Take home messages
Although durable remissions are observed in many patients treated with these agents, including those with poor risk features, it is becoming apparent that some patients will ultimately relapse.
Potential acquired resistance mechanisms identified to date include BTK (C481) and PLCG2 mutations in patients undergoing treatment with ibrutinib and a recurrent mutation at codon V101 within the BCL2 gene in patients treated with venetoclax.
The observation that some patients with acquired clinical resistance have extremely low allelic frequencies of BTK, PLCG2 or BCL2 mutations or, in some instances are absent for mutations in these genes, indicates that additional (epi)genetic aberrations must play a role in acquired resistance to novel agents.
Introduction
The exciting shift in the therapeutic management of chronic lymphocytic leukemia (CLL) can largely be attributed to regulatory approval, both in the US and Europe, of 2 oral kinase inhibitors, ibrutinib and idelalisib, as well as the B-cell lymphoma 2 (BCL2) antagonist, venetoclax. However, whilst durable remissions are observed in many patients treated with these agents, including those with poor risk features, it is becoming apparent that some patients will ultimately relapse. Understanding the distinct mechanisms driving resistance has the potential to lead to further targeted therapies to combat resistance, as well as the development of rational combinations to prevent the development of resistance.
Current State-of-the-art
Targeting the BcR pathway with kinase inhibitors: ibrutinib and idelalisib
Ibrutinib and Idelalisib are both orally bioavailable small molecule inhibitors that were approved for marketing back in 2014. Ibrutinib initially received accelerated US FDA approval for patients with relapsed or refractory CLL, but in 2016 the FDA expanded Ibrutinib's use to include treatment-naïve patients based on data emerging from the RESONATE trials indicating prolonged survival for ibrutinib-treated CLL patients in both the frontline and relapsed setting when compared with standard therapies.1–3 Idelalisib, in combination with rituximab, is approved for the treatment, of patients with relapsed/refractory CLL or high-risk CLL with defective TP53 (the latter only in Europe).4,5 Although both drugs operate as kinase inhibitors, their specific targets differ. Ibrutinib inactivates the Bruton agammaglobulinemia tyrosine kinase (BTK) through the formation of an irreversible covalent bond at the cysteine residue (C481), thereby inhibiting B-cell receptor (BCR) signaling and ultimately blocking the proliferation and survival of malignant B-cells.6,7 Idelalisib reversibly targets phosphatidylinositol 3-kinase delta (PI3Kδ) leading to inhibition of PI3K signaling and selectively inducing apoptosis in the CLL cells.4,5 Despite the success of these targeted agents, a subset of patients eventually develop drug resistance. With its widespread use in clinical practice the mechanisms of resistance to ibrutinib are now well characterized whereas definitive mechanisms driving resistance to idelalisib have yet to be described.
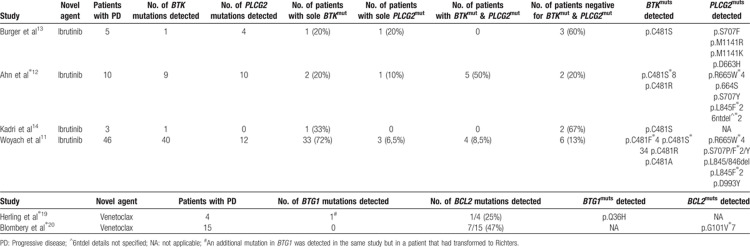
Woyach et al used whole-exome sequencing (WES) to investigate the genetic landscape of CLL patients relapsing on ibrutinib and were the first to discover acquired mutations in the BTK gene.8▪ Functional analyses demonstrated that the BTK gene mutations at C481, the ibrutinib binding site, reduce the binding affinity of ibrutinib for BTK thus leading to transient inhibition of BTK.8▪,9 In addition to BTK gene mutations, multiple mutations were detected in phospholipase Cg2 (PLCG2), a kinase located immediately downstream of BTK.8▪ Several PLCG2 mutations have been reported as gain-of-function, reactivating BCR signaling despite inactive BTK.10▪ Numerous studies have since confirmed that a substantial percentage of patients treated with ibrutinib acquire mutations in these genes and deep-sequencing of baseline samples have revealed the absence of BTK mutations prior to ibrutinib therapy, at least at a currently detectable level and within the specific tissue compartments studied, strongly indicating that these mutations are a predominant mechanism underlying resistance to ibrutinib.11,12▪,13,14 Studies of longitudinal samples have shed light on the acquisition of such mutations revealing that mutations within the BTK and/or PLCG2 gene are often present several months before an overt clinical relapse is observed.11,12▪,14 Intriguingly, a subset of patients who progress on ibrutinib carry BTK mutations in minor subclones and the precise manner in which BTK/PLCG2 mutations can drive resistance when present at such low allelic burden is not immediately clear.11,12▪,13,14 Finally, it is noteworthy that a subset of patients relapse on ibrutinib without acquiring mutations in either of these genes. The molecular alterations driving their resistance remain unknown but indicate that additional (epi)genetic aberrations must play a role in acquired resistance to BTK inhibitors.13
Despite significant efficacy, disease progression during idelalisib treatment is observed in CLL patients, and the underlying biological mechanisms for such relapses remains largely unknown. A small-scale WES study revealed that no mutations occurred at the drug-binding site, in the PI3K signaling pathway or in any related signaling pathway, indicating that there is no common mutational mechanism or single recurrent mutation that contributes to resistance.15 Scheffold et al performed in vivo modeling of resistance to PI3K in a mouse model and reported that all the resistant tumors had significant upregulation of genes involved in the integrin receptor complex.16 These preliminary studies indicate that resistance to Idelalisib may not be mediated by a recurrent mutation but instead stem from dysregulation of survival signaling; however, further research is required to corroborate these findings.
Venetoclax: the first BH3 mimetic to enter clinical routine
Like the aforementioned novel agents, venetoclax, is a first-in-class, highly selective and orally bioavailable small-molecule inhibitor, however, venetoclax is not a kinase inhibitor and instead targets BCL2, an apoptotic protein central to the regulation of programmed cell death. Venetoclax restores apoptosis and produces high overall response rates in heavily pretreated, high-risk CLL patients.17,18 Remarkably, treatment with venetoclax has yielded complete responses in a substantial proportion of patients, a phenomenon generally not observed with PI3K or BTK inhibitors. Venetoclax initially obtained accelerated approval for treatment of p53-deficient CLL patients who had failed or were unsuitable for treatment with kinase inhibitors, but has recently been granted approval for the treatment of CLL patients following at least one prior therapy irrespective of their TP53 status.
Table 1.
Potential Recurrent Resistance Mutations Identified in Sequencing Studies of Progressive CLL Patients Undergoing Either Ibrutinib or Venetoclax Treatment.
Two recent studies have shed light on why patients may stop responding to venetoclax. WES in a small cohort of del(17p) patients progressing on venetoclax identified a number of potential resistance-associated alterations, such as mutations in BTG1 or BRAF, homozygous deletion of CDKN2A/B and high-level focal amplification of CD274, however, causal relationships have yet to be established.19▪ More recently, a novel recurrent BCL2 mutation (G101 V) was identified in a cohort of CLL patients progressing on venetoclax.20▪ Functional analysis revealed that G101 V impairs binding of venetoclax to BCL2 preventing the drug from displacing proapoptotic molecules from BCL2 in cells and hence confers resistance. As evidenced for BTK C481 mutations, the G101 V BCL2 mutation was absent at baseline and was acquired several months before clinical disease progression.11,12▪,14,20▪ Finally, the wide range of subclonality observed for G101 V together with the finding that many relapsing patients did not harbor this change indicates that additional acquired changes confer resistance to venetoclax and these alternative mechanisms remain elusive.20▪
Future perspectives
Understanding the nature of disease progression has important implications. As patients are treated for longer with these drugs, it is inevitable that the incidence of relapse increases and hence the challenge of managing resistant disease and determining who is at risk for relapse is of paramount importance. It is vital to identify potential mechanisms of resistance, both for patient stratification and developing strategies to circumvent resistance, either before it develops or as it emerges. Whilst much work remains to be done, resistance mutations may serve as early biomarkers of disease progression prior to overt clinical relapse and hence their detection could facilitate early therapeutic intervention with different combination therapy to avert or reverse the relapse.
Footnotes
Citation: Sutton L-A. Mechanisms of Resistance to Targeted Therapies in Chronic Lymphocytic Leukemia. HemaSphere, 2019;00:00. http://dx.doi.org/10.1097/HS9.0000000000000240
Funding/support: None.
Disclosure: The author has receive honoraria from Abbvie, Gilead, and Janssen.
References
- 1.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373:2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia 2018; 32:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014; 123:3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011; 117:6287–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012; 119:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014; 370:2286–2294.One of the first reports detailing BTK and PLCG2 mutations in patients relapsing on ibrutinib [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng S, Guo A, Lu P, et al. Functional characterization of BTK(C481S) mutation that confers ibrutinib resistance: exploration of alternative kinase inhibitors. Leukemia 2015; 29:895–900. [DOI] [PubMed] [Google Scholar]
- 10▪.Jones D, Woyach JA, Zhao W, et al. PLCG2 C2 domain mutations co-occur with BTK and PLCG2 resistance mutations in chronic lymphocytic leukemia undergoing ibrutinib treatment. Leukemia 2017; 31:1645–1647.This study analyzes different recurrent PLCG2 variants and investigates their role in ibrutinib-resistant CLL [DOI] [PubMed] [Google Scholar]
- 11.Woyach JA, Ruppert AS, Guinn D, et al. BTK(C481S)-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J Clin Oncol 2017; 35:1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Ahn IE, Underbayev C, Albitar A, et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood 2017; 129:1469–1479.This large-scale study describes the presence of BTK and/or PLCG2 mutations several months before overt clinical progression [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger JA, Landau DA, Taylor-Weiner A, et al. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat Commun 2016; 7:11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadri S, Lee J, Fitzpatrick C, et al. Clonal evolution underlying leukemia progression and Richter transformation in patients with ibrutinib-relapsed CLL. Blood Adv 2017; 1:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghia P, Ljungström V, Tausch E, et al. Whole-exome sequencing revealed no recurrent mutations within the PI3K pathway in relapsed chronic lymphocytic leukemia patients progressing under idelalisib treatment. Blood 2016; 128:2770.27697770 [Google Scholar]
- 16.Scheffold A, Jebaraj Chelliah BM, Tausch E, et al. In vivo modeling of resistance to PI3Kd inhibitor treatment using EuTCL1-Tg tumor transfer model. Blood 2016; 128:190. [Google Scholar]
- 17.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 2016; 17:768–778. [DOI] [PubMed] [Google Scholar]
- 19▪.Herling CD, Abedpour N, Weiss J, et al. Clonal dynamics towards the development of venetoclax resistance in chronic lymphocytic leukemia. Nat Commun 2018; 9:727.This study is one of the first to use whole-exome sequencing to investigate the mutational landscape of patients relapsing on venetoclax [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Blombery P, Anderson MA, Gong JN, et al. Acquisition of the Recurrent Gly101Val Mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov 2019; 9:342–353.This study examines baseline and relapse samples from patients treated with venetoclax and provides the first description of an acquired recurrent mutation in BCL2 in relapsing cases [DOI] [PubMed] [Google Scholar]