Abstract
Background
Emergency or urgent surgery, which can be defined as surgery which must be done promptly to save life, limb or functional capacity, is associated with a high risk of bleeding and death. Antifibrinolytic agents, such as tranexamic acid, inhibit blood clot breakdown (fibrinolysis) and can reduce perioperative bleeding. Tranexamic acid has been shown to reduce the need for a blood transfusion in adult patients undergoing elective surgery but its effects in patients undergoing emergency or urgent surgery are unclear.
Objectives
To assess the effects of tranexamic acid on mortality, blood transfusion and thromboembolic events in adults undergoing emergency or urgent surgery.
Search methods
We searched the following electronic databases: the Cochrane Injuries Group Specialised Register (22 August 2012); Cochrane Central Register of Controlled Trials (2012, Issue 8 of 12); MEDLINE (OvidSP) (1950 to week 2 August 2012); PubMed (1 June 2012 to 22 August 2012); EMBASE (OvidSP) (1980 to Week 33 2012); ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 22 August 2012); ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to 22 August 2012). We also searched online trials registers on 22 August 2012 to identify unpublished studies.
Selection criteria
Randomised controlled trials comparing tranexamic acid with no tranexamic acid or placebo in adults undergoing emergency or urgent surgery.
Data collection and analysis
Two authors examined titles, abstracts and keywords of citations from the electronic databases for eligibility and extracted data for analysis and risk of bias assessment. Outcome measures of interest were mortality, receipt of a blood transfusion, units of blood transfused, reoperation, seizures and thromboembolic events (myocardial infarction, stroke, deep vein thrombosis and pulmonary embolism).
Main results
We identified five trials involving 372 people that met the inclusion criteria. Three trials (260 patients) contributed data to the analyses. The effect of tranexamic acid on mortality (RR 1.01; 95% CI 0.14 to 7.3) was uncertain. However, tranexamic acid reduced the probability of receiving a blood transfusion by 30% although the estimate was imprecise (RR 0.70; 95% CI 0.52 to 0.94). The effects on deep venous thrombosis (RR 2.29; 95% CI 0.68 to 7.66) and stroke (RR 2.79; 95% CI 0.12 to 67.10) were uncertain. There were no events of pulmonary embolism or myocardial infarction. None of the trials reported units of blood transfused, reoperation or seizure outcomes.
Authors' conclusions
There is evidence that tranexamic acid reduces blood transfusion in patients undergoing emergency or urgent surgery. There is a need for a large pragmatic clinical trial to assess the effects of routine use of tranexamic acid on mortality in a heterogeneous group of patients receiving urgent and emergency surgery.
Plain language summary
Tranexamic acid (an antifibrinolytic agent) for reducing mortality in emergency and urgent surgery
Emergency or urgent surgery, which can be defined as surgery which must be done promptly to save life, limb, or functional capacity, is associated with a high risk of bleeding and death. Antifibrinolytic drugs, such as tranexamic acid, promote blood clotting by preventing blood clots from breaking down. Previous studies have shown that this drug reduces the need for blood transfusion in patients undergoing elective surgery. The authors of this review searched for randomised controlled trials assessing the effects of tranexamic acid in patients undergoing urgent or emergency surgery.
The results of this review show that tranexamic acid reduces the probability that a patient will receive a blood transfusion by around 30%. The effect of tranexamic acid on other important outcomes, such as death, remains uncertain. The authors conclude that larger studies should be done to assess the effects of tranexamic acid on relevant outcomes such as death in patients undergoing all types of emergency and urgent surgery.
Background
Description of the condition
An estimated 234 million people worldwide undergo major surgical procedures every year, of which almost seven million have major complications and including one million who die (Weiser 2008). Emergency or urgent surgery, which can be defined as a surgery which must be done promptly to save life, limb or functional capacity, is associated with a high risk of bleeding and death (Karkouti 2004; Pearse 2006).
Although there are no accurate figures regarding the epidemiology of emergency surgeries, it is estimated that they represent a larger relative proportion in low‐ and middle‐income countries in comparison to high‐income countries. The 'Disease control priorities in developing countries' project estimated the burden of disease for conditions that require surgery. It found that low‐ and middle‐income regions present the highest burden of disease for surgical conditions, and the highest proportion of emergency surgical conditions, such as injuries or obstetric complications (Debas 2006). For example, surgical conditions in Africa represent 38 disability adjusted life years (DALYs) per 1000 population, of which 60% are emergencies; while for Europe surgical conditions represent 25 DALYs per 1000 population of which only 30% are emergencies (Debas 2006). Although perioperative mortality has been declining over the past 50 years, the mortality rate is twice as high in countries with a low human development index (Bainbridge 2012).
Bleeding is a leading complication of surgery, and has been shown to be associated with increased mortality (Karkouti 2004). In addition, perioperative bleeding is one of the main indications of a blood transfusion, which is associated with further complications (Goodnough 2008). Although the safety of red blood cell transfusion has improved dramatically over past decades, major complications (such as haemolysis, infection with HIV or hepatitis viruses, and acute lung injury) are still possible (Klein 2007). This knowledge has led to increased interest in the identification of interventions for reducing perioperative bleeding and the need for blood transfusion.
Description of the intervention
To reduce perioperative bleeding, the effects of antifibrinolytic drugs such as epsilon aminocaproic acid (EACA) and tranexamic acid (TXA) have been studied extensively. Antifibrinolytics promote blood clotting by preventing blood clots from breaking down. TXA and EACA are synthetic derivatives of the amino acid lysine that act as inhibitors of fibrinolysis by blocking the lysine binding sites on plasminogen molecules and inhibiting the formation of plasmin (Okamoto 1997). TXA is about 10 times more potent and binds much more strongly to both the strong and weak sites of the plasminogen molecule than EACA (Mannucci 2007).
How the intervention might work
TXA has been shown to reduce the need for a blood transfusion in adult patients scheduled for elective surgery. A Cochrane review showed that, compared with placebo, TXA reduced the probability of receiving a red blood cell transfusion by 39% (risk ratio (RR) 0.61; 95% confidence interval (CI) 0.53 to 0.70), and was associated with a non‐significant reduction in risk of reoperation (RR 0.80; 95% CI 0.55 to 1.17) and mortality (RR 0.60; 95% CI 0.33 to 1.10) (Henry 2011). However, this review did not include trials assessing the effect of TXA use in emergency surgery.
Why it is important to do this review
It is important to evaluate the effect of TXA in emergency surgery as these procedures are associated with a higher risk of bleeding and mortality. In addition, the recent CRASH‐2 trial assessed the effects of TXA in 20,211 trauma patients and found a reduction in all‐cause mortality (RR 0.91; 95% CI 0.85 to 0.97) and death due to bleeding (RR 0.85; 95% CI 0.76 to 0.96) with TXA use (CRASH‐2 2010). Almost half of the patients in the CRASH‐2 trial required surgery many of whom might have been in the emergency setting, which raises the possibility that TXA could be effective in emergency surgery.
Objectives
To assess the effects of tranexamic acid on mortality, blood transfusion and thromboembolic events in adult patients undergoing emergency or urgent surgery.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were included in this review.
Types of participants
Adult patients (over 18 years old) undergoing emergency or urgent surgery. We considered the surgery as 'emergency or urgent' if the authors used this definition, if the surgeries were conducted within 48 hours of hospital admission, or if because of the nature of the condition it is implicitly understood that patients will require emergency surgery (for example long bone fracture, hip fracture or acute aortic dissection).
Types of interventions
Tranexamic acid compared to placebo or no tranexamic acid.
Types of outcome measures
Primary outcomes
All‐cause mortality at the end of follow‐up
Secondary outcomes
Myocardial infarction
Stroke
Deep vein thrombosis
Pulmonary embolism
Seizure
Renal failure
Reoperation
Blood transfusion
Units of blood transfused
Search methods for identification of studies
We did not limit the searches by date, language or publication status.
Electronic searches
We searched the following electronic databases:
Cochrane Injuries Group Specialised Register (22 August 2012);
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) (2012, Issue 8 of 12);
MEDLINE (OvidSP) (1950 to week 2 August 2012);
EMBASE (OvidSP) (1980 to Week 33 2012);
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 22 August 2012);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to 22 August 2012).
The search strategies are listed in Appendix 1.
Searching other resources
Pfizer website (https://www.pfizer.com/research/) (accessed 22nd August 2012).
We checked the reference lists of relevant trials and other published reviews.
WHO Clinical Trials Registry Portal (http://apps.who.int/trialsearch/Default.aspx) (accessed 22 August 2012) to identify unpublished completed or ongoing studies.
Data collection and analysis
We conducted this review according to the published Cochrane protocol (Perel 2012).
Selection of studies
Two authors (PP and KK) examined the titles, abstracts and keywords of the citations from the electronic databases for eligibility. We obtained the full‐text of all potentially relevant records. Because the definition of emergency surgery is challenging, a third author (CM) independently (and blinded from the trial results) assessed whether or not the selected studies included emergency surgeries.
Data extraction and management
Two authors (PP and KK) extracted data on the number, age and sex of trial participants, type of surgery, dose and timing of tranexamic acid, type of comparator and outcome data. There were no disagreements about the inclusion of studies.
Assessment of risk of bias in included studies
Two authors (PP and KK) assessed the risk of bias in included studies using the Cochrane Collaboration's tool described in Higgins 2011. The following domains were assessed for each study: sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. We attempted to obtain the protocols for the included studies in order to assess selective outcome reporting bias. A risk of bias table was completed, incorporating a description of the study's performance against each of the above domains and our overall judgment of the risk of bias for each entry, as 'low risk’, ‘high risk’ or ‘unclear risk’ of bias. As the risk of bias for blinding and incomplete outcome data may vary according to outcome, we assessed this separately for mortality, transfusion and adverse events. There were no disagreements between the review authors regarding the risk of bias assessment.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios (RRs) and 95% confidence intervals (CI) for each trial. For the continuous outcome 'units of blood transfused', we planned to calculate the mean difference (MD) and 95% CI. If the results were reported in millilitres we planned to convert them into units by dividing by 300.
Unit of analysis issues
The patient was the unit of analysis.
Dealing with missing data
We contacted the original study investigators to obtain missing data, but did not receive any replies.
Assessment of heterogeneity
We examined trial characteristics in terms of participants, interventions and outcomes for evidence of clinical heterogeneity. Statistical heterogeneity was examined by both the I2 statistic and Chi2 test. The I2 statistic describes the percentage of total variation across studies due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity; substantial heterogeneity is considered to exist when I2 > 50% (Higgins 2011). For the Chi2 test, a P value of < 0.10 was used to indicate the presence of statistically significant heterogeneity.
Assessment of reporting biases
We planned to investigate the presence of reporting (publication) bias using funnel plots if there were at least 10 studies included in the review for the same outcome.
Data synthesis
We pooled the data using the fixed‐effect model as we judged that the trials were clinically and statistically homogenous.
Subgroup analysis and investigation of heterogeneity
We did not plan to conduct any subgroup analyses.
Sensitivity analysis
We planned to conduct a sensitivity analysis according to the risk of bias judgement for allocation concealment ('high' versus 'unclear' versus 'low'), and for fixed‐effect versus random‐effects models for data synthesis.
Results
Description of studies
Results of the search
We screened 7252 records and 128 full‐texts were assessed for eligibility. A total of five trials, involving 372 patients, met the inclusion criteria. No ongoing studies were identified. The process of reviewing studies for inclusion in the review is outlined in a flow diagram (Figure 1).
1.
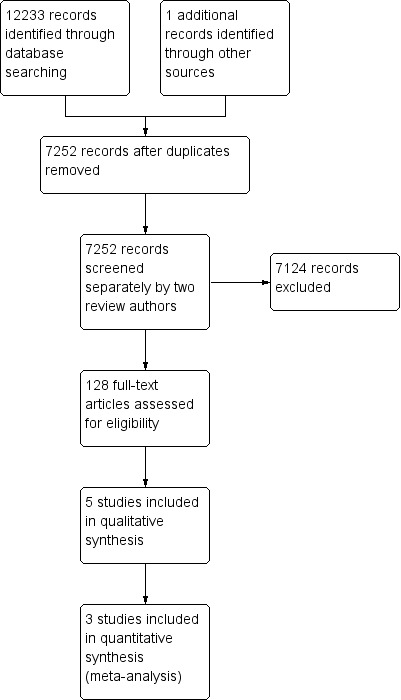
Study flow diagram.
Included studies
Two trials involved patients with hip fracture, two trials involved patients with femur fracture, and one trial involved patients undergoing emergency coronary artery bypass grafts. Two trials were conducted in Iran, one in Turkey, one in France and one in India. All of the trials compared tranexamic versus no tranexamic acid, one trial also included two more comparison arms, desmopressin or tranexamic plus desmopressin.
Of the five trials included in this review, we were able to obtain mortality and transfusion data for three trials; data on deep venous thrombosis for two trials; and data on pulmonary embolism, myocardial infarction and stroke for one trial. None of the trial reports provided data on the number of units of blood transfused.
Two trials were reported as conference abstracts and although we contacted the authors we were unable to obtain further details about their methods or results.
Further details of the five included trials are presented in the 'Characteristics of included studies' table.
Risk of bias in included studies
Two figures show our assessment of the risk of bias for the included trials (Figure 2 and Figure 3).
2.
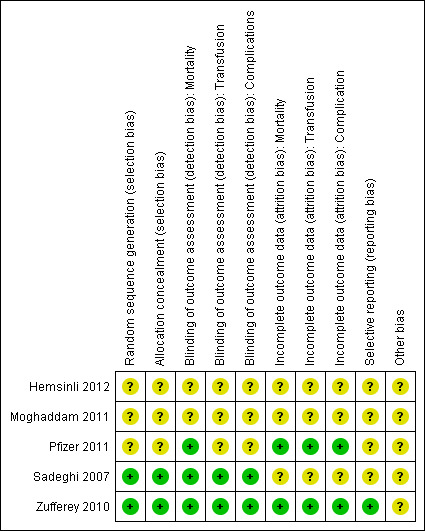
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
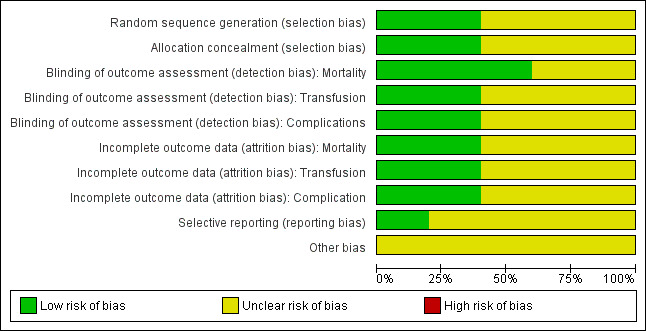
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Five studies are included in this review.
Allocation
Random sequence generation and allocation concealment were judged to be low risk of bias in two trials and unclear for the remaining three trials.
Blinding
Blinding was judged as low risk of bias for mortality in three trials, and low risk of bias in two trials for the other outcomes.
Incomplete outcome data
Incomplete outcome data were judged as low risk of bias in two trials for all outcomes.
Selective reporting
We were unable to retrieve any of the trials' protocols so selective reporting bias was judged as unclear for all five trials.
Other potential sources of bias
We did not identify any other sources of bias among the included trials.
Effects of interventions
Tranexamic acid versus no tranexamic acid
Mortality
1.1. Analysis.
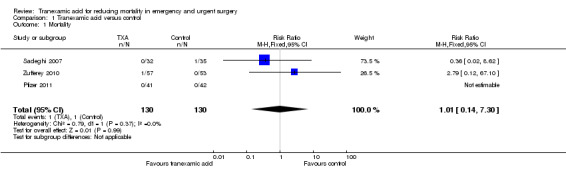
Comparison 1 Tranexamic acid versus control, Outcome 1 Mortality.
Three trials reported data on mortality, including a total of two deaths among 260 people. The pooled risk ratio (RR) was 1.01 (95% CI 0.14 to 7.3). Heterogeneity: Chi² = 0.79, df = 1 (P = 0.37); I² = 0%.
Transfusion
1.2. Analysis.
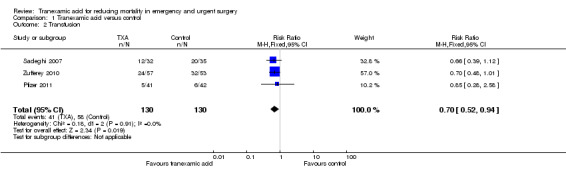
Comparison 1 Tranexamic acid versus control, Outcome 2 Transfusion.
Three trials reported data on transfusion, and 99 people received a transfusion from a total of 260 people. The pooled RR was 0.70 (95% CI 0.52 to 0.94). Heterogeneity: Chi² = 0.18, df = 2 (P = 0.91); I² = 0%. There were no data reported on the amount of blood transfused.
Deep venous thrombosis (DVT)
1.3. Analysis.
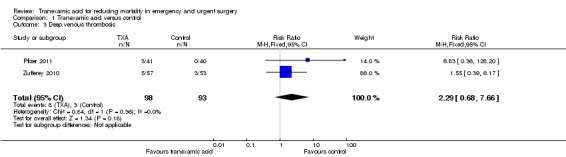
Comparison 1 Tranexamic acid versus control, Outcome 3 Deep venous thrombosis.
Two trials reported data on DVT, including a total of 11 thromboses among 191 people. The pooled RR was 2.29 (95% CI 0.68 to 7.66). Heterogeneity: Chi² = 0.84, df = 1 (P = 0.36); I² = 0%.
Other outcomes
Analysis 1.4; Analysis 1.5; Analysis 1.6
1.4. Analysis.
Comparison 1 Tranexamic acid versus control, Outcome 4 Pulmonary embolism.
1.5. Analysis.
Comparison 1 Tranexamic acid versus control, Outcome 5 Myocardial Infarction.
1.6. Analysis.
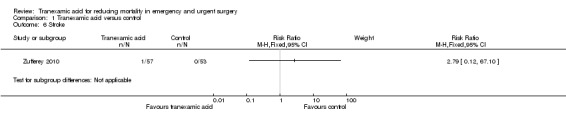
Comparison 1 Tranexamic acid versus control, Outcome 6 Stroke.
One trial (Zufferey 2010) including 110 people reported data on pulmonary embolism, myocardial infarction and stroke. Only one stroke occurred among the 57 people allocated to tranexamic acid (RR 2.79; 95% CI 0.12 to 67.10).
There were no pulmonary embolism or myocardial infarction events. There were no data reported on reoperation or seizures.
Sensitivity analysis
A sensitivity analysis was conducted for the pooled analysis of transfusion as it was the only analysis which included at least three studies, a criterion specified in the protocol for this review. The RR remained unchanged when using a random‐effects model, and when we included only trials with low risk of bias for allocation concealment the RR was 0.47 (95% CI 0.26 to 0.85).
Discussion
Summary of main results
We identified five trials evaluating the effects of tranexamic acid use in emergency or urgent surgery, but only three trials provided data for the prespecified outcomes. Although the effect estimate is imprecise, there is evidence that tranexamic acid reduces blood transfusion in patients undergoing emergency or urgent surgery. Two of the three studies that contributed data on transfusions had low risk of bias for allocation concealment and blinding thus increasing our confidence in the results. The small sample sizes and low event rates in the included trials prevent us from drawing reliable conclusions about the effect of tranexamic acid on any of the other outcomes considered in this review.
Overall completeness and applicability of evidence
Although emergency and urgent surgery are common procedures for different conditions we only found trials evaluating the effect of tranexamic acid in three surgical conditions; hip fracture, femur fracture, and emergency coronary artery bypass grafts. Two included trials were reported as abstracts and despite attempts to contact the authors we did not receive any reply and we were unable to obtain sufficient data from the abstract for inclusion in the analyses.
Quality of the evidence
Overall the risk of bias was low for the three trials that contributed data to the pooled analyses.
Potential biases in the review process
Although we systematically searched a range of databases for published and unpublished trials, as with any systematic review, we cannot exclude the possibility that some trials were missed. The fact that there is no clear consensus for the definition of emergency and urgent surgery is a potential further limitation.
Agreements and disagreements with other studies or reviews
The findings of this systematic review are consistent with the results of the Cochrane systematic review of the effects of antifibrinolytic agents for patients undergoing elective surgery (Henry 2011).
Authors' conclusions
Implications for practice.
Taking into account the evidence provided in this review, and in the context of other evidence such as from the review by Henry 2011, we can conclude that there is evidence that tranexamic acid reduces blood transfusion in patients undergoing emergency or urgent surgery. However, uncertainty remains about the effect on other clinically relevant outcomes.
Implications for research.
Although uncertainty remains about the effect of tranexamic acid on clinically relevant outcomes, the recent CRASH‐2 trial results showing that tranexamic acid reduces mortality in bleeding trauma patients and the evidence that tranexamic acid reduces blood transfusion in surgery suggest that it is most plausible that tranexamic could reduce mortality in patients undergoing emergency surgery. Therefore, there is a need for a large pragmatic clinical trial of the effect of routine use of tranexamic acid in a heterogeneous group of urgent and emergency surgical patients.
What's new
| Date | Event | Description |
|---|---|---|
| 12 February 2013 | Amended | Copy edits made to text. |
Acknowledgements
The authors wish to thank Karen Blackhall for designing and performing the database searches.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library)
#1MeSH descriptor Antifibrinolytic Agents explode all trees #2(anti‐fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti‐fibrinolysin* or antiplasmin* or anti‐plasmin* ):ab,ti or ((plasmin or fibrinolysis) near3 inhibitor*):ab,ti #3MeSH descriptor Tranexamic Acid explode all trees #4(tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or anvitoff or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA):ab,ti #5(#1 OR #2 OR #3 OR #4)
MEDLINE (OvidSP)
1 exp Antifibrinolytic Agents/ 2 (anti‐fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti‐fibrinolysin* or antiplasmin* or anti‐plasmin* or ((plasmin or fibrinolysis) adj3 inhibitor*)).ab,ti. 3 exp Tranexamic Acid/ 4 (tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or anvitoff or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA).ab,ti. 5 or/1‐4 6 randomi?ed.ab,ti. 7 randomized controlled trial.pt. 8 controlled clinical trial.pt. 9 placebo.ab. 10 clinical trials as topic.sh. 11 randomly.ab. 12 trial.ti. 13 6 or 7 or 8 or 9 or 10 or 11 or 12 14 (animals not (humans and animals)).sh. 15 13 not 14 16 5 and 15
EMBASE (OvidSP)
1.exp Antifibrinolytic Agent/ 2.(anti‐fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti‐fibrinolysin* or antiplasmin* or anti‐plasmin* or ((plasmin or fibrinolysis) adj3 inhibitor*)).ab,ti. 3.exp Tranexamic Acid/ 4.(tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or anvitoff or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA).ab,ti. 5.or/1‐4 6.exp Randomized Controlled Trial/ 7.exp controlled clinical trial/ 8.randomi?ed.ab,ti. 9.placebo.ab. 10.*Clinical Trial/ 11.randomly.ab. 12.trial.ti. 13.6 or 7 or 8 or 9 or 10 or 11 or 12 14.exp animal/ not (exp human/ and exp animal/) 15.13 not 14 16.5 and 15
ISI Web of Science: Science Citation Index Expanded, ISI Web of Science: Conference Proceedings Citation Index‐Science
#1 Topic=(anti‐fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti‐fibrinolysin* or antiplasmin* or anti‐plasmin*) #2 Topic=(((plasmin or fibrinolysis) near3 inhibitor*)) #3 Topic=(tranexamic or 'Cyclohexanecarboxylic Acid*' or Methylamine* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or 'kabi 2161' or transamin* or exacyl or amchafibrin or anvitoff or spotof or cyklokapron or 'ugurol oramino methylcyclohexane carboxylate' or 'aminomethylcyclohexanecarbonic acid' or 'aminomethylcyclohexanecarboxylic acid') #4 Topic=(AMCHA or amchafibrin or amikapron or 'aminomethyl cyclohexane carboxylic acid' or 'aminomethyl cyclohexanecarboxylic acid' or 'aminomethylcyclohexane carbonic acid' or 'aminomethylcyclohexane carboxylic acid' or 'aminomethylcyclohexanecarbonic acid' or 'aminomethylcyclohexanecarboxylic acid' or 'aminomethylcyclohexanocarboxylic acid' or 'aminomethylcyclohexanoic acid' or amstat or anvitoff or cl‐65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA) #5 (#1 or #2 or #3 or #4)
Data and analyses
Comparison 1. Tranexamic acid versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.30] |
| 2 Transfusion | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.94] |
| 3 Deep venous thrombosis | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [0.68, 7.66] |
| 4 Pulmonary embolism | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Myocardial Infarction | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Stroke | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hemsinli 2012.
| Methods | Prospective randomised double blind study | |
| Participants | Population: 54 patients taking dual antiplatelet therapy who underwent emergency cardiac surgery (CABG) Setting: Turkey Date the study was conducted: not reported |
|
| Interventions | Four arms Group 1 (n=18) received tranexamic acid (10 mg/kg, 30 min as induction + 1mg/kg,10 h infusion) Group 2 (n=16) received tranexamic acid and desmopressin (0.3 µg/kg, in 20 min iv infusion) Group 3 (n=10) received desmopressin infusion Group 4 (n=10) was the control group "regular infusions" |
|
| Outcomes | Blood loss and transfusion. No deaths were reported. | |
| Notes | The report is an abstract and does not present the results for blood transfusion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough data reported |
| Allocation concealment (selection bias) | Unclear risk | Not enough data reported |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Not enough data reported |
| Blinding of outcome assessment (detection bias) Transfusion | Unclear risk | Not enough data reported |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | Not enough data reported |
| Incomplete outcome data (attrition bias) Mortality | Unclear risk | Not enough data reported |
| Incomplete outcome data (attrition bias) Transfusion | Unclear risk | Not enough data reported |
| Incomplete outcome data (attrition bias) Complication | Unclear risk | Not enough data reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | ‐ |
Moghaddam 2011.
| Methods | Double blind clinical trial | |
| Participants | Population: 60 patients with femoral bone fracture Setting: Iran Date the study was conducted: not reported |
|
| Interventions | Intervention: tranexamic acid 10 mg/kg bolus dose and 1 mg/kg infusion during operation Control: saline (same dose) |
|
| Outcomes | Blood loss and blood transfusion. No deaths reported. | |
| Notes | The report is an abstract and does not present how many patients were randomised in each group so no data can be analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough data reported |
| Allocation concealment (selection bias) | Unclear risk | Not enough data reported |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Not enough data reported |
| Blinding of outcome assessment (detection bias) Transfusion | Unclear risk | Not enough data reported |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | Not enough data reported |
| Incomplete outcome data (attrition bias) Mortality | Unclear risk | Not enough data reported |
| Incomplete outcome data (attrition bias) Transfusion | Unclear risk | Not enough data reported |
| Incomplete outcome data (attrition bias) Complication | Unclear risk | Not enough data reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | ‐ |
Pfizer 2011.
| Methods | Prospective open label phase 4 randomised controlled trial | |
| Participants | Population: 81 patients between 18 and 50 years of age who underwent surgery for fracture shaft femur Setting: India Date the study was conducted: 2009‐2010 |
|
| Interventions | Intervention: tranexamic acid slow IV 15mg/kg 15 mins before surgery, followed by 2nd dose 3 hrs later and a 3rd dose 3 hrs later Control: standard care |
|
| Outcomes | Primary outcome: blood loss; secondary outcome: deep venous thrombosis. Pulmonary embolism, transfusion and deaths were also reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Quote: Open label Review authors' judgement: low risk of bias for mortality |
| Blinding of outcome assessment (detection bias) Transfusion | Unclear risk | Quote: Open label |
| Blinding of outcome assessment (detection bias) Complications | Unclear risk | Quote: Open label |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Quote: Intention to treat analysis and 17% loss to follow‐up |
| Incomplete outcome data (attrition bias) Transfusion | Low risk | Quote: Intention to treat analysis and 17% loss to follow‐up |
| Incomplete outcome data (attrition bias) Complication | Low risk | Quote: Intention to treat analysis and 17% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | ‐ |
Sadeghi 2007.
| Methods | Prospective, randomised, double blinded study | |
| Participants | Population: 67 patients undergoing hip fracture surgery Setting: Iran Date the study was conducted: 2004‐2005 |
|
| Interventions | Intervention: tranexamic acid single bolus dose of 15 mg kg–1 administered intravenously at induction of anaesthesia Control: the same volume of saline solution was infused |
|
| Outcomes | Blood loss, blood transfusion, thrombotic complications and neurological deficits. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Patients were randomised using a random number technique |
| Allocation concealment (selection bias) | Low risk | Quote: The correct treatment option was assured by means of coded infusion syringes prepared by hospital pharmacy not involved otherwise in the study |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Quote: Caring personnel both the staff of the operating room and the intensive care unit (ICU) were blinded regarding the type and nature of treatment |
| Blinding of outcome assessment (detection bias) Transfusion | Low risk | Quote: Caring personnel both the staff of the operating room and the intensive care unit (ICU) were blinded regarding the type and nature of treatment |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: Caring personnel both the staff of the operating room and the intensive care unit (ICU) were blinded regarding the type and nature of treatment |
| Incomplete outcome data (attrition bias) Mortality | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Transfusion | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Complication | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | ‐ |
Zufferey 2010.
| Methods | Double blinded randomised trial | |
| Participants | Population: 110 patients with isolated hip fracture Setting: France Date the study was conducted: 2005‐2006 |
|
| Interventions | Intervention: tranexamic acid 15 mg kg‐1 given at skin incision and 3 h later Control: saline at the same dose |
|
| Outcomes | Primary outcome: transfusion; secondary outcomes: bacterial infection, blood loss, deep venous thrombosis, pulmonary embolism, stroke, acute coronary syndrome and death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: Central allocation by a telephone system |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Quote: Masking was ensured by the administration of apparently identical saline drips. Patient caregivers, investigators collecting the data, safety monitoring board, and members of the adjudication committee remained unaware of study‐groups assignments |
| Blinding of outcome assessment (detection bias) Transfusion | Low risk | Quote: Masking was ensured by the administration of apparently identical saline drips. Patient caregivers, investigators collecting the data, safety monitoring board, and members of the adjudication committee remained unaware of study‐groups assignments |
| Blinding of outcome assessment (detection bias) Complications | Low risk | Quote: Masking was ensured by the administration of apparently identical saline drips. Patient caregivers, investigators collecting the data, safety monitoring board, and members of the adjudication committee remained unaware of study‐groups assignments |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Quote: All the analyses were based on the intention‐to‐treat principle and no reported loss to follow‐up |
| Incomplete outcome data (attrition bias) Transfusion | Low risk | Quote: All the analyses were based on the intention‐to‐treat principle and no reported loss to follow‐up |
| Incomplete outcome data (attrition bias) Complication | Low risk | Quote: All the analyses were based on the intention‐to‐treat principle and no reported loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Quote: All the analyses were conducted according to a pre specified statistical analysis plan |
| Other bias | Unclear risk | ‐ |
Differences between protocol and review
In the review's objective we included primary (mortality) and secondary outcomes (blood transfusion and thromboembolic events), while in the protocol's objective we only mentioned mortality.
Contributions of authors
PP, KK and IR conceived the study. KK and PP screened the search output. CM independently (and blinded from outcomes) confirmed whether or not the studies selected included emergency surgeries. KK and PP extracted data. PP carried out the analyses. PP wrote the first draft and all the authors contributed to the final manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Review Incentive Scheme, Department of Health, UK.
Declarations of interest
PP, KK, CM: none known.
IR: LSHTM has received funds from pharmaceutical companies to pay for the drug and placebo used in RCTs of tranexamic acid in acute severe bleeding. These funds are declared in the relevant publications.
Edited (no change to conclusions)
References
References to studies included in this review
Hemsinli 2012 {published data only}
- Hemsinli D, Pulathan Z, Altun G, Yasar Guven K, Civelek A. The effect of tranexamic acid and desmopressin acetate infusion on coagulation parameters in patients operated under dual antiplatelet therapy. 8th International Congress of Update in Cardiology and Cardiovascular Surgery Antalya Turkey. Carden Jennings Publishing Co. Ltd, 2012; Vol. 15:S21‐2.
Moghaddam 2011 {published data only}
- Moghaddam MJ, Darabi E, Sheikholeslamy F. Effect of tranexamic acid in decreasing need to transfusion in hip fracture surgery. European Journal of Anaesthesiology 2011;28:89. [Google Scholar]
Pfizer 2011 {published data only}
- Pfizer. Prospective randomised phase IV open label comparative study of tranexamic acid plus standard care versus standard care for the reduction of blood loss in subjects undergoing surgery for long bone fracture. PhRMA Web Synopsis. Protocol B1461005 2011.
Sadeghi 2007 {published data only}
- Sadeghi M, Mehr‐Aein A. Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective randomized double blind study in 67 patients. Acta Medica Iranica 2007;45(6):437‐42. [Google Scholar]
Zufferey 2010 {published data only}
- Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albadalejo P, et al. Tranexamic acid in hip fracture surgery: a randomized controlled trial. British Journal of Anaesthesia 2010;104(1):23‐30. [DOI] [PubMed] [Google Scholar]
Additional references
Bainbridge 2012
- Bainbridge D, Martin J, Arango M, Cheng D. Perioperative and anaesthetic‐related mortality in developed and developing countries: a systematic review and meta‐analysis. Lancet 2012;380:1075–81. [DOI] [PubMed] [Google Scholar]
CRASH‐2 2010
- The CRASH‐2 Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet 2010;376:23‐32. [DOI] [PubMed] [Google Scholar]
Debas 2006
- Debas HT, Gosselin R, McCord C, Thind A. Surgery. Disease control priorities in developing countries. 2nd Edition. New York: Oxford University Press, 2006:1245‐60. [Google Scholar]
Goodnough 2008
- Goodnough LT, Shander A. Risk and complications of blood transfusions: optimizing outcomes for patients with chemotherapy induced anemia. Advanced Studies in Medicine 2008;8(10):357‐62. [Google Scholar]
Henry 2011
- Henry David A, Carless Paul A, Moxey Annette J, O'Connell D, Stokes Barrie J, Fergusson Dean A, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2011, issue 3. [DOI: 10.1002/14651858.CD001886.pub4; CD001886] [DOI] [PubMed]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Karkouti 2004
- Karkouti K, Wijeysundera DN, Yau TM, Beattie WS, Abdelnaem E, McCluskey SA, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 2004;44(10):1453‐62. [DOI] [PubMed] [Google Scholar]
Klein 2007
- Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet 2007;370:415‐26. [DOI] [PubMed] [Google Scholar]
Mannucci 2007
- Mannucci PM. Prevention and treatment of major blood loss. New England Journal of Medicine 2007;356:2301‐11. [DOI] [PubMed] [Google Scholar]
Okamoto 1997
- Okamoto S, Hijikata‐Okunomiya A, Wanaka K, Okada Y, Okamoto U. Enzyme controlling medicines: introduction. Seminars in thrombosis and hemostasis 1997;23:493‐501. [DOI] [PubMed] [Google Scholar]
Pearse 2006
- Pearse RM, Harrison DA, James P, Watson D, Hinds C, Rhodes A, et al. Identification and characterisation of the high‐risk surgical population in the United Kingdom. Critical Care 2006;10:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiser 2008
- Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372(9633):139‐44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Perel 2012
- Perel P, Ker K, Morales UCH, Roberts I. Tranexamic acid for reducing mortality in emergency and urgent surgery. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2012, issue 11. [DOI: 10.1002/14651858.CD010245; CD010245] [DOI] [PMC free article] [PubMed]


