the mandibular first molars. Fluorescent protein expression was observed using an FITC/Texas Red dual filter cube on a Zeiss Axiovert 200 M microscope (Zeiss, Oberkochen, Germany). The same sections were subsequently stained with hematoxylin/eosin for routine histologic evaluation and were photographed using an Axiocam MRc digital camera (Zeiss).
Proliferation Assay
BrdU immunohistochemical analysis was completed using the Staining Kit (Zymed Laboratories–Invitrogen Corporation, Carlsbad, Calif) in accordance with the manufacturer's instructions. Sections were counterstained with hematoxylin. Negative controls were obtained by omitting the primary antibody step and incubating sections with blocking solution. Sections were viewed by light microscopy using a Zeiss Axiovert 200 M microscope, and images of proliferating cells in the PDL were taken using an Axiocam MRc digital camera.
Histomorphometric Analysis
To quantify BrdU, a rectangular box of fixed area was superimposed on 20× images of each section, and a labeling index (number of BrdU-positive cells/total number of cells) was calculated on the tension and compression sides in the furcation area of the mandibular first molars. Quantification of transgene expression (3.6Col1-GFPtopaz and BSP-GFPtopaz) was performed in the same manner. For each animal, three to five consecutive sections were counted by a calibrated blinded examiner. The average then was calculated and was used for the BrdU or the GFP labeling index for that animal.
Statistical Analysis
Data were evaluated by using GraphPad Prism (GraphPad Software Inc, La Jolla, Calif). The difference between loaded tension and compression sides compared with corresponding sides of the control molars was determined by a Mann-Whitney U-test. A P value < .05 was considered statistically significant.
RESULTS
Cell Proliferation and Viability
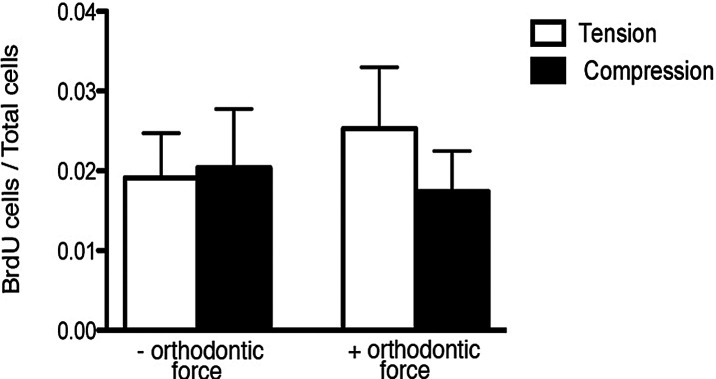
After 6 hours in culture, orthodontic force applied on the first molars produced a narrowing side-compression area and a widening side-tension area of the PDL (Figure 1B,D). The morphology of PDL cells showed well-demarcated nuclei, which suggests that the periodontium sustained its viability for this period in culture (Figure 1C,E). Proliferating cells were dispersed throughout the PDL of unloaded and loaded mandibles (Figure 2). The BrdU labeling index was increased in the tension and compression areas of loaded molars compared with unloaded molars (Figure 3).
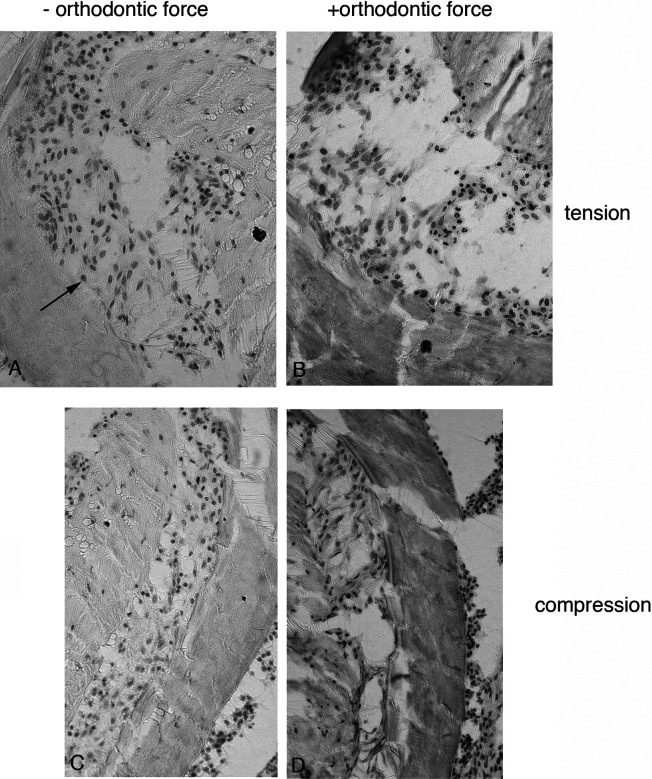
Figure 2.
BrdU-stained sections showing the proliferating cells (black arrow) on tension (A and B) and compression (C and D) sides of loaded (B and D) and unloaded molars (A and C).
Figure 3.
In vitro BrdU labeling index. Analysis of proliferation by BrdU staining was performed in the furcation of the first molar in areas exposed to compression or tension. Points represent the mean and SEM (n = 3) for mice of loaded and unloaded controls. * P < .05.
Activation of Osteoblastic Transgenes 3.6Col1-GFPtopaz and BSP-GFPtopaz
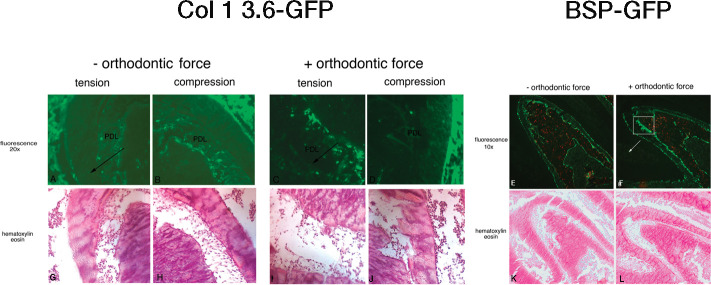
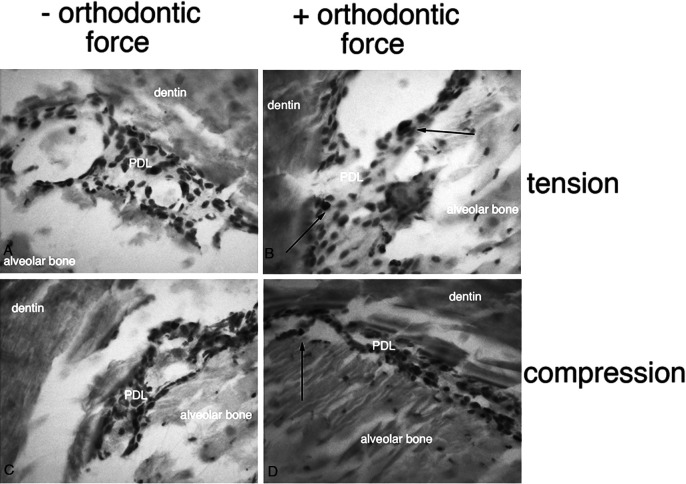
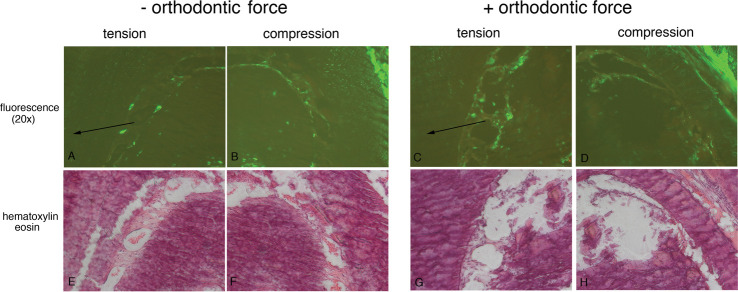
Activation of GFP transgenes during the first 6 hours of orthodontic force application revealed an increase in 3.6Col1-GFP expression in tension and decreased expression in the compression area of loaded first molars (Figures 4C,D) compared with unloaded controls (Figures 4A,B). Newly formed 3.6Col1-GFPtopaz cells were dispersed throughout the PDL, but cells with the strongest transgene expression appear primarily on the PDL–alveolar bone line. Quantification of 3.6Col1-GFP showed a significant increase in the tension area and a significant decrease in the compression area compared with unloaded controls (Figure 4). Hematoxylin/eosin sections show similar PDL space on both sides of the unloaded molars (Figure 4G,H) in contrast to the widened PDL on the tension side and the narrow PDL on the compressed side of the loaded molars (Figure 4I,J).
Figure 4.
Fluorescent images (A through F) and hematoxylin/eosin stainings (G through L) of sagittal sections of organ cultured hemisected mandibles from 3.6Col1-GFP and BSP-GFP transgenic mice (unloaded [A, C, and E] and loaded for 6 hours [B, D, and F] first molars). Arrows signify the direction of force.
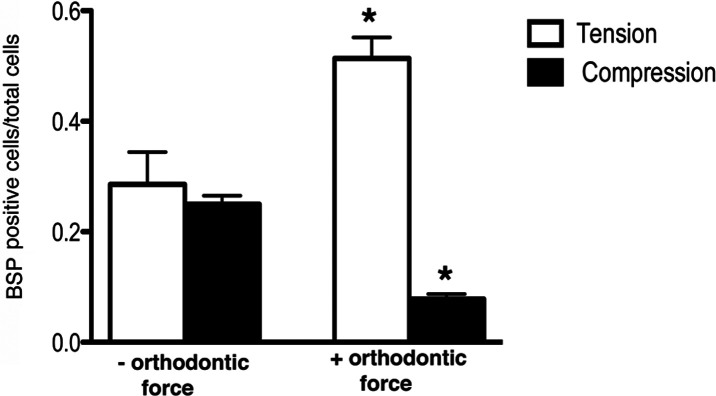
Orthodontic tooth movement also caused a significant increase in BSP-GFP expression in the tension area of the PDL compared with controls (Figures 4E,F and 5) that was restricted to the PDL–bone surface (Figure 4F, box area). Decreased BSP-GFP expression was observed on the compressed side (Figures 4E,F and 6). Hematoxylin/eosin sections show similar PDL space on both sides of the furcation of the unloaded molar (Figure 4K) compared with well-demarcated PDL differences between compression and tension sides in the loaded molar (Figure 4L).
Figure 5.
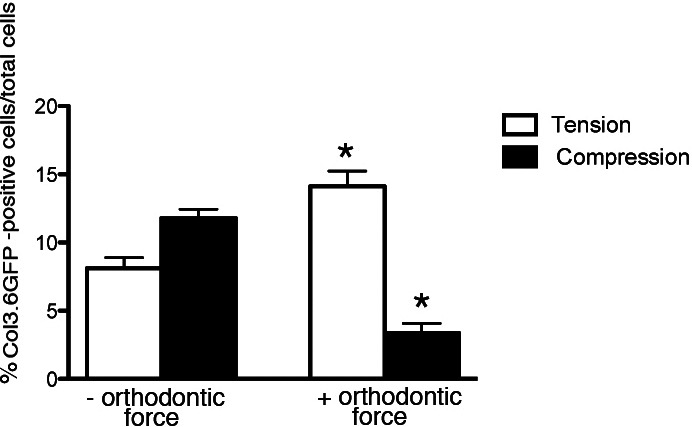
In vitro 3.6Col1-GFP labeling index. Expression of 3.6Col1-GFP was analyzed in the furcation of the first molar in areas exposed to compression or tension. Points represent the mean and SEM (n = 3) for mice of loaded and unloaded controls. * P < .05.
Figure 6.
In vitro BSP-GFP labeling index. BSP-GFP expression was analyzed in the furcation of the first molar in areas exposed to compression and tension. Points represent the mean and SEM (n = 3) for mice of loaded and unloaded controls. * P < .05.
In Vivo Findings
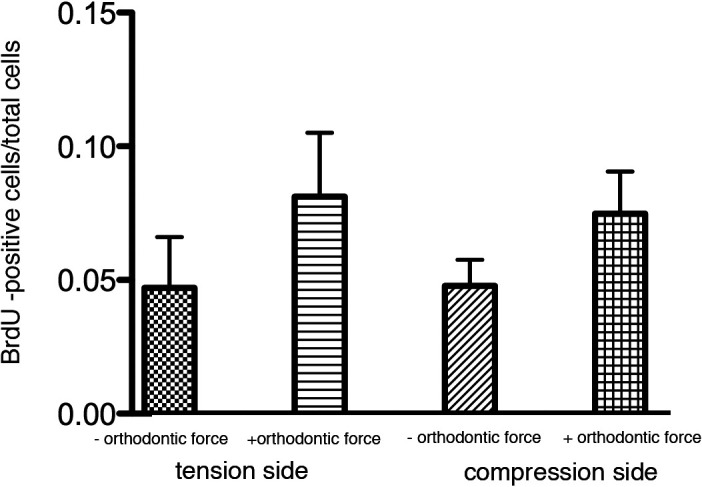
BrdU immunostained cells were found in both compression and tension areas (Figure 7). Proliferating cells were spread within the PDL, as well as on the PDL–alveolar bone line. Only an occasional BrdU-positive cell was observed in the PDL of unloaded molars (Figures 7 and 8).
Figure 7.
BrdU stained sections show proliferating cells (black arrows) on tension (A and B) and compression (C and D) sides of loaded (B and D) and unloaded molars (A and C).
Figure 8.
In vivo BrdU labeling index. Proliferation by BrdU staining was analyzed in the furcation of the first molar in areas exposed to compression or tension. Points represent the mean and SEM (n = 3) for mice. * P < .05.
The 3.6Col1-GFP activated cells were increased in the tension area of loaded molars compared with unloaded control molars (Figures 9A,C,E,G, and 10). These fibroblast-like cells (Figure 11) were embedded within the PDL, but very active cells were detected on the bone surface. On the other hand, these active cells were reduced in compression areas compared with the nonloaded contralateral tooth (Figures 9B,D,F,H, and 10).
Figure 9.
In vivo fluorescent images (A, B, C, and D) and hematoxylin/eosin stainings (E, F, G, and H) of sagittal sections of mandibles from 3.6Col1-GFP transgenic mice exposed to orthodontic force (C, D, G, and H) or serving as controls (A, B, E, and F). Arrows depict direction of force.
Figure 10.
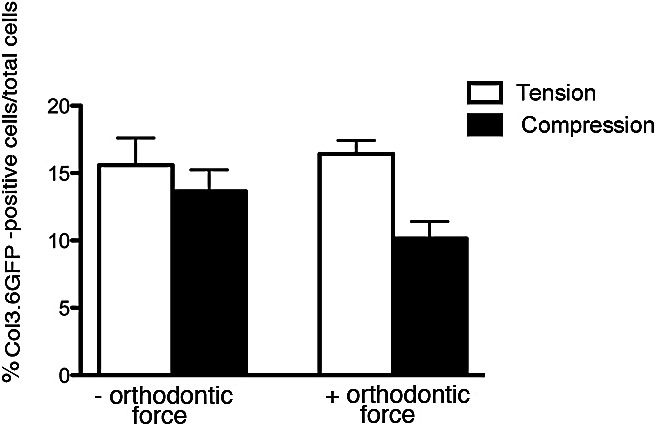
In vivo 3.6Col1-GFP labeling index. Expression of 3.6 Col1-GFP was analyzed in the furcation of the first molar in areas exposed to compression or tension. Points represent the mean and SEM (n = 3) for mice. * P < .05.
Figure 11.
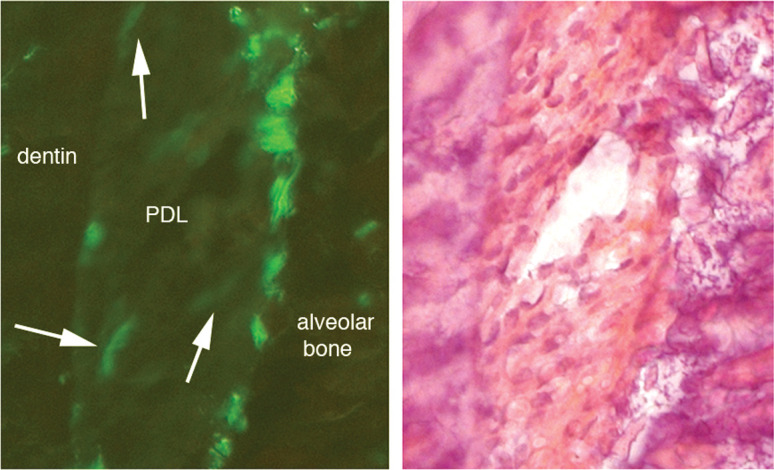
(A) Fibroblastic-like cells in the PDL (white arrows) in the tension side of in vivo 3.6Col1-GFP mice. (B) Corresponding hematoxylin/eosin stainings show the alveolar bone, the PDL, and dentin.
DISCUSSION
A novel mouse orthodontic mandibular organ culture model was developed to track early cellular changes after application of an orthodontic force. The PDL–alveolar bone complex allows a unique view of osteoblast differentiation, with undifferentiated osteoblast precursors within the PDL, periosteal osteoblasts lining the alveolar bone surface, and osteocytes within the alveolar bone.15 We believe that this model may be useful for studying the effects of mechanical loading on osteoblast differentiation, in addition to orthodontic tooth movement. One weakness of this model is the limited duration of the organ culture. In our organ culture model, basal GFP expression and cell viability decreased after 12 hours in culture (data not shown). In contrast, in the previously described rat orthodontic organ culture model, cell viability was maintained after 5 days of culture.14 Differences may be related to the fact that in the rat organ culture model, 2 mm transverse sections of the mandible were used, whereas in this study, entire hemisected mouse mandibles were used.
Cells expressing proteins of type I collagen and bone sialoprotein define the early and later stages of osteoblastic differentiation, respectively.5,6,9,10 Orthodontic forces were able to induce 3.6Col1-GFP and BSP-GFP expression in the tension sides. This increase was clearly evident in the areas along the bone surface, consistent with a similar study in which high Col1a1 mRNA expression, determined by in situ hybridization, was present in osteoblasts along the bone surface of mouse first molars after 6 days of mechanical loading with a spring.11 In another similar study evaluating early mRNA expression of BSP and Col1a1 on the tension side with the use of in situ hybridization, an increase in Col1a1 expression after 1 day and a faint signal of BSP, which was more evident by day 3, were found.12 Moreover, consistent with our findings, Anastasi et al.13 found an increase in Col1a1 on the tension side after 3 hours of orthodontic force on human premolars that were extracted and analyzed with immunofluorescence.
Our findings on the compression side of the PDL during orthodontic tooth movement revealed a significant decrease in 3.6Col1-GFP and BSP-GFP expression in vitro and a similar tendency in the in vivo model. PDL cells on the compression side undergo a “hylanization” process during orthodontic tooth movement, in which cells undergo cell death and the compressed PDL histologically resembles hylaline cartilage.16 Loss of 3.6Col1-GFP and BSP-GFP on the compression side may represent the initial step in the hyalinization process or cell death.
Similar expression of 3.6Col1-GFP was noted on the tension and compression sides in vivo and in vitro, despite the fact that older mice were used in the in vivo experiments. Older mice were used because of the technical difficulty that we experienced when placing orthodontic springs in 21-day-old mice. We believe that this age difference had no impact on the results, although an age-matched experiment conducted in the future may help to clarify this issue.
CONCLUSION
A mouse orthodontic tooth movement organ culture model has been developed to study the effects of orthodontic forces on periodontal tissues. Upon examining 3.6Col1-GFP and BSP-GFP expression, we found that after 6 hours of orthodontic force, a significant increase in 3.6Col1-GFP and BSP-GFP expression was noted on the tension side, as well as a significant decrease in their expression on the compression side.
Acknowledgments
This work was supported by a National Institute of Health grant #5U24DEO16495.
REFERENCES
- 1.Storey E. The nature of tooth movement. Am J Orthod. 1973;63:292–314. doi: 10.1016/0002-9416(73)90353-9. [DOI] [PubMed] [Google Scholar]
- 2.Hosoya A, Ninomiya T, Hiraga T, et al. Alveolar bone regeneration of subcutaneously transplanted rat molar. Bone. 2008;42:350–357. doi: 10.1016/j.bone.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Mukai M, Yoshimine Y, Akamine A, Maeda K. Bone-like nodules formed in vitro by rat periodontal ligament cells. Cell Tissue Res. 1993;271:453–460. doi: 10.1007/BF02913727. [DOI] [PubMed] [Google Scholar]
- 4.Aubin J. E, Liu F, Malaval L, Gupta A. K. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- 5.Dacic S, Kalajzic I, Visnjic D, Lichtler A. C, Rowe D. W. Col1a1-driven transgenic markers of osteoblast lineage progression. J Bone Miner Res. 2001;16:1228–1236. doi: 10.1359/jbmr.2001.16.7.1228. [DOI] [PubMed] [Google Scholar]
- 6.Kalajzic Z, Liu P, Kalajzic I, et al. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone. 2002;31:654–660. doi: 10.1016/s8756-3282(02)00912-2. [DOI] [PubMed] [Google Scholar]
- 7.Rowe D. W. Viewing problems in bone biology from the perspective of lineage identification. J Musculoskelet Neuronal Interact. 2005;5:350–352. [PubMed] [Google Scholar]
- 8.Maye P, Stover M. L, Liu Y, Rowe D. W, Gong S, Lichtler A. C. A BAC-bacterial recombination method to generate physically linked multiple gene reporter DNA constructs. BMC Biotechnol. 2009;9:20. doi: 10.1186/1472-6750-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalajzic I, Kalajzic Z, Kaliterna M, et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Ganss B, Kim R. H, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 11.Pavlin D, Dove S. B, Zadro R, Gluhak-Heinrich J. Mechanical loading stimulates differentiation of periodontal osteoblasts in a mouse osteoinduction model: effect on type I collagen and alkaline phosphatase genes. Calcif Tissue Int. 2000;67:163–172. doi: 10.1007/s00223001105. [DOI] [PubMed] [Google Scholar]
- 12.Domon S, Shimokawa H, Yamaguchi S, Soma K. Temporal and spatial mRNA expression of bone sialoprotein and type I collagen during rodent tooth movement. Eur J Orthod. 2001;23:339–348. doi: 10.1093/ejo/23.4.339. [DOI] [PubMed] [Google Scholar]
- 13.Anastasi G, Cordasco G, Matarese G, et al. An immunohistochemical, histological, and electron-microscopic study of the human periodontal ligament during orthodontic treatment. Int J Mol Med. 2008;21:545–554. [PubMed] [Google Scholar]
- 14.Dhopatkar A. A, Sloan A. J, Rock W. P, Cooper P. R, Smith A. J. British Orthodontic Society, Chapman Prize Winner 2003. A novel in vitro culture model to investigate the reaction of the dentine-pulp complex to orthodontic force. J Orthod. 2005;32:122–132. doi: 10.1179/146531205225020979. [DOI] [PubMed] [Google Scholar]
- 15.Pavlin D, Goldman E. S, Gluhak-Heinrich J, Magness M, Zadro R. Orthodontically stressed periodontium of transgenic mouse as a model for studying mechanical response in bone: the effect on the number of osteoblasts. Clin Orthod Res. 2000;3:55–66. [PubMed] [Google Scholar]
- 16.von Bohl M, Kuijpers-Jagtman A. M. Hyalinization during orthodontic tooth movement: a systematic review on tissue reactions. Eur J Orthod. 2009;31:30–36. doi: 10.1093/ejo/cjn080. [DOI] [PubMed] [Google Scholar]