Abstract
Postviral gastroparesis has been described in children, but it has not yet been attributed to SARS-CoV-2 infection. Our case report describes a teenager with abdominal pain, early satiety, and vomiting who likely had an asymptomatic SARS-CoV-2 infection 2 months before presentation. Through investigation of epidemiologic links, antibody testing, and clinical course, it is hypothesized that her significant reduction in gastric emptying was due to postviral gastroparesis secondary to SARS-CoV-2. She was treated with supportive care and prokinetic agents. The patient demonstrated symptom resolution and near normalization of gastric emptying by the time of 1 month follow up.
Keywords: SARS-CoV-2, virus, motility, child, postinfectious, COVID-19
INTRODUCTION
Postviral gastroparesis can occur following a variety of viral infections and is characterized by symptoms such as vomiting, abdominal pain, nausea, weight loss, and early satiety (1). Gastroparesis following SARS-CoV-2 infection has not yet been reported in the pediatric population. Here, we present a case of a 16-year-old female with gastroparesis after infection with SARS-CoV-2.
CASE
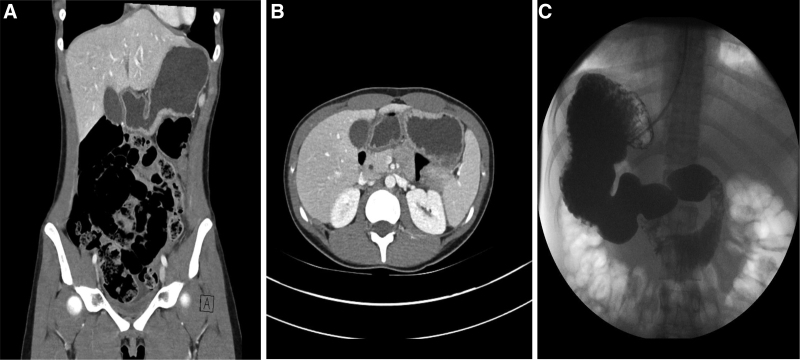
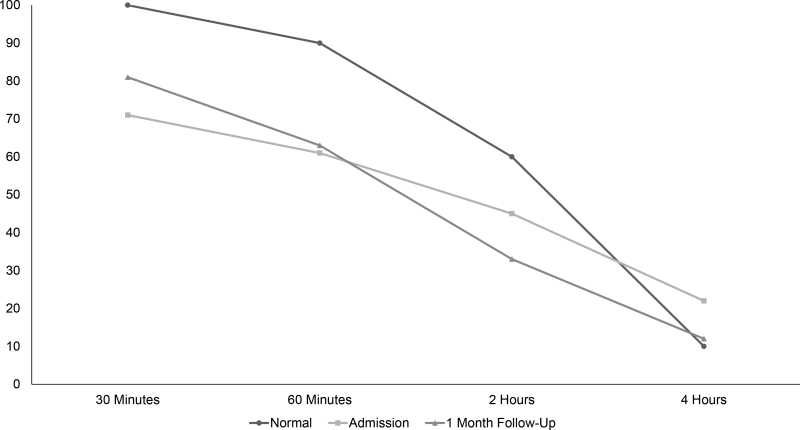
A 16-year-old female with no significant medical history presented with several days of epigastric pain, anorexia, and nonbloody, nonbilious emesis in November 2020. She was not on any medications at the time of symptom onset. She had contact with multiple close family members in the same household who had SARS-CoV-2-induced respiratory illness approximately 2 months prior to her presentation. At presentation, a routine preadmission SARS-CoV-2 PCR was positive. She did not have any respiratory symptoms at admission. Due to the severity of her pain and presence of vomiting, a CT scan was performed and revealed significant gastric distension with normal bowel gas pattern (Fig. 1). A contrast upper gastrointestinal study showed no evidence of anatomic gastric outlet obstruction or malrotation but did show poor forward peristalsis of contrast in the stomach and proximal duodenum. A four-hour gastric emptying scintigraphy study revealed delayed gastric emptying, with 78% gastric emptying at fourth hour (normal ≥ 90%) (Fig. 2). An upper endoscopy revealed normal mucosa in the esophagus, stomach, and duodenum. Biopsies obtained from the antrum were normal. Nasogastric tube decompression led to prompt improvement in symptoms. Use of intravenous metoclopramide led to successful advancement of diet. SARS-CoV2 antibody (IgG) test was positive. She was discharged to home on oral erythromycin therapy as well as dietary modifications used to treat gastroparesis. She continued to improve, with a near-normal gastric emptying study (88% gastric emptying at 4 hour) at 1 month postdischarge, at which time erythromycin was discontinued without return of symptoms.
FIGURE 1.
Persistently distended stomach with poor forward motility seen on imaging. The patient’s stomach, distended with liquid and debris despite over 17 hours of fasting, is shown on CT (A, B) and upper GI series (C). The upper GI series additionally noted poor forward peristalsis in the stomach and duodenum.
FIGURE 2.
Delayed gastric emptying after COVID-19 infection. Residual percentage of stomach technetium-99 activity is depicted at the listed times after ingestion of a labeled meal, along with the corresponding upper limit of normal at our institution. Maximum values in normal subjects are 100% at 30 minutes, 90% at 1 hour, 60% at 2 hours, and 10% at 4 hours. (If abnormal at 4 hours, 10–15% residual is considered mildly delayed, 15–35% moderately delayed, and >35% markedly delayed emptying.).
DISCUSSION
Gastroparesis refers to a delay in gastric emptying that is not caused by a mechanical obstruction. Pediatric patients with gastroparesis most often present with symptoms of vomiting, abdominal pain, nausea, weight loss, and early satiety (2). Four-hour gastric emptying scintigraphy has proven useful in demonstrating objectively decreased motility, although it lacks standardization in how it is conducted and interpreted (3,4). The potential causes of gastroparesis in the pediatric population include idiopathic, postinfectious, dysautonomia, connective tissue disorders, fundoplication, and others. In a recent study, postinfectious gastroparesis accounted for 11% of all cases. A viral etiology is suspected when a viral prodrome is followed by acute-onset symptomatology as previously described. The most frequent documented viral cases of gastroparesis are Epstein-Barr virus, cytomegalovirus, rotavirus, and Norwalk virus (1,5). A viral etiology is considered in gastroparesis patients when their illness is characterized by an acute onset, initial severe illness, and slow resolution toward a satisfactory quality of life (6). Patients are usually treated similarly to other patients with gastroparesis including lifestyle and dietary changes, and prokinetic agents.
Coronavirus Disease 2019 (COVID-19) is a disease caused by infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). It is known to cause a wide range of symptoms, but presentations frequently involve fever, cough, dyspnea, and headache. Gastrointestinal symptoms have also been reported with SARS-CoV2 infection; and some patients have reported symptoms after resolution of their acute infectious stage (7). To the best of our knowledge, there have been no reported cases of children with delayed gastric emptying in the setting of active SARS-CoV-2 infection. There has only been a single documented case of post-COVID-19 gastroparesis in a 37-year-old female with a history of diabetic gastroparesis (8). Our case represents unusual sequelae of COVID-19 infection that may occur with SARS-CoV-2, as with other viral infections. Although direct causation cannot be established in our case, a positive SARS-CoV-2 antibody test obtained during this admission, the aforementioned positive household contacts and improvement in symptoms and follow-up gastric emptying study strongly suggests a diagnosis of postviral gastroparesis following asymptomatic SARS-CoV-2 infection. The authors feel that the positive SARS-CoV-2 RNA PCR was likely due to prolonged viral shedding following an asymptomatic infection about 2 months before admission (9). Unfortunately, cycle threshold values were not available at our institution to conclusively prove that this was not an asymptomatic reinfection (10). The authors feel that routine investigation into recent SARS-CoV-2 infection (or exposure) or positive SARS-CoV-2 antibody test in children diagnosed with gastroparesis may help identify similar pediatric patients with SARS-CoV-2-induced postviral gastroparesis, and help strengthen this association.
ACKNOWLEDGMENTS
Informed consent was obtained from the patient’s guardian for this report
Footnotes
C.M.R. was primarily responsible for drafting the text of this case report as well as literature and chart review. J.M.M. cared for the patient reported here as a fellow and was secondarily responsible for drafting the text of this case report as well as literature and chart review. M.F.G. and S.D. were responsible for reading the radiologic studies described here and identified and provided the specific images used in Figure 1 of this case report. S.S.K. cared for the patient reported here as the attending physician and was secondarily responsible for drafting the text of this case report as well as literature and chart review.
The authors report no conflicts of interest.
REFERENCES
- 1.Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751–754. [DOI] [PubMed] [Google Scholar]
- 2.Waseem S, Islam S, Kahn G, et al. Spectrum of gastroparesis in children. J Pediatr Gastroenterol Nutr. 2012;55:166–172. [DOI] [PubMed] [Google Scholar]
- 3.Abell TL, Camilleri M, Donohoe K, et al.; American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. [DOI] [PubMed] [Google Scholar]
- 4.Wolfson S, Wilhelm Z, Opekun AR, et al. Clinical characterization of pediatric gastroparesis using a four-hour gastric emptying scintigraphy standard. J Pediatr Gastroenterol Nutr. 2021;72:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meeroff JC, Schreiber DS, Trier JS, et al. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370–373. [DOI] [PubMed] [Google Scholar]
- 6.Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis–clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501–1504. [PubMed] [Google Scholar]
- 7.Cholankeril G, Podboy A, Aivaliotis VI, et al. High prevalence of concurrent gastrointestinal manifestations in patients with severe acute respiratory syndrome coronavirus 2: early experience from California. Gastroenterology. 2020;159:775–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J, Bhuta R, Baig K, et al. COVID-19 infection manifesting as a severe gastroparesis flare: a case report. Medicine (Baltimore). 2021;100:e25467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aranha C, Patel V, Bhor V, et al. Cycle threshold values in RT-PCR to determine dynamics of SARS-CoV-2 viral load: an approach to reduce the isolation period for COVID-19 patients. J Med Virol. 2021;93:6794–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]