Abstract
The in vitro activity of Syn-2869 was compared with that of amphotericin B and itraconazole. MICs for 100 isolates of pathogenic molds belonging to 12 species were determined by a broth microdilution adaptation of the method recommended by the National Committee for Clinical Laboratory Standards. Syn-2869 and itraconazole showed comparable, good activity against the dematiaceous molds Cladophialophora bantiana, Cladophialophora carrionii, Exophiala dermatitidis, Fonsecaea pedrosoi, Phialophora parasitica, and Ramichloridium mackenziei. Neither of the azole agents was active against the hyaline molds Fusarium solani, Scedosporium prolificans, and Scopulariopsis brevicaulis, but both were more active than amphotericin B against Scedosporium apiospermum. The MICs of the three agents were comparable for the mucoraceous mold Absidia corymbifera, but Syn-2869 appeared to be the least active against the dimorphic mold Sporothrix schenckii. Our results suggest that Syn-2869 could be effective against a range of mold infections in humans.
The incidence of invasive mold infections is increasing, largely because of the rising number of immunocompromised patients (2, 19, 30). Although Aspergillus spp. are still the commonest causes of mold infection in these individuals, a growing number of other organisms, including Fusarium and Scedosporium spp., have been reported to cause lethal infection (2, 19, 30). Until recently, amphotericin B was the only effective agent against many mold infections, despite the fact that its use is seriously limited by nephrotoxicity and other side effects (11). Lipid-based preparations have reduced the toxicity but not significantly increased the efficacy of amphotericin B (16, 21, 23). In 1990 the triazole agent itraconazole became available, and it has since been used successfully to treat many patients with mold infections such as aspergillosis (4) and phaeohyphomycosis (29). However, not all mold infections respond to treatment with amphotericin B or itraconazole (3, 12), and there is a continuing need for new antifungal agents with a broad spectrum of action.
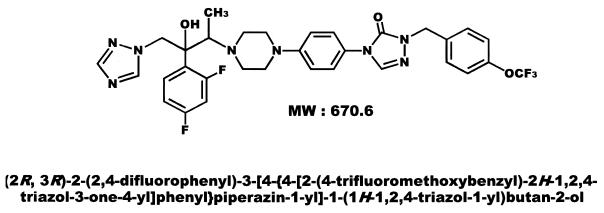
Syn-2869 (Fig. 1) is a new triazole antifungal agent (1) which has been reported to have potent in vitro and in vivo activity against isolates of Aspergillus spp., Candida spp., and Cryptococcus neoformans (9, 10, 13, 27, 28). To evaluate the potential usefulness of Syn-2869 in other infections, we compared its activity in vitro against 12 species of emerging and less common mold pathogens with the activities of amphotericin B and itraconazole. The in vitro testing method we employed was a microdilution adaptation of the standard broth macrodilution reference method of the National Committee for Clinical Laboratory Standards (NCCLS) (8, 20).
FIG. 1.
Chemical structure of Syn-2869.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998.)
Test isolates.
A total of 100 isolates were tested. These comprised 10 each of Absidia corymbifera, Cladophialophora bantiana, Exophiala dermatitidis, Fonsecaea pedrosoi, Fusarium solani, Phialophora parasitica, Scedosporium apiospermum, and Sporothrix schenckii and five each of Cladophialophora carrionii, Ramichloridium mackenziei, Scedosporium prolificans, and Scopulariopsis brevicaulis. The isolates tested came from the United Kingdom National Collection of Pathogenic Fungi (NCPF), held at the Mycology Reference Laboratory, Bristol, United Kingdom. Two reference strains, Aspergillus fumigatus NCPF 7097 and A. fumigatus NCPF 7100, were included in each batch of tests to ensure quality control.
Isolates were retrieved from storage in liquid nitrogen or water, subcultured on plates of Oxoid Sabouraud dextrose agar (Unipath Ltd., Basingstoke, United Kingdom) supplemented with 0.5% (wt/vol) chloramphenicol, and incubated at 30°C until adequate growth was obtained. To induce spore formation, the isolates were subcultured on slopes of Oxoid potato dextrose agar and incubated at 35°C for 7 days (8). Isolates of F. solani were incubated at 35°C for 2 to 3 days and then at 28 to 30°C for 4 to 5 days.
Antifungal agents.
Syn-2869 was obtained from SynPhar Laboratories Inc., Edmonton, Alberta, Canada, itraconazole was obtained from Janssen Research Foundation, Beerse, Belgium, and amphotericin B was obtained from Sigma Chemical Co. (St. Louis, Mo.). Stock solutions of Syn-2869 and itraconazole were prepared in polyethylene glycol 400, with the aid of heating to 70°C. Amphotericin B was dissolved in dimethyl sulfoxide. Further dilutions were made with RPMI 1640 medium (with l-glutamine, without bicarbonate) (Sigma), buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (Sigma). The recommendations stated in NCCLS document M27-A were followed for the dilution of each antifungal agent (20). The antifungal agents were tested over a final concentration range of 0.03 to 16 μg/ml.
Antifungal susceptibility testing.
Broth microdilution MICs were determined in 96-well, round-bottom microtiter plates, with a final volume of 200 μl per well. Spore suspensions were prepared in RPMI 1640 medium and adjusted to a final inoculum concentration of 0.4 × 104 to 5 × 104 spores/ml (8). The plates were incubated at 35°C and read after 24 h (A. corymbifera) or 48 h. The growth in each well was compared with that of the controls. The MIC was defined for amphotericin B as the lowest concentration at which there was complete inhibition of growth and for Syn-2869 and itraconazole as the lowest concentration at which there was prominent or complete inhibition of growth.
Results.
The in vitro activities of Syn-2869, itraconazole, and amphotericin B against the 100 mold isolates are summarized in Table 1. The data are presented as MIC ranges and, where appropriate, as the drug concentrations required to inhibit 50% and 90% of the isolates of each species (MIC50 and MIC90). In each batch of tests, the MICs of amphotericin B and itraconazole for the control strains were within the accepted limits.
TABLE 1.
In vitro activities of Syn-2869, itraconazole, and amphotericin B against 100 pathogenic mold isolates
| Organism (no. of isolates) | Agenta | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | MIC50 | MIC90 | ||
| Absidia corymbifera (10) | Syn-2869 | 0.25–1 | 0.5 | 0.5 |
| ITR | 0.25–0.5 | 0.25 | 0.5 | |
| AMB | 0.06–0.25 | 0.25 | 0.25 | |
| Cladophialophora bantiana (10) | Syn-2869 | ≤0.03–1 | 0.06 | 0.12 |
| ITR | ≤0.03–0.25 | 0.06 | 0.12 | |
| AMB | 0.25–0.5 | 0.25 | 0.5 | |
| Cladophialophora carrionii (5) | Syn-2869 | ≤0.03–0.12 | ||
| ITR | 0.06–0.25 | |||
| AMB | 0.25–4 | |||
| Exophiala dermatitidis (10) | Syn-2869 | 0.12–1 | 0.25 | 0.5 |
| ITR | 0.12–0.5 | 0.25 | 0.5 | |
| AMB | 0.12–1 | 0.5 | 1 | |
| Fonsecaea pedrosoi (10) | Syn-2869 | 0.06–0.5 | 0.25 | 0.25 |
| ITR | 0.12–0.25 | 0.12 | 0.25 | |
| AMB | 0.5–2 | 1 | 1 | |
| Fusarium solani (10) | Syn-2869 | >16 | >16 | >16 |
| ITR | >16 | >16 | >16 | |
| AMB | 1–2 | 1 | 2 | |
| Phialophora parasitica (10) | Syn-2869 | 0.5 | 0.5 | 0.5 |
| ITR | 1–2 | 1 | 2 | |
| AMB | 0.12–2 | 1 | 2 | |
| Ramichloridium mackenziei (5) | Syn-2869 | 0.12–0.5 | ||
| ITR | 0.12–0.25 | |||
| AMB | 4–>16 | |||
| Scedosporium apiospermum (10) | Syn-2869 | 0.5–2 | 1 | 1 |
| ITR | 0.25–4 | 1 | 4 | |
| AMB | 1.0–>16 | 2 | 8 | |
| Scedosporium prolificans (5) | Syn-2869 | >16 | ||
| ITR | >16 | |||
| AMB | 2–>16 | |||
| Scopulariopsis brevicaulis (5) | Syn-2869 | >16 | ||
| ITR | >16 | |||
| AMB | 2–16 | |||
| Sporothrix schenckii (10) | Syn-2869 | ≤0.03–>16 | 0.5 | >16 |
| ITR | 0.06–>16 | 0.5 | 4 | |
| AMB | 0.5–4 | 2 | 4 | |
| Aspergillus fumigatus NCPF 7097b | Syn-2869 | 0.5 | ||
| ITR | 0.12–0.5 | |||
| AMB | 0.5–1 | |||
| Aspergillus fumigatus NCPF 7100b | Syn-2869 | 0.5–1 | ||
| ITR | 4–>16 | |||
| AMB | 1–2 | |||
ITR, itraconazole; AMB, amphotericin B.
Quality control isolate.
Both Syn-2869 and itraconazole were more active than amphotericin B against the dematiaceous molds C. bantiana, C. carrionii, E. dermatitidis, F. pedrosoi, and R. mackenziei. However, Syn-2869 was more active than the other two agents against P. parasitica. The MIC50 and the MIC90 of each of the three agents were comparable for the mucoraceous mold A. corymbifera, but Syn-2869 appeared to be the least active against the dimorphic mold S. schenckii. Neither of the azole agents was active against the hyaline molds F. solani, S. prolificans, and S. brevicaulis, but both were more active than amphotericin B against S. apiospermum.
Discussion.
Although aspergillosis is still the commonest mold infection in immunocompromised patients, an increasing number of other environmental molds are being implicated as the cause of significant human infection (2, 19, 30). Among the more important of these emerging pathogens are Fusarium and Scedosporium spp., many isolates of which appear to be resistant to amphotericin B or itraconazole (3, 12, 17, 24). Dematiaceous molds, such as Cladophialophora, Exophiala, and Phialophora spp., have long been recognized as important causes of subcutaneous infection following traumatic inoculation, but they have also begun to emerge as important causes of deep fungal infection. These brown-pigmented molds are often susceptible to amphotericin B in vitro, as well as to triazole antifungal agents, such as itraconazole and voriconazole (6, 14, 18, 25). However, patients with these infections often fail to respond to currently available antifungal agents (26), and there is a need for new compounds.
Our results suggest that Syn-2869 is a broad-spectrum antifungal agent, effective in vitro against a wide range of organisms, including the mucoraceous mold A. corymbifera and the amphotericin B-resistant mold S. apiospermum. Like two other investigational triazoles, SCH 56592 and voriconazole, Syn-2869 was active against a range of dematiaceous molds but ineffective against the hyaline molds F. solani and S. prolificans (6, 7, 14). Unlike voriconazole, Syn-2869 appears to be active against the mucoraceous mold A. corymbifera (14). However, some caution must be exercised in making any conclusions regarding the relative potencies of the different triazole agents. Many of the molds studied in this investigation are uncommon causes of human infection, and the number of isolates available for testing was limited. The differences in MICs between the agents might have been more or less evident had larger numbers of isolates of some molds been tested.
It remains to be seen to what extent the low MICs seen with Syn-2869 in this and other investigations (9, 13, 28) will be predictive of clinical outcome. A standardized method has been developed for determining the MICs of five antifungal agents for Candida spp. and C. neoformans (20). In addition, interpretive breakpoints for Candida spp. have been proposed for itraconazole and fluconazole on the basis of a comparison of the clinical outcome of treatment with the MICs of the agents for the organisms isolated (26). Although standardization of antifungal susceptibility testing of molds is at a less advanced stage, a multicenter study involving 11 laboratories and 30 isolates showed a high level of agreement among the MICs of amphotericin B and itraconazole, determined by a broth microdilution adaptation of the NCCLS M27 method (8). In addition, correlations between antifungal drug susceptibilities of some molds in vitro and treatment outcomes in animal models of infection have been reported (5, 22). However, further studies will be required before firm conclusions can be drawn.
Initial pharmacokinetic data for mice indicate that Syn-2869 is well absorbed after oral administration (15). It has a serum half-life of about 6 h in rabbits, which is shorter than that of itraconazole, but has a higher tissue-to-plasma ratio than the older compound (15).
In conclusion, our results demonstrate that Syn-2869 is effective against a range of emerging and less common mold pathogens in vitro. Based on these findings and the favorable results from animal models in the treatment of aspergillosis, candidiasis, and cryptococcosis (10, 27), this triazole compound deserves further in vitro and in vivo investigation.
Acknowledgments
This study was partially supported by a grant from SynPhar Laboratories Inc.
REFERENCES
- 1.Abel M D, Bathini Y, Ha C, Furukawa T, Kasitu G, Khan J, Micetich R G, Nguyen D Q, Salama S M, Samari G, Sidhu I, Spevak P, Unemi N. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Syn-2869, novel broad-spectrum antifungal triazole: synthesis and structure activity relationships, abstr. F-148; p. 270. [Google Scholar]
- 2.Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992;14(Suppl. 1):S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 3.Berenguer J, Rodriguez-Tudela J L, Richard C, Alvarez M, Sanz M A, Gaztelurrutia L, Ayats J, Martinez-Suarez J V the Scedosporium prolificans Spanish Study Group. Deep infections caused by Scedosporium prolificans: a report on 16 cases in Spain and a review of the literature. Medicine. 1997;76:256–265. doi: 10.1097/00005792-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Denning D W, Lee J Y, Hostetler J S, Pappas P, Kauffman C A, Dewsnup D H, Galgiani J N, Graybill J R, Sugar A M, Catanzaro A, Gallis H, Perfect J R, Dockery B, Dismukes W E, Stevens D A. NIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97:135–144. doi: 10.1016/0002-9343(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 5.Denning D W, Radford S A, Oakley K L, Hall L, Johnson E M, Warnock D W. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome of Aspergillus fumigatus infection. J Antimicrob Chemother. 1997;40:401–414. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198–202. doi: 10.1128/jcm.36.1.198-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fothergill A W, Salama S M, Rinaldi M G. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. An in vitro head-to-head comparison of Syn2836, Syn2869, Syn2903, Syn2921, amphotericin B, fluconazole, and itraconazole against a spectrum of 90 clinically-significant fungi, abstr. J-117; p. 484. [Google Scholar]
- 10.Furukawa T, Saito H, Uji T, Nishida K, Higashitani F, Unemi N, Yamaguchi H. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. In vivo activity of Syn2869, a novel antifungal triazole in murine models of deep mycosis, abstr. F-149; p. 270. [Google Scholar]
- 11.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 12.Gamis A S, Gudnason T, Giebink G S, Ramsay N K C. Disseminated infection with Fusarium in recipients of bone marrow transplants. Rev Infect Dis. 1991;13:1077–1088. doi: 10.1093/clinids/13.6.1077. [DOI] [PubMed] [Google Scholar]
- 13.Gibb A P, Van Den Elzen H. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Activity of Syn2869, fluconazole, and itraconazole against 139 consecutive yeast isolates from normally-sterile sites, abstr. F-147; p. 270. [Google Scholar]
- 14.Johnson E M, Szekely A, Warnock D W. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J Antimicrob Chemother. 1998;42:741–745. doi: 10.1093/jac/42.6.741. [DOI] [PubMed] [Google Scholar]
- 15.Khan J K, Montaseri H, Poglod M, Bu H Z, Salama S, Micetich R G, Daneshtalab M. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Pharmacokinetics and oral bioavailability of novel triazole derivative Syn2869—a potent new antifungal agent and its comparison with itraconazole, abstr. F-152; p. 271. [Google Scholar]
- 16.Lister J. Amphotericin B lipid complex (Abelcet) in the treatment of invasive mycoses: the North American experience. Eur J Haematol. 1996;56(Suppl. 57):18–23. doi: 10.1111/j.1600-0609.1996.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 17.Lutwick L I, Galgiani J N, Johnson R H, Stevens D A. Visceral fungal infections due to Petriellidium boydii (Allesheria boydii). In vitro drug sensitivity studies. Am J Med. 1976;61:632–640. doi: 10.1016/0002-9343(76)90141-8. [DOI] [PubMed] [Google Scholar]
- 18.McGinnis M R, Pasarell L. In vitro testing of susceptibilities of filamentous ascomycetes to voriconazole, itraconazole, and amphotericin B, with consideration of phylogenetic implications. J Clin Microbiol. 1998;36:2353–2355. doi: 10.1128/jcm.36.8.2353-2355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison V A, Haake R J, Weisdorf D J. Non-Candida fungal infections after bone marrow transplantation: risk factors and outcome. Am J Med. 1994;96:497–503. doi: 10.1016/0002-9343(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 21.Ng T C, Denning D W. Liposomal amphotericin B (AmBisome) therapy in invasive fungal infections. Arch Intern Med. 1995;155:1093–1098. [PubMed] [Google Scholar]
- 22.Odds F C, Van Gerven F, Espinel-Ingroff A, Bartlett M S, Ghannoum M A, Lancaster M V, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother. 1998;42:282–288. doi: 10.1128/aac.42.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oppenheim B A, Herbrecht R, Kusne S. The safety and efficacy of amphotericin B colloidal dispersion in the treatment of invasive mycoses. Clin Infect Dis. 1995;21:1145–1153. doi: 10.1093/clinids/21.5.1145. [DOI] [PubMed] [Google Scholar]
- 24.Pujol I, Guarro J, Gene J, Sala J. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J Antimicrob Chemother. 1997;39:163–167. doi: 10.1093/jac/39.2.163. [DOI] [PubMed] [Google Scholar]
- 25.Radford S A, Johnson E M, Warnock D W. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob Agents Chemother. 1997;41:841–843. doi: 10.1128/aac.41.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole and candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 27.Salama S M, Atwal H, Gandhi A, Khan J, Montaseri H, Poglod M, Micetich R G, Daneshtalab M. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Syn2869, a new potent antifungal triazole: in vivo efficacy against systemic infections of Torulopsis glabrata and Cryptococcus neoformans in immunosuppressed mice, abstr. F-151; p. 271. [Google Scholar]
- 28.Salama S M, Gandhi A, Atwal H, Simon J, Khan J, Micetich R G, Daneshtalab M. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Syn2869, a new potent antifungal triazole for the treatment of systemic and superficial fungal infections: in vitro activity against clinical isolates of yeast and dermatphytes [sic], abstr. F-150; p. 270. [Google Scholar]
- 29.Sharkey P K, Graybill J R, Rinaldi M G, Stevens D A, Tucker R M, Peterie J D, Hoeprich P D, Greer D L, Frenkel L, Counts G W, Goodrich J, Zellner S, Bradsher R W, van der Horst C M, Israel K, Pankey G A, Barranco C P. Itraconazole treatment of phaeohyphomycosis. J Am Acad Dermatol. 1990;23:577–586. doi: 10.1016/0190-9622(90)70259-k. [DOI] [PubMed] [Google Scholar]
- 30.Vartivarian S E, Anaissie E J, Bodey G P. Emerging fungal pathogens in immunocompromised patients: classification, diagnosis, and management. Clin Infect Dis. 1993;17(Suppl. 2):S487–S491. doi: 10.1093/clinids/17.supplement_2.s487. [DOI] [PubMed] [Google Scholar]