Take home messages
To understand the main interplay between coagulation system and inflammation and to recognize the key invasive infectious agents causing typical abnormalities in activation of blood platelets, coagulation and fibrinolysis
To capture, monitor and follow-up the clinical and laboratory phenotype and the management related to the pathophysiology of inflammation.
Introduction
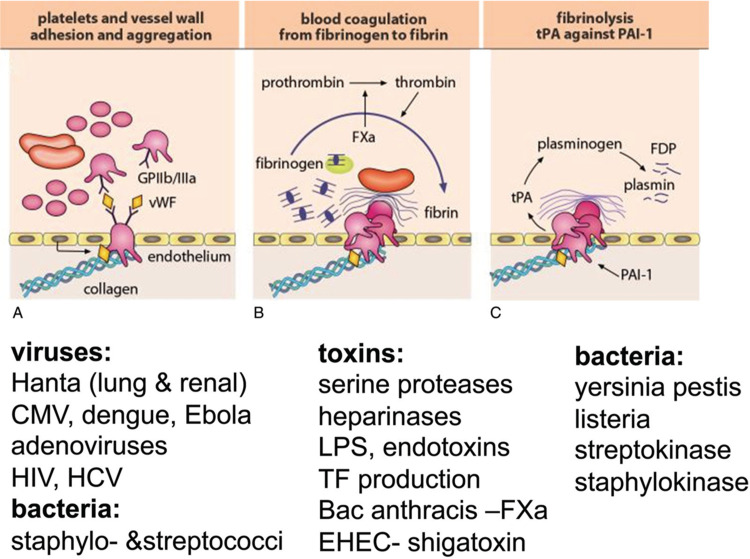
Inflammation induced by infection results in local tissue damaged followed by systemic endothelial injury, adhesion of platelets and activation of the coagulation cascade. In addition, during infection and inflammation the local control of coagulation fails, triggering the vicious circle of coagulation activating inflammation and vice versa. The hemostatic system represents the first and the most immediate element, programming tissue response to injury and continuum of inflammation, angiogenesis, stromal recruitment and repair.1▪ Endothelium is under continuous interphase with blood flow. From blood the pathogens and inflammatory mediators invade organ(s), causing vascular damage, microthrombi and multi(organ) failure (eg, sepsis).2▪ Upon escape of the local regulation of coagulation, typical clinical entities include thrombotic microangiopathy (hemolytic anemia, thrombocytopenia and microthrombi), complement (membrane attack complex) interplay and disseminated intravascular coagulation (DIC).3▪,4 Inflammation can influence all the three phases of hemostasis and their regulation: (1) megakaryocytes and platelets, (2) coagulation and (3) fibrinolysis (Fig. 1). This short review will provide examples, and some clinical management opportunities in these three phases.
Figure 1.
Coagulation targets of some pathogen as examples. Coagulation occurs sequentially: (A) platelet adhesion and activation, (B) thrombin generation and fibrin formation and (C) degradation of fibrin by fibrinolysis. Some infectious agents at each step are presented as examples. CMV = cytomegalovirus, EHEC = Entero Hemolytic Escherichia Coli, HCV = hepatitis C virus, HIV= human immunodeficiency virus, LPS = lipopolysaccharide, TF= tissue factor.
Current state of the art
The interplay between infection/inflammation and coagulation disorders
During bacterial infection, dual activation of platelets and macrophages can eradicate bacteria in liver.5 Strong leukocyte-derived proteolytic enzymes will cleave the anticoagulant and protective endothelial layer of glycosaminoglycans (GAG, ie, heparan sulfate, perlecans and syndecans), exposing von Willebrand factor (VWF), collagen and laminin to trigger platelet deposition, thrombin and fibrin formation (Fig. 1).6
Endothelial cells will constitutively secrete VWF, and VWF-size cleaving and controlling ADAMTS-13 enzyme to maintain VWF homeostasis. Infection may consume ADAMTS-13 or lead to antibody formation to exhaust or inactivate its capacity. This can result in the development of thrombotic thrombocytopenic purpura (TTP), or hemolytic uremic syndromeHUS or atypical, aHUS causing, thrombosis formation in the microvasculature (brain, kidneys) due to platelet- and VWF-rich deposition and complement activation.7 Platelets can also bind to endothelial derived-VWF and generated fibrin on the endothelium (thrombocytopenia, “consumption”). Thrombocytopenia, during DIC, may refer to reduced survival in association with severely ill infected patients. The timely therapy of the infection and/or inflammation, including regulation of coagulation may break this vicious loop.8▪ The main tools to limit excessive coagulation in treating and preventing thrombosis include mainly acetylsalicylic acid, or low-molecular weight heparin (LMWH). If a coagulation defect, that is, low antithrombin is noted and corrected early the balance of coagulation will tilt to a more physiological position.8▪,9
The role of megakaryocytes, platelets and thrombin in infection and inflammation
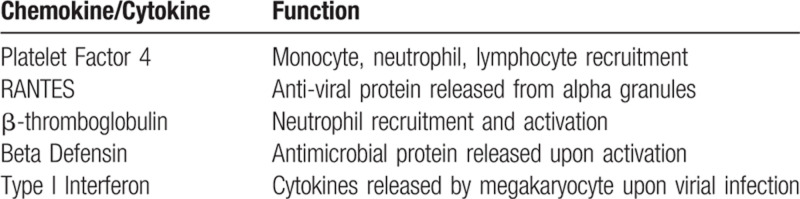
While the hemostatic roles of platelets are well recognized, emerging data demonstrate that megakaryocytes and platelets possess diverse and dynamic functions that also mediate inflammatory and immune responses (Table 1).10–12 Many infection processes result in thrombocytopenia due to enhanced destruction of megakaryocytes and platelets.13–15
Table 1.
Immune-Related Chemokines/Cytokines Released by Megakaryocytes.
However, megakaryocytes and platelets possess a multitude of innate immune tools, including toll-like receptors, to recognize pathogens, and Fc receptors, which recognize immune complexes.10 In addition, platelets contain many antimicrobial agents, including antimicrobial peptides and beta-defensins, which directly act on bacterial pathogens.10,12,16 Platelets also release chemokines such as platelet factor 4, RANTES and β-thromboglobulin, which increase leukocyte recruitment and survival during viral infections, and can reduce HIV infection by directly interacting with the viral envelope.17–19
Recently, megakaryocytes have been shown to play significant roles in fighting infections. For example, megakaryocytes possess major histocompatibility complex (MHC) I and are capable of endocytosing endogenous antigen.20 Upon processing the antigen through the proteasome, megakaryocytes can present antigens in an MHC-I dependent manner to activate CD8+T cells. In addition, viral infections such as influenza and dengue virus significantly alter the transcriptome of megakaryocyte and platelets, resulting in expression of novel anti-viral molecules such as interferon-induced transmembrane 3 (IFITM3).21 Induction of IFITM3 in megakaryocytes and surrounding hemopoietic stem cells reduces viral infection and appear to be mediated through Type I interferon release from the megakaryocyte.
Thrombin activates protease-activated receptors on platelets and endothelial cells and generates fibrin. Thrombin activation of platelets results in release of chemokines and platelet microbicidal proteins to reduce the spread of infection, while fibrin formation allows pathogens to be trapped and cleared by leukocytes.16,22,23▪ Both reduced thrombin generation and enhanced fibrinolysis in mouse models increase susceptibility to bacterial infections, suggesting thrombin, platelets and megakaryocytes play critical roles in stemming the spread of infection, while maintaining hemostasis.24
The effect of pathogen-host interactions on coagulation
Some intriguing observations suggest fibrin formation in addition to its role in ceasing bleeding, recruits macrophages (CD11/18) to limit pathogens from spreading.25 For instance, streptococci and staphylococci will invade the fibrin and surroundings by secreting strepto- and staphylokinase, which will activate matrix metalloproteinases (MMPs) and plasmin to allow the penetration of these pathogens (Fig. 1). Also, severe vascular damage may lead to tissue hemorrhaging, which platelets resist, while hematomas provide a growth media to the bacteria. Listeria sepsis in immunocompromised patients underlines the importance of fibrin (ogen) in limiting the infection from spreading.26 Hantaviruses trigger activation of fibrinolysis in relation to coagulation activity, simultaneously causing temporarily thrombocytopenia.27 Dengue hemorrhagic fever also induces hyperfibrinolysis and thrombocytopenia through destruction of platelets and megakaryocytes.14,15,28
Influence of Inflammation on coagulation responses
As examples of inflammation, vasculitis and atherosclerosis both impact the coagulation system. Immunological ANCA-vasculitis activates both coagulation and fibrinolysis, which associate with impairment of renal function.29 In individuals with atherosclerosis and impaired distal perfusion of leg arteries, the functional severity of vascular disease relates to the levels of fibrinogen, thrombin-antithrombin complexes and D-dimer.30 Finally, in the management of allogenic stem cell transplantation the outcome and later graft-versus-host disease are affected by the early and longitudinal maladapted regulation of coagulation.31,32 Thus, reduced protein C activity and enhanced thrombin generation, and high FVIII levels, refer to impaired protective effects of endothelium during the transplantation recovery.
Future clinical perspectives
The main approach is to observe the symptoms and signs and rapidly target the causative pathogen and individualize immune- and supportive therapy, including thromboprophylaxis, to eliminate the trigger of inflammation and coagulation disorder (8). A stepwise laboratory follow-up alongside clinical hemostasis abnormalities includes blood cell counts, C-reactive protein assessing the extent of inflammation, antithrombin, fibrinogen, coagulation screening tests of prothrombin time (PT) and activated partial thromboplastin time (APTT), thrombin time, FVIII/VWF, and D-dimer will give a broad overall picture of the potential deficiencies, over-activities (eg, ISTH DIC score), and their tendencies upon active patient management and recovery. Intravenous Vitamin K administration (1-5 mg, 0.15 mg being the daily requirement) corrects PT (FII, FVII, FIX, FX, protein C and S), if the liver synthesis is impaired due to consumption and poor access to vitamin K limited by antibiotics and malabsorption. Short APTT and thrombin time refer to enhanced contact pathway and thrombin activity. The higher the fibrinogen, the poorer the fibrinolytic capacity (D-dimer trends). FVIII/VWF indicates the extent of endothelial damage and low antithrombin may tilt the balance towards uncontrolled thrombin generation. In the future, the global hemostasis assessment, including thrombin generation capacity, will likely aid in the patient management.
Footnotes
Citation: Lassila R, Campbell R. Management of coagulation disorders in severe inflammation. HemaSphere, 2019;00:00. http://dx.doi.org/10.1097/HS9.0000000000000238
The authors have indicated they have no potential conflicts of interest to disclose.
References
- Magnus N, D’Asti E, Meehan B, et al. Oncogenes and the coagulation system--forces that modulate dormant and aggressive states in cancer. Thromb Res 2014; 133 (Suppl 2):S1–9.The hemostatic system represents the first and the most immediate element in the program of tissue response to injury and is a part of continuum of inflammation, angiogenesis, stromal recruitment and repair. [DOI] [PubMed] [Google Scholar]
- Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care 2019; 23:16.It is hypothesized that dysfunctions of glycocalyx, mainly resulting from its degradation, have a role in the early diagnosis and prognosis of sepsis; and restoration of the glycocalyx is a potential therapeutic target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H. New concepts of thrombotic thrombocytopenic purpura and a strategy to prevent its relapse. J Hematol Thrombo Dis 2014; 2: Each disorder needs to be evaluated individually in four types of pathogenetic contributions: a trigger of VWF-platelet thrombosis in patients with pre-existing TTP or TMA in patients with pre-existing aHUS; defective regulation of the immune system with the emergence of B-cell clones producing ADAMTS13 inhibitors (TTP) or CFH antibodies (aHUS); a cause of TMA via mechanisms other than defective complement regulation; and a cause of other types of pathology (fibrin-platelet thrombosis, vasculitis/vasculopathy or intravascular clusters of neoplastic cells). [Google Scholar]
- 4.Aird WC. Vascular bed-specific thrombosis. J Thromb Haemost 2007; 5 (Suppl 1):283–291. [DOI] [PubMed] [Google Scholar]
- 5.Wong CH, Jenne CN, Petri B, et al. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol 2013; 14:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayanan S. Multifunctional roles of thrombin. Ann Clin Lab Sci 1999; 29:275–280. [PubMed] [Google Scholar]
- 7.Kremer Hovinga JA, Lammle B. Role of ADAMTS13 in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2012; 2012:610–616. [DOI] [PubMed] [Google Scholar]
- Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care 2006; 10:222.Coagulation abnormalities may significantly contribute to morbidity and mortality and require prompt analysis to establish the underlying cause and to initiate corrective and supportive treatment [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care 2013; 17:R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Front Immunol 2014; 5:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost 2003; 1:1897–1905. [DOI] [PubMed] [Google Scholar]
- 12.Morrell CN, Aggrey AA, Chapman LM, et al. Emerging roles for platelets as immune and inflammatory cells. Blood 2014; 123:2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franchini M, Veneri D, Lippi G. Thrombocytopenia and infections. Expert Rev Hematol 2017; 10:99–106. [DOI] [PubMed] [Google Scholar]
- 14.Noisakran S, Onlamoon N, Hsiao HM, et al. Infection of bone marrow cells by dengue virus in vivo. Exp Hematol 2012; 40: 250-259 e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojha A, Nandi D, Batra H, et al. Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci Rep 2017; 7:41697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraemer BF, Campbell RA, Schwertz H, et al. Novel anti-bacterial activities of beta-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog 2011; 7:e1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocchi F, DeVico AL, Garzino-Demo A, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995; 270:1811–1815. [DOI] [PubMed] [Google Scholar]
- 18.Klinger MH, Wilhelm D, Bubel S, et al. Immunocytochemical localization of the chemokines RANTES and MIP-1 alpha within human platelets and their release during storage. Int Arch Allergy Immunol 1995; 107:541–546. [DOI] [PubMed] [Google Scholar]
- 19.Auerbach DJ, Lin Y, Miao H, et al. Identification of the platelet-derived chemokine CXCL4/PF-4 as a broad-spectrum HIV-1 inhibitor. Proc Natl Acad Sci U S A 2012; 109:9569–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zufferey A, Speck ER, Machlus KR, et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv 2017; 1:1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell RA, Schwertz H, Hottz ED, et al. Human megakaryocytes possess intrinsic anti-viral immunity through regulated induction of IFITM3. Blood 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun 2002; 70:6524–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci 2010; 67:525–544.These studies, and complementary studies with other human pathogens, illustrate that plasminogen and fibrinogen are extremely effective modifiers of the inflammatory response in vivo and critical determinants of bacterial virulence and host defence [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H, Wang X, Degen JL, et al. Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood 2009; 113:1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost 2007; 5 (Suppl 1):24–31. [DOI] [PubMed] [Google Scholar]
- 26.Mullarky IK, Szaba FM, Berggren KN, et al. Infection-stimulated fibrin deposition controls hemorrhage and limits hepatic bacterial growth during listeriosis. Infect Immun 2005; 73:3888–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laine O, Makela S, Mustonen J, et al. Platelet ligands and ADAMTS13 during Puumala hantavirus infection and associated thrombocytopenia. Blood Coagul Fibrinolysis 2011; 22:468–472. [DOI] [PubMed] [Google Scholar]
- 28.Marchi R, Nagaswami C, Weisel JW. Fibrin formation and lysis studies in dengue virus infection. Blood Coagul Fibrinolysis 2009; 20:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salmela A, Ekstrand A, Joutsi-Korhonen L, et al. Activation of endothelium, coagulation and fibrinolysis is enhanced and associates with renal anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2015; 30 (Suppl 1):i53–59. [DOI] [PubMed] [Google Scholar]
- 30.Lassila R, Peltonen S, Lepantalo M, et al. Severity of peripheral atherosclerosis is associated with fibrinogen and degradation of cross-linked fibrin. Arterioscler Thromb 1993; 13:1738–1742. [DOI] [PubMed] [Google Scholar]
- 31.Pinomaki A, Volin L, Joutsi-Korhonen L, et al. Early thrombin generation and impaired fibrinolysis after SCT associate with acute GVHD. Bone Marrow Transplant 2010; 45:730–737. [DOI] [PubMed] [Google Scholar]
- 32.Przybyla B, Pinomaki A, Petaja J, et al. Coordinated responses of natural anticoagulants to allogeneic stem cell transplantation and acute GVHD - A longitudinal study. PLoS One 2017; 12:e0190007. [DOI] [PMC free article] [PubMed] [Google Scholar]