Take home messages
Platelets are involved in recruitment and activation of neutrophils in thrombosis and inflammation
The interplay of platelets and neutrophils triggers neutrophil extracellular trap formation (NETs)
NETs in turn provide a scaffold for platelets adhesion and induce sustained platelet activation
Introduction
Neutrophils and platelets are among the most numerous cells in the blood and their function is tightly interconnected. Both are considered to be terminally differentiated effector cells with specific tasks. However, over the recent years it is increasingly recognized that both can only fulfill their functions in inflammation and thrombosis in a mutual, reinforcing interplay, where platelet activation serves as the initial trigger for a vicious circle. This review will focus on the interface between platelets and neutrophils, which is now accepted as a central driving force of immunothrombosis.1 Whereas thrombosis is defined as vessel occlusion due to activation of platelets and the coagulation system, immunothrombosis refers to thrombosis as an intrinsic element of innate immunity.
Current state of the art
Neutrophils and platelets are both considered to be first responders to pathogens and disruption of vascular integrity, respectively. However, these processes are not only linked on a pathophysiologic levels, but also on a cellular level. In the setting of thrombosis, platelets are rapidly recruited to the injured or activated endothelium. This relies on a multistep mechanism involving platelet rolling, tethering, adhesion, and aggregation. While contacting adhesive endothelial cells or subendothelial matrix, platelets also interact with neutrophils. If platelet recruitment to the endothelium is disrupted, also neutrophil accumulation is impaired underlining the functional interdependence of these cell types.2▪ On a molecular level, this is mediated by P-Selectin and PSGL-1 (P-Selectin glycoprotein ligand 1), which is not only involved in neutrophil rolling, but also intravascular crawling. PSGL-1 clusters at the leading edge of neutrophils actively scanning the environment for platelets allowing simultaneous interaction with them and endothelial cells.3 On the other hand, not only neutrophils have the capacity to migrate inside the vasculature: Also, platelets are equipped with the machinery to migrate and do that in an actomyosin-dependent manner once they overcome the adhesive forces of fibrinogen.4▪ The pathophysiologic relevance of the process is that migrating platelets bundle the adhesive substrate on their surface, which enables them to also collect bacteria. These platelet-bacteria bundles are then presented to neutrophils within the blood stream, which are activated and phagocytose these complexes. As a result, platelet migration contributes to organ damage in sepsis through interactions with neutrophils.
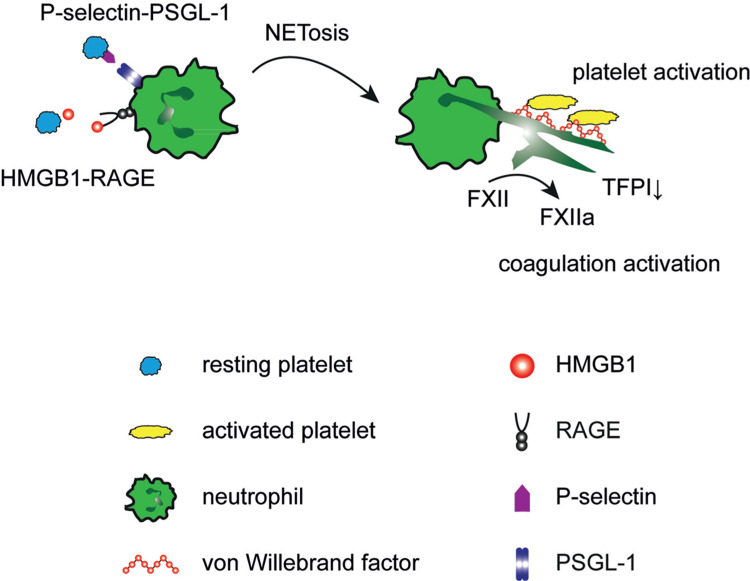
The physical interactions of neutrophils and platelets do not remain without consequences: activated platelets are essential triggers for neutrophil extracellular trap (NET) formation. These consist of nuclear DNA, which is spilled out in a coordinated process distinct from apoptotic and necrotic cell death.5 The DNA-histone backbone is decorated with neutrophil granule proteins and has strong bactericidal properties. In sepsis, platelets sense inflammatory mediators through TLR4 (Toll-like receptor 4), resulting in their activation and binding to neutrophils. This leads to NET formation allowing trapping of bacteria within the microcirculation of the liver and lungs.6▪ In addition, migrating platelets loaded with gathered bacteria are strong inducers of NETosis, contributing to their detrimental effects.4▪ But also during viral infections such as influenza and HIV, NETs are present and involved in neutralization of viruses.7,8 However, platelet-triggered NET formation not only contributes to the defense against invading pathogens, but also to thrombosis: NETs are present in both venous and arterial human thrombi, suggesting a clinical relevance.9,10 In this setting, platelet-derived P-Selectin induces NETosis through binding to PSGL-1 on neutrophils.11 Moreover, the prototypical danger associated molecular pattern HMGB1 (High-Mobility-Group-Protein B1) facilitates this process. Although platelets are an anucleated cell type, they contain this DNA binding protein, which has chemotactic and leukocyte activating properties.12,13▪ Upon activation, platelets release HMGB1, which triggers NET formation by RAGE (receptor for advanced glycation end products) in vitro as well as in vivo, thereby propagating obstructive clot formation.12,13▪
Even though the lifespan of netting neutrophils is limited, the crosstalk with platelets does not end there: NETs provide a platform for platelet activation and coagulation and thereby markedly enhance their prothrombotic properties (Figure 1). This makes them an essential interface between thrombosis and inflammation in thrombosis.2 The exposed DNA strands bind von-Willebrand factor and are an adhesive substrate for platelet adhesion, resulting in their activation and aggregation.14,15▪ This is one mechanism, how NETs promote venous thrombosis in vivo.2 In addition, they also have marked effects on coagulation: neutrophil elastase – which is present on NETs - degrades tissue factor pathway inhibitor. Through this, neutrophils disinhibit the activation of the extrinsic coagulation pathway and increase thrombin formation.16 In addition, NETs provide a negatively charged surface and catalyse the binding and activation of factor XII, which initiates the intrinsic coagulation cascade. Histones – as the backbone of NETs – also directly activate platelets and foster clot formation.17 Interestingly, it has recently been recognized, that the prothrombotic and proinflammatory effects of NETs are counteracted and limited by endogenous DNAses, in particular DNase1 and DNase1-like 3.18 DNase is also exploited therapeutically to disrupt NETs and NETosis and thrombus formation can also be impaired through inhibition of peptidylarginine deiminase 4.2,19 But also the well-known anticoagulant heparin affects NETs through its high affinity to histones, resulting in disruption of NETs. This anti-inflammatory effect of heparin is beneficial in the setting of thrombotic conditions.15▪,20
Figure 1.
Mutual activation of platelets and neutrophils. Left: Platelets induce neutrophil extracellular trap formation (NETosis) through HMGB1 binding to RAGE as well as P-Selectin-PSGL-1 interaction (Left). Right: NETs in turn promote platelet activation and coagulation by formation of activated factor XII (FXIIa) and degradation of tissue factor pathway inhibitor (TFPI).
Future perspectives
Targeting the detrimental side of platelet-neutrophil crosstalk provides a promising approach in many inflammatory diseases and thrombosis. However, this interaction is also essential to safeguard vascular integrity and host defense, making a general disruption of platelet-neutrophil communication potentially harmful. Therefore, a better understanding of the disease-specific mechanisms and mediators is mandatory to ensure beneficial effects of targeted disruption of this crosstalk.
Footnotes
Citation: Stark K. Platelet-Neutrophil Crosstalk and Netosis. HemaSphere, 2019;00:00. http://dx.doi.org/10.1097/HS9.0000000000000231
The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013; 13:34–45. [DOI] [PubMed] [Google Scholar]
- von Bruhl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012; 209:819–835.Showed the relevance of platelet-neutrophil crosstalk and NETosis in venous thrombosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sreeramkumar V, Adrover JM, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014; 346:1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner F, Ahmad Z, Rosenberger G, et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell 2017; 171:1368–1382. e23.The migratory properties of platelets were revealed as well as the biological relevance in collecting bacteria. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–1535. [DOI] [PubMed] [Google Scholar]
- Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nature Med 2007; 13:463–469.Provides evidence for platelet-induced NET formation in sepsis. [DOI] [PubMed] [Google Scholar]
- 7.Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 2011; 179:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saitoh T, Komano J, Saitoh Y, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012; 12:109–116. [DOI] [PubMed] [Google Scholar]
- 9.Riegger J, Byrne RA, Joner M, et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J 2016; 37:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savchenko AS, Martinod K, Seidman MA, et al. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost: JTH 2014; 12:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etulain J, Martinod K, Wong SL, et al. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015; 126:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maugeri N, Campana L, Gavina M, et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost: JTH 2014; 12:2074–2088. [DOI] [PubMed] [Google Scholar]
- Stark K, Philippi V, Stockhausen S, et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 2016; 128:2435–2449.Identified HMGB1 as platelet-derived trigger for neutrophil activation and NETosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brill A, Fuchs TA, Savchenko AS, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost: JTH 2012; 10:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010; 107:15880–15885.Revealed the platelet-activating effects of extracellular DNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010; 16:887–896. [DOI] [PubMed] [Google Scholar]
- 17.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011; 118:1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez-Alcazar M, Rangaswamy C, Panda R, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017; 358:1202–1206. [DOI] [PubMed] [Google Scholar]
- 19.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A 2013; 110:8674–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal PK, Starr T, Gertler MM. Neutralization of heparin by histone and its subfractions. Thromb Res 1983; 31:69–79. [DOI] [PubMed] [Google Scholar]