Take home messages
Treatment should be offered only to symptomatic patients and selected according to the individual patient and disease presentation.
Rituximab in combination with cyclophosphamide and dexamethasone or bendamustine are highly effective regimens with manageable toxicity and are considered standard treatment options in first line treatment.
Ibrutinib monotherapy or in combination is effective an may be a valid option in patients unfit for immuno-chemotherapy although a continuous treatment compared to a time limited one should be considered.
Treatment outcome may be affected by MYD88 and CXCR4 mutations. In the future a better understanding of their impact on treatment outcome may lead to a more targeted approach.
Introduction
Waldenstrom's Macroglobulinemia (WM) treatment options, considering the disease rarity, have been derived from phase II study data with the exception of a few phase III clinical trials. Similarly to other indolent lymphomas, asymptomatic patients should not receive treatment as there are no data to support early initiation of therapy over a watch and wait strategy. Considering the wide range of clinical presentations there is no single standard treatment so when making decisions physicians must tailor treatment according to patient's age, comorbidities, and disease characteristics. Furthermore, medication tolerance and avoidance of long-term toxic effects including the possible development of treatment-related secondary malignancies should be considered.
Current state of the art
Symptomatic patients with symptoms related to tissue infiltration by neoplastic cells, such as fatigue, recurrent fever, night sweats, cytopenias, lymphadenopathy, organomegaly, bulky extramedullary disease, or IgM-related complications should be treated. Although the level of monoclonal IgM per se had not been considered an indication to start therapy the last ESMO guidelines suggested that patients with an IgM level >60 g/L should receive treatment based on a study indicating the imminent risk of symptomatic hyperviscosity.1▪ Certain situations, like symptomatic hyperviscosity, should be considered a clinical emergency. When hyperviscosity is present, plasma exchange is the therapy of choice followed by systemic treatment. Considering the risk of “flare” after rituximab, defined as the transient increase of IgM serum level typically occurring after 1 to 4 months, this agent should be avoided in case of hyperviscosity syndrome and introduced when the serum IgM is <4000 mg/dL.2
In the elderly population, single-agent treatment may be considered a suitable approach. In one of the few randomized phase III trials, oral fludarabine was superior to chlorambucil in terms of progression-free survival (PFS) and overall survival (OS) with a lower cumulative incidence of secondary malignancies.3 Monotherapy with these agents should be considered only in patients with comorbidities that may preclude treatment with immuno-chemotherapy and in patients with low tumor burden considering the slow kinetics of response. Rituximab monotherapy, which is well tolerated but associated with modest responses, represents a valid option in the presence of immunologic disorders related to WM, such as symptomatic cryoglobulinemia, hemolytic anemia or isolated IgM related peripheral neuropathy.4
The combinations rituximab, dexamethasone, and cyclophosphamide (DRC) or rituximab bendamustine (BR) are currently considered standard first-line options.
DRC appears to be highly effective, with an overall response rate (ORR) of 83%, 7% complete remissions (CRs), 35 months median PFS and a prolonged median time to next treatment of 51 months but most importantly with a favorable toxic profile.5▪ However, this regimen does not allow, rapid disease control due to the long median time to response of 4.1 months. In patients with high tumor burden, the combination of BR may be preferred. BR was first compared to R-CHOP in a phase III trial also including patients with WM and although ORR was similar between the 2 regimens, BR was superior in terms of tolerability and PFS (69.5 vs 28.1 months).6▪
There is not a direct comparison between DRC and BR. A retrospective study at first line demonstrated a similar ORR with a trend for a longer PFS with BR (2-year PFS 88% with BR and 61% with DRC P = 0.07).7 Importantly, the activity of BR and DRC appeared to be unaffected by patients’ MYD88 mutation status. BR, as reported by the FILO group, has the advantage of schedule modification in terms of dosage reduction and seems to have the same outcome when administered for 6 or fewer courses (2 years PFS 87% vs 88%).8
Fludarabine, pentostatine or cladribine based immuno-chemotherapy allows the achievement of good quality responses and prolonged PFS.9,10 Despite the high efficacy of purine analog-based treatment, it should be avoided as first-line due to the significant incidence of myelotoxicity, immunosuppression and possible impact on stem cell harvest. Furthermore, although data from literature are still controversial,3,11 there is general concern to avoid purine analogs because of the risk of secondary malignancies development.
Bortezomib has prominent efficacy even as WM monotherapy. Both bortezomib rituximab with or without dexamethasone (BDR) have shown to be highly active.12▪,13 A long term follow-up of the phase II study of BDR showed a median PFS of 3.5 years and an OS rate of 66% at 7 years.12▪ The major advantages of bortezomib combinations are rapid reduction of IgM level and low myelotoxicity, so they may be indicated when there is an immediate need for disease control such as a very high IgM level or hyperviscosity. Furthermore, bortezomib unlike other treatments seems to overcome the negative impact of CXCR4 mutations on survival.14 An important consideration in selecting initial therapy with bortezomib is the possible development of neuropathy; therefore, it should be given subcutaneously preferably at weekly intervals and used with caution in neuropathic patients.
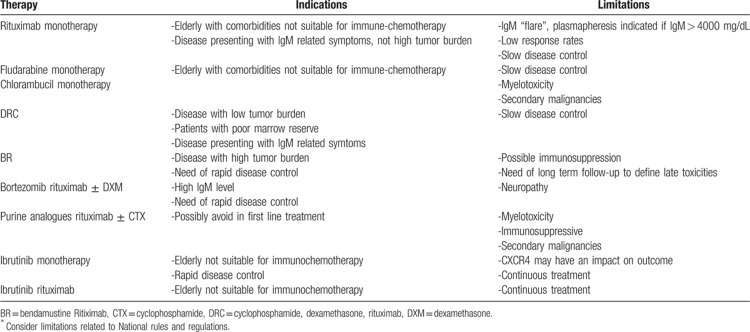
Ibrutinib is the first drug to receive approval for WM even in treatment-naïve patients. Clinically meaningful and importantly rapid responses were seen with ibrutinib monotherapy in 30 treatment-naïve patients with an 18 months PFS of 92%.15 The combination with rituximab in a cohort of untreated patients enrolled in the phase III randomized iNNOVATE Study demonstrated a significantly higher ORR, deeper responses, and superior PFS when compared to rituximab alone. The CXCR4 mutation exerts a negative impact when ibrutinib is administered as monotherapy but the addition of rituximab appears to overcome this negative prognostic feature.16▪ Several unique toxicities, including hemorrhagic episodes and atrial fibrillation mostly during the first 6 months, may develop during ibrutinib treatment due to its off-target effects. Ibrutinib may be a valid option at first line in patients unfit for immunochemotherapy although a continuous treatment compared to a time-limited one should be considered. Suggested therapies in previously untreated patients are summarized in Table 1.
Table 1.
Suggested Therapy in Treatment Naïve ∗Patients
Future directions
The expanding therapeutic armamentarium is aimed at finding more effective combination treatments and agents with a better toxicity profile to avoid short- and long-term complications. Furthermore, the effect of MYD88 and CXCR4 mutational status on the different types of therapy has not yet been clearly elucidated.
Whether the addition of bortezomib to the standard of treatment DRC may prolong PFS is the objective of a large ongoing randomized European trial (NCT01788020). The second-generation proteasome inhibitors, carfilzomib, and ixazomib, in combination with rituximab, have already shown promising results leading to deep responses translating in better PFS.17,18 Large prospective comparative trials are warranted to support their use. Second generation BTK inhibitors, acalabrutinib, zanubrutinib, are under development.19,20
At present individual treatment decisions are mostly based on comparisons of phase II data suggesting different approaches according to patients’ age and disease characteristics. In the future, it would be desirable to base therapeutic choices on results of larger trials that may help to direct the use molecular markers to develop personalized medicine in this field.
Footnotes
Citation: Tedeschi A, Frustaci AM. Waldenstrom's macroglobulinemia front line treatment. HemaSphere, 2019;00:00. http://dx.doi.org/10.1097/HS9.0000000000000222
Funding/support: None.
The authors declare no conflicts of interest.
References
- 1▪.Kastritis E, Leblond V, Dimopoulos MA, et al. Waldenstrom's macroglobulinaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29 (Suppl 4):iv41–iv50.ESMO guidelines addressing indications for treatment initiation and therapy. [DOI] [PubMed] [Google Scholar]
- 2.Gustine JN, Meid K, Toni D, et al. Serum IgM level as a predictor of symptomatic hypervisosity in patients with Waldenstrom macroglobulinemia. Br J Haematol 2017; 177:717–725. [DOI] [PubMed] [Google Scholar]
- 3.Leblond V, Johnson S, Chevret S, et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenstrom macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol 2013; 31:301–307. [DOI] [PubMed] [Google Scholar]
- 4.D'Sa S, Kersten MJ, Castillo JJ, et al. Investigation and management of IgM and Waldenstrom associated peripheral neuropathies. Recommendations from the IWWM-8 consensu panel. Br J Haematol 2017; 176:728–742. [DOI] [PubMed] [Google Scholar]
- 5▪.Kastritis E, Gavriatopoulou M, Kyrtsonis MC, et al. Dexamethasone, rituximab, and cyclophosphamide as primary treatment of Waldenstrom macroglobulinemia: final analysis of a phase 2 study. Blood 2015; 126:1392–1394.Update of the phase II study on DRC in first line treatment. [DOI] [PubMed] [Google Scholar]
- 6▪.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patientswith indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013; 381:1203–1210.Phase III randomized trial demonstrating the better toxic profile and outcome in patients treated with BR compared to R-CHOP. [DOI] [PubMed] [Google Scholar]
- 7.Paludo J, Abeykoon JP, Shreders A, et al. Bendamustine and rituximab (BR) versus dexamethasone, rituximab, and cyclophosphamide (DRC) in patients with Waldenström macroglobulinemia. Annal Hematol 2018; 97:1417–1425. [DOI] [PubMed] [Google Scholar]
- 8.Laribi K, Poulain S, Willems L, et al. Bendamustine plus rituximab in newly-diagnosed Waldenstrom macroglobulinemia patients. A study on behalf of the French Innovative Leukaemia Organization (FILO). Br J Haematol 2018; 103:1–3. [DOI] [PubMed] [Google Scholar]
- 9.Olszewski AJ, Chen C, Gutman R, et al. Comparative outcomes of immunochemotherapy regimens in Waldenstrom macroglobulinemia. Br J Haematol 2017; 179:106–115. [DOI] [PubMed] [Google Scholar]
- 10.Herth I, Hensel M, Rieger M, et al. Pentostatin, cyclophosphamide and rituximab is a safe and effective treatment in patients with Waldenstrom's macroglobulinemia. Leuk Lymphoma 2015; 56:97–102. [DOI] [PubMed] [Google Scholar]
- 11.Leleu X, Soumerai J, Roccaro A, et al. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenström macroglobulinemia treated with nucleoside analogs. J Clin Oncol 2009; 10:250–255. [DOI] [PubMed] [Google Scholar]
- 12▪.Gavriatopoulou M, Garcia-Sanz R, Kastritis E, et al. BDR in newly diagnosed patients with WM: final analysis of a phase 2 study after a minimum follow-up of 6 years. Blood 2017; 129:456–459.Update at 6 years of the phase II study on bortezomib dexamethasone rituximab in first line treatment. [DOI] [PubMed] [Google Scholar]
- 13.Ghobrial IM, Xie W, Padmanabhan S, et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenstrom macroglobulinemia. Am J Hematol 2010; 85:670–674. [DOI] [PubMed] [Google Scholar]
- 14.Sklavenitis-Pistofidis R, Capelletti M, Liu CJ, et al. Bortezomib overcomes the negative impact of CXCR4 mutations on survival of Waldenstrom's macroglobulinemia patients. Blood 2018; 132:2608–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treon SP, Gustine J, Meid K, et al. Ibrutinib monotherapy in symptomatic, treatment naïve patients with Waldenstrom macroglobulinemia. J Clin Oncol 2018; 36:2755–2761. [DOI] [PubMed] [Google Scholar]
- 16▪.Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenstrom's macroglobulinemia. N Eng J Med 2018; 378:2399–2410.Phase III randomized trial demonstrating the efficacy of the combination ibrutinib rituximab. [DOI] [PubMed] [Google Scholar]
- 17.Treon Sp, Tripsas CK, Meid K, et al. Carfilzomib, rituximab, dexamethasone (CaRD) treatment offers a neuropathy-sparyng approach for treating Waldenstrom macroglobulinemia. Blood 2014; 124:503–510. [DOI] [PubMed] [Google Scholar]
- 18.Castillo JJ, Meid K, Gustine JN, et al. Prospective clinical trial of ixazomib dexamethasone and rituximab as primary tehrapy in Waldenstrom's macroglobulinemia. Clin Cancer Res 2018; 24:3247–3252. [DOI] [PubMed] [Google Scholar]
- 19.Owen R, McCarthy H, Rule S, et al. Acalabrutinib in patients with Waldenstrom macrpglobulinemia (WM). J Clin Oncol 2018; 36:7501–7501. (abstract). [Google Scholar]
- 20.Tam CS, Le Blonde V, Novotny W, et al. A head to head face III study comparing zanabrutinib versus ibrutinib in patients with Waldenstrom macroglobulinemia. Future Oncol 2018; 14:2229–2237. [DOI] [PubMed] [Google Scholar]