Abstract
Background
Vitamin A deficiency (VAD) is a major public health problem in low‐ and middle‐income countries, affecting 190 million children under five years of age and leading to many adverse health consequences, including death. Based on prior evidence and a previous version of this review, the World Health Organization has continued to recommend vitamin A supplementation (VAS) for children aged 6 to 59 months. The last version of this review was published in 2017, and this is an updated version of that review.
Objectives
To assess the effects of vitamin A supplementation (VAS) for preventing morbidity and mortality in children aged six months to five years.
Search methods
We searched CENTRAL, MEDLINE, Embase, six other databases, and two trials registers up to March 2021. We also checked reference lists and contacted relevant organisations and researchers to identify additional studies.
Selection criteria
Randomised controlled trials (RCTs) and cluster‐RCTs evaluating the effect of synthetic VAS in children aged six months to five years living in the community. We excluded studies involving children in hospital and children with disease or infection. We also excluded studies evaluating the effects of food fortification, consumption of vitamin A rich foods, or beta‐carotene supplementation.
Data collection and analysis
For this update, two review authors independently assessed studies for inclusion resolving discrepancies by discussion. We performed meta‐analyses for outcomes, including all‐cause and cause‐specific mortality, disease, vision, and side effects. We used the GRADE approach to assess the quality of the evidence.
Main results
The updated search identified no new RCTs.
We identified 47 studies, involving approximately 1,223,856 children. Studies were set in 19 countries: 30 (63%) in Asia, 16 of these in India; 8 (17%) in Africa; 7 (15%) in Latin America, and 2 (4%) in Australia. About one‐third of the studies were in urban/periurban settings, and half were in rural settings; the remaining studies did not clearly report settings. Most studies included equal numbers of girls and boys and lasted about one year. The mean age of the children was about 33 months. The included studies were at variable overall risk of bias; however, evidence for the primary outcome was at low risk of bias.
A meta‐analysis for all‐cause mortality included 19 trials (1,202,382 children). At longest follow‐up, there was a 12% observed reduction in the risk of all‐cause mortality for VAS compared with control using a fixed‐effect model (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.83 to 0.93; high‐certainty evidence).
Nine trials reported mortality due to diarrhoea and showed a 12% overall reduction for VAS (RR 0.88, 95% CI 0.79 to 0.98; 1,098,538 children; high‐certainty evidence). There was no evidence of a difference for VAS on mortality due to measles (RR 0.88, 95% CI 0.69 to 1.11; 6 studies, 1,088,261 children; low‐certainty evidence), respiratory disease (RR 0.98, 95% CI 0.86 to 1.12; 9 studies, 1,098,538 children; low‐certainty evidence), and meningitis. VAS reduced the incidence of diarrhoea (RR 0.85, 95% CI 0.82 to 0.87; 15 studies, 77,946 children; low‐certainty evidence), measles (RR 0.50, 95% CI 0.37 to 0.67; 6 studies, 19,566 children; moderate‐certainty evidence), Bitot's spots (RR 0.42, 95% CI 0.33 to 0.53; 5 studies, 1,063,278 children; moderate‐certainty evidence), night blindness (RR 0.32, 95% CI 0.21 to 0.50; 2 studies, 22,972 children; moderate‐certainty evidence), and VAD (RR 0.71, 95% CI 0.65 to 0.78; 4 studies, 2262 children, moderate‐certainty evidence). However, there was no evidence of a difference on incidence of respiratory disease (RR 0.99, 95% CI 0.92 to 1.06; 11 studies, 27,540 children; low‐certainty evidence) or hospitalisations due to diarrhoea or pneumonia. There was an increased risk of vomiting within the first 48 hours of VAS (RR 1.97, 95% CI 1.44 to 2.69; 4 studies, 10,541 children; moderate‐certainty evidence).
Authors' conclusions
This update identified no new eligible studies and the conclusions remain the same. VAS is associated with a clinically meaningful reduction in morbidity and mortality in children. Further placebo‐controlled trials of VAS in children between six months and five years of age would not change the conclusions of this review, although studies that compare different doses and delivery mechanisms are needed. In populations with documented VAD, it would be unethical to conduct placebo‐controlled trials.
Keywords: Child; Child, Preschool; Female; Humans; Male; Diarrhea; Diarrhea/chemically induced; Dietary Supplements; Measles; Measles/chemically induced; Measles/complications; Morbidity; Respiration Disorders; Vitamin A; Vitamin A/therapeutic use; Vitamin A Deficiency; Vitamin A Deficiency/epidemiology; Vitamin A Deficiency/prevention & control
Plain language summary
Vitamin A supplementation for preventing disease and death in children aged six months to five years
Background
Vitamin A deficiency (VAD) is a major public health problem in low‐ and middle‐income countries, affecting 190 million children under five years of age. VAD predisposes children to increased risk of a range of problems, including respiratory diseases, diarrhoea, measles, and vision problems, and it can lead to death. Previous studies show that giving synthetic vitamin A supplementation (VAS) to children aged six months to five years who are at risk of VAD can reduce the risk of death and some diseases. This is an update of the previous review.
Review question
This review evaluated the effect of synthetic VAS compared to placebo (dummy tablet) or no intervention for preventing illness and death in children aged six months to five years.
Review methods
We searched different databases that contain both published and unpublished results of medical studies. The literature search was updated in March 2021. We included only randomised control trials (RCTs: a study in which participants are randomly allocated to one or more treatments); these are considered the best form of experimental studies in research literature. We combined the results mathematically to obtain overall estimates of effectiveness of VAS against illness and death.
Study characteristics
The update identified no new studies. The review includes 47 RCTs representing 1,223,856 children. Studies took place in 19 countries: 30 (63%) in Asia, 16 of which were in India; 8 (17%) in Africa; 7 (15%) in Latin America, and 2 (4%) in Australia. About one‐third of the studies were in urban/periurban settings, and half were in rural settings; the remaining studies did not clearly report settings. The average age of the children was about 33 months. Most studies included equal numbers of boys and girls and lasted about one year. The quality of the included studies was variable; however, it was unlikely that death rates were influenced by potential errors in the conduct of the studies.
Key results
The data on the effect of VAS for the prevention of death were available from 19 of the included studies, and the combined results indicate that VAS reduces overall risk of death and death due to diarrhoea by 12%. VAS does not specifically reduce death due to measles, respiratory infections, or meningitis, but it can reduce new occurrences of diarrhoea and measles. There was no effect on incidence of respiratory disease or admissions to hospital due to diarrhoea or pneumonia. Giving oral synthetic vitamin A to children at risk of VAD reduces the risk of night blindness and tiny flakes of protein in the eye called Bitot's spots. It also improves levels of vitamin A in their blood. The only reported side effect was risk of vomiting within 48 hours of taking vitamin A in large doses, as recommended by the World Health Organization.
Certainty of evidence
We rated the overall certainty of evidence using the GRADE approach, which considers methodological flaws within studies, consistency in reporting of results across studies, extent to which results apply to other settings, and effectiveness of treatments. Based on these criteria, we judged the overall certainty of evidence to be high for benefits of VAS against overall risk of death and death due to diarrhoea. For the other outcomes, we rated the evidence as low or moderate. One large, recently conducted study, which included about one million children, did not show any effect of VAS; however, when this study was combined with other, well‐conducted studies, VAS still had beneficial effects for the prevention of death and illness. In summary, VAS can reduce risk of illness and death in children aged 6 to 59 months of age who are at risk of VAD. This update did not identify and new eligible studies and the conclusions remain the same.
Summary of findings
Summary of findings 1. Vitamin A supplementation versus placebo or usual care for preventing morbidity and mortality in children from 6 months to 5 years of age.
| Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age | ||||||
|
Patient or population: children aged between 6 months and 5 years Intervention: vitamin A supplementation Comparison: placebo or usual care Setting: low‐ and middle‐income countries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | VAS | |||||
|
All‐cause mortality Follow‐up: 12–96 weeks |
Study population | RR 0.88 (0.83 to 0.93) | 1,202,382 (19 studies) |
⊕⊕⊕⊕ Highb | Random‐effects RR 0.76 (95% CI 0.66 to 0.88) |
|
| 26 per 1000a |
23 per 1000 (22 to 24) |
|||||
|
Mortality due to diarrhoea Follow‐up: 48–104 weeks |
Study population | RR 0.88 (0.79 to 0.98) | 1,098,538 (9 studies) | ⊕⊕⊕⊕
Highb |
Total number of participants reflects number randomised to studies. The analysis combined cumulative risk and risk per 1000 years' follow‐up. | |
| 8 per 1000a |
7 per 1000 (6 to 8) |
|||||
|
Mortality due to measles Follow‐up: 52–104 weeks |
Study population | RR 0.88 (0.69 to 1.11) | 1,088,261 (6 studies) | ⊕⊕⊖⊖ Lowc,d |
Total number of participants reflects number randomised to studies. The analysis combined cumulative risk and risk per 1000 years' follow‐up. | |
| 2 per 10,000a |
2 per 1000 (1 to 2) |
|||||
|
Mortality due to LRTI Follow‐up: 48–104 weeks |
Study population | RR 0.98 (0.86 to 1.12) | 1,098,538 (9 studies) | ⊕⊕⊖⊖ Lowc,d | Total number of participants reflects number randomised to studies. The analysis combined cumulative risk and risk per 1000 years' follow‐up. | |
| 4 per 10,000a |
4 per 1000 (3 to 5) |
|||||
|
Diarrhoea incidence Mean episodes per child per year Follow‐up: 24–60 weeks |
Study population |
RR 0.85 (95% CI 0.82 to 0.87) |
77,946 (15 studies) | ⊕⊕⊖⊖ Lowc,f | — | |
| Mean episodes of diarrhoea in control group: 4.0 per child per yeare | VAS led to 3 fewer episodes of diarrhoea per child per year (3 to 4 fewer episodes) | |||||
|
Measles incidence Mean episodes of measles per child per year Follow‐up: mean 52 weeks |
Study population |
RR 0.50 (95% CI 0.37 to 0.67) |
19,566 (6 studies) | ⊕⊕⊕⊖ Moderatec |
— | |
| Mean episodes of measles in control group: 0.2 per child per yeare | VAS led to 0.015 fewer episodes per child per year (0.019 events fewer per child to 0.01 events fewer per child) | |||||
|
LRTI incidence Mean episodes per child per year Follow‐up: mean 52 weeks |
Study population |
RR 0.99 (95% CI 0.92 to 1.06) |
27,540 (11 studies) | ⊕⊕⊖⊖ Lowc,d |
— | |
| Mean episodes of LRTI in control group: 0.1 episodes per child per yeare | VAS led to 0.1 more episodes of LRTI per child per year (0.1 fewer episodes to 0.1 more episodes) | |||||
|
Bitot's spots incidence Follow‐up: mean 80.72 weeks |
Study population | RR 0.42 (95% CI 0.33 to 0.53) | 1,063,278 (5 studies) | ⊕⊕⊕⊖ Moderatec |
— | |
| 35 per 1000a |
15 per 1000 (12 to 19) |
|||||
|
Night blindness incidence Follow‐up: 52 to 68 weeks |
Study population | RR 0.32 (95% CI 0.21 to 0.50) | 22,972 (2 studies) |
⊕⊕⊕⊖ Moderatec |
— | |
| 4 per 1000g |
1 per 1000 (1 to 2) |
|||||
|
Vomiting Follow‐up: 0.14–52 weeks |
Study population | RR 1.97 (95% CI 1.44 to 2.69) | 10,541 (4 studies) | ⊕⊕⊕⊖ Moderatec | — | |
| 31 per 1000g |
61 per 1000 (45 to 83) |
|||||
|
Vitamin A deficiency Follow‐up: mean 54.5 weeks |
Study population | RR 0.71 (95% CI 0.65 to 0.78) | 2262 (4 studies) | ⊕⊕⊕⊖ Moderatec | — | |
| 509 per 1000g |
361 per 1000 (331 to 397) |
|||||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DEVTA: deworming and enhanced vitamin A; LRTI: lower respiratory tract infection; RR: risk ratio; VAS: vitamin A supplementation. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aBased on control group mortality risk in DEVTA trial 2013. bWe acknowledge that the addition of DEVTA trial 2013 results decreased the overall effect size for this outcome compared to previous analysis for this review. However, we consider that vitamin A has robust effects on mortality as the direction of effect is in favour of intervention in most of the studies and summary estimate remains statistically significant irrespective of the use of random‐ or fixed‐effect models for meta‐analysis. cDowngraded one level due to serious risk of bias of included studies in analysis (concerns with randomisation procedures, completeness, and reporting of outcome data in the included studies). dDowngraded one level due to serious imprecision (wide CIs around the pooled effect estimate suggest both appreciable benefit and harm with vitamin A). eBased on control event rate in Chowdhury 2002. fDowngraded one level due to serious inconsistency (I2 = 94%, and the results of Herrera 1992; Lie 1993; and Chowdhury 2002 demonstrated clear evidence of benefit and were discordant with the results of the other studies). gRisk based on control event rates from the included studies.
Background
Description of the condition
Vitamin A is required for normal functioning of the visual system, maintenance of cell function for growth, epithelial integrity, red blood cell production, immunity, and reproduction (Sommer 1996). Vitamin A deficiency (VAD) impairs body functions and may cause death. Adverse health consequences may also include xerophthalmia (dry eyes), susceptibility to infection, stunting, and anaemia (Sommer 1996; Rice 2004). Chronic VAD may develop when animal sources and fortified foods are limited, for example in diets that rely heavily on vegetables and fruits (Ramakrishnan 2002). In poor societies, especially in low‐income countries, dietary deficiency can begin very early in life, when colostrum is discarded or when breastfeeding is inadequate (Haskell 1999).
VAD is interconnected with a deprived ecological, social, and economic environment. People with VAD may be exposed to measles, diarrhoea, and respiratory diseases (Sommer 2002; Rice 2004). When these problems are comorbid, depressed appetite and poor absorption may lower intake of vitamin A, while excessive metabolism and excretion may deplete body stores (Alvarez 1995; Mitra 1998). This combination of poor diet and infection leads to a vicious cycle that particularly affects young children and pregnant or lactating mothers (Sommer 2002; West 2003).
VAD is common in low‐ and middle‐income countries. About 19.1 million pregnant women and 190 million children under five years of age have VAD (i.e. serum retinol less than 0.70 µmol/L), representing about 33% of children under five years of age in populations at risk of VAD (WHO 2009). Based on biochemical VAD in young children, 122 countries have a moderate‐to‐severe public health problem (WHO 2009).
Data on global trends in VAD suggest that it remains widely prevalent in South Asia and Sub‐Saharan Africa, while rates have significantly fallen in Southeast Asia and Latin America (Stevens 2015). Deaths attributable to VAD have almost disappeared in many regions of the world, suggesting the need to revisit supplementation strategies according to population needs (Stevens 2015).
Description of the intervention
Vitamin A is a term used for a subclass of retinoic acids, a family of lipid‐soluble compounds (Bates 1995). Vitamin A is found in two main forms: provitamin A carotenoids and preformed vitamin A. Provitamin A carotenoids are found in plants; beta‐carotene is the only one that is metabolised by mammals into vitamin A. Though fruits and vegetables are nutritious in other ways, normal dietary intake of plants may not deliver adequate amounts of vitamin A because the intestinal carotenoid‐to‐retinol conversion ratio varies with type of food, ranging from 6:1 to 26:1 (US Institute of Medicine 2001; van Lieshout 2005). Consequently, VAD can exist in places with high vegetable and fruit consumption (West 2002). Preformed vitamin A (retinol, retinal, retinoic acid, and retinyl esters) is the most active form of vitamin A and is found in animal sources. Supplements usually use preformed vitamin A (Shenai 1993; Bates 1995).
How the intervention might work
Vitamin A is an essential nutrient; it cannot be synthesised by the human body and must therefore come from dietary sources (Bates 1995). Oral vitamin A supplementation (VAS) and food fortification are the most direct methods for providing vitamin A to people whose diets are deficient.
Vitamin A has been described as an anti‐infectious vitamin because of its role in regulating human immune function (Green 1928). Early studies in animals and humans revealed an association between VAD and increased susceptibility to infections (Semba 1999). In addition to its preventive and therapeutic effect against xerophthalmia (Sommer 1996), prophylactic VAS in apparently healthy children (over six months of age) residing in low‐income countries may reduce childhood mortality by as much as 30% (Beaton 1993; Fawzi 1993; Glasziou 1993), particularly by reducing diarrhoea and measles mortality.
Side effects of VAS are rare in children aged six months or older; however, vitamin A toxicity can develop if large amounts of vitamin A are used over a prolonged period of time. Symptoms of toxicity include liver damage, headaches, vomiting, skin desquamation, bone abnormalities, joint pain, and alopecia (Smith 1976). A very high single dose can also cause transient acute toxic symptoms that may include a bulging fontanelle in children under one year of age, headaches, vomiting, diarrhoea, loss of appetite, and irritability. Toxicity from ingestion of food sources with preformed vitamin A is rare (Hathcock 1997).
Why it is important to do this review
The last version of this review was published in 2017 (Imdad 2017), and an update is needed to assess for any additional evidence that may have been published. The update considers whether new, potentially eligible evidence has become available since the publication of the original review (Imdad 2010a). A separate Cochrane Review has evaluated the therapeutic role of vitamin A for measles (Huiming 2005), while another has focused on non‐measles pneumonia (Ni 2005). Different Cochrane Reviews in a variety of subpopulations of children and mothers are also evaluating the prophylactic role of vitamin A (Chen 2008; Darlow 2011; Gogia 2011; Wiysonge 2011; McCauley 2015; Bello 2016; Imdad 2016; Haider 2017; Hombali 2019).
Objectives
To assess the effects of vitamin A supplementation (VAS) for preventing morbidity and mortality in children aged six months to five years.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐RCTs evaluating the effect of synthetic VAS in children aged six months to five years. We included data from the first period of cross‐over studies only. We considered studies for inclusion irrespective of publication status or language of publication.
We excluded quasi‐RCTs, with the exception of two studies (see Differences between protocol and review).
Types of participants
Children living in the community and aged six months to five years at the time of recruitment were eligible for inclusion. We excluded children in hospital and children with disease or infection.
We contacted trial authors to determine if the study population included some participants who were not eligible for this review (e.g. children over five years of age) and requested disaggregated data. If such data were not available, we included studies if most participants (51% or more) met the inclusion criteria. If this could not be determined and the participants met the inclusion criteria on average (e.g. the mean age was within the eligible range), we included these trials.
Types of interventions
Synthetic oral VAS compared to either placebo or treatment‐as‐usual control groups, including trials of various doses and frequencies. Co‐interventions (e.g. multiple vitamin or mineral supplementation) must have been identical in both groups. We excluded studies evaluating the effects of food fortification, consumption of foods rich in vitamin A, and beta‐carotene supplementation.
If a trial included more than one eligible intervention group (e.g. different doses), we combined the groups for the main analysis, although we treated the groups separately for subgroup analyses where appropriate. If a trial included multiple control groups (e.g. both placebo and treatment as usual), we selected the control group that most closely replicated the non‐specific treatment of the intervention group (i.e. placebo).
Types of outcome measures
We extracted data on the outcomes listed below. In studies reporting more than one measure of an outcome, we combined measures for meta‐analysis using the methods described in Data synthesis.
Primary outcomes
All‐cause mortality.
Secondary outcomes
-
fCause‐specific mortality due to:
diarrhoea;
measles;
meningitis; and
lower respiratory tract infection (LRTI).
-
Cause‐specific morbidity (i.e. incidence and prevalence) due to:
diarrhoea;
measles;
malaria;
meningitis;
LRTI;
Bitot's spots;
night blindness;
xerophthalmia; and
hospitalisation.
Side effects (e.g. vomiting or diarrhoea following supplementation).
Vitamin A deficiency (VAD) status (based on serum retinol level).
We combined pneumonia and LRTI outcomes post hoc. Pneumonia is a type of LRTI, and most of the studies did not test for pneumonia specifically (using specific clinical criteria). In the event a study reported both pneumonia and LRTI outcomes, we extracted the LRTI outcome data to combine with other studies.
Search methods for identification of studies
Electronic searches
For this update, we searched the databases and trials registers listed below in March 2021. Searches were limited to the period 2016 onwards, in order to identify new studies published since the previous version of the review Appendix 1. Details of the previous search strategies are available in Imdad 2010a and Imdad 2017.
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 3, 2021) in the Cochrane Library (searched 5 March 2021).
MEDLINE Ovid (2016 to 8 March 2021).
Embase Elsevier (2016 to 8 March 2021).
Science Citation Index Web of Science (2016 to 8 March 2021).
Conference Proceedings Citation Index – Science Web of Science (2016 to 8 March 2021).
Cochrane Database of Systematic Reviews (CDSR; Issue 3, 2021) in the Cochrane Library (searched 5 March 2021).
ClinicalTrials.gov (clinicaltrials.gov; searched 8 March 2021).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch; searched 8 March 2021).
Global Index Medicus (contains WPRIM, LILACS, IMSEAR, IMEMR, AIM; www.globalindexmedicus.net/; searched 8 March 2021).
Scopus Elsevier (1966 to 8 March 2021).
We applied no language limits to the searches.
Searching other resources
We checked the reference lists of reviews and included and excluded studies to identify additional citations. We also contacted organisations and researchers.
Data collection and analysis
Selection of studies
For this update, two review authors (from MH, AR, and JS) independently screened titles and abstracts through Covidence (Covidence) for inclusion in the review. They discarded clearly irrelevant records and obtained the full‐text reports of those deemed potentially eligible or where more information was needed to determine eligibility. Two review authors (from MH, AR, and JS) then independently screened all full‐text reports. At both stages, differences of opinions about suitability for inclusion were resolved by discussion and through consultation with a senior review author (AI).
For the previous version of the review (Imdad 2017), two people (AI (member of the review author team) and JD or RS (recruited to assist with data extraction)) independently screened titles and abstracts and full‐text reports for inclusion in the review. We resolved differences of opinion about suitability for inclusion by discussion and through consultation with a third review author (EMW).
For both reviews, we recorded our final decisions in a PRISMA diagram (Moher 2009).
Data extraction and management
In the previous version of the review (Imdad 2017), two people (AI (member of the review author team) and JD or RS (recruited to assist with data extraction)) used a prepiloted data extraction sheet to independently extract the data below from each eligible study. Review authors resolved discrepancies through discussion.
-
General:
year of study;
location (country, urban/rural);
method of recruitment;
inclusion criteria;
unit of analysis; and
risk of bias (see Assessment of risk of bias in included studies).
-
Participants:
sociodemographic characteristics (age, sex); and
comorbidities.
-
For each intervention and comparison group of interest:
dosage;
duration;
frequency; and
co‐intervention (if any).
-
For each outcome of interest:
time points collected and reported;
definition;
validity;
unit of measurement (if relevant); and
loss to follow‐up.
The main analyses included the longest reported follow‐up in each study. We grouped outcomes according to follow‐up period (0 to 12 months; 13 to 60 months, and greater than 60 months since randomisation); when trials reported multiple time points for a period, we extracted the longest outcome interval in a given period.
Assessment of risk of bias in included studies
In previous versions of the review (Imdad 2017), two people (AI (member of the review author team) and JD or RS (recruited to assist with data extraction)) independently assessed the risk of bias within each included study using the Cochrane risk of bias tool (Higgins 2011a). Discrepancies in assessment were resolved by discussion. For all studies, we assessed the following domains: sequence generation; allocation concealment; blinding of participants and providers; blinding of outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. We specifically looked for the possibility of performance bias (differential treatment of the intervention and control groups) and detection bias (e.g. differential effort to locate death records for the intervention and control groups). Findings are discussed in the Risk of bias in included studies section and included in the risk of bias tables of the Characteristics of included studies table. We considered an overall risk of bias assessment for GRADE analysis based on the nature of the outcome and how the risk of bias might change the direction of effect.
Measures of treatment effect
We measured morbidity in different ways, and we combined all available data whenever possible. For example, for diarrhoea, we included all types of diarrhoea (mild, moderate, and severe). In the case of pneumonia, we included lower (but not upper) respiratory tract infection.
To avoid review author bias, we predetermined the order of preference for extracting outcomes when data were available in several formats. For studies that randomised individuals, we gave preference to data that required the least manipulation by authors or inference by review authors. We extracted raw values (e.g. means and standard deviations) rather than calculated effect sizes (e.g. Cohen's d). For mortality data, we gave preference to denominators in the following order: number with definite outcome known (or imputed as described below), number randomised, and child‐years. For other dichotomous outcomes to which both survivors and non‐survivors may have contributed data (e.g. incidence of measles), we gave preference to child‐years, number with definite outcome known, and number randomised.
In the case of cluster‐RCTs, we used either adjusted estimates reported by the trial authors or raw data, and we inflated the standard error (SE) using the procedures described in the Unit of analysis issues section.
Unit of analysis issues
Cluster‐randomised trials
In studies randomising units rather than the individual (i.e. clusters), trials should present results with controls for clustering (e.g. robust SEs or hierarchical linear models). We analysed clustered data using the procedures outlined in Higgins 2011b.
Where results did not control for clustering, we contacted trial authors to request an estimate of the intracluster correlation coefficient (ICC). If the trial authors were unable to provide an ICC, we calculated the ICC using design effects calculated previously (Beaton 1993), and we estimated the ICC for studies that did not publish a value (see section on 'Unit of randomisation' under Included studies). For estimated values, we conducted sensitivity analyses using larger and smaller design effects to determine if the results were robust (see Sensitivity analysis).
Multiple‐arm trials
For multiple‐arm trials, we grouped data so that the only difference between the groups was VAS. For example, if a trial had four arms (vitamin A plus zinc, zinc alone, vitamin A alone, and placebo), we included it as two comparisons: vitamin A plus zinc versus zinc alone and vitamin A alone versus placebo. In multiple‐arm trials using two different doses of vitamin A, we combined the two groups to avoid double‐counting the participants in the control group.
Cross‐over trials
For cross‐over trials, we included the data from the first phase of the trial only.
Dealing with missing data
Differential dropout can lead to biased estimates of effect size, and bias may arise if reasons for dropout differ across groups.
We described missing data, including dropouts and reasons for dropout, when given. If data were missing for some cases, or if reasons for dropout were not reported, we contacted the trial authors. When analyses considered completers and controlled for dropout (e.g. imputed using regression methods), we extracted the latter.
Assessment of heterogeneity
We assessed included studies for clinical heterogeneity by comparing the distribution of important factors such as study participants, study setting, dose, and duration of intervention and co‐interventions. We assessed methodological heterogeneity by comparing data included in the risk of bias tables (see Characteristics of included studies table). We assessed statistical heterogeneity by visual inspection of forest plots, the Chi2 test (and P value), and the I2 statistic. If the P value was less than 0.10 and the I2 exceeded 50%, we considered heterogeneity to be substantial. We also reported Tau2 – an estimate of between‐study variance.
Assessment of reporting biases
To assess the possibility of small‐study bias, we drew funnel plots for outcomes with 10 or more studies and compared random‐effects estimates to fixed‐effect estimates (see Sensitivity analysis).
Data synthesis
We performed meta‐analysis using Review Manager 5 software (Review Manager 2014). When data were in several formats that we could not combine directly in Review Manager 5, we used the generic inverse variance (GIV) option. This was meant to handle the scenario when only summary estimates (such as the risk ratio (RR)) were available and no numbers for nominators and denominators were available to calculate the summary estimate. In this case, it would not be possible to pool that study with other studies using conventional methods. Hence, we used GIV, which does not require input of data in the form of nominators and denominators of intervention and control group, but the log of effect size (e.g. RR) and SE. We entered data into the built‐in calculator in Review Manager 5 to calculate the log of RR and their SE.
We reported all outcomes with 95% confidence intervals (CIs) and weighted overall effects by the inverse of variance using a fixed‐effect model. Although there might be some differences across trials (e.g. dose and population), the biological mechanism should be similar. We explored differences through analyses described elsewhere (Mayo‐Wilson 2011).
For dichotomous outcomes, we calculated the overall RR. For incidence data, we combined RRs (events per child) and rate ratios (events per child‐year) because these ratios use the same scale and can be interpreted in the same way for these studies (the duration of studies was relatively short, that is, median duration was one year or less).
In some cases, we estimated time at risk, as when trial authors reported incidence rate, duration of study, and number of children in each group.
We decided post hoc that we would pool incidence and prevalence data for morbidity separately. The primary difference between incidence and prevalence data is time at risk. Incidence data covers the time (prospectively) while prevalence data is a snapshot of a condition at one point in time. Therefore, we thought that combining incidence and prevalence data was not appropriate.
For continuous outcomes, we calculated Hedges g.
Subgroup analysis and investigation of heterogeneity
Effectiveness of the intervention may differ across members of populations (e.g. due to differences in baseline vitamin A status) and may be affected by other interventions (e.g. immunisation or deficiency of other micronutrients). For example, neonatal VAS is thought to have different effects in Asia compared with Africa (Klemm 2009). Unlike trial‐level factors (such as dose), associations between individual‐level moderators (such as VAS) and outcomes should be analysed using individual patient data from RCTs and observational studies. With two exceptions, we did not include subgroup analyses based on individual‐level moderators in this review, as such analyses are at high risk of ecological fallacy (lack of variation between studies would not indicate there was no variation within them). We included subgroups of age and sex; trials commonly report separate effects for these groups. We performed subgroup analyses when disaggregated data were available for groups within studies or between studies.
We prespecified the following subgroup analyses, and explored differences using the Chi2 test in Review Manager 5 (Review Manager 2014).
Dose: standard (up to 100,000 IU for children aged six to 11 months, and 200,000 IU for children aged 12 months to five years) versus high (greater than standard).
Frequency: high (doses more than once in six months) versus low (one dose every six months or six‐plus‐month interval).
Location: continent.
Age: six to 12 months versus one to five years.
Sex: boys versus girls.
Sensitivity analysis
We performed the following sensitivity analyses.
To test for bias, we repeated the primary analysis without studies at high risk of bias for sequence generation.
To test for small‐study bias, we repeated the analysis using a random‐effects model (as the assumption for this model is that effect is not identical across studies, and included studies are considered a 'random' sample of all the possible studies on the topic) and drew funnel plots for all outcomes with 10 or more studies.
To test the robustness of results when using imputed ICCs, we conducted sensitivity analyses using larger and smaller design effects (post hoc sensitivity analysis described under the 'Unit of randomisation' subheading in the Included studies section below).
Summary of findings and assessment of the certainty of the evidence
In the previous version of this review, in collaboration with the Cochrane Editorial Unit, two review authors (AI and EMW) assessed the overall certainty of evidence using the GRADE approach (Guyatt 2011). Any discrepancies in assessment were resolved by discussion. The GRADE assessment was based on five criteria: limitations in the design and implementation of available studies, imprecision of results, inconsistency of results, indirectness of study results, and publication bias.
We assessed the certainty of evidence as 'high', 'moderate', 'low', or 'very low' for each of the following outcomes: all‐cause mortality; mortality due to diarrhoea, measles, and LRTI; incidence of diarrhoea, measles, LRTI, Bitot's spots, and night blindness; vomiting; and VAD status. The main comparison was VAS versus placebo or usual care. We presented our certainty ratings and results in Table 1; our reasons for the certainty rating are available in footnotes of the table. We downgraded the certainty of evidence up to three levels. All the outcomes reported in summary of findings table were reported at longest follow‐up.
Results
Description of studies
Results of the search
For this update, electronic searches identified 3859 records; 3545 records remained after removal of duplicates. From these, we identified 39 potentially relevant citations and reviewed the full texts. We excluded all 39 reports; no new eligible studies were found in this update (Figure 1). For more information, see Excluded studies. One study is awaiting classification (Aklamati 2006); no additional details were available since the publication of the last review to facilitate a decision on the eligibility of this study for inclusion in the review. We found no new eligible studies for this update, so there are no changes to number of included studies (47 included studies (from 106 reports). The details of the searches for previous versions of this review are available elsewhere (Imdad 2010a; Imdad 2017).
1.
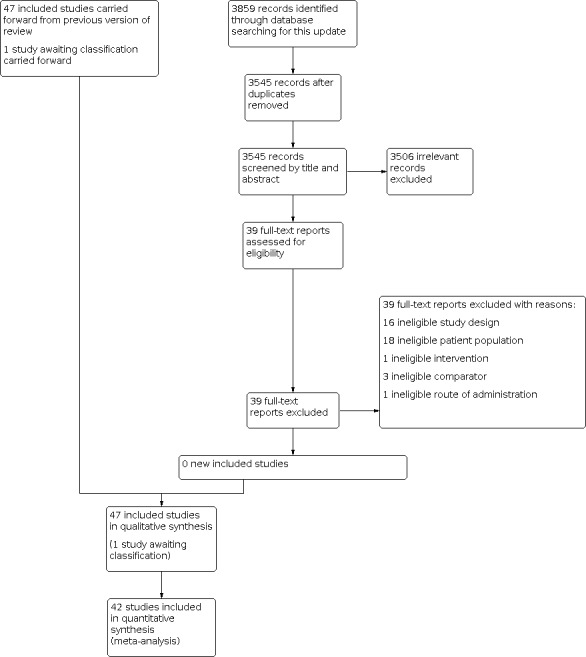
Study flow diagram.
Included studies
Study design
Three of the 47 included studies in this review were factorial design studies. Factorial design studies typically test more than one intervention in different combinations in a single study. For meta‐analysis, we included each such study as two discrete data sets (with intervention and comparison group differentiated by VAS only) and counted them as one study overall (Reddy 1986a and Reddy 1986b; Long 2006a and Long 2006b; Chen 2013a and Chen 2013b). Further details are available below under the subheading 'Multiple‐arms trials'. More than one report was available for 19 (40%) trials. Where multiple reports existed for an included trial, we extracted data from all reports in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Further information about individual studies is available in the Characteristics of included studies table.
Forty‐two trials (89%) reported data that could be included in a meta‐analysis; five trials reported outcomes that were not relevant to the review (Albert 2003; Cherian 2003), data that were not available by group (Lima 2014), or data that were incomplete (van Agtmaal 1988; Smith 1999).
We excluded quasi‐RCTs with the exception of Herrera 1992 and Stansfield 1993; we made this decision post hoc (Differences between protocol and review). Given the design of the interventions and the placebos as well as steps to blind those administering the sequence, we do not think these studies are meaningfully different from RCTs. Herrera 1992 assigned participants alternately by household, while Stansfield 1993 used a random starting point and alternating distribution of red or green tablets. Lack of a truly random sequence was not related to other sources of bias (e.g. performance bias) because individuals delivering the capsules had no ongoing contact with participants, and the manufacturer (Roche) held the code until the study was completed. Though post hoc, we made the decision to include these studies before extracting data or conducting analyses; we conducted a sensitivity analysis to determine if the decision had any impact on the results, which it did not (see 'Sensitivity analysis' subheading, under 'Primary outcome: all‐cause mortality' in Effects of interventions section).
Unit of randomisation
Cluster‐randomised trials
Two studies randomised participants by household, and we treated participants as if they were individually randomised (Herrera 1992; Stansfield 1993). We conducted a sensitivity analysis for all‐cause mortality using ICCs of 0 and 0.010 for studies estimating the mean design effect.
We used previously reported design effects from Beaton 1993 to calculate ICCs for clustered studies (Sommer 1986; Rahmathullah 1990; Vijayaraghavan 1990; West 1991; Daulaire 1992; Ross 1993 SURVIVAL). The ICCs were consistently around 0.002. We imputed an ICC value of 0.002 for the single study that did not account for clustering in the original analysis (DEVTA trial 2013).
Multiple‐arm trials
Fifteen (31%) trials had multiple arms, nine of which were relevant to this review (Reddy 1986a; Florentino 1990; Benn 1997; Smith 1999; Rahman 2001; Long 2006a; Lin 2009; Chen 2013a; DEVTA trial 2013).
Seven trials used factorial designs, combining vitamin A with other treatments such as zinc (Smith 1999; Rahman 2001; Albert 2003; Long 2006a), deworming (Reddy 1986a; DEVTA trial 2013), or iron (Chen 2013a); we extracted data for comparisons that differed only in the provision of vitamin A (e.g. vitamin A versus placebo; and vitamin A plus zinc versus zinc alone). One trial provided no raw data, and we could not identify outcome data for an eligible comparison (Rahman 2001). One study combined different doses (Florentino 1990).
Location/setting
Studies were set in 19 countries: 30 (63%) in Asia, including 16 in India; eight (17%) in Africa (Herrera 1992; Ross 1993 HEALTH; Ross 1993 SURVIVAL; Stabell 1995; Benn 1997; Donnen 1998; Shankar 1999; Fisker 2014); seven (15%) in Latin America (Stansfield 1993; Barreto 1994; Sempértegui 1999; Smith 1999; Long 2006a; Long 2007; Lima 2014), and two (4%) in Australia (Pinnock 1986; Pinnock 1988). Eighteen (38%) studies were conducted in urban/periurban settings, and 26 (55%) were in rural settings. Three studies did not explicitly describe their urban or rural setting.
Time
Eleven studies continued for five years or more (Vijayaraghavan 1990; West 1991; Herrera 1992; Dibley 1996; Pant 1996; Shankar 1999; Chowdhury 2002; Long 2006a; Long 2007; DEVTA trial 2013; Ross 1993 SURVIVAL); the remainder of the studies had a duration of about one year or less. In the event that a single study reported data at more than one time point, we used the data from the longest interval in the overall analysis.
Sample size
Trials assigned approximately 1,223,856 participants, with sample sizes ranging between 35 participants in van Agtmaal 1988 to approximately 1 million participants in DEVTA trial 2013. The 42 trials that could be analysed included 1,223,607 participants (99.9% of children included in the review).
The 11 largest studies randomised about 1,200,214 children, 98.06% of participants in the review (Sommer 1986; Rahmathullah 1990; Vijayaraghavan 1990; West 1991; Daulaire 1992; Herrera 1992; Ross 1993 SURVIVAL; Stansfield 1993; Agarwal 1995; Pant 1996; DEVTA trial 2013).
Allocation ratio
Thirty‐nine (83%) studies evenly allocated participants to the intervention and control groups. In eight (17%) studies, the number assigned to each group was unclear (Reddy 1986a; Ross 1993 HEALTH; Ross 1993 SURVIVAL; Stansfield 1993; Biswas 1994; Dibley 1996; Ramakrishnan 1995; Pant 1996).
Participants
Twenty studies categorically excluded children with clinical signs of VAD (such as xerophthalmia and Bitot's spots), while 23 studies did not clearly mention vitamin A. Four studies allowed children who had clinical signs of VAD (Rahmathullah 1990; West 1991; Daulaire 1992; DEVTA trial 2013). Only one trial mentioned biochemical VAD as an inclusion criterion (Albert 2003).
Age
Twenty‐one (44%) studies reported mean age, which was 33 months across the studies.
Sex
Thirty‐five (74%) studies reported sex. Most assigned approximately equal numbers of boys and girls. Three studies favoured boys by more than 10% (Semba 1991; Cherian 2001; Lin 2008). The median percentage of boys in the studies was 51%.
Comparisons
Seven (14%) studies compared VAS to treatment as usual (Sommer 1986; van Agtmaal 1988; West 1991; Daulaire 1992; Pant 1996; Donnen 1998; DEVTA trial 2013). Forty (85%) studies compared VAS to placebo. One large trial reported not using a placebo because it was forbidden by government (Sommer 1986).
Dose
All studies used large doses of vitamin A in the range of 50,000 IU to 200,000 IU (one IU = 0.3 μg), depending on the age of participants, except for five studies that used small doses, that is, 3866 IU three times a week (Pinnock 1988), 8333 IU once a week (Rahmathullah 1990), 10,000 IU weekly (Sempértegui 1999; Smith 1999), or 25,000 IU every two weeks (Chen 2013a and Chen 2013b; considered as one study). Some studies had two different dosing regimens for younger children (50,000 IU or 100,000 IU for ages six to 11 months) and older children (100,000 IU or 200,000 IU for ages one year or older).
Frequency
Participants received the large doses (50,000 IU to 200,000 IU) every four to six months, either once or more, depending on the study duration. Studies that used smaller doses gave them more frequently (see under 'Dose' above).
Route
Retinol palmitate was the most commonly used compound to deliver vitamin A, and all studies used the oral route for supplementation.
Excluded studies
We excluded 57 studies from this review. We excluded 18 studies from previous versions of this review (Imdad 2010a; Imdad 2017), and 39 from this review. We list all excluded studies in the Characteristics of excluded studies table with reasons for exclusion. Of the 39 studies excluded from this version of the review, 16 were because of ineligible study design, 18 due to ineligible patient population, one due to ineligible intervention, three due to ineligible comparator, and one due to ineligible route of administration.
Studies awaiting assessment
We could not assess one trial reported in a conference abstract (Aklamati 2006). It appeared to meet the inclusion criteria but reported unclear results. For example, the study included 36 children and reported an outcome of 1.2% of 17; though one child out of 17 is nearly 6%. To the best of our knowledge, the complete results have not been published as yet. See Characteristics of studies awaiting classification table for more information.
Risk of bias in included studies
For each study, we assessed seven domains of methodological bias listed in the Assessment of risk of bias in included studies section and rated them at high, low, or unclear risk. See Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
All included studies were RCTs or quasi‐RCTs. Twenty (42%) studies specified the method of randomisation and were at low risk of bias for sequence generation. Twenty‐four studies were at unclear risk. Three (6%) studies, including 42,660 participants (3% of those included in the review), were at high risk of bias in this domain (Herrera 1992; Stansfield 1993; Arya 2000). One of these studies described assignment as random (Arya 2000), but participants may have been assigned in order of arrival at hospital, which would not qualify as truly random.
Allocation concealment
We rated 21 studies at low risk of bias and 25 studies (53%) at unclear risk of bias. We judged one study to be at high risk of bias for allocation concealment (Daulaire 1992), as authors reported in correspondence that they had made no effort to conceal the allocation.
Blinding
Blinding of participants
Thirty‐two (68%) studies described efforts to blind participants, and we considered them at low risk of bias for blinding of participants. We deemed 12 (25%) studies at unclear risk of bias (Reddy 1986a; Sommer 1986; van Agtmaal 1988; Agarwal 1995; Kartasasmita 1995; Stabell 1995; Pant 1996; Donnen 1998; Smith 1999; Cherian 2001; Chowdhury 2002; Cherian 2003). We judged three studies at high risk of bias (Daulaire 1992; Lin 2009; DEVTA trial 2013).
Blinding of providers
In some trials, staff delivering the intervention also conducted assessments. We considered 31 (65%) studies at low risk of bias for blinding of providers. The risk was unclear in 13 (27%) studies (Reddy 1986a; Sommer 1986; van Agtmaal 1988; Agarwal 1995; Kartasasmita 1995; Stabell 1995; Pant 1996; Donnen 1998; Smith 1999; Cherian 2001; Chowdhury 2002; Cherian 2003; Lin 2008). We considered three studies at high risk of bias (Daulaire 1992; Lin 2009; DEVTA trial 2013).
Blinding of outcome assessors
Thirty (63%) studies had low risk of bias. The risk was unclear in 14 (30%) studies (Reddy 1986a; Sommer 1986; van Agtmaal 1988; Semba 1991; Agarwal 1995; Kartasasmita 1995; Stabell 1995; Pant 1996; Donnen 1998; Smith 1999; Cherian 2001; Chowdhury 2002; Cherian 2003; Lin 2008). We assessed three (6%) studies at high risk of bias for blinding of outcome assessors (Daulaire 1992; Lin 2009; DEVTA trial 2013).
Incomplete outcome data
For incomplete outcome data, we judged 26 (55%) studies at low risk of bias. The risk was unclear in 12 (25%) studies (Sinha 1976; Reddy 1986a; Sommer 1986; Vijayaraghavan 1990; West 1991; Ross 1993 HEALTH; Ross 1993 SURVIVAL; Agarwal 1995; Stabell 1995; Venkatarao 1996; Smith 1999; Cherian 2001). We rated nine (19%) studies at high risk of bias (van Agtmaal 1988; Lie 1993; Kartasasmita 1995; Semba 1995; Pant 1996; Bahl 1999; Arya 2000; Chowdhury 2002; Cherian 2003). The primary reason for a high‐risk rating was a lack of explanation for attrition in intervention and control group.
Selective reporting
Most of the trials in the review included multiple outcome measures, and positive results are more likely to be included in reports than negative results. Only seven (14%) studies appeared to be free of selective outcome reporting (Florentino 1990; Rahmathullah 1990; West 1991; Dibley 1996; Benn 1997; DEVTA trial 2013; Fisker 2014). We judged 26 (55%) studies at unclear risk of bias and 14 (29%) studies at high risk of bias (Pinnock 1988; van Agtmaal 1988; Vijayaraghavan 1990; Ross 1993 HEALTH; Ross 1993 SURVIVAL; Stansfield 1993; Ramakrishnan 1995; Pant 1996; Bahl 1999; Arya 2000; Cherian 2003; Lin 2008; Lin 2009; Lima 2014).
Most of the studies did not cite a published protocol, which is why we assessed a large proportion of studies at unclear risk of bias.
Other potential sources of bias
We extracted other potential sources of bias and noted them in the Characteristics of included studies table, but none were likely to influence the results of the review in a meaningful way.
Effects of interventions
See: Table 1
We present the results for each outcome below, summarising the main outcomes in Table 1.
We did not conduct all of our planned subgroup analyses. For the primary outcome, only one study used a non‐standard dose and frequency. Other analyses with more than 10 studies contained significantly fewer participants (e.g. the analysis of serum level included fewer than 7000 children). Consequently, we did not conduct subgroup analyses for dose and frequency because the analyses were clearly underpowered, and any effects would be attributable to chance. Results of the attempted subgroup analyses are listed in Table 2. We performed sensitivity analyses for all‐cause mortality and incidence due to diarrhoea and vitamin A serum levels only, as most analyses contained a small number of studies.
1. Subgroup and sensitivity analyses.
| Outcome or subgroup | Studies | Heterogeneity | Statistical method | Effect estimate |
Test for subgroup differences (P value) |
| All‐cause mortality | |||||
| All‐cause mortality, outcomes < 1 year since randomisation | 13 | Chi2 = 34.29, df = 12; P < 0.001; I2 = 65% | Risk ratio (GIV, fixed, 95% CI) | 0.83 (0.75 to 0.92) | NA |
| All‐cause mortality, outcomes 13–59 months since randomisation | 6 | Chi2 = 15.75, df = 5; P < 0.001; I2 = 68% | Risk ratio (GIV, fixed, 95% CI) | 0.88 (0.81 to 0.97) | NA |
| All‐cause mortality at longest follow‐up (subgroup analysis): Asia | 12 | Chi2 = 42.65, df = 10; P < 0.001; I2 = 77% | Risk ratio (GIV, fixed, 95% CI) | 0.90 (0.84 to 0.96) | 0.83 |
| All‐cause mortality at longest follow‐up (subgroup analysis): Africa | 6 | Chi2 = 10.06, df = 5; P = 0.07; I2 = 50% | Risk ratio (GIV, fixed, 95% CI) | 0.86 (0.75 to 0.98) | |
| All‐cause mortality at longest follow‐up (subgroup analysis): Latin America | 1 | NA | Risk ratio (GIV, fixed, 95% CI) | 1.00 (0.14 to 7.08) | |
| All‐cause mortality at longest follow‐up, by national child mortality rate (subgroup analysis): high (> 40/1000) | 17 | Chi2 = 53.07, df = 16 (P < 0.001; I2 = 70% | Risk ratio (GIV, fixed, 95% CI) | 0.89 (0.84 to 0.94) | 0.9 |
| All‐cause mortality at longest follow‐up, by national child mortality rate (subgroup analysis): low (< 40/1000) | 2 | NA | Risk ratio (GIV, fixed, 95% CI) | 1.00 (0.14 to 7.08) | |
| All‐cause mortality at longest follow‐up (sensitivity analysis): random‐effects model | 19 | Tau2 = 0.04; Chi2 = 44.00, df = 17; P = 0.001; I2 = 61% | Risk ratio (GIV, fixed, 95% CI) | 0.76 (0.66 to 0.88) | NA |
| All‐cause mortality at longest follow‐up (sensitivity analysis): without DEVTA trial | 18 | Chi2 = 30.38, df = 16; P = 0.02; I2 = 47% | Risk ratio (GIV, fixed, 95% CI) | 0.77 (0.70 to 0.84) | NA |
| All‐cause mortality at longest follow‐up (sensitivity analysis): ICC = 0.002 (assumes no impact of clustering for studies with unknown ICC) | 19 | Chi2 = 57.02, df = 16; P < 0.001; I2 = 72% | Risk ratio (GIV, fixed, 95% CI) | 0.89 (0.84, 0.94) | NA |
| All‐cause mortality at longest follow‐up (sensitivity analysis): ICC = 0.010 (assumes high impact of clustering for studies with unknown ICC) | 19 | Chi2 = 47.87, df = 16; P < 0.001; I2 = 67% |
Risk ratio (GIV, fixed, 95% CI) | 0.89 (0.84 to 0.94) | NA |
| Cause‐specific mortality | |||||
| Mortality due to diarrhoea, outcomes < 1 year since randomisation | 6 | Chi2 = 5.23, df = 5; P = 0.39; I2 = 4% | Risk ratio (GIV, fixed, 95% CI) | 0.76 (0.61 to 0.95) | NA |
| Mortality due to measles, outcomes < 1 year since randomisation | 4 | Chi2 = 0.52, df = 3; P = 0.91; I2 = 0% | Risk ratio (GIV, fixed, 95% CI) | 0.85 (0.52 to 1.37) | NA |
| Mortality due to meningitis, outcomes < 1 year since randomisation | 1 | NA | Risk ratio (GIV, fixed, 95% CI) | 5.79 (0.22 to 153.24) | NA |
| Mortality due to LRTI, outcomes < 1 year since randomisation | 6 | Chi2 = 5.66, df = 5; P = 0.34; I2 = 12% | Risk ratio (GIV, fixed, 95% CI) | 0.66 (0.40 to 1.10) | NA |
| Cause‐specific morbidity | |||||
| Diarrhoea incidence at longest follow‐up (sensitivity analysis): analysis without Lie 1993 and Chowdhury 2002 | 13 | Heterogeneity: Chi2 = 30.71, df = 12; P = 0.002; I2 = 61% | Risk ratio (GIV, fixed, 95% CI) | 0.96 (0.93 to 1.00) | NA |
| Diarrhoea incidence, outcomes < 1 year since randomisation | 13 | Chi2 = 51.64, df = 11; P < 0.001; I2 = 79% | Risk ratio (GIV, fixed, 95% CI) | 0.93 (0.89 to 0.96) | NA |
| Diarrhoea incidence at longest follow‐up (sensitivity analysis): random‐effects model | 15 | Tau2 = 0.07; Chi2 = 219.04, df = 14; P < 0.001; I2 = 94% | Risk ratio (GIV, random, 95% CI) | 0.84 (0.73, 0.98) | NA |
| Measles incidence, outcomes < 1 year since randomisation | 5 | Chi2 = 0.24, df = 4; P = 0.99; I2 = 0% | Risk ratio (GIV, fixed, 95% CI) | 0.54 (0.36 to 0.80) | NA |
| Malaria incidence, outcomes 1 + years since randomisation (subgroup analysis): age | 1 | NA | Risk ratio (M‐H, fixed, 95% CI) | 0.73 (0.60 to 0.88) | NA |
| LRTI Incidence, outcomes < 1 year since randomisation | 11 | Chi2 = 5.23, df = 8; P = 0.73; I2 = 0% | Risk ratio (GIV, fixed, 95% CI) | 0.96 (0.89 to 1.04) | NA |
| Bitot's spots incidence, outcomes < 1 year since randomisation | 1 | NA | Risk ratio (GIV, fixed, 95% CI) | 0.93 (0.76 to 1.14) | NA |
| Bitot's spots prevalence, outcomes < 1 year since randomisation | 3 | Chi2 = 6.06, df = 2; P = 0.05; I2 = 67% | Risk ratio (GIV, fixed, 95% CI) | 0.43 (0.33 to 0.56) | NA |
| Night blindness prevalence, outcomes < 1 year since randomisation | 1 | NA | Risk ratio (GIV, fixed, 95% CI) | 0.30 (0.17 to 0.52) | NA |
| Xerophthalmia incidence, outcomes < 1 year since randomisation | 2 | NA | Risk ratio (GIV, fixed, 95% CI) | 0.88 (0.72 to 1.07) | NA |
| Vitamin A deficiency status | |||||
| Vitamin A serum retinol level, outcomes < 1 year since randomisation | 11 | Chi2 = 178.42, df = 10; P < 0.001; I2 = 94% | Standardised mean difference (GIV, fixed, 95% CI) | 0.45 (0.37 to 0.53) | NA |
| Vitamin A serum retinol level at longest follow‐up (sensitivity analysis): random‐effects model | 14 | Tau2 = 0.13; Chi2 = 278.45, df = 14; P < 0.001; I2 = 95% | Standardised mean difference (GIV, random, 95% CI) | 0.50 (0.30 to 0.70) | NA |
CI: confidence interval; df: degrees of freedom; GIV: generic inverse variance; LRTI: lower respiratory tract infection; M‐H: mantel Haenszel method; NA: not applicable.
Primary outcome
All‐cause mortality
Nineteen trials involved 1,202,382 children (98.25% of the children included in the review) in an overall analysis (using data from the last follow‐up for trials measuring outcomes multiple times) (Sommer 1986; Rahmathullah 1990; Vijayaraghavan 1990; West 1991; Daulaire 1992; Herrera 1992; Ross 1993 HEALTH; Ross 1993 SURVIVAL; Barreto 1994; Agarwal 1995; Dibley 1996; Pant 1996; Venkatarao 1996; Benn 1997; Donnen 1998; Chowdhury 2002; Lin 2008; DEVTA trial 2013; Fisker 2014). One trial reported no events (Lin 2008).
Vitamin A was associated with a 12% reduction in all‐cause mortality (RR 0.88, 95% CI 0.83 to 0.93; Analysis 1.1; Figure 3), though there was moderate heterogeneity (Chi2 = 44.00, degrees of freedom (df) = 17; P < 0.001; I2 = 61%). We judged the certainty of evidence to be high (see Table 1).
1.1. Analysis.

Comparison 1: Vitamin A versus control, Outcome 1: All‐cause mortality at longest follow‐up
3.

Forest plot of comparison: 1 Vitamin A versus control, outcome: 1.1 All‐cause mortality at longest follow‐up.
The effect during the first year postrandomisation was similar based on data available from 13 studies (RR 0.83, 95% CI 0.75 to 0.92), and the statistical heterogeneity was similar (Chi2 = 34.29, df = 12; P < 0.001; I2 = 65%). Only six (12%) studies measured mortality between 13 and 59 months, and the effect was similar (RR 0.88, 95% CI 0.81 to 0.97, 6 studies), with moderate and significant statistical heterogeneity (Chi2 = 15.75, df = 5; P = 0.008; I2 = 68%). See Table 2.
Subgroup analyses
Dose and frequency
Only one study reporting all‐cause mortality did not use the standard dose and frequency recommended by WHO: Rahmathullah 1990 used a weekly dose for 52 weeks. We did not conduct the planned subgroup analyses.
Location
Twelve studies were set in Asia (RR 0.90, 95% CI 0.84 to 0.96), six in Africa (RR 0.86, 95% CI 0.75 to 0.98), and one in Latin America (RR 1.00, 95% CI 0.14 to 7.08). These results were not different based on location (P = 0.83). See Table 2.
Age
Five studies reported separate effects for children aged six to 12 months (RR 0.59, 95% CI 0.43 to 0.82; Analysis 1.2.1) and children aged one to five years (RR 0.68, 95% CI 0.57 to 0.81; Analysis 1.2.2) (Sommer 1986; Rahmathullah 1990; West 1991; Daulaire 1992; Benn 1997). The subgroups did not differ from each other (P = 0.46). Notably, both effect estimates were larger than the overall result from 19 trials reporting mortality.
1.2. Analysis.

Comparison 1: Vitamin A versus control, Outcome 2: All‐cause mortality at longest follow‐up (subgroup analysis): age
Sex
Seven studies reported separate effects for boys (RR 0.96, 95% CI 0.89 to 1.04; Analysis 1.3.1) and girls (RR 0.90, 95% CI 0.84 to 0.97; Analysis 1.3.2). The effects were not different based on sex (P = 0.22) (Sommer 1986; West 1991; Daulaire 1992; Herrera 1992; Lin 2008; DEVTA trial 2013; Fisker 2014).
1.3. Analysis.

Comparison 1: Vitamin A versus control, Outcome 3: All‐cause mortality at longest follow‐up (subgroup analysis): sex
Child mortality
Seventeen studies from countries with high child mortality showed a similar effect as the overall estimate (RR 0.89, 95% CI 0.84 to 0.94), and two studies from countries with low child mortality showed no combined effect for VAS (RR 1.00, 95% CI 0.14 to 7.08). See Table 2.
Sensitivity analyses
Bias
Of the studies at high risk of bias due to sequence generation, only Herrera 1992 contributed to the main mortality analysis and reported no effect (RR 1.06, 95% CI 0.82 to 1.37), indicating that this study was not likely to influence the results in a positive direction.
To test for small‐study bias, we repeated the analysis using a random‐effects model. The overall estimate was larger than the fixed‐effect estimate (RR 0.76, 95% CI 0.66 to 0.88, 19 studies; heterogeneity: Tau2 = 0.04; Chi2 = 44.00, df = 17; P < 0.001; I2 = 61%); however, CIs overlapped with estimates from the fixed‐effect model. The apparent increase in effect size suggests that heterogeneity might be explained by relatively small studies compared to larger studies, as exclusion of the DEVTA trial 2013 reduced the heterogeneity (from Chi2 = 44.00, df = 17; P < 0.001; I2 = 61% to Chi2 = 30.38, df = 16; P = 0.02; I2 = 47%). See Table 2.
Design effects in cluster trials
Known ICCs were consistent. For three studies for which the ICC was not known, we estimated the ICC as 0.002 and adjusted SEs using this value and the mean cluster size. To determine if this decision had any impact on the results, we repeated the primary analysis using a much larger and much smaller ICC estimate. The size of the effect was slightly smaller when these trials were treated as if they had randomised individuals (RR 0.89, 95% CI 0.84 to 0.94, 19 studies). The effect was virtually unchanged when we increased the ICC to 0.01 (RR 0.89, 95% CI 0.84 to 0.94, 19 studies). See Table 2. These results indicate that over‐weighting these three studies in the analysis would not impact the conclusions of this review; further inflating their SEs would increase the size of the effect estimate.
Secondary outcomes
Cause‐specific mortality
Diarrhoea
Nine studies reported a combined 12% reduction in mortality due to diarrhoea (RR 0.88, 95% CI 0.79 to 0.98; 1,098,538 children; Analysis 1.4; Rahmathullah 1990; Daulaire 1992; Herrera 1992; Ross 1993 SURVIVAL; Agarwal 1995; Venkatarao 1996; Chowdhury 2002; DEVTA trial 2013; Fisker 2014), with no important heterogeneity (Chi2 = 10.15, df = 8; P = 0.25; I2 = 21%). We judged the certainty of this evidence to be high (Table 1). Results for diarrhoea mortality reported within one year of randomisation showed similar results (0.76, 95% CI 0.61 to 0.95; 6 studies; Table 2).
1.4. Analysis.

Comparison 1: Vitamin A versus control, Outcome 4: Mortality due to diarrhoea at longest follow‐up
Measles
Six studies reported a lower risk of mortality due to measles (RR 0.88, 95% CI 0.69 to 1.11; 1,088,261 children; low‐certainty evidence; Analysis 1.5; Rahmathullah 1990; Daulaire 1992; Herrera 1992; Ross 1993 SURVIVAL; Agarwal 1995; DEVTA trial 2013). There was no important heterogeneity (Chi2 = 0.66, df = 5; P = 0.99; I2 = 0%). We judged the certainty of this evidence as low (Table 1). One‐year postrandomisation results were similar (RR 0.85, 95% CI 0.52 to 1.37; 4 studies; Table 2).
1.5. Analysis.

Comparison 1: Vitamin A versus control, Outcome 5: Mortality due to measles at longest follow‐up
Meningitis
Three studies reported a lower risk of mortality due to meningitis, but the CI around the summary estimate was imprecise and included a null effect (RR 0.57, 95% CI 0.17 to 1.88; Analysis 1.6; Ross 1993 SURVIVAL; Agarwal 1995; Chowdhury 2002). There was no important heterogeneity (Chi2 = 0.75, df = 2; P = 0.69; I2 = 0%). Only one study reported data within one‐year postrandomisation, with results that were imprecise and the CI included a null effect (RR 5.79, 95% CI 0.22 to 153.24; Table 2).
1.6. Analysis.

Comparison 1: Vitamin A versus control, Outcome 6: Mortality due to meningitis at longest follow‐up
Lower respiratory tract infection
Nine studies found no evidence of a difference between the intervention and placebo group (RR 0.98, 95% CI 0.86 to 1.12; 1,098,538 children; Analysis 1.7; Rahmathullah 1990; Daulaire 1992; Herrera 1992; Ross 1993 SURVIVAL; Agarwal 1995; Venkatarao 1996; Chowdhury 2002; DEVTA trial 2013; Fisker 2014). There was no important heterogeneity (Chi2 = 9.70, df = 8; P = 0.29; I2 = 18%). We judged the certainty of evidence as low (Table 1). A combined result for one‐year postrandomisation showed imprecise results and the CI included a null effect (RR 0.66, 95% CI 0.40 to 1.10; 6 studies; Table 2).
1.7. Analysis.

Comparison 1: Vitamin A versus control, Outcome 7: Mortality due to lower respiratory tract infection (LRTI) at longest follow‐up
Cause‐specific morbidity
Diarrhoea
Meta‐analyses
Fifteen studies reported a 15% decrease in diarrhoea incidence (RR 0.85, 95% CI 0.82 to 0.87; 77,946 children; Analysis 1.8; Figure 4; Florentino 1990; Herrera 1992; Lie 1993; Barreto 1994; Biswas 1994; Ramakrishnan 1995; Dibley 1996; Venkatarao 1996; Sempértegui 1999; Shankar 1999; Arya 2000; Chowdhury 2002; Long 2007; Chen 2013a and Chen 2013b (counted as one study); Fisker 2014), though statistical heterogeneity was substantial (Chi2 = 219.04, df = 14; P < 0.001; I2 = 94%). We judged this evidence to be of low certainty (Table 1).
1.8. Analysis.

Comparison 1: Vitamin A versus control, Outcome 8: Diarrhoea incidence at longest follow‐up
4.

Forest plot of comparison: 1 Vitamin A versus control, outcome: 1.8 Diarrhoea incidence at longest follow‐up.
Two studies were responsible for most of the heterogeneity and accounted for most of the overall effect (Lie 1993; Chowdhury 2002). Exclusion of these studies reduced the I2 statistic from 94% to 61%, and the overall effect almost disappeared (RR 0.96, 95% CI 0.93 to 1.00; see Table 2). The observed heterogeneity may be due to measurement error or differences in the effects of VAS across populations and settings. For example, VAS may reduce susceptibility to particular infections that are prevalent in some places but not others.
Thirteen studies that reported data for within one‐year postrandomisation showed a small effect (RR 0.93, 95% CI 0.89 to 0.96; Table 2).
Three studies reported no protective effect on diarrhoea prevalence (RR 1.06, 95% CI 1.03 to 1.10; Analysis 1.9; Stansfield 1993; Long 2006a and Long 2006b (counted as one study); DEVTA trial 2013), though statistical heterogeneity was substantial (Chi2 = 28.91, df = 3; P < 0.001; I2 = 90%).
1.9. Analysis.

Comparison 1: Vitamin A versus control, Outcome 9: Diarrhoea prevalence at longest follow‐up
Sensitivity analysis
To test for small‐study bias, we repeated the analysis using a random‐effects model. The overall estimate was identical to the fixed‐effect estimate, though the CI widened compared to the fixed‐effect model, suggesting that heterogeneity is not explained by small studies reporting larger effects (RR 0.84, 95% CI 0.73 to 0.98; 15 studies; Table 2). The funnel plot we produced was dominated by two studies accounting for 74% of the overall effect (Figure 5), and the plot was relatively flat.
5.

Funnel plot of comparison: 1 Vitamin A versus control, outcome: 1.1 All‐cause mortality at longest follow‐up.
With regard to the design effects in cluster trials, no ICCs were imputed, so a sensitivity analysis was not required.
Measles
Six studies reported a 50% decrease in measles incidence (RR 0.50, 95% CI 0.37 to 0.67; 19,566 children; Analysis 1.10; Figure 6; Herrera 1992; Barreto 1994; Semba 1995; Benn 1997; Bahl 1999; Chowdhury 2002), with no important heterogeneity (Chi2 = 0.55, df = 5; P = 0.99; I2 = 0%). We judged this evidence to be of moderate certainty (Table 1).
1.10. Analysis.

Comparison 1: Vitamin A versus control, Outcome 10: Measles incidence at longest follow‐up
6.

Forest plot of comparison: 1 Vitamin A versus control, outcome: 1.12 Measles incidence at longest follow‐up.
A combined effect from studies that reported measles incidence within one‐year postrandomisation showed similar results (RR 0.54, 95% CI 0.36 to 0.80; 5 studies; Table 2).
There were no studies that reported data on prevalence of measles.
Malaria
One study reported a 27% reduction in malaria incidence at follow‐up (RR 0.73, 95% CI 0.60 to 0.88; see the illustrative forest plot in Analysis 1.11 and Table 2; Shankar 1999).
1.11. Analysis.

Comparison 1: Vitamin A versus control, Outcome 11: Malaria incidence at longest follow‐up
Two studies reported data on malaria prevalence; the combined effect was imprecise and the CI around the summary estimate included a null effect (RR 0.73, 95% CI 0.41 to 1.28; Analysis 1.12; Ross 1993 HEALTH; Ross 1993 SURVIVAL), and there was no important heterogeneity (Chi2 = 0.02, df = 1; P = 0.88; I2 = 0%).
1.12. Analysis.

Comparison 1: Vitamin A versus control, Outcome 12: Malaria prevalence at longest follow‐up
Meningitis
There were no studies that reported incidence or prevalence data for meningitis.
Lower respiratory tract infection
Eleven studies reported no combined effect for VAS on LRTI incidence (RR 0.99, 95% CI 0.92 to 1.06; 27,540 children; Analysis 1.13; Rahmathullah 1990; Lie 1993; Barreto 1994; Biswas 1994; Kartasasmita 1995; Venkatarao 1996; Sempértegui 1999; Chowdhury 2002; Long 2007; Chen 2013a and Chen 2013b (considered as one study); Fisker 2014), with no important heterogeneity (Chi2 = 11.35, df = 9; P = 0.25; I2 = 21%). We judged the certainty of evidence to be low (Table 1).
1.13. Analysis.

Comparison 1: Vitamin A versus control, Outcome 13: LRTI incidence at longest follow‐up
Eleven studies that reported data on LRTI incidence within one‐year postrandomisation showed similar results (RR 0.96, 95% CI 0.89 to 1.04; Table 2).
Two trials with two relevant comparisons reported LRTI prevalence; the combined result suggests benefit for VAS (RR 0.60, 95% CI 0.45 to 0.81; Analysis 1.14; Long 2006a; DEVTA trial 2013).
1.14. Analysis.

Comparison 1: Vitamin A versus control, Outcome 14: LRTI prevalence at longest follow‐up
Bitot's spots
Herrera 1992 reported no effect on Bitot's spots incidence (RR 0.93, 95% CI 0.76 to 1.14; Table 2).
Five trials reported a 58% reduction in Bitot's spots prevalence (RR 0.42, 95% CI 0.33 to 0.53; 1,063,278 children; Analysis 1.15; Sinha 1976; Sommer 1986; West 1991; Pant 1996; DEVTA trial 2013), with substantial heterogeneity (Chi2 = 7.89, df = 4; P = 0.10; I2 = 49%). We judged this evidence to be of moderate certainty (Table 1).
1.15. Analysis.

Comparison 1: Vitamin A versus control, Outcome 15: Bitot's spots prevalence at longest follow‐up
Three studies reported data within one‐year postrandomisation, and combined results were similar (RR 0.43, 95% CI 0.33 to 0.56; Table 2).
Night blindness
Herrera 1992 reported a 47% reduction in night blindness incidence (RR 0.53, 95% CI 0.28 to 0.99), as shown in the illustrative forest plot in Analysis 1.16.
1.16. Analysis.

Comparison 1: Vitamin A versus control, Outcome 16: Night blindness incidence at longest follow‐up
Sommer 1986 and West 1991 reported a 68% reduction in night blindness prevalence (RR 0.32, 95% CI 0.21 to 0.50; 22,972 children; Analysis 1.17), with no heterogeneity (Chi2 = 0.19, df = 1; P = 0.66; I2 = 0%). We judged the certainty of evidence to be moderate (Table 1).
1.17. Analysis.

Comparison 1: Vitamin A versus control, Outcome 17: Night blindness prevalence at longest follow‐up
One study reported prevalence within one‐year postrandomisation, and results were similar (RR 0.30, 95% CI 0.17 to 0.52; Table 2).
Xerophthalmia
Three trials reported no combined effect on xerophthalmia incidence (RR 0.85, 95% CI 0.70 to 1.03; Analysis 1.18; West 1991; Herrera 1992; Barreto 1994), though statistical heterogeneity was substantial (Chi2 = 2.69, df = 1; P = 0.10; I2 = 63%).
1.18. Analysis.

Comparison 1: Vitamin A versus control, Outcome 18: Xerophthalmia incidence at longest follow‐up
Two studies reported data for one‐year postrandomisation, and results were similar (RR 0.88, 95% CI 0.72 to 1.07; Table 2).
Sommer 1986 and West 1991 reported a 69% reduction in xerophthalmia prevalence (RR 0.31, 95% CI 0.22 to 0.45; Analysis 1.19), with no statistical heterogeneity (Chi2 = 0.22, df = 1; P = 0.64; I2 = 0%).
1.19. Analysis.

Comparison 1: Vitamin A versus control, Outcome 19: Xerophthalmia prevalence at longest follow‐up
Hospitalisation
Ross 1993 HEALTH reported the likelihood of hospitalisations; however, results were imprecise and the CI around the summary estimate included a null effect (RR 0.64, 95% CI 0.40 to 1.02; see the illustrative forest plot in Analysis 1.20).
1.20. Analysis.

Comparison 1: Vitamin A versus control, Outcome 20: Hospitalisation: number of children hospitalised once or more at longest follow‐up
Lie 1993 reported inconclusive evidence on hospitalisation due to diarrhoea (RR 0.25, 95% CI 0.01 to 6.11; see the illustrative forest plot in Analysis 1.21) and hospitalisation due to LRTI (RR 0.11, 95% CI 0.01 to 2.06; see the illustrative forest plot in Analysis 1.22).
1.21. Analysis.

Comparison 1: Vitamin A versus control, Outcome 21: Hospitalisation due to diarrhoea at longest follow‐up
1.22. Analysis.

Comparison 1: Vitamin A versus control, Outcome 22: Hospitalisation due to LRTI at longest follow‐up
Side effects
We assessed two short‐term side effects: vomiting (within 48 hours) and bulging fontanelle.
Four trials reported an increase in risk of vomiting (RR 1.97, 95% CI 1.44 to 2.69; 10,541 children; Analysis 1.23; Sinha 1976; Florentino 1990; Arya 2000; Fisker 2014), with substantial statistical heterogeneity (Chi2 = 9.51, df = 3; P = 0.02; I2 = 68%). We judged this evidence to be of moderate certainty (Table 1).
1.23. Analysis.

Comparison 1: Vitamin A versus control, Outcome 23: Side effect: vomiting
Four trials reported bulging fontanelle side effects, but the only two that had enough data to enable analysis reported no effect (RR 1.24, 95% CI 0.74 to 2.08; Analysis 1.24; Stabell 1995; Bahl 1999; Arya 2000; Fisker 2014). Most studies included children over one year of age and would not have assessed this side effect.
1.24. Analysis.

Comparison 1: Vitamin A versus control, Outcome 24: Side effect: bulging fontanelle
Vitamin A deficiency status
Meta‐analyses
We assessed two indices of VAD: number deficient and serum retinol level.
Four trials reported a 29% reduction in the number of VAD children (RR 0.71, 95% CI 0.65 to 0.78; 2262 children; Analysis 1.25; Ross 1993 HEALTH; Dibley 1996; Shankar 1999; Cherian 2001); however, statistical heterogeneity was substantial (Chi2 = 13.58, df = 3; P = 0.004; I2 = 78%). We judged this evidence to be of moderate certainty (Table 1).
1.25. Analysis.

Comparison 1: Vitamin A versus control, Outcome 25: Vitamin A deficiency status: number deficient at longest follow‐up
Fourteen trials reported data on vitamin A serum retinol level at follow‐up, including one factorial study contributing two comparisons (Pinnock 1986; Reddy 1986a and Reddy 1986b (considered as one study); Pinnock 1988; Semba 1991; Lie 1993; Ross 1993 HEALTH; Ross 1993 SURVIVAL; Kartasasmita 1995; Dibley 1996; Sempértegui 1999; Shankar 1999; Cherian 2001; Lin 2009; DEVTA trial 2013). Vitamin A serum levels were higher in the vitamin A group (standardised mean difference (SMD) 0.26, 95% CI 0.22 to 0.30; Analysis 1.26); however, statistical heterogeneity was substantial (Chi2 = 278.45, df = 14; P < 0.001; I2 = 95%).
1.26. Analysis.

Comparison 1: Vitamin A versus control, Outcome 26: Vitamin A deficiency status: vitamin A serum retinol level at longest follow‐up
Eleven studies reported data within one‐year postrandomisation and results showed a relatively modest effect (RR 0.45, 95% CI 0.37 to 0.53; Table 2).
Sensitivity analysis
No studies reporting VAD status were at high risk of bias for sequence generation.
To test for small‐study bias, we repeated the analysis using a random‐effects model. The overall estimate was considerably larger than the fixed‐effect estimate, suggesting small studies report larger effects (SMD 0.50, 95% CI 0.30 to 0.70; 14 studies; Table 2).
The funnel plot that we produced was highly asymmetrical (data not shown).
With regard to the design effects in cluster trials, no ICCs were imputed, so a sensitivity analysis was not required.
Discussion
Summary of main results
For this update, there were no new eligible RCTs.
In the previous version (Imdad 2017), despite the addition of newer studies, notably the large study from India (DEVTA trial 2013), VAS was still associated with a reduction in all‐cause mortality of 12%. There was some statistical heterogeneity in the pooled data, and a sensitivity analysis using a random‐effects model changed the effect size from 12% to 24%; however, the CIs overlapped with that of the fixed‐effect model. Whatever method of analysis we used, vitamin A had a significant and clinically meaningful effect, so supplementation should be offered to children in populations at risk of VAD.
Even though the exact mechanism of vitamin A against mortality is unclear, at least some of its protective effect stems from reductions in death due to diarrhoea and measles. The overall effect for mortality due to measles was not significant, as not all the studies that reported all‐cause mortality reported measles‐specific mortality; however, the therapeutic effects of VAS in reducing measles‐related mortality and morbidity are well established (Huiming 2005). Furthermore, VAS resulted in reduced incidence of diarrhoea and measles. Other reviews have shown that the therapeutic use of VAD may prevent acute diarrhoea from becoming chronic (Imdad 2010b). Together, these results suggest that reductions in diarrhoea and measles are potential pathways in the reduction of all‐cause mortality.
In addition to reducing death and illness, VAS reduces night blindness and potential precursors to blindness, namely Bitot's spots and xerophthalmia.
Few studies reported data about side effects, including vomiting, bulging fontanelle, and diarrhoea soon after receiving the intervention. VAS may increase short‐term vomiting almost two‐fold.
Overall completeness and applicability of evidence
This review systematically assessed both mortality and morbidity associated with VAS. This update does not include any new eligible study, so the results are the same in terms of the effectiveness of VAS for reducing mortality, morbidity, and nutrition‐related blindness.
All included studies reporting all‐cause mortality were set in low‐ to middle‐income countries. Given that a large proportion of the included studies (20/47) specifically excluded children with VAD, and vitamin A status was unclear in 23, it is likely that the effectiveness of VAS may be even more effective for children in low‐ and middle‐income countries who are at risk of VAD. The primary analysis was based on 19 trials from different countries and locations. It included 1,202,382 children randomised. The risk of selective reporting for the primary outcome appeared minimal. Statistical heterogeneity suggested that the magnitude of the effect may differ across settings and populations, possibly due to the extent of VAD or the availability of other nutrients. For example, dietary intake of vitamin A will differ across locations, and the effects of supplementation may be smaller in places with greater access to foods rich in vitamin A. Concomitant nutrient deficiencies may also impair the bioavailability of the supplements, since some of these nutrients (including fat, protein, and zinc) could be limiting factors for the absorption and utilisation of vitamin A, which is lipid‐soluble (Villamor 2000). Comorbid illnesses could also reduce absorption of vitamin A; that is, if vitamin A reduces mortality by reducing susceptibility to particular pathogens, differences in the prevalence of disease, sanitation, etc. might contribute to heterogeneity in outcomes across trials.
Analyses for many of the cause‐specific mortality and morbidity outcomes were consistent in favour of VAS. A general weakness of many interventions is the under‐reporting of implementation data, such as the core components of an intervention, the degree to which they are delivered in practice, and what aspects of the trial may have influenced implementation (Mayo‐Wilson 2007). In theory, the putative effect of this intervention relies little on the relationship between the provider and participant, but it is essential that large‐scale interventions effectively distribute capsules that have been stored properly and remain active. Additionally, the degree to which children were treated for morbidities across trials might influence incidence and prevalence data collected in various trials, and this could contribute to heterogeneity.
This review suggests some ways in which vitamin A might work, but it does not describe how the effects of vitamin A might differ across subpopulations. The included trials did not report the data required for such analyses, and we decided a priori not to include subgroup analyses based on individual‐level moderators for reasons described in the section on Subgroup analysis and investigation of heterogeneity. A more detailed investigation of heterogeneity would require individual participant data and possibly information on vitamin A status at the individual or population level. Co‐interventions, including other nutrients or vaccinations, might interact with vitamin A, but we were unable to review possible interaction effects. We were also unable to compare HIV‐positive children to HIV‐negative children, though there is a separate Cochrane Review that specifically considered the effectiveness of VAS in people infected with HIV, and the results were similar (Irlam 2010). Subgroup analyses by geographic region included few studies; some disaggregated data by sex and age, but these were not representative of the studies overall or the results. Subgroup results were neither significant nor meaningful, and they are vulnerable to reporting bias (i.e. differences are more likely to be reported than similarities). Though a review with individual participant data could be informative, systematic reviews are not the best method for answering all questions, and other studies might explain why results are sometimes different. In any case, the observed effects are so large that heterogeneity may be unimportant; vitamin A should be given to children whether it reduces childhood mortality by 7% or 17%.
Quality of the evidence
This update included no new eligible studies; however, previously, this review included 47 studies and an estimated 1,223,856 children. This is the largest review of VAS for children to date.
In certain studies, it was impossible to assess allocation concealment. Efforts to blind participants and providers suggest the overall risk of bias is minimal, and any impact on the primary outcome (all‐cause mortality) is likely to be small.
In some trials, children interacted with researchers or clinicians who were aware of their assignment. We judged three studies at high risk of performance bias, mostly because of failure to adequately blind participants, providers, and outcome assessors (Daulaire 1992; Lin 2009; DEVTA trial 2013). We considered bias due to inadequate blinding to be low and, if anything, likely to underestimate effects; for example, a teacher would be more likely to give extra food to a child receiving the placebo rather than the reverse.
Missing data are much more likely to influence secondary analyses than the primary outcome. Results for all‐cause mortality are known for over 98% of randomised participants. Of the 19 (40%) studies that reported this outcome, we judged seven at unclear risk of bias, but four of these had minimal attrition (Vijayaraghavan 1990; Ross 1993 HEALTH; Ross 1993 SURVIVAL; Venkatarao 1996. The others failed to report reasons for dropout. Two studies did not adequately manage missing data, but together these studies contributed only 5% to the pooled estimate (Pant 1996; Chowdhury 2002).
The DEVTA trial 2013, which included about one million children, found a small benefit for VAS. These findings generated controversy because many experts believe that the methods for the delivery of the intervention and the assessment of the primary outcome (i.e. all‐cause mortality) were not rigorous (Habicht 2013; Mannar 2013; Mayo‐Wilson 2013; Sloan 2013; Sommer 2013). For example, investigators did not count children at baseline or obtain informed consent, and methods of follow‐up and data collection were not vigorous (Mannar 2013; Sommer 2013). In this cluster‐RCT, vitamin A capsules were distributed by Anganwadi workers who had contact with only 26% of the children living in the study area (Sommer 2013). In reply to this extensive criticism, authors of DEVTA emphasised that results of this trial need to be interpreted alongside previously published studies (Peto 2013). In the updated analysis of 19 trials for all‐cause mortality for this review, DEVTA accounted for 61.7% of the combined effect in a fixed‐effect analysis. A sensitivity analysis using a random‐effects model found a 24% reduction in mortality, essentially the same as our original estimate (RR 0.76, 95% CI 0.69 to 0.83), published previously (Imdad 2010a). Thus, VAS appears to have a robust effect on risk of death in children, which is clinically meaningful and important for policy. Unsurprisingly, the effect of VAS may be reduced when the intervention is not delivered with fidelity.
In summary, the primary outcome was at low risk of bias, and the size and the significance of the effect cannot be explained by bias. While there was some evidence of small‐study bias for secondary outcomes, further research is unlikely to change the conclusion that VAS, delivered with high quality and coverage, prevents death among children aged six to 59 months in low‐ and middle‐income countries. Despite sensitivity analyses and attempts to explain sources of heterogeneity by comparing the characteristics of the studies, we could not explain reasons for these differences across trials. Observational studies might investigate the mechanisms by which vitamin A reduces mortality.
Potential biases in the review process
This review used clearly specified inclusion and exclusion criteria, a comprehensive search strategy for the identification of relevant studies, and prespecified subgroup and sensitivity analyses to explore heterogeneity. We also described the post hoc decision to include two quasi‐RCTs (Herrera 1992; Stansfield 1993). Only Herrera 1992 contributed data to the primary outcome of all‐cause mortality, and sensitivity analyses demonstrated that exclusion of this study did not change the results significantly.
We combined RRs (events per child) and rate ratios (events per child‐year) for incidence data. Strictly speaking these two ratios have different interpretations; however, we consider that the included studies used the same scale, and outcomes are less likely to be biased by use of denominator. For the primary outcome of all‐cause mortality, there were three studies where the denominator was time at risk (Ross 1993 HEALTH; Dibley 1996; Fisker 2014), and exclusion of these studies did not change the results.
For three trials with multiple arms, we included each such study as two comparisons (Reddy 1986a and Reddy 1986b; Long 2006a and Long 2006b; Chen 2013a and Chen 2013b). We acknowledge that results for comparisons from the same study may be correlated; however, this is unlikely to affect the results of our analysis because each group was counted only once (i.e. we would have obtained the same overall result by combining the eligible treatment groups and the eligible control groups).
The comprehensive search strategy was devised to minimise publication bias by searching for both published and unpublished studies, though none of the included studies were unpublished. While studies with positive results are more likely to be published than studies with negative results, studies large enough to influence this review are very likely to be published. One study awaiting assessment was too small to affect any analysis (Aklamati 2006).
Some secondary outcomes did not contain a majority of the children randomised in the review, and these results may be vulnerable to selective outcome reporting bias.
Agreements and disagreements with other studies or reviews
Our results are consistent with the results of other reviews assessing a similar question, though the magnitude of the reduction in risk of death was smaller. Glasziou 1993 reported a 30% reduction in all‐cause mortality, and Beaton 1993 reported a 23% reduction. Fawzi 1993 used an odds ratio (OR) rather than RR as the measure of association, so the reported reduction is not directly comparable (OR 0.70, 95% CI 0.56 to 0.87).
Authors' conclusions
Implications for practice.
Over the years, the prevalence of vitamin A deficiency (VAD) has decreased; however, it is still widely prevalent in Southeast Asia and Sub‐Saharan Africa (Stevens 2015). Even though no new eligible study was included in this update, the previous versions of this review showed that synthetic vitamin A supplementation (VAS) reduced disease and death in children aged six to 59 months (Imdad 2010a; Imdad 2017). However, we acknowledge that synthetic VAS may not be the long‐term solution to control VAD. Fortification, food distribution programmes, and horticultural developments may provide more permanent relief. For example, vitamin A could be added to rice or growers may aim to increase access to agricultural products such as orange‐fleshed sweet potato (Klemm 2010; Klemm 2016). Furthermore, if vitamin A reduces mortality by preventing measles, widespread vaccination will reduce the relative contribution of VAS. Until such long‐term solutions are in place, supplementation should continue. As access to vitamin A increases, it will be important to continue to identify at‐risk groups and deliver supplements to them (Bhutta 2015).
The World Health Organization (WHO) currently recommends VAS to children between six and 59 months of age, in a dose of 100,000 IU for children aged six to 12 months and a dose of 200,000 IU for children aged one to five years, every six months. Based on existing literature, we suggest continuing this policy for children under five years of age in areas at risk of VAD. However, the global policy for universal VAS must be revisited for populations where VAD no longer remains a public health issue and VAD‐associated deaths have markedly declined (Stevens 2015).
Implications for research.
The effectiveness of VAS for preventing mortality is well established. The primary results in this review are robust and clinically meaningful. Further placebo‐controlled studies would be unethical.
Nevertheless, this review does not answer a number of important questions. There was little variation in dosing among studies reporting the primary outcome. One trial used weekly doses and estimated a 54% reduction in all‐cause mortality (Rahmathullah 1990). It would be ethical to conduct trials in which participants receive different doses of vitamin A that are likely to be beneficial, some of which could lead to larger benefits than those observed so far, and might lead to fewer side effects (e.g. vomiting).
Reductions in mortality are likely related to reduced incidence and severity of diarrhoea and measles. The effects of VAS on relevant pathogens and disease pathways are not well understood, and these could be examined in observational studies or in trials of other interventions for these problems.
Growth and other developmental outcomes are less important than mortality, and few studies have looked into these questions. These outcomes could be added to future versions of this review. Observational studies might elucidate the relationship (if any) between vitamin A and growth.
Despite the primary effect, observed increases in vitamin A serum levels were small. That said, serum level may be a poor indicator of status and may not be related to more meaningful outcomes such as mortality or blindness (WHO 2009). In addition, oral synthetic VAS supplementation may not be the best pathway for delivery. For example, absorption may be better in protein carriers compared to carbohydrate carriers. Further studies might compare synthetic supplementation to fortification or other delivery mechanisms.
Two additional Cochrane Reviews cover the preventive aspect of VAS for infants less than six months of age: one investigated the effects of vitamin A during the neonatal period (Haider 2017), while another focused on infants aged one to six months (Imdad 2016). Further reviews might investigate different delivery channels, including food supplementation and improved access to food or social programmes to increase uptake of vitamin A‐rich foods. Several studies have investigated VAS for pregnant and lactating mothers; these and other efforts to promote delivery of vitamin A (e.g. by increased rates and duration of breastfeeding) may require further attention.
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2021 | New search has been performed | Review updated following a new search in March 2021 |
| 4 August 2021 | New citation required but conclusions have not changed | No new studies found. Conclusions remain unchanged. |
History
Protocol first published: Issue 5, 2010 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 23 November 2017 | Amended | Added additional sentences to the Excluded studies section that further describe studies excluded from the review. |
| 13 January 2017 | New search has been performed | Updated following a new search in March 2016. |
| 13 January 2017 | New citation required but conclusions have not changed | We included four new studies. |
| 7 December 2010 | Amended | Edited to correct typographical errors and improve readability. |
Acknowledgements
We thank the editorial group at Cochrane Developmental Psychosocial and Learning Problems. We are thankful to Olivia Tsistinas for developing the search strategy. We thank Kurt Herzer and Mohammad Yawar Yakoob for their contributions to the first version of the review (Imdad 2010a), and Jai Das and Renee Sharma for their contributions to the last version of this review (Imdad 2017).
The CRG Editorial Team are grateful to Anne Lawson for copy editing and proofreading this update.
Appendices
Appendix 1. Search strategies 2021
Cochrane CENTRAL
#1 MeSH descriptor: [Vitamin A] explode all trees #2 "Vitamin A" or retinol* or "Aquasol A" or retinal #3 #1 OR #2 #4 MeSH descriptor: [Child] explode all trees #5 MeSH descriptor: [Infant] explode all trees #6 (baby OR babies OR infant* OR toddler* OR child* OR (pre NEXT school*) OR preschool* OR pre‐school* OR girl* OR boy*) #7 #4 OR #5 OR #6 #8 #3 AND #7 Publication Date 2016 to 2021
MEDLINE Ovid (R)
1 exp Vitamin A/ 2 (retinol$ or retinal$ or aquasol a or vitamin a).tw. 3 1 or 2 4 exp Infant/ 5 exp Child/ 6 (baby or babies or infant$ or toddler$ or child$ or girl$ or boy$ or pre school$ or pre‐school$ or preschool$) 7 4 or 5 or 6 8 exp placebos/ 9 randomized controlled trial.pt. 10 controlled clinical trial.pt. 11 randomi#ed.ab. 12 placebo$.ab. 13 drug therapy.fs. 14 randomly.ab. 15 random.ab. 16 trial.ab. 17 trials.ab. 18 group.ab. 19 groups.ab. 20 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21 3 and 7 and 20 22 exp animals/ not humans.sh. 23 21 not 22 24 limit 19 to yr="2016 ‐Current"
Ovid MEDLINE(R) and In‐Process, In‐Data‐Review & Other Non‐Indexed Citations
1 (retinol$ or retinal$ or aquasol a or vitamin a).tw. 2 (baby or babies or infant$ or toddler$ or child$ or girl$ or boy$ or pre school$ or pre‐school$ or preschool$).tw. 3 1 and 2 4 random$.tw. 5 placebo$.tw. 6 trial.tw. 7 trials.tw. 8 group.tw. 9 groups.tw. 10 (crossover or cross‐over).tw. 11 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 12 prospective.tw. 13 factorial$.tw. 14 assign$.ab. 15 allocat$.ab. 16 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17 3 and 16 18 remove duplicates from 17 19 limit 16 to yr="2016 ‐Current"
Embase Elsevier
#1 'retinol'/exp #2 retinol* OR retinal* OR 'aquasol a' OR 'vitamin a' #3 #1 OR #2 #4 'child'/exp #5 baby OR babies OR infant* OR toddler* OR child* OR girl* OR boy* OR 'pre school*' OR preschool* #6 #4 OR #5 #7 'randomized controlled trial'/exp #8 'controlled clinical trial'/exp #9 'single blind procedure'/exp #10 'double blind procedure'/exp #11 'triple blind procedure'/exp #12 'crossover procedure'/exp #13 crossover OR 'cross over' #14 (singl* OR doubl* OR tripl* OR trebl*) NEXT/1 (blind* OR mask*) #15 'placebo'/exp #16 placebo* #17 prospective #18 factorial* #19 random* #20 assign*:ab #21 allocat*:ab #22 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #3 AND #6 AND #22 #24 #23 NOT ([animals]/lim NOT [humans]/lim) #25 #24 AND [2016‐2021]/py #26 #25 NOT [medline]/lim
Web of Science (Science Citation Index and Conference Proceedings Citation Index – Science)
#1 TS=(retinol OR "vitamin a") #2 TS=(baby OR babies OR infant* OR toddler* OR child* OR girl* OR boy* OR "pre school*" OR pre‐school* OR preschool*) #3 #2 AND #1 #4 TS=(random* OR placebo* OR trial OR trials ) #5 #4 AND #3 #6 #5 Timespan=2016‐2021
Cochrane Database of Systematic Reviews
#1 MeSH descriptor: [Vitamin A] explode all trees #2 "Vitamin A" or retinol* or "Aquasol A" or retinal #3 #1 OR #2 #4 MeSH descriptor: [Child] explode all trees #5 MeSH descriptor: [Infant] explode all trees #6 (baby OR babies OR infant* OR toddler* OR child* OR (pre NEXT school*) OR preschool* OR pre‐school* OR girl* OR boy*) #7 #4 OR #5 OR #6 #8 #3 AND #7 Publication Date 01/01/16 to 02/03/2021
ClinicalTrials.gov
Advanced search: Intervention: Vitamin A AND Age Group: child
World Health Organization International Clinical Trials Registry Platform
Searched from standard search page
(Vitamin A AND child) OR (Vitamin A AND babies) OR (Vitamin A AND infants)
Global Index Medicus (WPRIM, LILACS, IMSEAR, IMEMR, AIM)
(mh:(retinol)) OR (("vitamin a" OR retinol* OR "aquasol a" OR retinal*)) AND ((mh:(child)) OR ((mh:(infant))) OR ((baby OR babies OR child* OR infant* OR toddler* OR girl* OR boy* OR preschool* OR "pre school*" OR pre‐school*))) AND ((mh:(placebos)) OR ((random* OR placebo* OR trial OR trials OR group OR groups OR crossover OR cross‐over OR prospective OR factorial* ) OR ((ab:(assign* OR allocat*))) OR ((singl* OR doubl* OR tripl* OR trebl*) AND ((blind* OR mask*))))) AND NOT ((mh:(animals)) AND NOT ((mh:(animals)) AND ((mh:(humans))))) AND (year_cluster:[2016 TO 2021])
Scopus Elsevier
( ( TITLE‐ABS‐KEY ( retinol* OR retinal* OR "aquasol a" OR "vitamin a" ) ) AND ( TITLE‐ABS‐KEY ( baby OR babies OR infant* OR toddler* OR child* OR girl* OR boy* OR "pre school*" OR preschool* OR pre‐school* ) ) ) AND ( ( TITLE‐ABS‐KEY ( random* OR placebo* OR trial OR trials OR group OR groups OR crossover OR cross‐over OR prospective OR factorial* ) ) OR ( TITLE‐ABS‐KEY ( singl* OR doubl* OR tripl* OR trebl* ) PRE/1 TITLE‐ABS‐KEY ( blind* OR mask* ) ) OR ( ABS ( assign* OR allocat* ) ) ) AND NOT INDEX ( medline ) AND ( LIMIT‐TO ( PUBYEAR , 2021 ) OR LIMIT‐TO ( PUBYEAR , 2020 ) OR LIMIT‐TO ( PUBYEAR , 2019 ) OR LIMIT‐TO ( PUBYEAR , 2018 ) OR LIMIT‐TO ( PUBYEAR , 2017 ) OR LIMIT‐TO ( PUBYEAR , 2016 ) OR LIMIT‐TO ( PUBYEAR , 2015 ) OR LIMIT‐TO ( PUBYEAR, 2014 ) )
Data and analyses
Comparison 1. Vitamin A versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality at longest follow‐up | 19 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.83, 0.93] | |
| 1.2 All‐cause mortality at longest follow‐up (subgroup analysis): age | 5 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Aged 6–12 months | 4 | Risk Ratio (IV, Fixed, 95% CI) | 0.59 [0.43, 0.82] | |
| 1.2.2 Aged 1–5 years | 4 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.57, 0.81] | |
| 1.3 All‐cause mortality at longest follow‐up (subgroup analysis): sex | 7 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Boys | 7 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.89, 1.04] | |
| 1.3.2 Girls | 7 | Risk Ratio (IV, Fixed, 95% CI) | 0.90 [0.84, 0.97] | |
| 1.4 Mortality due to diarrhoea at longest follow‐up | 9 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.79, 0.98] | |
| 1.5 Mortality due to measles at longest follow‐up | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.69, 1.11] | |
| 1.6 Mortality due to meningitis at longest follow‐up | 3 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.17, 1.88] | |
| 1.7 Mortality due to lower respiratory tract infection (LRTI) at longest follow‐up | 9 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.86, 1.12] | |
| 1.8 Diarrhoea incidence at longest follow‐up | 16 | Risk Ratio (IV, Fixed, 95% CI) | 0.85 [0.82, 0.87] | |
| 1.9 Diarrhoea prevalence at longest follow‐up | 4 | Risk Ratio (IV, Fixed, 95% CI) | 1.06 [1.03, 1.10] | |
| 1.10 Measles incidence at longest follow‐up | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.50 [0.37, 0.67] | |
| 1.11 Malaria incidence at longest follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.12 Malaria prevalence at longest follow‐up | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.41, 1.28] | |
| 1.13 LRTI incidence at longest follow‐up | 12 | Risk Ratio (IV, Fixed, 95% CI) | 0.99 [0.92, 1.06] | |
| 1.14 LRTI prevalence at longest follow‐up | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.60 [0.45, 0.81] | |
| 1.15 Bitot's spots prevalence at longest follow‐up | 5 | Risk Ratio (IV, Fixed, 95% CI) | 0.42 [0.33, 0.53] | |
| 1.16 Night blindness incidence at longest follow‐up | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.17 Night blindness prevalence at longest follow‐up | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.32 [0.21, 0.50] | |
| 1.18 Xerophthalmia incidence at longest follow‐up | 3 | Risk Ratio (IV, Fixed, 95% CI) | 0.85 [0.70, 1.03] | |
| 1.19 Xerophthalmia prevalence at longest follow‐up | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.31 [0.22, 0.45] | |
| 1.20 Hospitalisation: number of children hospitalised once or more at longest follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.21 Hospitalisation due to diarrhoea at longest follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.22 Hospitalisation due to LRTI at longest follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.23 Side effect: vomiting | 4 | 4427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.44, 2.69] |
| 1.24 Side effect: bulging fontanelle | 4 | 2318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.74, 2.08] |
| 1.25 Vitamin A deficiency status: number deficient at longest follow‐up | 4 | 2262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 1.26 Vitamin A deficiency status: vitamin A serum retinol level at longest follow‐up | 15 | 11788 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.22, 0.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 1995.
| Study characteristics | ||
| Methods | Cluster‐randomised trial conducted in Uttar Pradesh, India | |
| Participants |
Eligibility: children aged < 6 years Excluded: children with xerophthalmia Sample: 16 clusters (subcentres) were randomly selected and divided into 4 subdivisions (4 subcentres in each), with drugs A (vitamin A) and B (placebo) distributed in 2 each randomly. At the end of the study, investigators found that vitamin A was distributed in 3 subdivisions (12 subcentres) and placebo in 1 only (4 subcentres) by mistake. 17,778 children were approached but only 15,247 children were included in the final analysis based on the fact that they received ≥ 1 dose of vitamin A |
|
| Interventions |
Experimental group: vitamin A 50,000 IU + vitamin E 10 IU for children aged 1–6 months and vitamin A 100,000 IU and vitamin E 20 IU for children aged 7–72 months Control group: placebo Study duration: intervention delivered every 4 months for 12 months |
|
| Outcomes | All‐cause and cause‐specific mortality due to diarrhoea, pneumonia, measles, and meningitis | |
| Notes | The trial was conducted in 2 phases. The first phase consisted of 15 months (i.e. 3 months for registration and 12 months for intervention and measurement of relevant outcomes). In the second phase, mortality was measured in a subsample of initially included children, exactly 12 months after termination of first phase. The cause of death was assigned using a verbal autopsy tool. Baseline mortality rates for children < 6 years of age were 27.7 for the intervention group and 23.3 per 1000 for the control group, with significant differences in the 2 groups (P < 0.01). According to WHO, India is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Out of the total 43 subcentres, 16 were randomly selected, four subdivisions (4 subcentres in each) were made and drugs A and B distributed in two each randomly". Comment: authors did not specify the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Other bias | Unclear risk | Comment: insufficient information to permit judgement. |
Albert 2003.
| Study characteristics | ||
| Methods | Factorial design, individually randomised trial conducted in Dhaka, Bangladesh | |
| Participants |
Eligibility: children aged 2–5 years of either sex, VAD (serum retinol level < 20 mg/dL; and nutritional status corresponding to a weight‐for‐age score that was 61% of the median National Center for Health Standards standard Excluded: children who had received VAS during the preceding 6 months or with history of night blindness or sickness due to underlying illnesses such as diarrhoea or respiratory tract infections Sample: 256 children |
|
| Interventions | 4 intervention groups Experimental group I: vitamin A. 5 mL vitamin A syrup 200,000 IU once a week before administration of the first dose of the vaccine and received 5 mL of a placebo syrup every day for 42 days starting 3 weeks before administration of the first dose of vaccine and ending 1 week after the second dose of vaccine Experimental group II: zinc. 5 mL zinc acetate syrup (containing 20 mg of elemental zinc) daily + single dose of a placebo syrup, according to the same schedule used for the children in the A group Experimental group III: vitamin A + zinc Control group: placebo |
|
| Outcomes | Vibriocidal antibody response to cholera vaccine | |
| Notes | No clinical outcomes were available so no data were included in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Bottles of syrup were serially numbered according to the randomizations list". Comment: most likely done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomizations code was broken after completion of the study". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "The zinc syrup and its placebo syrup looked very similar, as did the vitamin A syrup and its placebo syrup". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "The randomization code was broken after completion of the study". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "The randomization code was broken after completion of the study". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: minimal attrition. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no trial registration number was available. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Arya 2000.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in New Delhi, India | |
| Participants |
Eligibility: infants aged 9–12 months attending the immunisation clinic of Safdarjung hospital in New Delhi Excluded: sick infants requiring hospitalisation Sample: 256 infants; 128 in vitamin A group, 128 in placebo group. Mean age 9 months |
|
| Interventions |
Experimental group: single‐dose vitamin A 100,000 IU in arachis oil Control group: placebo in peanut oil Both vitamin A and placebo were administered at the time of measles vaccination. At end of study, vitamin A group received placebo, and placebo group received vitamin A. |
|
| Outcomes | Incidence of side effects in first 24 hours (vomiting, loose stools, fever, irritability, bulging fontanelle) | |
| Notes | Study participants were not significantly different in sex, age, weight distribution, and nutritional status at the baseline. The baseline prevalence of vomiting, loose stools, fever, and irritability during the 24 hours prior to dosing was similar in both groups. 97.3% of the included infants had normal serum retinol level before the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "The infants were randomised … according to the order of arrival at hospital. Randomisation was done by the nurse who gave measles vaccine to these children". Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: children were randomised according to their entry into hospital. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "This double‐blind, randomised … supplied in small dark bottles marked '1' and '2' ". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "This double‐blind, randomised … supplied in small dark bottles marked '1' and '2' " |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "This double‐blind, randomised … supplied in small dark bottles marked '1' and '2' … Two clinicians examined each of the infants at both first and second visits. Neither clinician knew the bottle code". |
| Incomplete outcome data (attrition bias) | High risk | Comment: 39 (15.2%) infants were lost to follow‐up with similar distribution in both the groups. Reasons for loss to follow‐up not given. |
| Selective reporting (reporting bias) | High risk | Comment: methods described that the clinicians did physical examinations and recorded weight, nutritional status, any signs of VAD, heart rate, respiratory rate, temperature, and systemic examination, especially neurological examination including the state of the fontanelle, reflexes, motor and sensory functions, etc. But bulging fontanelle not reported as an outcome, or other variables mentioned in the results. |
| Other bias | Low risk | Comment: no other apparent bias. |
Bahl 1999.
| Study characteristics | ||
| Methods | Individually randomised study conducted in an urban slum of Delhi, India | |
| Participants |
Eligibility: infants aged 6–9 months enrolled into study when they turned 9 months old Excluded: infants with history of measles, contact with a case of measles or measles immunisation, or had received a dose of vitamin A in the previous 4 months; serious illness requiring hospitalisation or having clinical signs of VAD (i.e. xerophthalmia, Bitot's spots, etc.) Sample: 618 infants; 309 in vitamin A group, 309 in control group. 50% boys |
|
| Interventions |
Experimental group: single‐dose vitamin A 30 mg (100,000 IU) in the form of retinol palmitate Control group: soybean oil Follow‐up: 4 months |
|
| Outcomes | Antibody response to measles vaccine, incidence of measles during study period, and side effects (e.g. vomiting, drowsiness, etc.) in first 48 hours | |
| Notes | The primary objective of the study was to determine the response to measles vaccine when administered along with vitamin A at 9 months of age. The study found no significant difference in antibody titres between groups 3 months after the administration of intervention. The baseline prevalence of clinical VAD in children aged 1–5 years in the study area was 3.5% and that of biochemical VAD was 37%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Infants were randomly assigned to receive vitamin A or a placebo by using a simple randomisation scheme with random permuted blocks of size eight, i.e. four infants each out of every eight infants enrolled were randomised to receive vitamin A or a placebo". Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "This scheme ensured that all infants received 30 mg vitamin A by 12 mo [months] of age without interfering with the double‐blind design of the study". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: adequate masking of vitamin A and placebo should have meant that providers were adequately blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: adequate masking of vitamin A and placebo should have meant that outcome assessors were adequately blinded. |
| Incomplete outcome data (attrition bias) | High risk | Comment: losses to follow‐up and exclusions described. Missing data excluded from the analysis. It is not possible to ascertain whether the exclusion of data from 17% of participants (equally distributed between treatment groups) would have impacted on the results. The investigators stated that the reason for their exclusion was that a follow‐up serum sample could not be ascertained. |
| Selective reporting (reporting bias) | High risk | Comment: data on harms are incompletely disclosed in the study report. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Barreto 1994.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in Serrinha, Brazil | |
| Participants |
Eligibility: children aged 6–48 months Excluded: presence of xerophthalmia or measles infection within the previous 30 days; children who received a high‐dose VAS in previous 6 months or had weight‐for‐age < 60% of the statistical median Sample: 1240 children; 620 in vitamin A group, 620 in placebo group. Mean age 28 months. 52% boys |
|
| Interventions |
Experimental group: vitamin A 100,000 IU for children aged < 12 months and 200,000 IU for children aged > 12 months Control group: placebo Study duration: intervention delivered every 4 months for 1 year |
|
| Outcomes | All‐cause mortality, incidence and prevalence of diarrhoea and respiratory tract disease, incidence of measles and xerophthalmia | |
| Notes | Study area had inadequate public health services. A previous survey in the area showed a biochemical deficiency (serum vitamin A concentration < 0.35 mmol/L) rate of 7.4% in children of this age group. According to WHO criteria, VAD should be considered a public health problem in this area. The surveillance for morbidity outcome was performed 3 times/week for 1 year, so the recall period was 48–72 hours. We used data for incidence of measles and xerophthalmia from account of attrition in study results section. According to WHO, Brazil does not have a high child mortality rate (i.e. < 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Children were randomly assigned to receive vitamin A or placebo four times‐at the start of the trial and every 4 months thereafter". Comment: authors did not specify the method of sequence generation. |
| Allocation concealment (selection bias) | Low risk |
Quote: "… only an external investigator had the codes for the individually wrapped and numbered capsules". Comment: although specific details were not disclosed, the available information suggested that allocation was adequately concealed. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "The gelatinous capsules of vitamin A and placebo (supplied by Hoffman La Roche) were identical in appearance and were unwrapped just before administration". Comment: study was double‐blind, with identical presentation and dosing of vitamin A and placebo. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "The gelatinous capsules of vitamin A and placebo (supplied by Hoffman La Roche) were identical in appearance and were unwrapped just before administration". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk |
Quote: "The study was kept double‐blind and only an external investigator had the codes for the individually wrapped and numbered capsules". Comment: if the assessors were not involved in the allocation process as suggested by the available information, outcome assessors were likely to have been blinded to treatment group assignment. |
| Incomplete outcome data (attrition bias) | Low risk |
Quote: "The total loss in follow‐up time was 10.3%, equally distributed between the study groups". Comment: the rate of attrition was balanced between groups and was primarily attributable to migration. On that basis, attrition bias is not likely to have impacted on the results of the review. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol for the study was not available and, as such, this aspect of the reporting of the study could not be assessed. |
| Other bias | Low risk | Comment: study appeared free of other potential bias. |
Benn 1997.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in Belem and Mindra, 2 districts in Bissau, Guinea‐Bissau | |
| Participants |
Eligibility: infants aged 6–9 months Excluded: children with signs of xerophthalmia; history of previous VAS; history of measles infection before 9 months of age, or who had a positive haemagglutinin‐inhibition assay titre at 9 months of age; infants reported to have had measles at 9–18 months of age Sample: 462 infants. Mean age 8.7 months. 51% boys |
|
| Interventions | 3 intervention groups Group I: infants aged 6 months randomly allocated to receive either a dose of measles vaccine at 6 months and a dose of measles vaccine at 9 months together with vitamin A supplement or the same dosing of measles vaccine with placebo as the supplement Group II: infants randomly allocated either poliomyelitis vaccine at 6 months and a single dose of measles vaccine at 9 months with vitamin A supplement or the same vaccines with a placebo as the supplement Group III: infants aged > 7.5 months at the beginning of the study or who were not found at home until they reached the age of 7.5 months were included in the study at age 9 months and received a measles vaccine plus vitamin A or placebo supplement Vitamin A was in a single dose of 100,000 IU dissolved in 1 mL of vegetable oil along with 40 IU of vitamin E. Placebo was vitamin E 40 IU dissolved in 1 mL of vegetable oil |
|
| Outcomes | Antibody response to measles vaccine, all‐cause mortality, incidence of measles | |
| Notes | Primary objective of study was to calculate the antibody response to measles vaccine when given with vitamin A. The results for antibody response to measles vaccine showed no significant difference between the groups. The study concluded that simultaneous administration of measles vaccine and vitamin A had no negative effect on measles immunity. Similarly, VAS had no significant effect on immune response of CD4 and CD8 T‐cells in children without clinical VAD. Vitamin A or placebo was given only at 9 months of age in all 3 study groups. The only difference among the groups was the frequency and type of vaccine administered. Therefore, we added the data for all 3 intervention and placebo groups to report the outcomes of interest to our review. We primarily took data from trial flow diagram and calculated the effect sizes accordingly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation sequence was computer generated". |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was kept in sealed envelopes and only released when all clinical laboratory analyses were completed". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "… because of the young age of the participants, any difference in taste was irrelevant …". Comment: identical presentation; probably adequate. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "None of the staff involved knew whether the bottles contained vitamin A or placebo …". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk |
Quote: "None of the staff involved knew whether the bottles contained vitamin A or placebo …". Comment: blinding of treatment group assignment and treatment to study personnel likely to have been maintained throughout. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: number lost to follow‐up and those excluded were explicitly described and equal in both the groups. Loss to follow‐up exceeded the number of deaths and children with measles. Reasons for missing data (migration) probably unrelated to treatment. |
| Selective reporting (reporting bias) | Low risk | Comment: some evidence of selective outcome reporting around malaria; however, deaths and prevalence of measles reported. |
| Other bias | Unclear risk | Comment: authors reported imbalance in self‐reported disease in the children aged 6 months at baseline. It is unclear how big an impact this will have had as the variable is not specific. |
Biswas 1994.
| Study characteristics | ||
| Methods | Individually randomised, placebo‐controlled trial conducted in Gobinda‐Khatick slum area of eastern Kolkata (formerly Calcutta), India | |
| Participants |
Eligibility: children aged 12–71 months Excluded: children with signs of VAD (e.g. xerophthalmia) Sample: 180 children. Mean age and proportions of boys not specified |
|
| Interventions |
Experimental group: single‐dose vitamin A 200,000 IU in form of retinyl palmitate Control group: placebo Follow‐up: 6 months |
|
| Outcomes | Incidence of diarrhoea and acute respiratory tract infection | |
| Notes | The baseline age and nutritional characteristics were similar in both the groups. The surveillance for morbidity outcomes was performed twice monthly. For respiratory disease morbidity, we used data for LRTI only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "For each strata, a restricted randomisation list was prepared … a random permutated block of block length 6 was used". Comment: block randomisation by age and weight; probably done. |
| Allocation concealment (selection bias) | Low risk |
Quote: "… randomisation was done by a pharmacist of the drug manufacturing company". Comment: assuming that the pharmacist was independent of the study team, this was probably adequate. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "… identical (colour and taste) placebo. Both drug and placebo were prepared and dispensed in a single dose amber coloured glass ampoule by a local pharmaceutical company". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "For keeping the trial totally blinded to all participants (for example, patients, investigators, surveyor), randomisation was done by a pharmacist of the drug manufacturing company. Samples of drug (or placebo) were identified by the code number of the respective child". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "For keeping the trial totally blinded to all participants (for example, patients, investigators, surveyor), randomisation was done by a pharmacist of the drug manufacturing company. Samples of drug (or placebo) were identified by the code number of the respective child". |
| Incomplete outcome data (attrition bias) | Low risk |
Quote: "… data was analysed for 174 children due to attrition of 6 children for various reasons (for example, 5 children were hospitalised due to illnesses unrelated to the study objectives and the death of 1 child due post‐measles bronchopneumonia)". Comment: attrition was low and reported as unrelated to treatment. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol was not available to permit a clear judgement. Study aims were to measure diarrhoea and respiratory infection; both outcomes were reported in full in the study report. 1 child died and the treatment group assignment was not disclosed. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Chen 2013a.
| Study characteristics | ||
| Methods | Factorial design, individually randomised trial conducted in Chengdu City, China | |
| Participants |
Eligibility: children aged 3–6 years, apparently good health, haemoglobin concentration > 60 g/L, serum C‐reactive protein < 10 mg/L, parental or guardian's approval for participation and parental or guardian's agreement to avoid additional use of vitamin A and iron supplements during the investigation Excluded: children with evidence of recent acute or chronic illnesses or haemoglobin < 60 g/L (or both) Sample: 387 children |
|
| Interventions | 4 intervention groups Experimental group I: vitamin A 200,000 IU capsule (as retinol) just once initially Experimental group II: ferrous sulphate (elemental iron 1–2 mg/kg) once daily for 6 months Experimental group III: vitamin A 200,000 IU capsule once initially and ferrous sulphate (elemental iron 1–2 mg/kg) once daily for 6 months Control group: neither vitamin A nor ferrous sulphate |
|
| Outcomes | Incidence of diarrhoea and LRTI | |
| Notes | Study setting was a periurban area in Huayuan Town, Pixian County of Chengdu City, Sichuan Province, western China, from March to September 2011. Supplementation was given in schools. The paper did not have a study flow diagram. The data from the factorial design were included in 2 data sets. The first data set is the comparison between vitamin A and placebo (Chen 2013a), while the second data set is the comparison between vitamin A + iron vs iron only (Chen 2013b). The data for meta‐analysis was taken from table 2 and we calculated the rate ratio based on the number of events in the experimental and control groups with the denominator as person‐days at risk. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The RAND function of Excel (Microsoft, Redmond, WA, USA) was used to generate computer randomly permutated codes". |
| Allocation concealment (selection bias) | Low risk |
Quote: "The health care workers, outcome assessors, data analyst and children were not made aware of the intervention assignment until the completion of data analysis". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "Children were not made aware of the intervention". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "The health care workers, outcome assessors, data analyst and children were not made aware of the intervention …". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "… outcome assessors, data analyst and children were not made aware of the intervention …" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: loss to follow‐up was 13% and balanced in each group with similar reasons for attrition. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the trial registration number was not given. Authors did mention that they could not report some of the a priori mentioned serum biochemical markers, as they could not collect enough blood samples. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Chen 2013b.
| Study characteristics | ||
| Methods | — | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | Same as Chen 2013a above. | |
Cherian 2001.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in India | |
| Participants |
Eligibility: children aged 12–60 months with recurrent respiratory tract infections Excluded: children with mild or moderate asthma; who were receiving vitamin supplements or who had received a massive dose of vitamin A in the previous 6 months; with pre‐existing congenital heart disease, chronic lung disease, pulmonary tuberculosis or immunodeficiency disorders; receiving immunosuppressive drugs; with clinically apparent VAD Sample: 61 children; 30 in vitamin A group, 31 in placebo group. Mean age 35.7 months. 60.7% boys |
|
| Interventions |
Experimental group: single‐dose vitamin A 200,000 IU Control group: placebo in arachis oil Follow‐up: 6 months |
|
| Outcomes | Incidence of respiratory disease, mean vitamin A serum levels | |
| Notes | Definition of respiratory illness used was not specific enough. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Eligible children were randomly allocated to receive either 200,000 IU of vitamin A in arachis oil or a placebo containing arachis oil without vitamin A". Comment: details of sequence generation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk | Quote: "Eligible children were randomly allocated to receive either 200,000 IU of vitamin A in arachis oil or a placebo containing arachis oil without vitamin A". |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: not mentioned. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk |
Quote: "Of the 61 included children, seven (3 in the placebo group and four in vitamin A group) did not return for follow‐up" (second page). Comment: authors did not address the reasons for losses to follow‐up, and given the small size of this trial, bias may or may not be introduced depending on why the losses occurred by group. Given this lack of discussion, it is difficult to judge whether there is a low or high risk of bias, but it is likely to be high. |
| Selective reporting (reporting bias) | Unclear risk |
Quote: "Details of doctor or outpatient visits and hospital cough, wheezy breathing, shortness of breath and fever. Details of doctor or outpatient visits and hospital admissions during the study period were also recorded. During each monthly follow‐up visit, the entries in the monthly calendar were reviewed with the parent". Comment: hospitalisation data were not reported though they were collected. |
| Other bias | Unclear risk | Comment: very little information provided in the paper; difficult to assess. |
Cherian 2003.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in Vellor, India | |
| Participants |
Eligibility: infants aged 9–12 months Excluded: children with history of measles vaccination or an exanthematous illness; with moderate or severe malnutrition; clinical signs of VAD; known immune deficiency or receiving immunosuppressive therapy; received blood or blood products in the previous 6 months Sample: 395 infants; 198 in vitamin A group, 197 in placebo group. Mean age 9.8 months. 52% boys |
|
| Interventions |
Experimental group: single‐dose vitamin A 100,000 IU Control group: placebo Interventions provided at time of measles vaccination |
|
| Outcomes | Antibody response to measles vaccine | |
| Notes | Primary objective of study was to measure the antibody response to measles vaccine when given with and without vitamin A. Study found no significant inhibitory or enhancing influence on antibody response to measles vaccine when administered concomitantly with vitamin A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The infants who were immunized with monovalent measles vaccine were randomly assigned, in blocks of eight, to concomitantly receive 100,000 IU of vitamin A in arachis oil or a placebo containing carboxymethylcellulose prepared in the hospital pharmacy". Comment: authors did not specify the method of sequence generation. |
| Allocation concealment (selection bias) | Low risk |
Quote: "… arachis oil or a placebo containing carboxymethylcellulose prepared in the hospital pharmacy". Comment: probably done since hospital pharmacy was responsible for preparing the order of vitamin A and placebo, and unlikely to have been internal to the study team. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk |
Quote: "… Vitamin A in arachis oil or a placebo containing carboxymethylcellulose …" Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | High risk | Comment: the proportion of children providing adequate samples was low at 6 months, and there was insufficient detail about the reasons for missing data. |
| Selective reporting (reporting bias) | High risk | Comment: there was no mention of mortality or any morbidity of measles or diarrhoea. |
| Other bias | Unclear risk | Comment: insufficient information to permit judgement. |
Chowdhury 2002.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in urban slums of Chandigarh, India | |
| Participants |
Eligibility: children aged < 10 years Excluded: children with xerophthalmia and history of VAS Sample: 1520 children; 756 to vitamin A group, 759 to placebo group. Mean age 51 months. 50% boys |
|
| Interventions |
Experimental group: vitamin A 50,000 IU for children aged < 6 months; 100,000 IU for children aged 6–12 months and 200,000 IU for children aged > 1 year Control group: placebo Study duration: intervention given every 4 months for 15 months |
|
| Outcomes | All‐cause mortality; cause‐specific mortality due to diarrhoea, pneumonia, and meningitis; incidence of diarrhoea, pneumonia, and measles. Measurement of subclinical VAD status was by conjunctival impression cytology | |
| Notes | Baseline sociodemographic and anthropometric characteristics were similar in both the groups. Study population had a high prevalence of VAD. Children were contacted every 15 days by home visits to obtain information on morbidity and mortality. Study included children aged < 10 years; however, the mean age of the children was 51 months. Study methods were not explicitly described. According to WHO, India is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "From three slums of Chandigarh, 1520 non‐xerophthalmic children of less than 10 years of age were individually randomised in equal number to receive vitamin A or placebo". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk |
Quote: "An equivalent volume of arachis oil was given as placebo". Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | High risk | Comment: although attrition rates were balanced, the rates of mortality were lower than the rate of withdrawal. This could impact on the reliability of the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Other bias | Unclear risk | Comment: study not sufficiently reported in order to assess this item fully. |
Daulaire 1992.
| Study characteristics | ||
| Methods | Cluster‐randomised, non‐placebo‐controlled trial conducted in Jumla district, Nepal | |
| Participants |
Eligibility: children aged 1–59 months Sample: 16 clusters randomly assigned. 7197 children; 3786 in vitamin A group, 3411 in control group. 51% boys |
|
| Interventions |
Experimental group: single‐dose vitamin A 200,000 IU for children aged 12–59 months; 100,000 IU for children aged 6–12 months; and 50,000 IU for children aged < 6 months Control group: treatment as usual Follow‐up: 5 months |
|
| Outcomes | All‐cause mortality and cause‐specific mortality due to diarrhoea, pneumonia, and measles | |
| Notes | Study site was a remote, mountainous region of northwestern Nepal with a total population of about 80,000, with 12,000 children under 5 years of age. This area was considered as 1 of the poorest and most medically underserved areas of the country. Infant mortality rate was 189 deaths per 1000 live births and child (1–4 years) mortality rate was 52 per 1000 per year. Malnutrition was prevalent in the study area, and 26% of children aged 1–4 years were experiencing substantial malnutrition. A survey of 3651 children aged < 5 years showed active xerophthalmia in 1.3–2% of population and 1–5% among infants, which is high for this age group. Disaggregated data on mortality were available according to different age groups. We used data for children aged 6–59 months according to the objectives of our review. According to WHO, Nepal is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "We randomly selected by card eight of the 16 sub‐districts for vitamin A supplementation". Comment: probably done. |
| Allocation concealment (selection bias) | High risk |
Comment: author contacted and replied. Quote from author: "No effort was made to conceal the allocation sequence". |
| Blinding (performance bias and detection bias) Blinding of participants | High risk | Quote: "There was no placebo or blinding". |
| Blinding (performance bias and detection bias) Blinding of provider | High risk | Quote: "There was no placebo or blinding". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | High risk | Quote: "There was no placebo or blinding". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there was no loss to follow‐up; coverage of intervention described in detail. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
DEVTA trial 2013.
| Study characteristics | ||
| Methods | Factorial design, cluster‐randomised trial conducted in Northern India | |
| Participants |
Eligibility: children aged 1–6 years Sample: total clusters were 72; 36 clusters in vitamin A group, 36 in control group. Authors claimed to include 1 million children in the trial |
|
| Interventions |
Experimental group: vitamin A 200,000 IU every 6 months for 5 years. Vitamin A was supplemented on mass treatment days by village childcare workers. Capsules were open and poured into child's mouth Control group: no intervention Factorial design was:
|
|
| Outcomes | All‐cause mortality; cause‐specific mortality due to diarrhoea, pneumonia, measles, and malnutrition; mean vitamin A serum levels; prevalence of Bitot's spots, and measles and pneumonia morbidity | |
| Notes | Study was conducted in Uttar Pradesh, India. Study utilised the infrastructure of the Integrated Child Development Services, which maintains childcare centres called Anganwadi childcare centres across the state. The other intervention as part of the factorial design was albendazole for deworming. Study was approved by King George's Medical University. Surveillance for disease outcomes was performed every 6 months, and children were not selected randomly for that but chosen from Anganwadi childcare lists. Deaths were recorded by 18 full‐time, motorcycle village‐to‐village monitors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Neighbouring blocks (clusters), in groups of four (where possible in the same district), were randomly allocated in Oxford, UK", and "[a]part from the district each block was in, no relevant details of it were known to those generating the random allocation". Comment: most likely done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Apart from the district each block was in, no relevant details of it were known to those generating the random allocation". |
| Blinding (performance bias and detection bias) Blinding of participants | High risk | Comment: intervention was given on mass treatment days, and used no placebo tablets. So participants most likely were not blinded to treatment allocation. |
| Blinding (performance bias and detection bias) Blinding of provider | High risk | Comment: again, intervention was delivered on mass treatment days by AWC and treatment was known to Anganwadi childcare centres. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | High risk | Comment: outcomes assessors seemed aware of the treatment allocation and control, as parents were asked if their children received intervention on mass treatment days. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: loss to follow‐up was 2%. |
| Selective reporting (reporting bias) | Low risk | Comment: the trial was registered as NCT00222547, and prespecified outcomes were mentioned in protocol and analysed accordingly. |
| Other bias | High risk | Comment: there were concerns that surveillance for implementation of intervention and assessment of outcomes were not rigorous. |
Dibley 1996.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in 34 rural villages located on the southern coast of Central Java in Indonesia | |
| Participants |
Eligibility: children aged 6–47 months Excluded: children with cerebral palsy, epilepsy, flaccid paralysis, mental retardation, congenital or rheumatic heart disease were permanently excluded; those with weight‐for‐height > 3 standard deviations below the WHO growth reference mean or acute xerophthalmia were excluded for 1 cycle and treated with high‐dose vitamin A and then included Sample: 1405 children; 50.9% boys |
|
| Interventions |
Experimental group: vitamin A 206,000 IU in form of retinyl ester + vitamin E 37 IU for children aged > 12 months or 103,000 IU in form of retinyl ester + vitamin E 17 IU for children aged < 12 months of age Control group: placebo containing vitamin E 17 IU or 37 IU according to the age of the participant Study duration: intervention given every 4 months for 24 months Mean of 89% of the children received a treatment (vitamin A or placebo) |
|
| Outcomes | All‐cause mortality, incidence of diarrhoea and respiratory disease, mean vitamin A serum level, proportion of vitamin A deficient, growth | |
| Notes | Baseline demographic, clinical, and nutritional characteristics of the participants were the same, and the groups remained balanced at the start of each of the other 5 cycles. Children were visited every other day for 6 cycles. The longest recall period allowed was 4 days. Observed child‐days of ALRI of the vitamin A group was 280,186 and the control group was 273,630. According to WHO, Indonesia is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomization of the treatments was done with a 1:1 allocation ratio in blocks of eight, based on a table of random permutations of integers". Comment: likely to be adequate. |
| Allocation concealment (selection bias) | Low risk |
Quote: "All investigators, field and laboratory staff, and participants were masked to the treatment code". Quote: "The capsules were packaged in opaque blister packs with a unique treatment code". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "The oily contents of the vitamin A and placebo capsules were of similar taste and colour". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "All investigators, field and laboratory staff, and participants were masked to the treatment code". Comment: adequate allocation concealment and the identical presentation of placebo and vitamin A should have prevented providers becoming unblinded to treatment group assignment. Low risk of performance bias. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk |
Quote: "All investigators, field and laboratory staff, and participants were masked to the treatment code". Comment: adequate allocation concealment and the identical presentation of placebo and vitamin A should have prevented outcome assessors becoming unblinded to treatment group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: complete details of those excluded and lost to follow‐up with reason were described. There was a low and balanced number of withdrawals between groups. The analytical method account for the time on treatment (i.e. follow‐up time for each cycle), and this may have been adequate. |
| Selective reporting (reporting bias) | Low risk | Comment: lack of trial protocol hindered full assessment of this item. However, data on outcomes of relevance to the review were reported. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Donnen 1998.
| Study characteristics | ||
| Methods | Individually randomised, non‐placebo‐controlled trial conducted in South Kivu province of Congo | |
| Participants |
Eligibility: children aged 0–72 months. Children were recruited as soon they were discharged from Kotive Children's Hospital Excluded: none described Sample: 358 children; 118 in vitamin A group, 123 in mebendazole group, 117 in control group |
|
| Interventions | 3 intervention groups Experimental group I: vitamin A 100,000 IU as retinol palmitate for children aged < 1 year and 200,000 IU for children aged > 1 year Experimental group II: mebendazole for deworming Control group: observation Study duration: supplementation was repeated after 6 months and continued for 12 months |
|
| Outcomes | All‐cause mortality, growth, and incidence of diarrhoea and respiratory disease morbidity | |
| Notes | Morbidity surveillance was performed every 2 weeks for the first 3 months, then every 3 months until 12 months. Data on morbidity outcomes were presented as odds ratios based on generalised estimating equation models. As we were using the data as RRs, and no nominators were given in this study, we could not pool the data for diarrhoea and respiratory morbidity from this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "As soon as the children were discharged from the hospital, they were randomly assigned to one of the three groups". Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details available to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk | Comment: insufficient details available to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: insufficient details available to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: insufficient details available to make a judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: overall, 6% of the children were lost to follow‐up, with approximately equal proportions in each group. Some had died but it was not indicated how or from which group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient details available to make a judgement. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Fisker 2014.
| Study characteristics | ||
| Methods | Individually randomised, double‐blind trial conducted in Guinea‐Bissau | |
| Participants |
Eligibility: children aged 6–23 months Excluded: VAS within the preceding month; participation in another trial Sample: 7587 children |
|
| Interventions |
Experimental group: vitamin A 100,000 IU for children aged 6–11 months and 200,000 IU for children aged 12–23 months. Vitamin A bottles contained vegetable oil with vitamin A 200,000 IU as retinyl palmitate and vitamin E 40 IU per mL oil Control group: placebo in same liquid volume as the intervention group. Placebo bottles contained vitamin E 40 IU per mL oil. Supplementation given at time of vaccination. |
|
| Outcomes | All‐cause mortality, sex‐specific mortality, diarrhoea incidence, respiratory infection, adverse events | |
| Notes | Children who died because of accident were censored from mortality data analysis. We used the raw data to calculate the mortality and morbidity estimates (i.e. number of events in intervention group compared to control group, with denominators as time of follow‐up). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The mother then drew a lot from an envelope prepared by the study supervisor". Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Coded vitamin A and placebo supplements were prepared by Skanderborg Pharmacy, Denmark". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "The dark brown bottles contained 10 ml". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: both the interventions were placed in a similar bottle, so it was less likely that those provided knew the allocation. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: study investigators were unaware of allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 27 loss to follow‐up in vitamin A group and 21 in placebo group. Reason for attrition were given, and they were similar in both groups. |
| Selective reporting (reporting bias) | Low risk | Comment: the trial was registered (NCT00514891). All a priori outcomes were reported. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Florentino 1990.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in the municipalities of Pililla and Binangonan in the province of Rizal, Philippines | |
| Participants |
Eligibility: children aged 1–6 years Excluded: any child with clinical signs of VAD Sample: 2471 children. Mean age 3.4 years. 49.5% boys |
|
| Interventions | 3 intervention groups Experimental group I: single high‐dose vitamin A (200,000 IU) Experimental group II: single medium‐dose vitamin A (100,000 IU) Control group: placebo Follow‐up: 1 week. |
|
| Outcomes | Incidence of side effects within 1 week (nausea or vomiting (or both), headache, diarrhoea and fever) | |
| Notes | The study area had a high prevalence of malnutrition, and therefore VAD was likely to be prevalent. The study reported outcomes for the first 48 hours and within 1 week. We pooled the data for the first week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "By use of a double‐blind study design, children were randomly assigned to three treatment groups". Comment: no qualifying information on what 'randomly assigned' means. Difficult to assess sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details available to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "Neither the researchers and field workers nor the subjects knew the contents of the preparations; the code was kept confidential by Hoffman La Roche until after the analysis of the results was completed". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "Neither the researchers and field workers nor the subjects knew the contents of the preparations; the code was kept confidential by Hoffman La Roche until after the analysis of the results was completed". Comment: blinding adequate and performance bias unlikely to have influenced results. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "Neither the researchers and field workers nor the subjects knew the contents of the preparations; the code was kept confidential by Hoffman La Roche until after the analysis of the results was completed". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: complete details of those excluded and lost to follow‐up were provided. Only 76 children lost; differences slight between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: though not explicitly stated, all reported measured outcomes have data reported in results with sufficient clarity and explanation. |
| Other bias | Low risk | Comment: no other apparent bias was noted. |
Herrera 1992.
| Study characteristics | ||
| Methods | Cluster‐randomised trial conducted in 5 rural councils in northern Sudan | |
| Participants |
Eligibility: children aged 9–72 months Excluded: children with xerophthalmia Sample: randomisation by households. 28,753 children; 14,455 in vitamin A group, 14,298 in placebo group. 50.7% boys |
|
| Interventions |
Experimental group: vitamin A 200,000 IU of retinol palmitate + vitamin E 40 IU Control group: vitamin E 40 IU Study duration: intervention given every 6 months for 18 months. |
|
| Outcomes | All‐cause mortality; cause‐specific mortality due to diarrhoea, measles, respiratory disease; incidence of diarrhoea, respiratory disease, and measles; incidence of xerophthalmia, Bitot's spots, and night blindness | |
| Notes | Authors used non‐specific terms for describing cause of death (in table 4) such as "shortness of breath", "convulsions", and "fever", etc. We pooled data for "shortness of breath" under the heading of mortality due to LRTI. This is because it is highly unlikely that a child will die of an upper respiratory tract infection, and LRTI is a more general term than pneumonia to cover this, as it includes pneumonia as well. According to WHO, Sudan is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "Randomisation was done by household … Assignment to treatment group was achieved by the two interviewers visiting alternate households throughout the village. All eligible children in alternate households were assigned to receive, every 6 months, either a capsule of 60 mg (200 000 IU) of vitamin A and 40 mg (40 IU) of vitamin E or a capsule of 40 mg of vitamin E without vitamin A". Comment: did not appear to be randomised. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details available to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "The capsules were colour‐coded to avoid the possibility of mix ups, but none of the study team members was aware which was the experimental capsule and which was the placebo until the end of data collection. All eligible children in a household received capsules of the same colour". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "The capsules were colour‐coded to avoid the possibility of mix ups, but none of the study team members was aware which was the experimental capsule and which was the placebo until the end of data collection. All eligible children in a household received capsules of the same colour". Comment: performance bias unlikely given that trialists and staff were blinded during the intervention. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk |
Quote: "Only the manufacturer knew the contents of the capsules until after data collection and preliminary analysis of the results". Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk |
Comment: 3320 children did not receive 1 or 2 of the 3 vitamin A or placebo capsules. Most of this non‐compliant group consisted of children absent from the household at the time of follow‐up, whereas others had moved away or refused to take part further. As a group, the non‐compliant children tended to be from poorer households than those who continued in the study. However, there were no significant differences between vitamin A and placebo groups in the number of non‐compliant children or in their ages, sex or nutritional status. With respect to the variables relevant to the intervention, the losses to follow‐up were not significantly different from those who remained in the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: did not reference a protocol or trial registration number and did not state that all measured outcomes were reported. |
| Other bias | Unclear risk | Comment: insufficient details available to make a judgement. |
Kartasasmita 1995.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in a suburban community of city Bandung, Indonesia | |
| Participants |
Eligibility: children aged 12–54 months Excluded: not specified Sample: 269 children; 126 children in vitamin A group, 141 children in control group. Mean age 33 months. 51% boys |
|
| Interventions |
Experimental group: vitamin A 200,000 IU once every 6 months for 12 months Control group: placebo |
|
| Outcomes | Incidence of respiratory disease, mean serum retinol levels | |
| Notes | Authors presented data on respiratory outcomes according to severity of disease. We included data for "severe respiratory disease" only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The children were selected by randomised stratified sampling from the almost 2000 under‐fives residing in Cikutra". Comment: insufficient details available to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details provided to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk | Quote: "All children participated in an age‐ and sex‐matched randomised, double blind vitamin A supplementation programme by receiving vitamin A 200,000 IU or placebo capsules orally, at the start and at the 6th month of the study". |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Incomplete outcome data (attrition bias) | High risk | Comment: insufficient reporting of attrition/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Other bias | Unclear risk | Comment: methods of study were not described very clearly. |
Lie 1993.
| Study characteristics | ||
| Methods | Randomised trial conducted in a rural area of China | |
| Participants |
Eligibility: children aged 6 months to 3 years Sample: 198 children; 105 in vitamin A group, 81 in the placebo group. Mean age and proportion of boys not specified |
|
| Interventions |
Experimental group: vitamin A 200,000 IU for children aged > 12 months and 100,000 IU for children aged < 12 months Control group: placebo (vegetable oil) Study duration: interventions given every 4 months for 12 months |
|
| Outcomes | Incidence of diarrhoea and respiratory disease, all‐cause hospitalisations, diarrhoea‐specific hospitalisations, pneumonia‐specific hospitalisations, mean vitamin A serum levels | |
| Notes | Baseline serum levels of retinol were similar in both groups. Measurement of biochemical vitamin A levels in the study area fulfilled the WHO criterion for an action to be triggered at a public health level. Morbidity surveillance was performed twice a month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "198 children who were randomly assigned on a 3:2 allocation to treatment (105) and control (81) groups". Comment: no more information provided about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "Administration was double blind: neither parents nor doctors knew whether the child was in a treatment or control group". Comment: placebo capsules contained vegetable oil and were likely to have been indistinguishable from intervention. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "Placebo capsules contained vegetable oil and were likely to have been indistinguishable from intervention". Comment: in view of the adequate blinding procedures, performance bias was unlikely to have influenced the results. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "Data collected by doctors who were already blind to treatment group assignment". |
| Incomplete outcome data (attrition bias) | High risk | Comment: reasons for loss to follow‐up were not provided. The number randomised and those reported after loss to follow‐up did not match. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol of study was not available to permit a clear judgement. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Lima 2014.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in Fortaleza, the capital of the Ceara state in northeastern Brazil | |
| Participants |
Eligibility: children aged 2 months to 9 years Excluded: children with fever > 38 °C or exclusively breastfed Sample: 79 children; 39 in vitamin A group, 40 in control group. Mean age 43.3 months. 57% boys |
|
| Interventions |
Experimental group: vitamin A 100,000 IU in form of retinol palmitate for children aged < 12 months and 200,000 IU for children aged > 12 months Control group: vitamin E Supplements given at enrolment, 4 months and 8 months |
|
| Outcomes | Mean serum retinol levels, growth and adverse reactions to vitamin A | |
| Notes | Infant mortality rate in study area was 35/1000 live births. Primary objective of study was to measure the effect of vitamin A on barrier function of gastrointestinal tract. Study concluded that the prevalence of new parasitic infection, especially with Giardia species, was significantly decreased with vitamin A intervention, suggesting an immune regulatory modulation of this nutrient on parasitic intestinal infections. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: 79 children were randomly selected (using computer‐generated random numbers). |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Comment: the parent or guardian of the children, field study team and investigators were blinded to treatment agent. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: the parent or guardian of the children, field study team and investigators were blinded to treatment agent |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: the parent or guardian of the children, field study team and investigators were blinded to treatment agent. Indication that blinded field study teams assessed outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: after 12‐month follow‐up, 22 children were withdrawn from the study due to: change of address (16), parents or guardians did not co‐operate with the study (5), and had above the median z score for length or height at the time of the study initiation (1). The percentage of participants completing the study at 12 months was 72.2%. |
| Selective reporting (reporting bias) | High risk | Comment: the objective of study also included reporting of diarrhoea. Authors had reported the overall incidence of diarrhoea in the whole population but the figures were presented in a way that they could not be used in the meta‐analysis. |
| Other bias | Low risk | Comment: no other apparent bias observed. |
Lin 2008.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled trial conducted in Wuhan, an industrial centre in central region of China | |
| Participants |
Eligibility: children aged 2–7 years. Children were recruited from kindergarten in the area Excluded: children with fever, diarrhoea or a recent preventive injection; underweight children with BMI age‐ and sex‐specific 5th percentile of the first US National Health and Nutrition Examination Survey data; children whose protein or energy intake met Chinese RDA Sample: 105 children. Mean age 55 months. 61% boys |
|
| Interventions | 3 intervention groups. 2 consisted of children who were vitamin A deficient and 1 with children who were vitamin A sufficient. Experimental group I (vitamin A deficient): vitamin A 100,000 IU every month for 3 months Control group I (vitamin A deficient): placebo Control group II (vitamin A sufficient): placebo |
|
| Outcomes | All‐cause mortality, mean serum vitamin A levels | |
| Notes | We included data for vitamin A deficient children who were either supplemented with vitamin A or placebo. According to WHO, China does not have a high child mortality rate (i.e. < 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The remaining 70 vitamin A‐deficient children were randomly and equally divided into vitamin A deficient‐supplemented group and vitamin A‐deficient placebo group". Comment: the term 'randomised' is also used to describe a third group that is clearly matched. This may not be a randomised controlled trial. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "Children of vitamin A‐deficient‐supplemented group were given 100 000 IU (retinol equivalent) vitamin A capsules every 2 weeks for 3 months (Grubesic, 2004). Children of vitamin A‐sufficient placebo group and vitamin A‐deficient placebo group received placebo capsules in the same way". |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: although study was double randomised trial, no details of how blinding was achieved was described in the district. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no attrition reported. |
| Selective reporting (reporting bias) | High risk | Comment: main outcome data not reported in a manner that could be analysed. |
| Other bias | Unclear risk | Comment: as blinding was not described, potential performance bias and other sources of bias could not be assessed. |
Lin 2009.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in rural China | |
| Participants |
Eligibility: children aged 6 months to 7 years Excluded: children without informed consent or with acute and chronic diseases Sample: 132 children. Mean age 36.5 months. 50% boys |
|
| Interventions | 3 intervention groups Experimental group I: vitamin A 100,000 IU every month for 3 months Experimental group II: beta‐carotene Control group: placebo (biscuits) |
|
| Outcomes | Mean vitamin A serum levels | |
| Notes | We included the results for vitamin A group versus placebo only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The 50 severe vitamin A deficient children and 82 marginal vitamin A deficient children were randomly divided into three groups respectively by using a table with randomly assorted digits". Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no methods of allocation concealment are described in the text. |
| Blinding (performance bias and detection bias) Blinding of participants | High risk |
Quote: "Vitamin A intervening group were administered 100,000 IU vitamin A capsules … the beta‐carotene intervening group … was administered 4 mg purified beta‐carotene … dissolved in vegetable oil and dropped into a general little biscuit … the placebo group were just administered a general little biscuit". Comment: vitamin A and placebo were administered in 2 different forms. Vitamin A in capsule form while placebo was in the form of biscuits. |
| Blinding (performance bias and detection bias) Blinding of provider | High risk | Comment: vitamin A and placebo were administered in 2 different forms. Vitamin A in capsule form while placebo was in the form of biscuits. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | High risk | Comment: vitamin A and placebo were administered in 2 different forms. Vitamin A in capsule form while placebo was in the form of biscuits. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropouts reported, and numbers at baseline and follow‐up appeared to be the same. |
| Selective reporting (reporting bias) | High risk | Comment: use of clinic services, hospitalisation, cause‐specific morbidity not reported. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Long 2006a.
| Study characteristics | ||
| Methods | Factorial design, individually randomised trial conducted in La Magdalena Atlicpac, Mexico | |
| Participants |
Eligibility: children aged 6–15 months Excluded: children with diseases causing immunosuppression and any congenital or acquired alteration of the digestive tract that could alter the absorption of micronutrients; children taking vitamin supplements Sample: 786 children. Mean age 9.8 months. 51.7% boys |
|
| Interventions | 4 intervention groups Experimental group I: vitamin A 20,000 IU retinol every 2 months for children aged < 1 year or 45,000 IU for children aged > 1 year Experimental group II: daily dose equivalent to 20 mg elemental zinc as zinc methionine Experimental group III: zinc supplement + vitamin A as above Control group: placebo Study duration: interventions delivered every 2 months for 12 months |
|
| Outcomes | Diarrhoea and respiratory disease morbidity | |
| Notes | We included data of this factorial design trial in 2 sets. The first data set compared vitamin A vs placebo, and second set compared vitamin A + zinc vs zinc only. Data on respiratory morbidity were given with 3 definitions. We pooled the data for "cough + difficulty breathing" under the heading of LRTI. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was generated by using a random‐number table by project personnel from CENSIA, a division of the Mexican Ministry of Health". |
| Allocation concealment (selection bias) | Low risk | Quote: "These solutions were packaged in consecutively numbered, colour‐coded, opaque plastic droplet bottles to ensure that field personnel and the principal investigator were blinded". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "The vitamin A, zinc, and vitamin A + zinc supplements were prepared by personnel at the National Institute of Nutrition in 5‐mL solutions that were similar in taste and appearance". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "This double‐blind randomised trial … These solutions were packaged in consecutively numbered, color‐coded, opaque plastic droplet bottles to ensure that field personnel and the principal investigator were blinded". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk |
Quote: "This double‐blind randomised trial … These solutions were packaged in consecutively numbered, color‐coded, opaque plastic droplet bottles to ensure that field personnel and the principal investigator were blinded". Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: lost to follow‐up data given along with reasons for lost to follow‐up. 93 children were lost to follow‐up or excluded. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available so could not assess or make a judgement. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Long 2006b.
| Study characteristics | ||
| Methods | — | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | As above (Long 2006a). | |
Long 2007.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in Mexico | |
| Participants |
Eligibility: children aged 5–15 months Excluded: children who were immunosuppressed; had any congenital abnormality or chronic diarrhoea; history of VAS Sample: 195 children; 97 in vitamin A group, 98 in placebo group. 49.7% boys |
|
| Interventions |
Experimental group: vitamin A 20,000 IU for children aged < 12 months and 45,000 IU for children aged > 12 months Control group: placebo Study duration: intervention repeated every 2 months for 12 months |
|
| Outcomes | Incidence of diarrhoea and respiratory disease | |
| Notes | Baseline sociodemographic characteristics of study children and households were similar between groups. Children received monthly visits and referrals to the doctor, which appeared to exceed normal treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The randomisation sequence was generated by project personnel based at the National Institute of Public Health". Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Comment: personnel at the National Institute of Nutrition prepared the supplements to assure that field personnel and the principal investigator were unaware of treatment regimen. Children in the vitamin A and placebo groups received a 5 mL solution, from identical opaque plastic droplet bottles numbered consecutively, administered by the field team. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "Testing had been carried out at the National Institute of Nutrition to assure that the placebo and vitamin A water miscible solution were similar in taste, viscosity and colour". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "Personnel at the National Institute of Nutrition carried out the preparation of the supplements to assure that field personnel and the principal investigator were unaware of treatment regimen". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "Personnel at the National Institute of Nutrition carried out the preparation of the supplements to assure that field personnel and the principal investigator were unaware of treatment regimen". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: unclear what was done with data for 7 missing children, but dropout was small and similar between groups (4 in vitamin A group, 3 in control group). |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not referenced, though the grant applications may be available. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Pant 1996.
| Study characteristics | ||
| Methods | Cluster‐randomised trial in rural Nepal | |
| Participants |
Eligibility: children aged 6 months to 10 years Sample: from 100 potentially eligible cluster sites, 75 were randomised (approximately 25,301 children). Baseline data on the number in each treatment group, proportion of boys and mean age not provided |
|
| Interventions | 3 intervention groups Experimental group I: single‐dose vitamin A 100,000 IU for children aged 6–12 months and 200,000 IU for children aged 1–10 years via a capsule Control group I: control (not adequately described) Control group II: nutritional education Study duration: 24 months |
|
| Outcomes | All‐cause mortality and Bitot's spots | |
| Notes | No details on loss to follow‐up were given. Inclusion/exclusion criteria were inadequately described. No nominators/denominators were available for Bitot's spots. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using random number tables and the reference number for each block …". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: insufficient detail provided to make a judgement. |
| Incomplete outcome data (attrition bias) | High risk | Comment: no information given as regards how incomplete outcome data were addressed. |
| Selective reporting (reporting bias) | High risk | Comment: very specific outcomes reported. 5 types of examinations were administered to the children: ophthalmic, physical, anthropometric, blood and faecal; however, data in results were given only for prevalence of Bitot's spots and all‐cause mortality |
| Other bias | Unclear risk | Comment: insufficient detail provided to make a judgement. |
Pinnock 1986.
| Study characteristics | ||
| Methods | Individually randomised study in urban area of Australia | |
| Participants |
Eligibility: children aged 1–4 years of age in 3 general practices from Adelaide. Children with > 15 days of cough or 3 separate episodes of respiratory illness during the preceding 3 months were eligible Sample: 147 children. Mean age 39.3 months. 50% boys |
|
| Interventions |
Experimental group: vitamin 1160 μg as retinyl palmitate 3 times per week for 20 weeks Control group: placebo |
|
| Outcomes | Acute respiratory infections, pneumonia, mean serum vitamin A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomization of treatment was achieved by combining active and placebo bottles in a sequence, which was determined by consulting a table of random numbers, and numbering the bottles accordingly". Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "The placebo was a similarly constituted syrup omitting retinyl palmitate and labelled and bottled identically". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "All staff connected with the study remained blind to the identity of the child's medication". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "All staff connected with the study remained blind to the identity of the child's medication". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: a high rate of attrition, but reasons for withdrawal given and that there were no significant changes in the distribution of major potential confounding factors between the 2 groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available. |
| Other bias | Low risk | Comment: no other apparent bias observed. |
Pinnock 1988.
| Study characteristics | ||
| Methods | Individually randomised study in urban area of Australia | |
| Participants |
Eligibility: children aged 0–2 years with history of bronchiolitis and nasal culture positive for RSV Excluded: children taking vitamin A; children with cystic fibrosis, cardiopulmonary difficulties, major brain dysfunctions Sample: 206 children. Mean age 58 months. 60% boys |
|
| Interventions |
Experimental group: vitamin 4.2 mg per week as retinyl palmitate for 12 months Control group: placebo |
|
| Outcomes | Diarrhoea, diarrhoea‐related hospitalisation, acute respiratory infections, pneumonia, pneumonia‐related hospitalisation, mean serum vitamin A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomization was achieved by randomly allocating four of eight batch numbers to vitamin A supplement and the remaining four to placebo". Quote: "the batch number code was retained by the manufacturer". |
| Allocation concealment (selection bias) | Low risk |
Quote: "The batch number code was retained by the manufacturer. The bottles were then distributed sequentially according to batch number as children presented …". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "The placebo had an identical appearance and formulation except for the active ingredient". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "Both investigators and parents were blind as to the treatment status of the child". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "Both investigators and parents were blind as to the treatment status of the child … The batch number code was retained by the manufacturer". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: complete details of those excluded and lost to follow‐up were provided. |
| Selective reporting (reporting bias) | High risk | Comment: outcomes mentioned in methods not reported in results. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
Rahman 2001.
| Study characteristics | ||
| Methods | Individually randomised study conducted in an urban area of Bangladesh | |
| Participants |
Eligibility: children aged 12–35 months Excluded: children who had received vitamin A within previous 4 months; had severe malnutrition, with signs or symptoms of vitamin A or zinc deficiency; or with any systemic illness such as diarrhoea, respiratory infection, fever or any other illness that warranted medical intervention at the time of enrolment Sample: 800 children; 200 in each of 4 treatment groups. Mean age 23.5–24.2 months across treatment groups. 56% boys |
|
| Interventions | 4 intervention groups Group I: vitamin A 200,000 IU (60 mg) given as a single capsule at day 14 + placebo syrup daily for 14 days Group II: placebo capsule at day 14 + placebo syrup for 14 days Group III: vitamin A 200,000 IU (60 mg) given as a single capsule at day 14 + zinc syrup daily for 14 days Group IV: zinc syrup daily for 14 days + placebo capsule at day 14 Study duration: 6 months |
|
| Outcomes | Diarrhoea, acute respiratory infections, serum vitamin A levels and vitamin deficiency | |
| Notes | Data on treatment analysis were not presented. We have written to authors to request data on each treatment arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The children were randomly assigned by a person not involved in the study who used permuted blocks of random numbers". Comment: probably done. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Sets of 2 bottles and 1 capsule for each child were serially numbered … A local pharmaceutical company prepared the study syrups (zinc and placebo) which were supplied in identical 50‐mL bottles … The vitamin A and placebo capsules looked identical". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "The zinc and placebo syrups were supplied in bottles that looked identical, and the appearance and consistency of the syrups were similar. Vitamin A and placebo capsules were identical in appearance". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "The randomisation code was kept sealed until the completion of the study". Comment: identical presentation; probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "The treatment allocations were disclosed after the final analysis". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: data on loss to follow‐up given and also stated that the baseline characteristics of children who were excluded or lost to follow‐up were comparable to those of the children who continued in the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available. |
| Other bias | Low risk | Comment: no other apparent bias. |
Rahmathullah 1990.
| Study characteristics | ||
| Methods | Cluster‐randomised trial conducted in Trichy district of Tamil Nadu in southern India | |
| Participants |
Eligibility: children aged 6–60 months Sample: clustering unit was 'panchyat' (local government areas). 206 clusters were formed, and most consisted of 50–100 children. The included clusters had 15,419 children; 7764 in vitamin A group, 7655 in placebo group |
|
| Interventions |
Experimental group: weekly doses vitamin A 8333 IU + vitamin E 20 mg Control group: vitamin E 20 IU in peanut oil Any children diagnosed with xerophthalmia at baseline, mid‐term or final examination were given a high‐dose vitamin A (200,000 IU) and continued in study. Study duration: supplementations given for 52 weeks Children who missed 7 consecutive dosages were excluded from the analysis. |
|
| Outcomes | All‐cause mortality; cause‐specific mortality due to diarrhoea, measles, and respiratory disease; incidence of diarrhoea and respiratory disease morbidity | |
| Notes | Baseline characteristics of groups were similar in terms of age and sex, 1‐month history of diarrhoea and respiratory disease, anthropometric indexes of nutritional status, xerophthalmia status, 5‐year retrospective history of mortality of children aged < 5 years, household economic, household hygienic status and serum retinol levels. On average, > 90% of children were contacted each week, and the lowest coverage in any single week was 88%. 11% had clinical evidence of xerophthalmia, while about 38% had serum retinol concentrations < 0.35 mmol/L at baseline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The clusters were arranged according to population size; after a random start, they were assigned alternately to the treated or control groups". Comment: exact method of sequence generation was not provided. |
| Allocation concealment (selection bias) | Low risk | Quote: "… no one associated with the study was aware of the colour code, which was held by the Hoffmann‐LaRoche until the study ended". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "The appearance and taste of the solutions were identical … no one associated with the study was aware of the colour code, which was held by the Hoffmann‐LaRoche until the study ended". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "The appearance and taste of the solutions were identical … no one associated with the study was aware of the colour code, which was held by the Hoffmann‐LaRoche until the study ended … masked controlled …". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "The appearance and taste of the solutions were identical … no one associated with the study was aware of the colour code, which was held by the Hoffmann‐LaRoche until the study ended … masked controlled …". |
| Incomplete outcome data (attrition bias) | Low risk |
Quote: "There was no difference in rates of contact between the treated and control groups. The reasons for lack of contact included moving from the study area …". Comment: reasons for loss to follow‐up given with a note that there was no difference in contact rates between the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes given in results as mentioned in the methods section. |
| Other bias | Low risk | Comment: no other apparent bias. |
Ramakrishnan 1995.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in rural India | |
| Participants |
Eligibility: children aged 6–36 months Excluded: children with ophthalmic signs of xerophthalmia, serious diseases, or severe malnutrition (< 60% of weight‐for‐age or < 85% of height‐for‐age of the National Center for Health Statistics median), who received appropriate treatment, including vitamin A Sample: 583 children; 309 in vitamin A group, 274 in placebo group. Mean age 18.6 months. 49.9% boys |
|
| Interventions |
Experimental group: vitamin A 100,000 IU for children aged < 1 year and 200,000 IU for children aged > 1 year Control group: placebo Study duration: interventions given every 4 months for 12 months |
|
| Outcomes | Incidence of diarrhoea and respiratory disease | |
| Notes | Definition used for respiratory disease was too generalised to be included under LRTI. It mainly covered upper respiratory tract infections. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The study design was a randomised, double‐blind, placebo controlled intervention trial in which every 4 mo [months] the treatment group received a high‐dose vitamin A supplement and the control group received a placebo". Comment: insufficient detail provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "The study design was a randomised, double‐blind, placebo controlled intervention trial in which every 4 mo the treatment group received a high‐dose vitamin A supplement and the control group received a placebo". Comment: statement that blinding occurred, no further details provided. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "The study design was a randomised, double‐blind, placebo controlled intervention trial in which every 4 mo the treatment group received a high‐dose vitamin A supplement and the control group received a placebo". Comment: statement that blinding occurred, no further details provided. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk |
Quote: "The study design was a randomised, double‐blind, placebo controlled intervention trial in which every 4 mo the treatment group received a high‐dose vitamin A supplement and the control group received a placebo". Comment: statement that blinding occurred, no further details provided. |
| Incomplete outcome data (attrition bias) | Low risk |
Quote: "Out of the 660 children who were eligible, a final group of 592 children who had both pre‐ and post‐anthropometric measurements were used in this analysis. The losses at follow‐up due to migration (n = 50), death (n = 10) and incomplete measurements (n = 8) were similar for both groups". Comment: losses were not large and balanced between groups; unlikely to introduce substantial bias here. Clinically relevant impact unlikely. |
| Selective reporting (reporting bias) | High risk |
Quote: "The examination for ophthalmic signs of vitamin A deficiency, using WHO criteria (27), was conducted by trained ophthalmologists from the Department of Ophthalmology, CMCH, at baseline and at the end of the 1‐y follow‐up period. Blood samples were also taken (from finger pricks) at the beginning and the end of the study by using 250‐pt capillary tubes. Serum retinol concentrations were estimated by using reversed‐phase HPLC at the Wellcome Research Laboratory, CMCH, Vellore, using retinyl acetate and all trans‐retinol (Sigma Chemical Co, St Louis) as standards". Comment: though measured, serum retinol results were not reported. |
| Other bias | Low risk | Comment: no other apparent bias. |
Reddy 1986a.
| Study characteristics | ||
| Methods | Factorial design, individually randomised trial conducted in India | |
| Participants |
Eligibility: children aged 1–5 years Excluded: children without parental consent Sample: 487 children. Mean age and proportion of boys not reported |
|
| Interventions | 4 intervention groups Experimental group I: oral administration of L‐tetramisole 50 mg followed 3 days later by vitamin A 200,000 IU Experimental group II: vitamin A 200,000 IU Experimental group III: L‐tetramisole 50 mg orally Control group: placebo |
|
| Outcomes | Mean vitamin A serum levels | |
| Notes | Data included in 2 sets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "After the baseline survey, the children were assigned, randomly, into four groups, matched for age, anthropometry, serum vitamin A, and worm infestation and the following treatment was given". Comment: insufficient details provided to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk |
Quote: "After 6 months and 12 months, heights and weights were measured, clinical status was assessed and morbidity for the preceding one month was recorded. Finger‐prick blood samples were collected and serum vitamin A levels were estimated, stool samples were examined for the presence of ascaris ova and other parasites". Comment: authors did not report height or weights, or detailed data on clinical status or morbidity. |
| Other bias | Unclear risk | Comment: insufficient information to permit judgement. |
Reddy 1986b.
| Study characteristics | ||
| Methods | — | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | As Reddy 1986a above. | |
Ross 1993 HEALTH.
| Study characteristics | ||
| Methods | Randomised, double‐blind controlled trial conducted in Guinea savannah area of Ghana | |
| Participants |
Eligibility: children aged 6–59 months Excluded: children with active xerophthalmia or measles once they were confirmed Sample: 1455 children. 49.5% boys |
|
| Interventions |
Experimental group: vitamin A 200,000 IU retinol equivalent for children aged > 12 months or 100,000 IU for children aged 6–12 months Control group: placebo Study duration: interventions given every 4 months for 12 months |
|
| Outcomes | All‐cause mortality; mean daily prevalence of respiratory tract disease, diarrhoea, measles, malaria; mean vitamin A serum levels; all‐cause hospitalisations | |
| Notes | Study populations were rural and their main staple foods are deficient in carotenoids and vitamin A. VAD and xerophthalmia were recognised as problems locally. Children were visited weekly for 1 year. Children in the Health Study were followed up for 596 child‐years for vitamin A group and 589 for control group. According to WHO, Ghana is a country with a high child mortality rate (i.e. > 40/1000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Randomisation was blocked in both studies to ensure similar numbers of children in each group in each part of the study area". Comment: explicit methods for generating allocation sequence not available. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Randomisation was carried out in London by an independent statistician, who held the randomisation code and who also did an interim analysis of the mortality results from the Survival Study for the trial's data‐monitoring committee after a year of follow‐up". Comment: code was protected for the duration of the trial. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "Vitamin A and placebo were supplied by Hoffmann‐La‐Roche's Sight and Life Programme, and were similar in taste and colour. In the Survival Study, liquid vitamin A or placebo was supplied in opaque 150 mL bottles containing 20 IU/mL vitamin E alone (placebo) or plus 100,000 IU/mL retinol equivalent as retinyl palmitate (vitamin A) in purified peanut oil. Each bottle had a unique number, and was labelled with a cluster code before despatch to Ghana". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: as above; probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: in view of the blinding procedures in place elsewhere in the study, this was probably adequate. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: morbidity information was missing for 5–7% of the weekly follow‐up visits, owing to temporary absences of the study children or their mothers, but the missing data were equally distributed between groups. |
| Selective reporting (reporting bias) | High risk | Comment: there was an indication that xerophthalmia data were measured, but none were reported. No protocol available. |
| Other bias | Low risk | Comment: no other apparent bias. |
Ross 1993 SURVIVAL.
| Study characteristics | ||
| Methods | Cluster‐randomised trial conducted in Ghana | |
| Participants |
Eligibility: children aged 6–90 months Excluded: children with xerophthalmia Sample: 185 clusters that included 21,906 children. 51.5% boys |
|
| Interventions |
Experimental group: vitamin A 100,000 IU for children aged 6–11 months and 200,000 IU for older children + vitamin E 20 IU Control group: placebo + vitamin E 20 IU Study duration: intervention delivered every 4 months for 24 months |
|
| Outcomes | All‐cause mortality and cause‐specific mortality due to diarrhoea, respiratory disease, measles and meningitis; mean vitamin A serum levels; malaria prevalence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Randomisation was blocked in both studies to ensure similar numbers of children in each group in each part of the study area". Comment: explicit methods for generating allocation sequence not available. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Randomisation was carried out in London by an independent statistician, who held the randomisation code and who also did an interim analysis of the mortality results from the Survival Study for the trial's data‐monitoring committee after a year of follow‐up". Comment: code was protected for the duration of trial. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "Vitamin A and placebo were supplied by Hoffmann‐La‐Roche's Sight and Life Programme, and were similar in taste and colour. In the Survival Study, liquid vitamin A or placebo was supplied in opaque 150 mL bottles containing 20 IU/mL vitamin E alone (placebo) or plus 100,000 IU/mL retinol equivalent as retinyl palmitate (vitamin A) in purified peanut oil. Each bottle had a unique number, and was labelled with a cluster code before despatch to Ghana". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: as above; probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: in view of the blinding procedures in place elsewhere in the study, this was probably adequate. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 8.4% (1847) children lost to follow‐up and similar between groups. The reasons for losses to follow‐up were not provided. |
| Selective reporting (reporting bias) | High risk | Comment: authors collected data on night blindness, Bitot's spots and xerophthalmia, but did not report them. |
| Other bias | Unclear risk | Comment: the method for inflating the CIs was not well‐described. No ICC reported |
Semba 1991.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in Indonesia | |
| Participants |
Eligibility: children aged 3–6 years Excluded: children with median weight for age < 80% of the National Center for Health Statistics; children with serious illness were excluded from the study and treated appropriately Sample: 236 children. Mean age 58.9 months. 71.6% boys |
|
| Interventions | 4 intervention groups. 2 groups (vitamin A and placebo) had clinical signs of VAD, while 2 groups were clinically normal. Experimental groups: single‐dose vitamin A 60,000 μg of retinol equivalent Control groups: placebo Follow‐up: 1 month |
|
| Outcomes | Mean vitamin A serum levels | |
| Notes | The 2 vitamin A and 2 placebo groups were combined, respectively, for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "A double‐masked, randomised, placebo‐controlled, clinical trial involving 236 preschool children, age 3–6 years, was carried out at the outpatient clinic of the Cicendo Eye Hospital in Bandung, West Java, Indonesia". Comment: details of sequence generation not provided. |
| Allocation concealment (selection bias) | Low risk |
Quote: "The treatment code was broken after the conclusion of the study". Comment: allocation sequence appeared to have been protected. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "A double‐masked, randomised, placebo‐controlled, clinical trial involving 236 preschool children". Quote: "The vitamin A and placebo solutions were supplied in coded containers, and the identity of the solutions was known only to the manufacturer … The solutions were identical in colour, taste, smell and consistency". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: as above; providers likely to have been adequately blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: the provider administering vitamin A and the outcome assessor appear to be different individuals, and it is not clearly stated if the outcome assessors were also blinded to group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 232/236 children enrolled at baseline completed the study protocol (p. 102). |
| Selective reporting (reporting bias) | Unclear risk | Comment: did not reference a protocol or trial registration number and did not state that all measured outcomes were reported. |
| Other bias | Unclear risk | Comment: insufficient information to permit judgement. |
Semba 1995.
| Study characteristics | ||
| Methods | Individually randomised study in rural Indonesia | |
| Participants |
Eligibility: children aged 6 months at vaccination against measles Excluded: children who had measles previously Sample: 336 children. Baseline details on age and sex not provided |
|
| Interventions |
Experimental group: single‐dose vitamin A 100,000 IU Control group: placebo Vitamin A or placebo given with measles vaccine Study duration: 6 months |
|
| Outcomes | Measles | |
| Notes | Primary objective of study was to measure the antibody response to measles vaccine when given with vitamin A or placebo. Trialists found a significant decrease in seroconversion of measles vaccine in the vitamin A group compared to placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Treatment was assigned by random number table in blocks of ten". Comment: probably done. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Infants received identification numbers as they were enrolled in the study, and each identification number had an envelope with an identical capsule containing either vitamin A or placebo". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "Vitamin A, 100,000 IU, or placebo in identical capsules". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "Infants received identification numbers as they were enrolled in the study, and each identification number had an envelope with an identical capsule containing either vitamin A or placebo". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: as above; probably done. |
| Incomplete outcome data (attrition bias) | High risk |
Quote: "Follow‐up rates were 93% and 90% at one and six months post immunisation, respectively". Comment: the reasons for lost to follow‐up not given; only available‐case data given. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol not available. |
| Other bias | Unclear risk | Comment: inadequate information presented to assess this formally. |
Sempértegui 1999.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in the northwestern region of the Quito, Ecuador | |
| Participants |
Eligibility: children aged 6–36 months Excluded: children with clinical VAD, who did not reliably stay at home or at day care centres during weekdays or who had been given multivitamins in last 3 months Sample: 400 children; 200 in vitamin A group, 200 in placebo group. Mean age 21.1 months. 50% boys |
|
| Interventions |
Experimental group: weekly dose vitamin A 10,000 IU for 40 weeks Control group: weekly placebo for 40 weeks |
|
| Outcomes | Incidence of diarrhoea and respiratory disease morbidity, mean vitamin A serum levels | |
| Notes | The baseline study characteristics were comparable between groups. The study was conducted in a slum with substantial rates of malnutrition and subclinical VAD. Morbidity surveillance was done weekly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For random allocation of each child to treatment or placebo group the following procedure was performed. Identical flasks containing vitamin A or placebo were numbered from 1 to 400 by members of the study team in Boston, Massachusetts. The local Ethical Committee of the Ecuadorian Biotechnology Corporation in Quito did not know the identity of the active or placebo flasks, because they did not have the code. Then, this committee assigned each flask to a specific child from a random list by using a table of random numbers. After randomisation, the ethical committee received the confidential code from Boston". |
| Allocation concealment (selection bias) | Low risk | Quote: "After randomisation, the ethical committee received the confidential code from Boston and kept it for the remainder of the study, when it was revealed". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "Identical flasks containing vitamin A or placebo were numbered from 1 to 400 by members of the study team in Boston, Massachusetts". Comment: trial described as double‐blind; given procedures used for ensuring that intervention and placebo were identical, it is very likely that blinding of children was maintained. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Quote: "The syrups were administered at home and at day care centres by study researchers who were blinded to the presence or absence of active drug". |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: outcome assessors were the same as the providers, therefore blinded. |
| Incomplete outcome data (attrition bias) | Low risk |
Quote: "A total of 306 children finished the study, because 50 children from the supplement‐treated group and 44 from the non‐supplemented group were lost to follow‐up when their families moved to other neighbourhoods. Of all children, 70%, including those lost to follow‐up, accumulated > 30 weeks of observation … Children with incomplete follow‐up were distributed evenly in relation to the baseline variables". Comment: loss to follow‐up similar in magnitude in both groups and for similar reasons. Some lost still contributed data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol referred to but not referenced. Not explicitly stated if all measured outcomes were reported. |
| Other bias | Low risk | Comment: no other apparent bias was noted. |
Shankar 1999.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in Guinea Bissau | |
| Participants |
Eligibility: children aged 6–60 months who planned to reside within the study area for ≥ 1 year Excluded: children with ocular signs of VAD or history of night blindness Sample: 480 children; 239 in vitamin A group, 241 in control group. 51% boys |
|
| Interventions |
Experimental group: vitamin A 100,000 IU for children aged < 1 year and 200,000 IU for older children + vitamin E 20 IU Control group: placebo + vitamin E 20 IU Study duration: intervention given every 4 months for 13 months |
|
| Outcomes | Incidence of diarrhoea and malaria morbidity, mean vitamin A serum levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Within these strata, children were individually allocated vitamin A or placebo in blocks of four (two vitamin A, two placebo) by computer generated randomly permutated codes". |
| Allocation concealment (selection bias) | Low risk | Quote: "Capsules were encoded into four groups; two placebo and two vitamin A, and the code was kept offsite by personnel who were not involved in the study". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Comment: identical capsules, and allocation was concealed and code kept off site; described as double‐blind. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: as above; probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: unlikely that the trained village‐based morbidity worker knew the assignments, however, this is never stated explicitly. Probably done. |
| Incomplete outcome data (attrition bias) | Low risk |
Quote: "Cross sectional follow‐up rates for mid‐study and end of study were 428 of 480 (89%) and 410 of 480 (85%), respectively, and similar for vitamin A and placebo groups. During the trial two children dropped out, 66 moved out of the study area, and two died". Comment: intention‐to‐treat used. Missing outcome data balanced in numbers across groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not referenced and not stated that all measured outcomes were reported. Data at 7 months not completely reported. |
| Other bias | Low risk | Comment: no other apparent bias. |
Sinha 1976.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in India | |
| Participants |
Eligibility: children aged 2 months to 4.5 years Excluded: not described Sample: 306 children; 153 in vitamin A group, 153 in control group |
|
| Interventions |
Experimental group: vitamin A 200,000 IU every 4 months for 12 months Control group: placebo |
|
| Outcomes | Bitot's spots, side effects (vomiting) | |
| Notes | The people in the study population were extremely poor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The children were divided in two groups of 153 each (two of the children died in the 1st year and two left the village) and were matched for age, sex, socioeconomic status, and playmate contacts. One of the children of each matched pair was selected randomly for receiving vitamin A and the other child received a placebo". Comment: no detail about randomisation method provided. |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "In a separate laboratory, the designated 2‐ml dose of vitamin A or placebo for each child was put into a vial labelled with the child's number and the vials were then shipped to the field station for distribution. Neither the clinician nor the paramedical workers, who personally fed vitamin A and placebo or examined the children for the signs and symptoms of vitamin A deficiency, knew which children received vitamin A". Comment: insufficient details provided. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "Neither the clinician nor the paramedical workers, who personally fed vitamin A and placebo or examined the children for the signs and symptoms of vitamin A deficiency, knew which children received vitamin A". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "Neither the clinician nor the paramedical workers, who personally fed vitamin A and placebo or examined the children for the signs and symptoms of vitamin A deficiency, knew which children received vitamin A". Quote: "The placebo consisted of deodorized arachis oil which was coloured and favoured with orange to match exactly the vitamin A preparation". Comment: provider blinded. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Quote: "Neither the clinician nor the paramedical workers, who personally fed vitamin A and placebo or examined the children for the signs and symptoms of vitamin A deficiency, knew which children received vitamin A". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: based on the outcome data reported, it seems that no children dropped out (i.e. there were no losses); however, this could be because the authors were conducting an intention‐to‐treat analysis but never reported this. They were not explicit in this regard, as such the risk of bias due to incomplete outcome data was unclear. |
| Selective reporting (reporting bias) | Unclear risk | Comment: did not reference a protocol or trial registration number and did not state that all measured outcomes were reported. |
| Other bias | Low risk | Comment: no other apparent bias. |
Smith 1999.
| Study characteristics | ||
| Methods | Factorial design, individually randomised trial conducted in Belize | |
| Participants |
Eligibility: children aged 2.2–5.5 years Excluded: children with fever or serious respiratory illness Sample: 51 children. Mean age 46.3 months |
|
| Interventions | 4 intervention groups Experimental group I: vitamin A 10,000 IU Experimental group II: zinc 70 mg Experimental group III: vitamin A 10,000 IU + zinc 70 mg Control group: placebo Study duration: 6 months |
|
| Outcomes | Vitamin A serum level | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The children selected were randomly assigned to receive one of the following supplements once per week: placebo; Zn, 70 mg as Zn gluconate; vitamin A, 3030 RE as retinyl palmitate; or a combination of vitamin A and Zn". Comment: stated to be randomised, but no further data reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient details provided. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk |
Quote: "Supplements were ingested orally in an orange flavoured powder (10 g), Tangt (Kraft General Foods Inc, White Plains, NY 10625) prepared as a beverage dissolved in approximately 120 mL of water". Comment: stated to be "double‐blind" in the article keywords, but there appeared to be no details about blinding methods in the text. The intervention (or no intervention in the placebo group) were diluted in the same solution, so presumably all groups were identical. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: not adequately reported. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: not adequately reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient details provided; losses not accounted for by group and small sample size made this especially relevant. |
| Selective reporting (reporting bias) | Unclear risk | Comment: did not reference a protocol or trial registration number and did not state that all measured outcomes were reported. |
| Other bias | Unclear risk | Comment: insufficient details provided. |
Sommer 1986.
| Study characteristics | ||
| Methods | Cluster‐randomised trial conducted in a rural area of Indonesia | |
| Participants |
Eligibility: children aged 0–5 years Excluded: children with active xerophthalmia Sample: 29,236 children from 450 villages (cluster sites) in Java. 50% boys |
|
| Interventions |
Experimental group: vitamin A 200,000 IU capsules administered twice over the course of the study + vitamin E 40 IU Control group: no treatment (served as a waiting list control) Study duration: 9–13 months |
|
| Outcomes | Mortality, diarrhoea, Bitot's spots, night blindness, xerophthalmia | |
| Notes | ICC not reported (CIs from analyses reported to have been adjusted for design effect). TJL back‐calculated an ICC of 0.008307 from effect estimate provided in paper. Vitamin A was not intended to have been distributed to children aged < 12 months, but it would appear that some children aged 0–12 months received the vitamin A capsule. Outcome data were reported on a cohort of 0‐ to 12‐month‐old children. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "From a random start, 450 villages were systematically selected for the study; these were then randomised for capsule distribution after the baseline examination …". Comment: inadequate information provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: inadequate information was presented to assess this item in relation to timing of recruitment into the study. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk |
Quote: "The Government of Indonesia would not condone the use of placebos but field‐workers collecting demographic data were unaware that mortality was a research issue". Comment: described as a controlled study, without adequate description of what the control group received. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk |
Quote: "The Government of Indonesia would not condone the use of placebos but field‐workers collecting demographic data were unaware that mortality was a research issue". Comment: described as a controlled study, without adequate description of what the control group received. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk |
Quote: "The Government of Indonesia would not condone the use of placebos but field‐workers collecting demographic data were unaware that mortality was a research issue". Comment: described as a controlled study, without adequate description of what the control group received. |
| Incomplete outcome data (attrition bias) | Unclear risk |
Quote: "Follow‐up information was available on 89% of the programme children and 88.4% of the controls". Comment: authors indicated percentage remaining per group at follow‐up, but nothing more detailed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial protocol not available. |
| Other bias | Unclear risk | Comment: insufficient information to permit judgement. |
Stabell 1995.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in Guinea Bissau | |
| Participants |
Eligibility: children aged 6 months of age Sample: 68 children; 32 in vitamin A group and 36 in placebo group |
|
| Interventions |
Experimental group: vitamin A 100,000 IU at the time of measles vaccination at 6 and 9 months of age Control group: placebo |
|
| Outcomes | Side effects (bulging fontanelle) | |
| Notes | Denominator data not entirely clear in Table 1 of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Carrying out a double‐blinded, randomised, placebo‐controlled trial". Comment: sequence generation not mentioned in the paper. |
| Allocation concealment (selection bias) | Unclear risk | Comment: nothing mentioned regarding allocation concealment. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk | Comment: claimed it was blinded but no details provided. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: claimed it was blinded but no details provided. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk |
Quote from author: "Children were examined by one of us (CS) to see if their fontanelle was normal, sunken or bulging". Comment: appeared outcome assessors were the same people as the investigators. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: losses to follow‐up by group indicated but no detail provided. Unclear what losses actually occurred in Table 1. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol referenced or statement that all measured outcomes were reported. |
| Other bias | Unclear risk | Comment: short communication, insufficient detail to make an informed judgement. |
Stansfield 1993.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled trial conducted in northwest of Haiti | |
| Participants |
Eligibility: children aged 6–83 months Excluded: children with corneal changes consistent with VAD; with measles; those who had received vitamin A within the past 4 months Sample: 13,651 children. 49% boys |
|
| Interventions |
Experimental group: vitamin A 100,000 IU every 4 months for 3 distribution cycles for children aged 6–11 months and 200,000 IU for the older children Control group: placebo |
|
| Outcomes | 2‐week prevalence of signs of respiratory tract infections (cold, cough and rapid breathing, and diarrhoea) | |
| Notes | A slightly larger number of children (55%) were assigned to vitamin A group. There was a significant difference between groups with respect to age. Study area had a high prevalence of malnutrition and xerophthalmia in the study population. Children were visited every 2 weeks for 12 months. The respiratory disease morbidity was reported with respect to cold, cough and rapid breathing, which were too non‐specific for inclusion under umbrella of pneumonia or LRTI morbidity in our review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote from the author: "A random number generator was used to number the first household and the households were numbered sequentially thereafter. Every other household was given a green capsule, while the rest were given red capsules". Comment: alternate allocation. |
| Allocation concealment (selection bias) | Low risk | Quote from the author: "The manufacturer (Roche) held the code until the study was completed". |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "The colour code was held only by the manufacturer until the study was completed". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk |
Quote: "Before the study inquiries among health workers and community members had indicated no symbolism associated with or preference for either green or red". Comment: highly unlikely that providers would be biased about a single intervention. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk |
Quote: "The colour code was held only by the manufacturer until the study was completed". Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk |
Quote: "The frequency of non‐participation was essentially identical among children from even and odd‐numbered households". Comment: probably done. |
| Selective reporting (reporting bias) | High risk |
Quote: "We did not collect data on the impact of supplementation on vitamin A status, or on the incidence, duration, or severity of symptoms of infection". Comment: only mortality and morbidity outcomes given. Protocol not available. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
van Agtmaal 1988.
| Study characteristics | ||
| Methods | Individually randomised, non‐placebo trial conducted in Thailand | |
| Participants |
Eligibility: not described Excluded: not described Sample: 30 children; 14 in vitamin A group, 21 in control group. Mean age 3.1 years |
|
| Interventions |
Experimental group: single‐dose vitamin A 200,000 IU Control group: no supplement Follow‐up: 4 months |
|
| Outcomes | Mean vitamin A serum levels | |
| Notes | Children were recruited from 3 rural day care centres | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "After selection, 14 children were randomly supplemented with a single, oral dose of vitamin A (110 mg retinyl palmitate, 200,000 IU),according to WHO recommendations (9), and 21 children served as a control group". Comment: inadequate information provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: inadequate information provided. |
| Blinding (performance bias and detection bias) Blinding of participants | Unclear risk | Comment: inadequate information provided. |
| Blinding (performance bias and detection bias) Blinding of provider | Unclear risk | Comment: inadequate information provided. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Unclear risk | Comment: inadequate information provided. |
| Incomplete outcome data (attrition bias) | High risk |
Quote: "Due to the absence of some children at the different time points the number of data available for statistical analysis was less than the total number of children involved in this study … the number of children from whom complete data sets could be collected was rather low". Comment: no comprehensive data given on lost to follow‐up nor reasons for loss. |
| Selective reporting (reporting bias) | High risk | Comment: did not report data on serum retinol levels, which were collected/measured. |
| Other bias | Unclear risk | Comment: inadequate information provided. |
Venkatarao 1996.
| Study characteristics | ||
| Methods | Individually randomised trial conducted in India | |
| Participants |
Eligibility: infants aged 6 months Sample: 909 infants. 50% boys |
|
| Interventions | 3 intervention groups Group I: mother and infants both received vitamin A Group II: mother received vitamin A while infant received placebo Group III: both mother and infant received placebo Dose of vitamin A for infant was 200,000 IU |
|
| Outcomes | All‐cause mortality and cause‐specific mortality due to diarrhoea and respiratory disease; incidence of diarrhoea and respiratory disease morbidity | |
| Notes | We included the data for groups I vs II. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Each pair of subjects enrolled for the study was randomly allocated to one of the following three groups: (i) AA‐Both mother and infant received vitamin A, the former soon after delivery and the latter at 6 months; (ii) AP: mother received vitamin A but her infant received a placebo (Sesame oil); and (iii) PP: both mother and infant received placebo, the former Vitamin E and the latter Sesame oil". Comment: insufficient detail to form a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail to form a judgement. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk |
Quote: "At the age of 6 to 6Vi months, the infant was weighed again and given the appropriate syrup by the Medical Officer from coded bottles, supplied again by the Statistical Section at the Camp Office". Comment: probably done. |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: as above; probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: as above; probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk |
Quote: "4 each in the AA and AP groups and 5 in the PP group were withdrawn from the trial on medical grounds such as congenital abnormalities, epileptic fits or jaundice. Migration accounted for the loss of 34 infants in the AA group, 25 in the AP group and 20 in the PP group while 7, 9 and 7 were excluded due to other miscellaneous reasons. Of the remaining 263, 255 and 256 infants in the three group, 233 in the AA and 228 each in the AP and PP groups were followed‐up very regularly and form the basis for analyses in this report". Comment: they provided specific information about losses by group. However, it is unclear why 263 (group I), 255 (group II) and 256 infants (group III) that remain after attrition is described in the results as only 233 in the group I and 228 each in group II and III being used as the basis for analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: did not reference a protocol or trial registration number and did not state that all measured outcomes were reported. |
| Other bias | Low risk |
Quote: "Quality control of the morbidity data collected by the field investigators was undertaken throughout. As long recall periods pose problems, the collection of morbidity data was intensified from once a fortnight to once a week when the study had been in progress for 9 months". Comment: authors attempted to minimise other biases such as recall bias, though specific details of "quality control" were not provided. |
Vijayaraghavan 1990.
| Study characteristics | ||
| Methods | Cluster‐randomised study in rural India | |
| Participants |
Eligibility: children aged 1–5 years Excluded: children with corneal involvement Sample: 15,775 children in 84 clusters. 50.4% boys |
|
| Interventions |
Experimental group: vitamin A 200,000 IU given twice Control group: placebo (arachis oil) Study duration: unclear |
|
| Outcomes | Mortality, diarrhoea, acute respiratory infections, measles | |
| Notes | Respiratory infection has non‐specific definition of "clinically significant cough". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The villages were allocated randomly into two groups‐treatment and control". Comment: insufficient detail to form a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail provided. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "The trial was double blind: the investigators and medical officers did not know which were the treatment and which were the control areas. They were not aware whether the dose they were distributing was vitamin A or placebo. Decoding was done only after data had been collected". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: as above; probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: as above; probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: insufficient detail provided. |
| Selective reporting (reporting bias) | High risk | Comment: incidence of infections outcome not given with respect to vitamin A and control groups. Given according to the clinical vitamin A status of all the study children. |
| Other bias | Low risk | Comment: study appeared free of other bias. |
West 1991.
| Study characteristics | ||
| Methods | Cluster‐randomised study in rural Nepal | |
| Participants |
Eligibility: children aged 0–5 years; children with xerophthalmia Excluded: children who had recently participated in a vitamin A programme Sample: 28,630 children in 261 clusters. 51.3% boys |
|
| Interventions |
Experimental group I: vitamin A 100,000 IU for children aged 6–11 months and 200,000 IU for children aged ≥ 12 months administered 1–3 times + vitamin E 40 IU Experimental group II: very low dose vitamin A (1000 IU) + vitamin E 40 IU Study duration: 16 months |
|
| Outcomes | Mortality, cause‐specific mortality, Bitot's spots, night blindness, xerophthalmia | |
| Notes | ICC not disclosed, although study estimates reported to have been adjusted for the unit of allocation. Study had additional recruitment phases in second and third treatment cycles. 1807 children at 4 months and 2018 children at 8 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "After blocking on the local development area, the 261 wards were randomly assigned to receive vitamin A supplementation or placebos at 4‐month intervals". Comment: inadequately described to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "Both the investigators and communities were masked to the random assignment". Comment: study was a cluster‐designed trial and there was insufficient information to determine whether allocation occurred before or after treatment group assignment was known. |
| Blinding (performance bias and detection bias) Blinding of participants | Low risk | Quote: "The supplements were given as single‐dose gelatin capsules of identical taste and appearance". |
| Blinding (performance bias and detection bias) Blinding of provider | Low risk | Comment: as above; probably done. |
| Blinding (performance bias and detection bias) Blinding of outcome assessor | Low risk | Comment: as above; probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk |
Quote: "All analyses were carried out on an intention‐to‐treat basis. Computed mortality rates were based on child‐years of observation".
Quote: " … all children living in wards which received high dose vitamin A every 4 months were considered to have been treated with vitamin A, and all children living in wards which received placebo were considered 'untreated' ". Comment: the rates of withdrawal were balanced between groups and the data were analysed based on patient‐years of observation. The unclear reasons for withdrawals, variable duration of follow‐up due to more than recruitment cycle and the low rate of mortality in relation to the withdrawal rates mean that it is uncertain whether the study was at risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: complete data for all time points were available for the review. The last available observation reported in a follow‐up article gave an RR for mortality slightly higher than that for the 12‐month data given in the primary study report (0.74 versus 0.7). |
| Other bias | Low risk | Comment: a method for estimating the ICCs was reported in Katz 1995. |
ALRI: acute lower respiratory illness; BMI: body mass index; CENSIA: Centro Nacional para la Salud de la Infancia y la Adolescencia; CI: confidence interval; CMCH: Christian Medication College & Hospital; HPLC: high‐performance liquid chromatography; ICC: intracluster correlation coefficient; LRTI: lower respiratory tract infection; RAND: a function in Excel, which is used to generate a random number; RDA: recommended dietary allowance; RR: risk ratio; RSV: respiratory syncytial virus; VAD: vitamin A deficiency; VAS: vitamin A supplementation; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmad 2020 | Ineligible patient population |
| Ali 2017 | Ineligible patient population |
| Al‐Mekhlafi 2013 | Ineligible patient population (included children aged 7–12 years) |
| Bahl 1997 | Ineligible patient population (included children currently with diarrhoea) |
| Basu 2019 | Study included only very‐low birth weight infants |
| Benn 2017 | Ineligible study design |
| Berde 2019 | Ineligible study design |
| Bhaskaram 1997 | Ineligible study design (not a randomised controlled trial) |
| Bhattacharya 2017 | Ineligible study design |
| Bloem 1990 | Ineligible study design (not a randomised controlled trial); ineligible patient population (mean age of children was 6.6 years (range 3–9 years)) |
| Changezi 2017 | Ineligible study design |
| Chen 2012 | Ineligible comparator (all groups received vitamin A supplementation) |
| Chen 2016 | Ineligible patient population |
| Chhagan 2009 | Ineligible comparator (all groups received vitamin A supplementation) |
| Clermont 2017 | Ineligible study design |
| Edmond 2012 | Ineligible patient population (maternal vitamin A supplementation) |
| Fahmida 2007 | Ineligible patient population (included children aged 3–6 months) |
| Gannon 2014 | Ineligible intervention (vitamin A given with maize) |
| Guevara 2016 | Ineligible patient population |
| Handjieva‐Darlenska 2014 | Ineligible intervention |
| Handjieva‐Darlenska 2016 | Ineligible study design |
| Healy 2018 | Ineligible study design |
| Hoang 2021 | Ineligible patient population |
| Horton 2018 | Ineligible study design |
| Kahbazi 2019 | Ineligible patient population |
| Kartasurya 2012 | Ineligible comparator (vitamin A given to both groups) |
| Kartasurya 2020 | Ineligible comparator |
| Khan 2020 | Ineligible study design |
| Kheirkhah 2016 | Ineligible patient population |
| Koroma 2020 | Ineligible study design |
| Kothari 1991 | Ineligible study design (not a randomised controlled trial) |
| Kranz 2017 | Ineligible study design |
| Laillou 2021 | Ineligible study design |
| Lakshman 2011 | Ineligible study design |
| Liben 2019 | Ineligible study design |
| Longardt 2014 | Ineligible patient population |
| Murray 2016 | Ineligible study design |
| Nankabirwa 2011 | Ineligible study design (vitamin A supplementation not randomised) |
| NCT03383744 | Ineligible comparator |
| NCT04137354 | Comparison between high‐ and low‐dose vitamin A and iron. No placebo group without vitamin A |
| Oiye 2019 | Ineligible study design |
| Oliveira 2016 | Ineligible route of administration |
| Owusu‐Agyei 2013 | Ineligible comparator (vitamin A given to both groups) |
| Pacifici 2016 | Ineligible patient population |
| Patel 2019 | Ineligible patient population |
| Pimpin 2016 | Ineligible study design |
| Semba 1990 | Ineligible intervention (vitamin A given as a therapeutic intervention for Bitot's spots) |
| Semba 2005 | Ineligible patient population (study population consisted of children infected with HIV) |
| Smith 2016 | Ineligible patient population |
| Srinivasan 2016 | Ineligible study design |
| Sukmawati 2020 | Ineligible intervention |
| Tomiya 2017 | Ineligible patient population |
| Wu 2007 | Ineligible study design (not a randomised controlled trial) |
| Yakymenko 2011 | Ineligible comparator (vitamin A given to both groups) |
| Yang 2002 | Ineligible intervention (other micronutrients were supplemented with vitamin A and these supplements were not balanced out in the control group). It was difficult to disaggregate the effect of vitamin A. |
| Zhang 2016 | Ineligible patient population |
| Zhang 2018 | Ineligible study design |
Characteristics of studies awaiting classification [ordered by study ID]
Aklamati 2006.
| Methods | Individually randomised, placebo‐controlled trial conducted in Zambia, Africa |
| Participants | Boys aged 3–4 years 36 children; 19 in vitamin A group, 17 in placebo group |
| Interventions | Experimental group: single‐dose vitamin A 60 mg Control group: placebo |
| Outcomes | Mean plasma retinol levels, prevalence of fever, diarrhoea, rhinorrhoea, cough and malaria |
| Notes | Data were available only in the form of abstract, and the numbers did not match those given in the results section of abstract. We decided to wait for publication of this study before we include it in the review. |
Differences between protocol and review
The current version of the review is similar to the last version (Imdad 2017), except for minor changes and those that are mentioned in the sections below. Most of the changes mentioned below reflect differences between the protocol (Imdad 2010c), and the first version of the review (Imdad 2010a).
Differences between protocol and original review
Types of studies
We made a post hoc decision to include two studies in which participants were assigned using a quasi‐random method (Herrera 1992; Stansfield 1993). Given the design of the interventions and the placebos as well as steps to blind those administering the sequence, we did not think these studies were meaningfully different from RCTs. For more information, see Included studies section.
Types of outcome measures > secondary outcomes
We made a post hoc decision to include two new outcomes.
Hospitalisation.
Vitamin A deficiency status based on serum retinol level.
See Secondary outcomes section.
Differences between original review and last update
Electronic searches
The Global Health database, which we searched in the previous version of this review (Imdad 2010a), was no longer available to us.
The metaRegister of Controlled Trials (mRCT) was under review and unavailable at the time of searching, so we searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) instead.
-
We searched four additional databases for this update.
Science Citation Index.
Conference Proceedings Citation Index – Science.
Cochrane Database of Systematic Reviews.
Database of Abstracts of Reviews of Effects (DARE).
Differences between last update of the review and this version
Data collection and analysis
Electronic searches
For this update, we did not search DARE, as it was no longer available.
In this update, LILACS and AIM were searched via Global Index Medicus. WPRIM (Western Pacific Region Index Medicus), IMSEAR (Index Medicus for the South‐East Asia Region), and IMEMR (Index Medicus for the Eastern Mediterranean Region) were also searched as part of Global Index Medicus. Scopus was searched for this review. The 2021 Embase search was conducted in Embase Elsevier, not Embase Ovid.
Selection of studies
We used the software COVIDENCE for screening of titles for this update. This software was used to assist the team with double screening and management of citations.
Contributions of authors
AI and EMW contributed to the background section.
EMW and AI were primarily responsible for the methods section.
AS conducted the literature search.
JS, MH and AR reviewed citations for inclusion and resolved disagreements through consultation.
EMW and ZAB contributed to writing the discussion.
ZAB provided supervision and contributed to the writing and analyses.
AI is the guarantor of the review.
Sources of support
Internal sources
-
Aga Khan University, Karachi, Pakistan
Zulfiqar A Bhutta ‐ Faculty member
External sources
No sources of support provided
Declarations of interest
AI: reported that he has no conflicts of interest.
EMW: reported that he has no conflicts of interest.
MH: reported that she has no conflicts of interest.
AR: reported that she has no conflicts of interest.
JS: reported that she has no conflicts of interest.
AS: reported that she has no conflicts of interest.
ZB: reported that he has no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Agarwal 1995 {published data only}
- Agarwal DK, Pandey CM, Agarwal KN.Vitamin A administration and preschool child mortality. Nutrition Research 1995;15(5):669-80. [DOI: 10.1016/0271-5317(95)00034-G] [DOI] [Google Scholar]
Albert 2003 {published data only}
- Albert MJ, Qadri F, Wahed MA, Ahmed T, Rahman AS, Ahmed F, et al.Supplementation with zinc, but not vitamin A, improves seroconversion to vibriocidal antibody in children given an oral cholera vaccine. Journal of Infectious Diseases 2003;187(6):909-13. [DOI: 10.1086/368132] [PMID: ] [URL: www.jstor.org/stable/30085780] [DOI] [PubMed] [Google Scholar]
Arya 2000 {published data only}
- Arya S, Chellani H, Pandey J.Evaluation of safety of oral vitamin 'A' megadose co-administered with measles vaccination. Indian Pediatrics 2000;37(12):1341-7. [PMID: ] [PubMed] [Google Scholar]
Bahl 1999 {published data only}
- Bahl R, Kumar R, Bhandari N, Kant S, Srivastava R, Bhan MK.Vitamin A administered with measles vaccine to nine-month-old infants does not reduce vaccine immunogenicity. Journal of Nutrition 1999;129(8):1569-73. [DOI: 10.1093/jn/129.8.1569] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barreto 1994 {published data only}
- Andreozzi VL, Bailey TC, Nobre FF, Struchiner CJ, Barreto ML, Assis AM, et al.Random-effects models in investigating the effect of vitamin A in childhood diarrhea. Annals of Epidemiology 2006;16(4):241-7. [DOI: 10.1016/j.annepidem.2005.08.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Barreto ML, Santos LM, Assis AM, Araújo MP, Farenzena GG, Santos PA, et al.Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet 1994;344(8917):228-31. [DOI: 10.1016/s0140-6736(94)92998-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Benn 1997 {published data only}
- Benn CS, Aaby P, Balé C, Olsen J, Michaelsen KF, George E, et al.Randomised trial of effect of vitamin A supplementation on antibody response to measles vaccine in Guinea-Bissau, West Africa. Lancet 1997;350(9071):101-5. [DOI: 10.1016/S0140-6736(96)12019-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Benn CS, Lisse IM, Bale C, Michaelsen KF, Olsen J, Hedegaard K, et al.No strong long-term effect of vitamin A supplementation in infancy on CD4 and CD8 T-cell subsets. A community study from Guinea-Bissau, West Africa. Annals of Tropical Paediatrics 2000;20(4):259-64. [DOI: 10.1080/02724936.2000.11748145] [PMID: ] [DOI] [PubMed] [Google Scholar]
Biswas 1994 {published data only}
- Biswas R, Biswas AB, Manna B, Bhattacharya SK, Dey R, Sarkar S.Effect of vitamin A supplementation on diarrhoea and acute respiratory tract infection in children. A double blind placebo controlled trial in a Calcutta slum community. European Journal of Epidemiology 1994;10(1):57-61. [DOI: 10.1007/BF01717453] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2013a {published data only}
- Chen K, Chen XR, Zhang L, Luo HY, Gao N, Wang J, et al.Effect of simultaneous supplementation of vitamin A and iron on diarrheal and respiratory tract infection in preschool children in Chengdu City, China. Nutrition 2013;29(10):1197-203. [DOI: 10.1016/j.nut.2013.03.025] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chen K, Zhang L, Luo H, Wang J, Li Q, Mao M.Effect of vitamin A supplements on iron metabolic homeostasis for preschoolers. Chinese Journal of Preventive Medicine 2014;48(1):18-22. [PMID: ] [PubMed] [Google Scholar]
Chen 2013b {published data only}
- Chen K, Chen XR, Zhang L, Luo HY, Gao N, Wang J, et al.Effect of simultaneous supplementation of vitamin A and iron on diarrheal and respiratory tract infection in preschool children in Chengdu City, China. Nutrition 2013;29(10):1197-203. [DOI: 10.1016/j.nut.2013.03.025] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chen K, Zhang L, Luo H, Wang J, Li Q, Mao M.Effect of vitamin A supplements on iron metabolic homeostasis for preschoolers. Chinese Journal of Preventive Medicine 2014;48(1):18-22. [PMID: ] [PubMed] [Google Scholar]
Cherian 2001 {published data only}
- Cherian T, Ranjini EK, Balasubramaniam KA, Raghupathy P.Vitamin A supplementation in children with recurrent respiratory infections. Indian Pediatrics 2001;38(7):771-5. [PMID: ] [PubMed] [Google Scholar]
Cherian 2003 {published data only}
- Cherian T, Varkki S, Raghupathy P, Ratnam S, Chandra RK.Effect of vitamin A supplementation on the immune response to measles vaccination. Vaccine 2003;21(19-20):2418-20. [DOI: 10.1016/S0264-410X(03)00060-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chowdhury 2002 {published data only}
- Chowdhury S, Kumar R, Ganguly NK, Kumar L, Walia BN.Effect of vitamin A supplementation on childhood morbidity and mortality. Indian Journal of Medical Sciences 2002;56(6):259-64. [PMID: ] [PubMed] [Google Scholar]
Daulaire 1992 {published data only}
- Daulaire NM, Starbuck ES, Houston RM, Church MS, Stukel TA, Pandey MR.Childhood mortality after a high dose of vitamin A in a high risk population. BMJ 1992;304(6821):207-10. [DOI: 10.1136/bmj.304.6821.207] [PMCID: PMC1881470] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DEVTA trial 2013 {published data only}
- Awasthi S, Peto R, Bundy D, Read S, Kourellias K, Clark S, et al.Six-monthly vitamin A supplementation from 1 to 6 years of age. ILSI Micronutrient Forum; 2007 Apr 16-18, Istanbul, Turkey.
- Awasthi S, Peto R, Read S, Clark S, Pande V, Bundy D, et al.Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 2013;381(9876):1469-77. [DOI: 10.1016/S0140-6736(12)62125-4] [PMCID: PMC3647148] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Peto R, Read S, Richards SM, Pande V, Bundy D.Population deworming every 6 months with albendazole in 1 million pre-school children in North India: DEVTA, a cluster-randomised trial. Lancet 2013;381(9876):1478-86. [DOI: 10.1016/S0140-6736(12)62126-6] [PMCID: PMC3647147] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dibley 1996 {published data only}
- Dibley MJ, Sadjimin T, Kjolhede CL, Moulton LH.Vitamin A supplementation fails to reduce incidence of acute respiratory illness and diarrhea in preschool-age Indonesian children. Journal of Nutrition 1996;126(2):434-42. [DOI: 10.1093/jn/126.2.434] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hadi H, Dibley MJ, West KP Jr.Complex interactions with infection and diet may explain seasonal growth responses to vitamin A in preschool aged Indonesian children. European Journal of Clinical Nutrition 2004;58(7):990-9. [DOI: 10.1038/sj.ejcn.1601920] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hadi H, Stoltzfus RJ, Dibley MJ, Moulton LH, West KP Jr, Kjolhede CL, et al.Vitamin A supplementation selectively improves the linear growth of Indonesian preschool children: results from a randomized controlled trial. American Journal of Clinical Nutrition 2000;71(2):507-13. [DOI: 10.1093/ajcn/71.2.507] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hadi H, Stoltzfus RJ, Moulton LH, Dibley MJ, West KP Jr.Respiratory infections reduce the growth response to vitamin A supplementation in a randomized controlled trial. International Journal of Epidemiology 1999;28(5):874-81. [DOI: 10.1093/ije/28.5.874] [PMID: ] [DOI] [PubMed] [Google Scholar]
Donnen 1998 {published data only}
- Donnen P, Brasseur D, Dramaix M, Vertongen F, Zihindula M, Muhamiriza M, et al.Vitamin A supplementation but not deworming improves growth of malnourished preschool children in eastern Zaire. Journal of Nutrition 1998;128(8):1320-7. [DOI: 10.1093/jn/128.8.1320] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Donnen P, Dramaix M, Brasseur D, Zihindula M, Muhamiriza M, Hennart P.Malnourished children morbidity following vitamin A supplementation or deworming in Democratic Republic of Congo. Archives of Public Health 1998;56:109-24. [Google Scholar]
Fisker 2014 {published data only}
- Fisker AB, Bale C, Jørgensen MJ, Balde I, Hornshøj L, Bibby BM, et al.High-dose vitamin A supplementation administered with vaccinations after 6 months of age: sex-differential adverse reactions and morbidity. Vaccine 2013;31(31):3191-8. [DOI: 10.1016/j.vaccine.2013.04.072] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fisker AB, Bale C, Rodrigues A, Balde I, Fernandes M, Jørgensen MJ, et al.High-dose vitamin A with vaccination after 6 months of age: a randomized trial. Pediatrics 2014;134(3):e739-e48. [DOI: 10.1542/peds.2014-0550] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fisker AB, Bale C, Rodrigues A, Jørgensen MJ, Danneskiold-Sørensen N, Hornshørj L, et al.P-426 A randomised trial of high-dose vitamin A at vaccination contacts after 6 months of age: sex-differential effects on mortality. European Journal of Epidemiology 2013;28(Suppl 1):S229-30. [PMID: 10.1007/s10654-013-9820-0] [DOI] [Google Scholar]
- Jensen KJ, Fisker AB, Andersen A, Sartono E, Yazdanbakhsh M, Aaby P, et al.The effects of vitamin A supplementation with measles vaccine on leucocyte counts and in vitro cytokine production. British Journal of Nutrition 2016;115(4):619-28. [DOI: 10.1017/S0007114515004869] [PMID: ] [DOI] [PubMed] [Google Scholar]
Florentino 1990 {published data only}
- Florentino RF, Tanchoco CC, Ramos AC, Mendoza TS, Natividad EP, Tangco JB, et al.Tolerance of preschoolers to two dosage strengths of vitamin A preparation. American Journal of Clinical Nutrition 1990;52(4):694-700. [DOI: 10.1093/ajcn/52.4.694] [DOI] [PubMed] [Google Scholar]
Herrera 1992 {published data only}
- Fawzi WW, Herrera MG, Willett WC, El Amin A, Nestel P, Lipsitz S, et al.Vitamin A supplementation and dietary vitamin A in relation to the risk of xerophthalmia. American Journal of Clinical Nutrition 1993;58(3):385-91. [DOI: 10.1093/ajcn/58.3.385] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Herrera MG, Willett WC, Nestel P, El Amin A, Lipsitz S, et al.Dietary vitamin A intake and the risk of mortality among children. American Journal of Clinical Nutrition 1994;59(2):401-8. [PMID: 10.1093/ajcn/59.2.401] [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Herrera MG, Willett WC, Nestel P, El Amin A, Mohamed KA.Dietary vitamin A intake and the incidence of diarrhea and respiratory infection among Sudanese children. Journal of Nutrition 1995;125(5):1211-21. [DOI: 10.1093/jn/125.5.1211] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Herrera MG, Willett WC, Nestel P, El Amin A, Mohamed KA.The effect of vitamin A supplementation on the growth of preschool children in the Sudan. American Journal of Public Health 1997;87(8):1359-62. [DOI: 10.2105/ajph.87.8.1359] [PMCID: PMC1381102] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera MG, Nestel P, El Amin A, Fawzi WW, Mohamed KA, Weld L.Vitamin A supplementation and child survival. Lancet 1992;340(8814):267-71. [DOI: 10.1016/0140-6736(92)92357-l] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kartasasmita 1995 {published data only}
- Kartasasmita CB, Rosmayudi O, Deville W, Demedts M.Plasma retinol level, vitamin A supplementation and acute respiratory infections in children of 1–5 years old in a developing country. Tubercle and Lung Disease 1995;76(6):563-9. [DOI: 10.1016/0962-8479(95)90535-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lie 1993 {published data only}
- Lie C, Ying C, Wang EL, Brun T, Geissler C.Impact of large-dose vitamin A supplementation on childhood diarrhoea, respiratory disease and growth. European Journal of Clinical Nutrition 1993;47(2):88-96. [PMID: ] [PubMed] [Google Scholar]
Lima 2014 {published data only}
- Lima AA, Anstead GM, Zhang Q, Figueiredo IL, Soares AM, Mota RM, et al.Effects of glutamine alone or in combination with zinc and vitamin A on growth, intestinal barrier function, stress and satiety-related hormones in Brazilian shantytown children. Clinics 2014;69(4):225-33. [DOI: 10.6061/clinics/2014(04)02] [PMCID: PMC3971359] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima AA, Kvalsund MP, De Souza PP, Figueiredo IL, Soares AM, Mota RM, et al.Zinc, vitamin A, and glutamine supplementation in Brazilian shantytown children at risk for diarrhea results in sex-specific improvements in verbal learning. Clinics 2013;68(3):351-8. [DOI: 10.6061/clinics/2013(03)OA11] [PMCID: PMC3611743] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima AA, Soares AM, Lima NL, Mota RM, Maciel BL, Kvalsund MP, et al.Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2010;50(3):309-15. [DOI: 10.1097/MPG.0b013e3181a96489] [PMCID: PMC2830290] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin J, Song F, Yao P, Yang X, Li N, Sun S, et al.Effect of vitamin A supplementation on immune function of well-nourished children suffering from vitamin A deficiency in China. European Journal of Clinical Nutrition 2008;62(12):1412-8. [DOI: 10.1038/sj.ejcn.1602881] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2009 {published data only}
- Lin J, Lai X, Qin J, Song F, Zhang Y, Yao P, et al.Effect of beta-carotene supplementation on health and growth of vitamin A deficient children in China rural villages: a randomized controlled trial. e-SPEN, the European e-Journal of Clinical Nutrition and Metabolism 2009;4(1):e17-e21. [DOI: 10.1016/j.eclnm.2008.09.001] [DOI] [Google Scholar]
Long 2006a {published data only}
- Long K, Moran N, Santos J, Rosado J, Estrada-Garcia T.Impact of vitamin A and zinc on diarrheal E. Coli infections and associated diarrheal episodes among children in Mexico City, Mexico. Annals of Nutrition and Metabolism 2013;63(Suppl 1):170. [DOI: 10.1159/000354245] [URL: www.jstor.org/stable/48507351] [DOI] [Google Scholar]
- Long K, Vasan P, Raga H, Santos J, Rosado J, Mamun A.Household water access and sanitation as indicators of vitamin A and zinc efficacy on gut parasite resolution. Annals of Nutrition and Metabolism 2013;63(Suppl 1):221. [PMID: 10.1159/000354245] [PMID: ] [DOI] [Google Scholar]
- Long KZ, Montoya Y, Hertzmark E, Santos JI, Rosado JL.A double-blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. American Journal of Clinical Nutrition 2006;83(3):693-700. [DOI: 10.1093/ajcn.83.3.693] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rosado JL, Caamaño MC, Montoya YA, De Lourdes Solano MdL, Santos JI, Long KZ.Interaction of zinc or vitamin A supplementation and specific parasite infections on Mexican infants' growth: a randomized clinical trial. European Journal of Clinical Nutrition 2009;63(10):1176-84. [DOI: 10.1038/ejcn.2009.53] [PMID: ] [DOI] [PubMed] [Google Scholar]
Long 2006b {published data only}
- Long KZ, Montoya Y, Hertzmark E, Santos JI, Rosado JL.A double-blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. American Journal of Clinical Nutrition 2006;83(3):693-700. [DOI: 10.1093/ajcn.83.3.693] [PMID: ] [DOI] [PubMed] [Google Scholar]
Long 2007 {published data only}
- Long KZ, Estrada-Garcia T, Rosado JL, Santos JI, Haas M, Firestone M, et al.The effect of vitamin A supplementation on the intestinal immune response in Mexican children is modified by pathogen infections and diarrhea. Journal of Nutrition 2006;136(5):1365-70. [DOI: 10.1093/jn/136.5.1365] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Long KZ, Rosado JL, DuPont HL, Hertzmark E, Santos JI.Supplementation with vitamin A reduces watery diarrhoea and respiratory infections in Mexican children. British Journal of Nutrition 2007;97(2):337-43. [DOI: 10.1017/S0007114507257757] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Long KZ, Santos JI, Rosado JL, Lopez-Saucedo C, Thompson-Bonilla R, Abonce M, et al.Impact of vitamin A on selected gastrointestinal pathogen infections and associated diarrheal episodes among children in Mexico City, Mexico. Journal of Infectious Diseases 2006;194(9):1217-25. [DOI: 10.1086/508292] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pant 1996 {published data only}
- Pant CR, Pokharel GP, Curtale F, Pokhrel RP, Grosse RN, Lepkowski J, et al.Impact of nutrition education and mega-dose vitamin A supplementation on the health of children in Nepal. Bulletin of the World Health Organization 1996;74(5):533-45. [PMCID: PMC2486860] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Pokharel GP, Pant CR, Tilden RL, Pokhrel RP, Atmarita, Curtale F.Nutrition education and mega-dose vitamin A supplementation in Nepal. Indian Journal of Pediatrics 1998;65(4):547-55. [DOI: 10.1007/BF02730892] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pinnock 1986 {published data only}
- Pinnock CB, Douglas RM, Badcock NR.Vitamin A status in children who are prone to respiratory tract infections. Australian Paediatric Journal 1986;22(2):95-9. [DOI: 10.1111/j.1440-1754.1986.tb00197.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pinnock 1988 {published data only}
- Pinnock CB, Douglas RM, Martin AJ, Badcock NR.Vitamin A status of children with a history of respiratory syncytial virus infection in infancy. Australian Paediatric Journal 1988;24(5):286-9. [DOI: 10.1111/j.1440-1754.1988.tb01364.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rahman 2001 {published data only}
- Rahman MM, Tofail F, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO.Short-term supplementation with zinc and vitamin A has no significant effect on the growth of undernourished Bangladeshi children. American Journal of Clinical Nutrition 2002;75(1):87-91. [PMID: 10.1093/ajcn/75.1.87] [DOI] [PubMed] [Google Scholar]
- Rahman MM, Vermund SH, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO.Simultaneous zinc and vitamin A supplementation in Bangladeshi children: randomised double blind controlled trial. BMJ 2001;323(7308):314-8. [DOI: 10.1136/bmj.323.7308.314] [PMCID: PMC37318] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO.Synergistic effect of zinc and vitamin A on the biochemical indexes of vitamin A nutrition in children. American Journal of Clinical Nutrition 2002;75(1):92-8. [DOI: 10.1093/ajcn/75.1.92] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rahmathullah 1990 {published data only}
- Acharya SK.Vitamin A supplementation reduces childhood mortality. National Medical Journal of India 1991;4(4):187-9. [PMID: ] [PubMed] [Google Scholar]
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC, Ramaswamy K, Rahmathullah R, et al.Reduced mortality among children in southern India receiving a small weekly dose of vitamin A. New England Journal of Medicine 1990;323(14):929-35. [DOI: 10.1056/NEJM199010043231401] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC.Diarrhea, respiratory infections, and growth are not affected by a weekly low-dose vitamin A supplement: a masked, controlled field trial in children in southern India. American Journal of Clinical Nutrition 1991;54(3):568-77. [DOI: 10.1093/ajcn/54.3.568] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rahmathullah L.Effect of receiving a weekly dose of vitamin A equivalent to the recommended dietary allowances among pre school children on mortality in south India. Indian Journal of Pediatrics 1991;58(6):837-47. [DOI: 10.1007/BF02825447] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramakrishnan 1995 {published data only}
- Ramakrishnan U, Latham MC, Abel R, Frongillo EA Jr.Vitamin A supplementation and morbidity among preschool children in south India. American Journal of Clinical Nutrition 1995;61(6):1295-303. [DOI: 10.1093/ajcn/61.6.1295] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Latham MC, Abel R.Vitamin A supplementation does not improve growth of preschool children: a randomized, double-blind field trial in south India. Journal of Nutrition 1995;125(2):202-11. [DOI: 10.1093/jn/125.2.202] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reddy 1986a {published data only}
- Reddy V, Vijayaraghavan K, Mathur KK.Effect of deworming and vitamin A administration on serum vitamin A levels in preschool children. Journal of Tropical Pediatrics 1986;32(4):196-9. [DOI: 10.1093/tropej/32.4.196] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reddy 1986b {published data only}
- Reddy V, Vijayaraghavan K, Mathur KK.Effect of deworming and vitamin A administration on serum vitamin A levels in preschool children. Journal of Tropical Pediatrics 1986;32(4):196-9. [DOI: 10.1093/tropej/32.4.196] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ross 1993 HEALTH {published data only}
- Benn CS, Aaby P, Nielsen J, Binka FN, Ross DA.Does vitamin A supplementation interact with routine vaccinations? An analysis of the Ghana Vitamin A Supplementation Trial. American Journal of Clinical Nutrition 2009;90(3):629-39. [DOI: 10.3945/ajcn.2009.27477] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Binka FN, Ross DA, Morris SS, Kirkwood BR, Arthur P, Dollimore N, et al.Vitamin A supplementation and childhood malaria in northern Ghana. American Journal of Clinical Nutrition 1995;61(4):853-9. [DOI: 10.1093/ajcn/61.4.853] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Dollimore N, Cutts F, Binka FN, Ross DA, Morris SS, Smith PG.Measles incidence, case fatality, and delayed mortality in children with or without vitamin A supplementation in rural Ghana. American Journal of Epidemiology 1997;146(8):646-54. [DOI: 10.1093/oxfordjournals.aje.a009330] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Filteau SM, Morris SS, Raynes JG, Arthur P, Ross DA, Kirkwood BR, et al.Vitamin A supplementation, morbidity, and serum acute-phase proteins in young Ghanaian children. American Journal of Clinical Nutrition 1995;62(2):434-8. [DOI: 10.1093/ajcn/62.2.434] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Filteau SM, Morris SS, Tomkins AM, Arthur P, Kirkwood BR, Ross DA, et al.Lack of association between vitamin A status and measures of conjunctival epithelial integrity in young children in northern Ghana. European Journal of Clinical Nutrition 1994;48(9):669-77. [PMID: ] [PubMed] [Google Scholar]
- Ghana VAST Study Team, Ross DA, Dollimore N, Smith PG, Kirkwood BR, Arthur P, et al.Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 1993;342(8862):7-12. [PMID: ] [PubMed] [Google Scholar]
- Kirkwood BR, Ross DA, Arthur P, Morris SS, Dollimore N, Binka FN, et al.Effect of vitamin A supplementation on the growth of young children in northern Ghana. American Journal of Clinical Nutrition 1996;63(5):773-81. [PMID: 10.1093/ajcn/63.5.773] [DOI] [PubMed] [Google Scholar]
- Kirkwood BR, Ross DA, Arthur P, Morris SS, Dollimore N, Binka FN, et al.Effect of vitamin A supplementation on the growth of young children in northern Ghana. Early Human Development 1996;46(3):279. [DOI: 10.1016/0378-3782(96)88188-6] [DOI] [PubMed] [Google Scholar]
- Ross DA, Kirkwood BR, Binka FN, Arthur P, Dollimore N, Morris SS, et al.Child morbidity and mortality following vitamin A supplementation in Ghana: time since dosing, number of doses, and time of year. American Journal of Public Health 1995;85(9):1246-51. [DOI: 10.2105/ajph.85.9.1246] [PMCID: PMC1615567] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 1993 SURVIVAL {published data only}
- Benn CS, Aaby P, Nielsen J, Binka FN, Ross DA.Does vitamin A supplementation interact with routine vaccinations? An analysis of the Ghana Vitamin A Supplementation Trial. American Journal of Clinical Nutrition 2009;90(3):629-39. [DOI: 10.3945/ajcn.2009.27477] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Binka FN, Ross DA, Morris SS, Kirkwood BR, Arthur P, Dollimore N, et al.Vitamin A supplementation and childhood malaria in northern Ghana. American Journal of Clinical Nutrition 1995;61(4):853-9. [DOI: 10.1093/ajcn/61.4.853] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Dollimore N, Cutts F, Binka FN, Ross DA, Morris SS, Smith PG.Measles incidence, case fatality, and delayed mortality in children with or without vitamin A supplementation in rural Ghana. American Journal of Epidemiology 1997;146(8):646-54. [DOI: 10.1093/oxfordjournals.aje.a009330] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Filteau SM, Morris SS, Raynes JG, Arthur P, Ross DA, Kirkwood BR, et al.Vitamin A supplementation, morbidity, and serum acute-phase proteins in young Ghanaian children. American Journal of Clinical Nutrition 1995;62(2):434-8. [DOI: 10.1093/ajcn/62.2.434] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Filteau SM, Morris SS, Tomkins AM, Arthur P, Kirkwood BR, Ross DA, et al.Lack of association between vitamin A status and measures of conjunctival epithelial integrity in young children in northern Ghana. European Journal of Clinical Nutrition 1994;48(9):669-77. [PMID: ] [PubMed] [Google Scholar]
- Ghana VAST Study Team, Ross DA, Dollimore N, Smith PG, Kirkwood BR, Arthur P, et al.Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 1993;342(8862):7-12. [PMID: ] [PubMed] [Google Scholar]
- Kirkwood BR, Ross DA, Arthur P, Morris SS, Dollimore N, Binka FN, et al.Effect of vitamin A supplementation on the growth of young children in northern Ghana. American Journal of Clinical Nutrition 1996;63(5):773-81. [DOI: 10.1093/ajcn/63.5.773] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kirkwood BR, Ross DA, Arthur P, Morris SS, Dollimore N, Binka FN, et al.Effect of vitamin A supplementation on the growth of young children in northern Ghana. Early Human Development 1996;46(3):279. [DOI: 10.1016/0378-3782(96)88188-6] [DOI] [PubMed] [Google Scholar]
- Ross DA, Kirkwood BR, Binka FN, Arthur P, Dollimore N, Morris SS, et al.Child morbidity and mortality following vitamin A supplementation in Ghana: time since dosing, number of doses, and time of year. American Journal of Public Health 1995;85(9):1246-51. [DOI: 10.2105/ajph.85.9.1246] [PMCID: PMC1615567] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Semba 1991 {published data only}
- Semba R, Muhilal, Scott A, Natadisastra G, Wirasasmita S, Griffin D, et al.Immune status in children with mild vitamin A deficiency in Indonesia. Investigative Ophthalmology and Visual Science 1991;32(4):-. [URL: iovs.arvojournals.org/article.aspx?articleid=2178555] [Google Scholar]
- Semba RD, Muhilal, Scott AL, Natadisastra G, West KP Jr, Sommer A.Effect of vitamin A supplementation on immunoglobulin G subclass responses to tetanus toxoid in children. Clinical and Diagnostic Laboratory Immunology 1994;1(2):172-5. [DOI: 10.1128/cdli.1.2.172-175.1994] [PMCID: PMC368222] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Muhilal, Scott AL, Natadisastra G, Wirasasmita S, Mele L, et al.Depressed immune response to tetanus in children with vitamin A deficiency. Journal of Nutrition 1992;122(1):101-7. [DOI: 10.1093/jn/122.1.101] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Semba RD, Muhilal MP, West KP Jr, Winget M, Natadisastra G, Scott A, et al.Impact of vitamin A supplementation on hematological indicators of iron metabolism and protein status in children. Nutrition Research 1992;12(4-5):469-78. [DOI: 10.1016/S0271-5317(05)80017-X] [DOI] [Google Scholar]
Semba 1995 {published data only}
- Semba RD, Munasir Z, Beeler J, Akib A, Muhilal, Audet S, et al.Reduced seroconversion to measles in infants given vitamin A with measles vaccination. Lancet 1995;345(8961):1330-2. [DOI: 10.1016/s0140-6736(95)92536-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sempértegui 1999 {published data only}
- Sempértegui F, Estrella B, Camaniero V, Betancourt V, Izurieta R, Ortiz W, et al.The beneficial effects of weekly low-dose vitamin A supplementation on acute lower respiratory infections and diarrhea in Ecuadorian children. Pediatrics 1999;104(1):e1. [DOI: 10.1542/peds.104.1.e1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shankar 1999 {published data only}
- Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, et al.Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet 1999;354(9174):203-9. [DOI: 10.1016/S0140-6736(98)08293-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sinha 1976 {published data only}
- Sinha DP, Bang FB.The effect of massive doses of vitamin A on the signs of vitamin A deficiency in preschool children. American Journal of Clinical Nutrition 1976;29(1):110-5. [DOI: 10.1093/ajcn/29.1.110] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith JC, Makdani D, Hegar A, Rao D, Douglass LW.Vitamin A and zinc supplementation of preschool children. Journal of the American College of Nutrition 1999;18(3):213-22. [DOI: 10.1080/07315724.1999.10718854] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sommer 1986 {published data only}
- Abdeljaber MH, Monto AS, Tilden RL, Schork MA, Tarwotjo I.The impact of vitamin A supplementation on morbidity: a randomized community intervention trial. American Journal of Public Health 1991;81(12):1654-6. [DOI: 10.2105/ajph.81.12.1654] [PMCID: PMC1405285] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djunaedi E, Sommer A, Pandji A, Kusdiono, Taylor HR.Impact of vitamin A supplementation on xerophthalmia. A randomized controlled community trial. Archives of Ophthalmology 1988;106(2):218-22. [DOI: 10.1001/archopht.1988.01060130228033] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schmitz J, West KP Jr, Khatry SK, Wu L, LeClerq SC, Karna S, et al.Vitamin A supplementation in preschool children and risk of hearing loss as adolescents and young adults in rural Nepal: randomised trial cohort follow-up study. BMJ 2012;344:d7962. [DOI: 10.1136/bmj.d7962] [PMCID: PMC3254201] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Tarwotjo I, Djunaedi E, West KP Jr, Loeden AA, Tilden R, et al.Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet 1986;1(8491):1169-73. [DOI: 10.1016/s0140-6736(86)91157-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tielsch JM, West KP Jr.Cost and efficiency considerations in community-based trials of vitamin A in developing countries. Statistics in Medicine 1990;9(1-2):35-41; discussion 41-3. [DOI: 10.1002/sim.4780090110] [PMID: ] [DOI] [PubMed] [Google Scholar]
- West KP Jr, Djunaedi E, Pandji A, Kusdiono, Tarwotjo I, Sommer A.Vitamin A supplementation and growth: a randomized community trial. American Journal of Clinical Nutrition 1988;48(5):1257-64. [DOI: 10.1093/ajcn/48.5.1257] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stabell 1995 {published data only}
- Stabell C, Balé C, Pedro da Silva A, Olsen J, Aaby P.No evidence of fontanelle-bulging episodes after vitamin A supplementation of 6- and 9-month-old infants in Guinea Bissau. European Journal of Clinical Nutrition 1995;49(1):73-4. [PMID: ] [PubMed] [Google Scholar]
Stansfield 1993 {published data only}
- Stansfield SK, Pierre-Louis M, Lerebours G, Augustin A.Vitamin A supplementation and increased prevalence of childhood diarrhoea and acute respiratory infections. Lancet 1993;342(8871):578-82. [DOI: 10.1016/0140-6736(93)91410-N] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Agtmaal 1988 {published data only}
- Agtmaal EJ, Bloem MW, Speek AJ, Saowakontha S, Schreurs HP, Haeringen NJ.The effect of vitamin A supplementation on tear fluid retinol levels of marginally nourished preschool children. Current Eye Research 1988;7(1):43-8. [DOI: 10.3109/02713688809047018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Venkatarao 1996 {published data only}
- Venkatarao T, Ramakrishnan R, Nair NG, Radhakrishnan S, Sundaramoorthy L, Koya PK, et al.Effect of vitamin A supplementation to mother and infant on morbidity in infancy. Indian Pediatrics 1996;33(4):279-86. [PMID: ] [PubMed] [Google Scholar]
Vijayaraghavan 1990 {published data only}
- Vijayaraghavan K, Radhaiah G, Prakasam BS, Sarma KV, Reddy V.Effect of massive dose vitamin A on morbidity and mortality in Indian children. Lancet 1990;336(8727):1342-5. [DOI: 10.1016/0140-6736(90)92895-o] [PMID: ] [DOI] [PubMed] [Google Scholar]
West 1991 {published data only}
- Bishai D, Kumar KC, Waters H, Koenig M, Katz J, Khatry SK, et al.The impact of vitamin A supplementation on mortality inequalities among children in Nepal. Health Policy and Planning 2005;20(1):60-6. [PMID: 10.1093/heapol/czi007] [DOI] [PubMed] [Google Scholar]
- Katz J, West KP Jr, Khatry SK, Thapa MD, LeClerq SC, Pradhan EK, et al.Impact of vitamin A supplementation on prevalence and incidence of xerophthalmia in Nepal. Investigative Ophthalmology and Visual Science 1995;36(13):2577-83. [PMID: ] [PubMed] [Google Scholar]
- Pokhrel RP, Khatry SK, West KP Jr, Shrestha SR, Katz J, Pradhan EK, et al.Sustained reduction in child mortality with vitamin A in Nepal. Lancet 1994;343(8909):1368-9. [DOI: 10.1016/S0140-6736(94)92508-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shih JH, Lu SE.Analysis of failure time data with multilevel clustering, with application to the child vitamin a intervention trial in Nepal. Biometrics 2007;63(3):673-80. [DOI: 10.1111/j.1541-0420.2007.00756.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- West KP Jr, LeClerq SC, Shrestha SR, Wu LS, Pradhan EK, Khatry SK, et al.Effects of vitamin A on growth of vitamin A-deficient children: field studies in Nepal. Journal of Nutrition 1997;127(10):1957-65. [DOI: 10.1093/jn/127.10.1957] [PMID: ] [DOI] [PubMed] [Google Scholar]
- West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, et al.Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 1991;338(8759):67-71. [DOI: 10.1016/0140-6736(91)90070-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmad 2020 {published data only}
- Ahmad SM, Huda MN, Raqib R, Qadri F, Alam MJ, Afsar MN, et al.High-dose neonatal vitamin A supplementation to Bangladeshi infants increases the percentage of CCR9-positive Treg cells in infants with lower birthweight in early infancy, and decreases plasma sCD14 concentration and the prevalence of vitamin A deficiency at two years of age. Journal of Nutrition 2020;150(11):3005-12. [DOI: 10.1093/jn/nxaa260] [PMCID: PMC7675026 (available on 2021-09-16)] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2017 {published data only}
- Ali H, Hamadani J, Mehra S, Tofail F, Hasan MI, Shaikh S, et al.Effect of maternal antenatal and newborn supplementation with vitamin A on cognitive development of school-aged children in rural Bangladesh: a follow-up of a placebo-controlled, randomized trial. American Journal of Clinical Nutrition 2017;106(1):77-87. [DOI: 10.3945/ajcn.116.134478] [PMID: ] [DOI] [PubMed] [Google Scholar]
Al‐Mekhlafi 2013 {published data only}
- Al-Mekhlafi HM, Al-Zabedi EM, Al-Maktari MT, Atroosh WM, Al-Delaimy AK, Moktar N, et al.Effects of vitamin A supplementation on iron status indices and iron deficiency anaemia: a randomized controlled trial. Nutrients 2013;6(1):190-206. [DOI: 10.3390/nu6010190] [PMCID: PMC3916855] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mekhlafi HM, Anuar TS, Al-Zabedi EM, Al-Maktari MT, Mahdy MA, Ahmed A, et al.Does vitamin A supplementation protect schoolchildren from acquiring soil-transmitted helminthiasis? A randomized controlled trial. Parasites & Vectors 2014;7:367. [DOI: 10.1186/1756-3305-7-367] [PMCID: PMC4141119] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahl 1997 {published data only}
- Bahl R, Bhandari N, Taneja S, Bhan MK.The impact of vitamin A supplementation on physical growth of children is dependent on season. European Journal of Clinical Nutrition 1997;51(1):26-9. [DOI: 10.1038/sj.ejcn.1600352] [PMID: ] [DOI] [PubMed] [Google Scholar]
Basu 2019 {published data only}
- Basu S, Khanna P, Srivastava R, Kumar A.Oral vitamin A supplementation in very low birth weight neonates: a randomized controlled trial. European Journal of Pediatrics 2019;178(8):1255-65. [DOI] [PubMed] [Google Scholar]
Benn 2017 {published data only}
- Benn CS, Fisker AB, Aaby P.Serious danger signals: response to: the effect of neonatal vitamin A supplementation on morbidity and mortality at 12 months: a randomized trial. International Journal of Epidemiology 2017;46(5):1718-20. [DOI: 10.1093/ije/dyx139] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berde 2019 {published data only}
- Berde AS, Bester P, Kruger IM.Coverage and factors associated with vitamin A supplementation among children aged 6–59 months in twenty-three sub-Saharan African countries. Public Health Nutrition 2019;22(10):1770-6. [DOI: 10.1017/S1368980018004056] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhaskaram 1997 {published data only}
- Bhaskaram P, Rao KV.Enhancement in seroconversion to measles vaccine with simultaneous administration of vitamin A in 9-months-old Indian infants. Indian Journal of Pediatrics 1997;64(4):503-9. [DOI: 10.1007/BF02737757] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhattacharya 2017 {published data only}
- Bhattacharya S, Singh A.Time to revisit the strategy of massive vitamin A prophylaxis dose administration to the under five children in India – an analysis of available evidence. Clinical Nutrition ESPEN 2017;21:26-30. [DOI: 10.1016/j.clnesp.2017.07.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bloem 1990 {published data only}
- Bloem MW, Wedel M, Agtmaal EJ, Speek AJ, Saowakontha S, Schreurs WH.Vitamin A intervention: short-term effects of a single, oral, massive dose on iron metabolism. American Journal of Clinical Nutrition 1990;51(1):76-9. [DOI: 10.1093/ajcn/51.1.76] [PMID: ] [DOI] [PubMed] [Google Scholar]
Changezi 2017 {published data only}
- Changezi F, Lindberg L.Socio-economic determinants of vitamin A intake in children under 5 years of age: evidence from Pakistan. Journal of Human Nutrition and Dietetics 2017;30(5):615-20. [DOI: 10.1111/jhn.12450] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen K, Li TY, Chen L, Qu P, Liu YX.Effects of vitamin A, vitamin A plus iron and multiple micronutrient-fortified seasoning powder on preschool children in a suburb of Chongqing, China. Journal of Nutritional Science and Vitaminology 2008;54(6):440-7. [DOI: 10.3177/jnsv.54.440] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chen K, Liu YF, Chen L, Zhang X, Liu YX, Chen J, et al.Effects of vitamin A, vitamin A plus iron and multiple micronutrient-fortified seasoning powder on iron metabolic homeostasis. Chinese Journal of Pediatrics 2011;49(12):926-32. [PMID: ] [PubMed] [Google Scholar]
- Chen L, Liu YF, Gong M, Jiang W, Fan Z, Qu P, et al.Effects of vitamin A, vitamin A plus zinc, and multiple micronutrients on anemia in preschool children in Chongqing, China. Asia Pacific Journal of Clinical Nutrition 2012;21(1):3-11. [PMID: ] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen K, Xie HM, Tian W, Zheng X, Jiang AC.Effect of single-dose albendazole and vitamin A supplementation on the iron status of pre-school children in Sichuan, China. British Journal of Nutrition 2016;115(8):1415-23. [DOI: 10.1017/S0007114516000350] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chhagan 2009 {published data only}
- Chhagan MK, den Broeck J, Luabeya KK, Mpontshane N, Tomkins A, Bennish ML.Effect on longitudinal growth and anemia of zinc or multiple micronutrients added to vitamin A: a randomized controlled trial in children aged 6–24 months. BMC Public Health 2010;10:145. [DOI: 10.1186/1471-2458-10-145] [PMCID: PMC2847544] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhagan MK, den Broeck J, Luabeya KK, Mpontshane N, Tucker KL, Bennish ML.Effect of micronutrient supplementation on diarrhoeal disease among stunted children in rural South Africa. European Journal of Clinical Nutrition 2009;63(7):850-7. [DOI: 10.1038/ejcn.2008.78] [PMCID: PMC2705811] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clermont 2017 {published data only}
- Clermont A.The impact of eliminating within-country inequality in health coverage on maternal and child mortality: A Lives Saved Tool analysis. BMC Public Health 2017;17(Suppl 4):734. [DOI: 10.1186/s12889-017-4737-2] [PMCID: PMC5688502] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edmond 2012 {published data only}
- Edmond KL, Hurt L, Fenty J, Amenga-Etego S, Zandoh C, Hurt C, et al.Effect of vitamin A supplementation in women of reproductive age on cause-specific early and late infant mortality in rural Ghana: ObaapaVitA double-blind, cluster-randomised, placebo-controlled trial. BMJ Open 2012;2(1):e000658. [DOI: 10.1136/bmjopen-2011-000658] [PMCID: PMC3330261] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fahmida 2007 {published data only}
- Fahmida U, Rumawas JS, Utomo B, Patmonodewo S, Schultink W.Zinc-iron, but not zinc-alone supplementation, increased linear growth of stunted infants with low haemoglobin. Asia Pacific Journal of Clinical Nutrition 2007;16(2):301-9. [PMID: ] [PubMed] [Google Scholar]
Gannon 2014 {published data only}
- Gannon B, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N, et al.Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. American Journal of Clinical Nutrition 2014;100(6):1541-50. [DOI: 10.3945/ajcn.114.087379] [PMCID: PMC4232019] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guevara 2016 {published data only}
- Guevara D, Reyes S, Anarumba D, Lopez M, Cevallos S, Montenegro E, et al.Impact of milk based micronutrient supplementation on zinc, iron, and vitamin A deficiencies in school children in Quito-Ecuador. FASEB Journal 2016;30(Suppl 1):292.8. [DOI: 10.1096/fasebj.30.1_supplement.292.8] [DOI] [Google Scholar]
Handjieva‐Darlenska 2014 {published data only}
- Handjieva-Darlenska T, Hristova K, Singh RB.Antioxidant vitamins and the heart. World Heart Journal 2014;6(3):179-84. [URL: www.researchgate.net/publication/286291843_Antioxidant_vitamins_and_the_heart] [Google Scholar]
Handjieva‐Darlenska 2016 {published data only}
- Handjieva-Darlenska T, Hristova K, Singh RB.Antioxidant vitamins and the heart. Chronocardiology and Cardiac Research 2016;4:263-70. [Google Scholar]
Healy 2018 {published data only}
- Healy K, Palmer AC, Barffour MA, Schulze KJ, Siamusantu W, Chileshe J, et al.Nutritional status measures are correlated with pupillary responsiveness in Zambian children. Journal of Nutrition 2018;148(7):1160-6. [DOI: 10.1093/jn/nxy069] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoang 2021 {published data only}
- Hoang NT, Orellana L, Gibson RS, Le TD, Worsley A, Sinclair AJ, et al.Multiple micronutrient supplementation improves micronutrient status in primary school children in Hai Phong City, Vietnam: a randomised controlled trial. Scientific Reports 2021;11(1):3728. [DOI: ] [PMCID: PMC7881239] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horton 2018 {published data only}
- Horton S, Blum LS, Diouf M, Ndiaye B, Ndoye F, Niang K, et al.Delivering vitamin A supplements to children aged 6–59 months: comparing delivery through campaigns and through routine health services in Senegal. Current Developments in Nutrition 2018;2(4):nzy006. [DOI: 10.1093/cdn/nzy006] [PMCID: PMC6041955] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahbazi 2019 {published data only}
- Kahbazi M, Sharafkhah M, Yousefichaijan P, Taherahmadi H, Rafiei M, Kaviani P, et al.Vitamin A supplementation is effective for improving the clinical symptoms of urinary tract infections and reducing renal scarring in girls with acute pyelonephritis: a randomized, double-blind placebo-controlled, clinical trial study. Complimentary Therapies in Medicine 2019;42:429-37. [DOI: 10.1016/j.ctim.2018.12.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kartasurya 2012 {published data only}
- Kartasurya MI, Ahmed F, Subagio HW, Rahfiludin MZ, Marks GC.Zinc combined with vitamin A reduces upper respiratory tract infection morbidity in a randomised trial in preschool children in Indonesia. British Journal of Nutrition 2012;108(12):2251-60. [DOI: 10.1017/S0007114512000499] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kartasurya 2020 {published data only}
- Kartasurya MI, Marks GC, Ahmed F, Subagio HW, Rahfiludin MZ.Effect of zinc and vitamin A supplementation on immune responses in Indonesian pre-schoolers. Asia Pacific Journal of Clinical Nutrition 2020;29(4):732-42. [DOI: 10.6133/apjcn.202012_29(4).0008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Khan 2020 {published data only}
- Khan JR, Karim ME.A propensity score analysis of the effect of a single dose vitamin A supplementation on child hemoglobin status in Bangladesh. Child Care in Practice 2020. [DOI: 10.1080/13575279.2020.1837074] [DOI]
Kheirkhah 2016 {published data only}
- Kheirkhah D, Sharif MR, Honarpisheh P, Sharif A.The effects of vitamin A on acute watery diarrhea in children 1–5 years old. International Journal of Medical Research & Health Sciences 2016;5(12):228-32. [URL: www.indianjournals.com/ijor.aspx?target=ijor:ijmrhs&volume=5&issue=12&article=038] [Google Scholar]
Koroma 2020 {published data only}
- Koroma AS, Conteh SG, Bah M, Kamara HI, Turay M, Kandeh A, et al.Routine vitamin A supplementation and other high impact interventions in Sierra Leone. Maternal & Child Nutrition 2020;16(4):e13041. [DOI: 10.1111/mcn.13041] [PMCID: PMC7507363] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kothari 1991 {published data only}
- Kothari G.The effect of vitamin A prophylaxis on morbidity and mortality among children in urban slums in Bombay. Journal of Tropical Pediatrics 1991;37(3):141. [DOI: 10.1093/tropej/37.3.141] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kranz 2017 {published data only}
- Kranz S, Pimpin L, Fawzi W, Duggan C, Webb P, Mozaffarian D.Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food and Nutrition Bulletin 2017;38(2):260-6. [DOI: 10.1177/0379572117696663] [PMID: ] [DOI] [PubMed] [Google Scholar]
Laillou 2021 {published data only}
- Laillou A, Baye K, Zelalem M, Chitekwe S.Vitamin A supplementation and estimated number of averted child deaths in Ethiopia: 15 years in practice (2005–2019). Maternal & Child Nutrition 2021;17(3):e13132. [DOI: 10.1111/mcn.13132] [PMCID: PMC8189216] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakshman 2011 {published data only}
- Lakshman A.Vitamin A and zinc supplements for child survival – experiences and challenges ahead. In: Changer Vir S, editors(s). Public Health and Nutrition in Developing Countries. 1st edition. New Delhi (India): Woodhead Publishing India, 2011:772-94. [Google Scholar]
Liben 2019 {published data only}
- Liben ML, Wuneh AG, Shamie R, Kiros G.Factors associated with child survival in children admitted to outpatient therapeutic program at public health institutions in Afar Regional State, Ethiopia: a prospective cohort study. Journal of Health, Population and Nutrition 2019;38(1):35. [DOI: 10.1186/s41043-019-0193-1] [PMCID: PMC6882177] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Longardt 2014 {published data only}
- Longardt AC, Schmiedchen B, Raila J, Schweigert FJ, Obladen M, Bührer C, et al.Characterization of the vitamin A transport in preterm infants after repeated high-dose vitamin A injections. European Journal of Clinical Nutrition 2014;68(12):1300-4. [DOI: 10.1038/ejcn.2014.202] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murray 2016 {published data only}
- Murray JS, White J.Vitamin A supplementation in infants and children. Journal for Specialists in Pediatric Nursing 2016;21(4):212-7. [DOI: 10.1111/jspn.12156] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nankabirwa 2011 {published data only}
- Nankabirwa V, Tylleskar T, Nankunda J, Engebretsen I, Sommerfelt H, Tumwine J.I.I-002 Prevalence of malaria parasitaemia among infants in Uganda and its association with breastfeeding, peer counselling and vitamin A supplementation. Tropical Medicine & International Health 2011;16(Suppl 1):97. [DOI: 10.1111/j.1365-3156.2011.02861.x] [DOI] [Google Scholar]
- Nankabirwa V, Tylleskar T, Nankunda J, Engebretsen IM, Sommerfelt H, Tumwine JK.Malaria parasitaemia among infants and its association with breastfeeding peer counselling and vitamin A supplementation: a secondary analysis of a cluster randomized trial. PLoS One 2011;6(7):e21862. [DOI: 10.1371/journal.pone.0021862] [PMCID: PMC3131393] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03383744 {published data only}
- NCT03383744.Using stable isotopes to assess the effectiveness of vitamin A supplementation in Cameroon [Using stable isotope techniques to monitor and assess the vitamin A status of children susceptible to infection]. clinicaltrials.gov/ct2/show/NCT03383744 (first received 16 December 2017).
NCT04137354 {published data only}
- NCT04137354.Iron and vitamin A in school children (IronVitA). clinicaltrials.gov/ct2/show/NCT04137354 (first received 24 October 2019).
Oiye 2019 {published data only}
- Oiye S, Safari N, Anyango J, Arimi C, Nyawa B, Kimeu M, et al.Programmatic implications of some vitamin A supplementation and deworming determinants among children aged 6–59 months in resource-poor rural Kenya. Pan African Medical Journal 2019;32:96. [DOI: 10.11604/pamj.2019.32.96.17221] [PMCID: PMC6570821] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliveira 2016 {published data only}
- Oliveira CS, Sampaio P, Muniz PT, Cardoso MA, ENFAC Working Group.Multiple micronutrients in powder delivered through primary health care reduce iron and vitamin A deficiencies in young Amazonian children. Public Health Nutrition 2016;19(16):3039-47. [DOI: 10.1017/S1368980016001294] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Owusu‐Agyei 2013 {published data only}
- Owusu-Agyei S, Newton S, Mahama E, Febir LG, Ali M, Adjei K, et al.Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutrition Journal 2013;12:131. [DOI: 10.1186/1475-2891-12-131] [PMCID: PMC3850154] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pacifici 2016 {published data only}
- Pacifici GM.Effects of vitamin A in neonates and young infants. International Journal of Pediatrics 2016;4(2):1339-54. [DOI: 10.22038/IJP.2016.6445] [DOI] [Google Scholar]
Patel 2019 {published data only}
- Patel N, Penkert RR, Jones BG, Sealy RE, Surman SL, Sun Y, et al.Baseline serum vitamin A and D levels determine benefit of oral vitamin A & D supplements to humoral immune responses following pediatric influenza vaccination. Viruses 2019;11(10):907. [DOI: 10.3390/v11100907] [PMCID: PMC6832482] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pimpin 2016 {published data only}
- Pimpin L, Kranz S, Fawzi W, Duggan C, Mozaffarian D.The importance of vitamin A supplementation dose, frequency and duration on all-cause mortality in children under 5 years. FASEB Journal 2016;30(Suppl 1):671.6. [DOI: 10.1096/fasebj.30.1_supplement.671.6] [DOI] [Google Scholar]
Semba 1990 {published data only}
- Semba RD, Wirasasmita S, Natadisastra G, Muhilal, Sommer A.Response of Bitot's spots in preschool children to vitamin A treatment. American Journal of Ophthalmology 1990;110(4):416-20. [DOI: 10.1016/s0002-9394(14)77024-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Semba 2005 {published data only}
- Semba RD, Ndugwa C, Perry RT, Clark TD, Jackson JB, Melikian G, et al.Effect of periodic vitamin A supplementation on mortality and morbidity of human immunodeficiency virus-infected children in Uganda: a controlled clinical trial. Nutrition 2005;21(1):25-31. [DOI: 10.1016/j.nut.2004.10.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2016 {published data only}
- Smith ER, Muhihi A, Mshamu S, Sudfeld CR, Noor RA, Spiegelman D, et al.The effect of neonatal vitamin A supplementation on morbidity and mortality at 12 months: a randomized trial. International Journal of Epidemiology 2016;45(6):2112-21. [DOI: 10.1093/ije/dyw238] [PMCID: PMC5841838] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasan 2016 {published data only}
- Srinivasan P, Lawa HR, Rosado JL, Al Mamun A, Khatun M, Santos JI, et al.Household and personal factors are sources of heterogeneity in intestinal parasite clearance among Mexican children 6–15 months of age supplemented with vitamin A and zinc. Acta Tropica 2016;156:48-56. [DOI: 10.1016/j.actatropica.2015.12.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sukmawati 2020 {published data only}
- Sukmawati H, Sirajuddin.Assistance in child feeding influences the nutritional intake of stunting children: randomized control trial. Indian Journal of Forensic Medicine & Toxicology 2020;14(3):1948-52. [DOI: 10.37506/ijfmt.v14i3.10713] [DOI] [Google Scholar]
Tomiya 2017 {published data only}
- Tomiya MT, De Arruda IK, Da Silva Diniz A, Santana RA, Da Silveira KC, Andreto LM.The effect of vitamin A supplementation with 400 000 IU vs 200 000 IU on retinol concentrations in the breast milk: a randomized clinical trial. Clinical Nutrition 2017;36(1):100-6. [DOI: 10.1016/j.clnu.2015.11.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2007 {published data only}
- Wu Z, Lin L, Ouyang L.Impact of vitamin A on the immune function of infants. China Tropical Medicine 2007;7(4):540-1. [Google Scholar]
Yakymenko 2011 {published data only}
- Yakymenko D, Benn CS, Martins C, Diness BR, Fisker AB, Rodrigues A, et al.The impact of different doses of vitamin A supplementation on male and female mortality. A randomised trial from Guinea-Bissau. BMC Pediatrics 2011;11:77. [DOI: 10.1186/1471-2431-11-77] [PMCID: PMC3175170] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2002 {published data only}
- Yang YX, Han JH, Shao XP, He M, Bian LH, Wang Z, et al.Effect of micronutrient supplementation on the growth of preschool children in China. Biomedical and Environmental Sciences 2002;15(3):196-202. [PMID: ] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang X, Ding F, Li H, Zhao W, Jing H, Yan Y, et al.Low serum levels of vitamins A, D, and E are associated with recurrent respiratory tract infections in children living in Northern China: a case control study. PLoS One 2016;11(12):e0167689. [DOI: 10.1371/journal.pone.0167689] [PMCID: PMC5147939] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang ZH, Ni M, Hu Y.Current status of vitamin A deficiency in preschool children in Dongguan, China and the effect of vitamin A on serum ferritin and red blood cell parameters. Chinese Journal of Contemporary Pediatrics 2018;20(3):195-9. [DOI: 10.7499/j.issn.1008-8830.2018.03.006] [PMCID: PMC7389791] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aklamati 2006 {published data only}
- Aklamati E, Brown KH, Mulenga M, Kafwembe E, Peerson JM, Stephensen C, et al.Impact of high-dose vitamin A supplements on vitamin A status of 3–4 year old Zambian boys. FASEB Journal 2006;20(5):A1050. [DOI: 10.1096/fasebj.20.5.A1050] [DOI] [Google Scholar]
Additional references
Alvarez 1995
- Alvarez JO, Salazar-Lindo E, Kohatsu J, Miranda P, Stephensen CB.Urinary excretion of retinol in children with acute diarrhoea. American Journal of Clinical Nutrition 1995;61(6):1273-6. [DOI: 10.1093/ajcn/61.6.1273] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bates 1995
- Bates CJ.Vitamin A. Lancet 1995;345(8941):31-5. [DOI: 10.1016/s0140-6736(95)91157-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Beaton 1993
- Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, et al.Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries – nutrition policy discussion paper no. 13. www.unscn.org/layout/modules/resources/files/Policy_paper_No_13.pdf (accessed 23 March 2016).
Bello 2016
- Bello S, Meremikwu MM, Ejemot-Nwadiaro RI, Oduwole O.Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD007719. [DOI: 10.1002/14651858.CD007719.pub4] [PMCID: PMC8483617] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2015
- Bhutta ZA, Baker SK.Premature abandonment of global vitamin A supplementation programmes is not prudent! International Journal of Epidemiology 2015;44(1):297-9. [DOI: 10.1093/ije/dyu274] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2008
- Chen H, Zhuo Q, Yuan W, Wang J, Wu T.Vitamin A for preventing acute lower respiratory tract infections in children up to seven years of age. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD006090. [DOI: 10.1002/14651858.CD006090.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence.Version accessed 5 April 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Darlow 2011
- Darlow BA, Graham PJ.Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birthweight infants. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD000501. [DOI: 10.1002/14651858.CD000501.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fawzi 1993
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F.Vitamin A supplementation and child mortality. A meta-analysis. JAMA 1993;269(7):898-903. [DOI: 10.1001/jama.1993.03500070078033] [PMID: ] [DOI] [PubMed] [Google Scholar]
Glasziou 1993
- Glasziou PP, Mackerras DE.Vitamin A supplementation in infectious diseases: a meta-analysis. BMJ 1993;306(6874):366-70. [DOI: 10.1136/bmj.306.6874.366] [PMCID: PMC1676417] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gogia 2011
- Gogia S, Sachdev HS.Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD007480. [DOI: 10.1002/14651858.CD007480.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Green 1928
- Green HN, Mellanby E.Vitamin A as an anti-infective agent. British Medical Journal 1928;2(3537):691-6. [DOI: 10.1136/bmj.2.3537.691] [PMCID: PMC2456524] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Habicht 2013
- Habicht JP, Victora C.Vitamin A supplementation in Indian children. Lancet 2013;382(9892):592. [DOI: 10.1016/S0140-6736(13)61736-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Haider 2017
- Haider BA, Sharma R, Bhutta ZA.Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD006980. [DOI: 10.1002/14651858.CD006980.pub3] [PMCID: PMC6464547] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haskell 1999
- Haskell MJ, Brown KH.Maternal vitamin A nutriture and the vitamin A content of human milk. Journal of Mammary Gland Biology and Neoplasia 1999;4(3):243-57. [DOI: 10.1023/a:1018745812512] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hathcock 1997
- Hathcock JN.Vitamins and minerals: efficacy and safety. American Journal of Clinical Nutrition 1997;66(2):427-37. [DOI: 10.1093/ajcn/66.2.427] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG.Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hombali 2019
- Hombali AS, Solon JA, Venkatesh BT, Nair NS, Peña-Rosas JP.Fortification of staple foods with vitamin A for vitamin A deficiency. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD010068. [DOI: 10.1002/14651858.CD010068.pub2] [PMCID: PMC6509778] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huiming 2005
- Huiming Y, Chaomin W, Meng M, Yang H, Wan C, Mao M.Vitamin A for treating measles in children. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD001479. [DOI: 10.1002/14651858.CD001479.pub3] [PMCID: PMC7076287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imdad 2010b
- Imdad A, Yakoob MY, Haider BA, Bhutta ZA.Preventive and therapeutic effects of vitamin A supplementation on infant and childhood morbidity and mortality: a systematic review. In: Bhutta ZA, editors(s). Nutrition Interventions for Maternal and Child Health and Survival. Vol. 1. Karachi (Pakistan): Oxford University Press, 2010:125-39. [Google Scholar]
Imdad 2016
- Imdad A, Ahmed Z, Bhutta ZA.Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD007480. [DOI: 10.1002/14651858.CD007480.pub3] [PMCID: PMC6457829] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Irlam 2010
- Irlam JH, Visser MM, Rollins NN, Siegfried N.Micronutrient supplementation in children and adults with HIV infection. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD003650. [DOI: 10.1002/14651858.CD003650.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Katz 1995
- Katz J, West KP Jr, Khatry SK, Thapa MD, LeClerq SC, Pradhan EK, et al.Impact of vitamin A supplementation on prevalence and incidence of xerophthalmia in Nepal. Investigative Ophthalmology and Visual Science 1995;36(13):2577-83. [PMID: ] [PubMed] [Google Scholar]
Klemm 2009
- Klemm RD, West KJ, Tielsh J, Wu L, Katz J.TU54 poster presentation: pooled analysis of Asian newborn vitamin A supplementation trials to assess differential effects of early infant mortality. In: Micronutrients, Health and Development: Evidence-based Programs. Proceedings of the 2nd International Meeting of the Micronutrient Forum; 2009 May 12-15; Beijing, China. 2009.
Klemm 2010
- Klemm RD, West KP Jr, Palmer AC, Johnson Q, Randall P, Ranum P, et al.Vitamin A fortification of wheat flour: considerations and current recommendations. Food and Nutrition Bulletin 2010;31(1 Suppl):S47-61. [DOI: 10.1177/15648265100311S105] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klemm 2016
- Klemm RD, Palmer AC, Greig A, Engle-Stone R, Dalmiya N.A changing landscape for vitamin A programs: implications for optimal intervention packages, program monitoring, and safety. Food and Nutrition Bulletin 2016;37(2 Suppl):S75-86. [DOI: 10.1177/0379572116630481] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mannar 2013
- Mannar V, Schultink W, Spahn K.Vitamin A supplementation in Indian children. Lancet 2013;382(9892):591-2. [DOI: 10.1016/s0140-6736(13)61735-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mayo‐Wilson 2007
- Mayo-Wilson E.Reporting implementation in randomized trials: proposed additions to the consolidated standards of reporting trials statement. American Journal of Public Health 2007;97(4):630-3. [DOI: 10.2105/AJPH.2006.094169] [PMCID: PMC1829360] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayo‐Wilson 2011
- Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA.Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011;343:d5094. [DOI: 10.1136/bmj.d5094] [PMCID: PMC3162042] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayo‐Wilson 2013
- Mayo-Wilson E, Imdad A, Herzer K, Bhutta ZA.Vitamin A supplementation in Indian children. Lancet 2013;382(9892):594. [DOI: 10.1016/S0140-6736(13)61739-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
McCauley 2015
- McCauley ME, den Broek N, Dou L, Othman M.Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD008666. [DOI: 10.1002/14651858.CD008666.pub3] [PMCID: PMC7173731] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitra 1998
- Mitra AK, Alvarez JO, Guay-Woodford L, Fuchs GJ, Wahed MA, Stephenson CB.Urinary retinol excretion and kidney function in children with shigellosis. American Journal of Clinical Nutrition 1998;68(5):1095-103. [DOI: 10.1093/ajcn/68.5.1095] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMCID: PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni 2005
- Ni J, Wei J, Wu T.Vitamin A for non-measles pneumonia in children. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD003700. [DOI: 10.1002/14651858.CD003700.pub2] [PMCID: PMC6991929] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peto 2013
- Peto R, Awasthi S, Read S, Clark S, Bundy D.Vitamin A supplementation in Indian children – authors' reply. Lancet 2013;382(9892):594-6. [DOI: 10.1016/S0140-6736(13)61741-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramakrishnan 2002
- Ramakrishnan U, Darnton-Hill I.Assessment and control of vitamin A deficiency disorders. Journal of Nutrition 2002;132(9 Suppl):2947-53S. [DOI: 10.1093/jn/132.9.2947S] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rice 2004
- Rice AL, West KP Jr, Black RE.Vitamin A deficiency. In: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Vol. 1. Geneva (Switzerland): World Health Organization, 2004. [Google Scholar]
Semba 1999
- Semba RD.Vitamin A and immunity to viral, bacterial and protozoan infections. Proceedings of the Nutrition Society 1999;58(3):719-27. [DOI: 10.1017/s0029665199000944] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shenai 1993
- Shenai JP.Vitamin A. In: Tsang RC, Lucas A, Uauy R, editors(s). Nutritional Needs of the Preterm Infant: Scientific Basis and Practical Guidelines. Baltimore (MD): Williams and Wilkins, 1993:87-100. [Google Scholar]
Sloan 2013
- Sloan NL, Mitra SN.Vitamin A supplementation in Indian children. Lancet 2013;382(9892):593. [DOI: 10.1016/S0140-6736(13)61738-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 1976
- Smith FR, Goodman DS.Vitamin A transport in human vitamin A toxicity. New England Journal of Medicine 1976;294(15):805-8. [DOI: 10.1056/NEJM197604082941503] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sommer 1996
- Sommer A, West KP Jr.Vitamin A Deficiency: Health, Survival and Vision. New York (NY): Oxford University Press, 1996. [Google Scholar]
Sommer 2002
- Sommer A, Davidson FR, Annecy Accords.Assessment and control of vitamin A deficiency: the Annecy Accords. Journal of Nutrition 2002;132(9 Suppl):2845S-50S. [DOI: 10.1093/jn/132.9.2845S] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sommer 2013
- Sommer A, West KP Jr, Martorell R.Vitamin A supplementation in Indian children. Lancet 2013;382(9892):591. [DOI: 10.1016/S0140-6736(13)60645-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stevens 2015
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, et al.Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet. Global Health 2015;3(9):e528-36. [DOI: 10.1016/S2214-109X(15)00039-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
US Institute of Medicine 2001
- US Institute of Medicine.Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academy Press, 2001. [BOOKSHELF ID: NBK222310] [DOI: 10.17226/10026] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Lieshout 2005
- Lieshout M, De Pee S.Vitamin A equivalency estimates: understanding apparent differences. American Journal of Clinical Nutrition 2005;81(4):943-5; author reply 945-6. [DOI: 10.1093/ajcn/81.4.943] [PMID: ] [DOI] [PubMed] [Google Scholar]
Villamor 2000
West 2002
- West CE, Eilander A, Lieshout M.Consequences of revised estimates of carotenoid bioefficacy for dietary control of vitamin A deficiency in developing countries. Journal of Nutrition 2002;132(9 Suppl):2920-6S. [DOI: 10.1093/jn/132.9.2920S] [PMID: ] [DOI] [PubMed] [Google Scholar]
West 2003
- West KP Jr.Vitamin A deficiency disorders in children and women. Food and Nutrition Bulletin 2003;4(4 Suppl):S78-90. [DOI: 10.1177/15648265030244S204] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization.Global prevalence of vitamin A deficiency in populations at risk 1995–2005: WHO global database on vitamin A deficiency; 2009. apps.who.int/iris/bitstream/10665/44110/1/9789241598019_eng.pdf (accessed 23 March 2016).
Wiysonge 2011
- Wiysonge CS, Shey M, Kongnyuy EJ, Sterne JA, Brocklehurst P.Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No: CD003648. [DOI: 10.1002/14651858.CD003648.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Imdad 2010a
- Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA.Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD008524. [DOI: 10.1002/14651858.CD008524.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Imdad 2010c
- Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA.Vitamin A supplementation for preventing morbidity and mortality in children six months to five years of age. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD008524. [DOI: 10.1002/14651858.CD008524] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imdad 2017
- Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA.Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD008524. [DOI: 10.1002/14651858.CD008524.pub3] [PMCID: PMC6464706] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


