Abstract
Background:
Prior research, primarily with young adults, suggests transcranial direct current stimulation (tDCS) effects are driven by the primary excitatory and/or inhibitory neurotransmitters, glutamate and gamma-aminobutyric acid (GABA), respectively.
Objective/Hypothesis:
We examined the neurometabolic mechanisms of tDCS in older adults with and without mild cognitive impairment (MCI).
Method:
We used data from a double-blind, cross-over, randomized controlled trial (NCT01958437) in 32 older adults to evaluate High Definition (HD-) tDCS-induced changes in glutamate and GABA via magnetic resonance spectroscopy (MRS). Participants underwent MRS following two counterbalanced HD-tDCS sessions (one active, one sham) that targeted the right superior parietal cortex (center anode at P2) and delivered 2mA for 20 minutes.
Results:
Relative to sham, and when co-varying for MRS voxel overlap and right superior parietal volume, active HD-tDCS significantly increased GABA and decreased the ratio of glutamate to GABA. No changes were observed in a left prefrontal control MRS voxel. Although we did not find a significant correlation between strength of delivered current (measured via MRI-based computational modeling) and neurometabolite change, there was a robust positive relationship between the volume of right superior parietal cortex and neurometabolite change.
Conclusion:
Our preliminary findings of increased GABA and reduced glutamate/GABA ratio raise the possibility that (HD-)tDCS effects differ by age. Moreover, age- and disease-related regional brain volume loss may be especially important to consider when planning future studies. Replication would emphasize the importance of developing population-specific tDCS parameters that consider structural and physiologic changes associated with “normal” and pathological aging.
Keywords: transcranial electrical stimulation, magnetic resonance spectroscopy, neuromodulation, neurotransmitters, aging, mild cognitive impairment
Introduction
Growing evidence suggests transcranial direct current stimulation (tDCS) can modulate neural activity, affect a wide range of behavioral and cognitive processes, and ameliorate deficits in several neurologic and psychiatric disorders [1–5]. Although such findings are encouraging, relatively little is known about the basic neurobiological mechanisms through which tDCS exerts its effects, especially in vivo. A current belief is tDCS modulates cortical excitability via primary effects on glutamate (the primary excitatory neurotransmitter) and gamma-aminobutyric acid (GABA – the primary inhibitory neurotransmitter) [6–10].
Magnetic resonance spectroscopy (MRS) has emerged as a promising method for non-invasively quantifying such changes in neurotransmitter levels in vivo. Multiple studies conducted in healthy (and typically young) adult samples suggest tDCS may reduce GABA concentrations [11–15] and increase glutamate concentrations in regions under the anode [16], as well as reduce glutamate in regions under the cathode [11]. However, multiple other studies have failed to show such patterns after tDCS at the site of the anode or cathode [17–18], suggesting incomplete understanding of the factors that mediate or moderate tDCS effects.
Indeed, the existing literature in this area is limited in several important ways. First, nearly all existing studies assessing the effects of tDCS using MRS have been conducted in healthy, young adult samples (e.g., 11–13, 15, 16–18). This is problematic for advancing translation of tDCS in populations that may benefit such as those experiencing neurologic disease and injuries as well as those showing “normal” age-related cognitive decline. To date, only one known study combining tDCS and MRS in older adults has been published [14]. Consistent with the young and healthy adult literature, Antonenko and colleagues (2017) observed a significant reduction in levels of GABA at the site of the anode (left somatosensory region) following active versus sham stimulation in their healthy older adult sample. However, additional research is needed to replicate these findings and examine effects in non-sensorimotor cortices, especially in brain regions susceptible to age- and disease-related changes. This is particularly important because structural (i.e., atrophy) and physiologic changes, including declines in neurotransmitter levels [19], are characteristic of both normal and pathological aging processes, and could reasonably lead to variability in tDCS effects. We are not aware of any prior studies that have used MRS to quantify tDCS effects in older adults with cognitive impairment, despite the clear potential for clinical translation.
The current study used MRS data from a double-blind, cross-over design, randomized controlled trial (NCT01958437) that delivered active or sham High Definition (HD-) tDCS to the right parietal lobe; a region known to demonstrate age- and disease-related changes. We view the study as exploratory in nature given the modest sample size and lack of existing data in this content area. Study goals were to first evaluate the effects of HD-tDCS on local levels of glutamate and GABA, and ratios thereof, in a mixed sample consisting of cognitively intact, healthy Older Adult (HOA) and those with amnestic mild cognitive impairment (MCI). This goal inherently attempted to replicate findings demonstrated in younger adults and the single study conducted in healthy older adults [14]. The second goal was to examine the effect of electric field (EF) magnitude and targeted brain volume on MRS changes. The use of individualized modeling is especially important in older adults given the impact of reduced/variable brain volumes [20].
In achieving these goals, we address several key knowledge gaps and methodological shortcomings of the existing literature that are important to note here. First, we used HD-tDCS since it provides more focal stimulation delivery relative to the traditional pad-based approach used in prior studies [21–22]. HD-tDCS allowed us to place the MRS voxel in the region that theoretically received the greatest electric field (EF) (i.e., the region under the center anode). Importantly, we included a control voxel in the diagonal quadrant of the brain (i.e., left frontal cortex) since modeling indicated no current was delivered to this region. Second, because MRS voxel placement may vary across sessions even with skilled technologists [23], we adjusted for the overlap (or lack thereof) in voxel placement across sessions. Third, we examined variability in the magnitude of EF delivered to the targeted brain region using MRI based modeling, as well as brain volume of the stimulated region, in response to literature suggesting these factors may account for heterogeneity in stimulation effects [15, 24]. Relatedly, we included both cognitively intact older adults and those with a clinical diagnosis of amnestic MCI in order to explore how normal and pathological aging may differentially respond to tDCS. The parietal focus of stimulation in our study is particularly appropriate in these cohorts due to the well-established posterior to anterior shift in aging [e.g., 25] and the disease pathology that accumulates in this region early in the course of AD [e.g., 26]. Additionally, the parietal location allowed us to move past the typical focus on frontal, temporal and motor cortices in the existing tDCS-MRS literature. Finally, we used ratios of glutamate to GABA since prior literature suggests this combined approach provides a more comprehensive picture of neurophysiology [7, 27].
Materials and Methods
Participants
Data were available from thirty-two, right-handed and magnetic resonance imaging (MRI) -compatible older adults who completed a larger clinical trial (NCT01958437). Thirteen participants held a diagnosis of amnestic MCI (single or multiple domain) with a presumed AD etiology based on the Albert and colleagues [28] (i.e., subjective complaint, objective evidence of impairment, but intact everyday functioning), while the other nineteen were cognitively intact, referred to herein as Healthy Older Adults (HOA) (Table 1). Both participant groups were diagnosed during an interdisciplinary consensus conference that included neuropsychologists, neurologists, nurses, social workers, or other related disciplines. There were no significant differences between groups on demographic variables (i.e., age, sex, education). Exclusion criteria included history of other contributing neurological (i.e., epilepsy, moderate-severe traumatic brain injury) or medical conditions known to affect cognitive functioning, significant mental illness (e.g., moderate to severe depression, bipolar disorder, schizophrenia), sensory impairments that limited their ability to participate in the study, or a history of alcohol or drug abuse/dependence. Participants were right-handed based on the Edinburgh handedness inventory [29] and MRI-eligible based on American College of Radiology (ACR) safety guidelines [30].
Table 1.
Demographic data and cognitive functioning of healthy older adult and MCI groups.
| Demographic/Measure | Healthy Group (n=19) M (SD) | MCI Group (n=13) M (SD) | Group Differences |
|---|---|---|---|
| Age | 69.26 (6.73) | 71.15 (5.26) | t=−0.85, p=0.40 |
| Sex | 10m/9f | 10m/3f | χ2=1.94, p=0.27 |
| Education | 15.75 (1.92) | 16.25 (2.77) | t=−0.28, p=0.78 |
| MoCA (raw score) | 25.95 (2.50) | 23.08 (3.17) | t=2.86, p=0.008* |
| WTAR (Word Reading) | 115.74 (9.03) | 104.31 (12.53) | t=3.00, p=0.005* |
| RBANS Immediate Memory | 100.05 (16.51) | 76.38 (9.89) | t=4.62, p<0.001* |
| RBANS Visuospatial | 111.74 (14.21) | 95.62 (15.70) | t=3.02, p=0.005* |
| RBANS Language | 104.00 (10.38) | 92.69 (8.98) | t=3.19, p=0.003* |
| RBANS Attention | 111.47 (18.43) | 92.00 (28.47) | t=2.36, p=0.025* |
| RBANS Delayed Memory | 103.26 (13.33) | 73.15 (18.15) | t=5.42, p<0.001* |
| Trails A (z-score) | 0.69 (0.95) | 0.19 (0.93) | t=1.47, p=0.15 |
| Trails B (z-score) | 0.70 (1.05) | 0.02 (1.56) | t=1.47, p=0.15 |
All test scores represent standard scores (M=100, SD=15), unless otherwise specified. Tests marked with an asterisk (*) denote significant group differences at p<0.05; MCI=mild cognitive impairment; m=male; f= female.
Procedures
Following written informed consent, participants underwent a brief neuropsychological protocol (Table 1). Eligible participants were randomized to receive sham HD-tDCS followed by active, or the opposite order. Randomization used a sealed envelope method, in which the stimulation order was pre-determined and represented by unique six-digit codes for each session. Envelopes were sealed, shuffled, and then numbered (different envelopes for MCI and HOA) by a staff member who was not involved with this study. Our study team member opened the sealed envelope at the start of the first session. The structure of each session was identical as both started with HD-tDCS and then proceeded to MRI scanning, which included MRS. At least 48 hours separated tDCS sessions 1 and 2 (Figure 1a), consistent with washout periods used in prior studies [e.g., 11] and well beyond the known effects arising from a single session [31].
Figure 1.
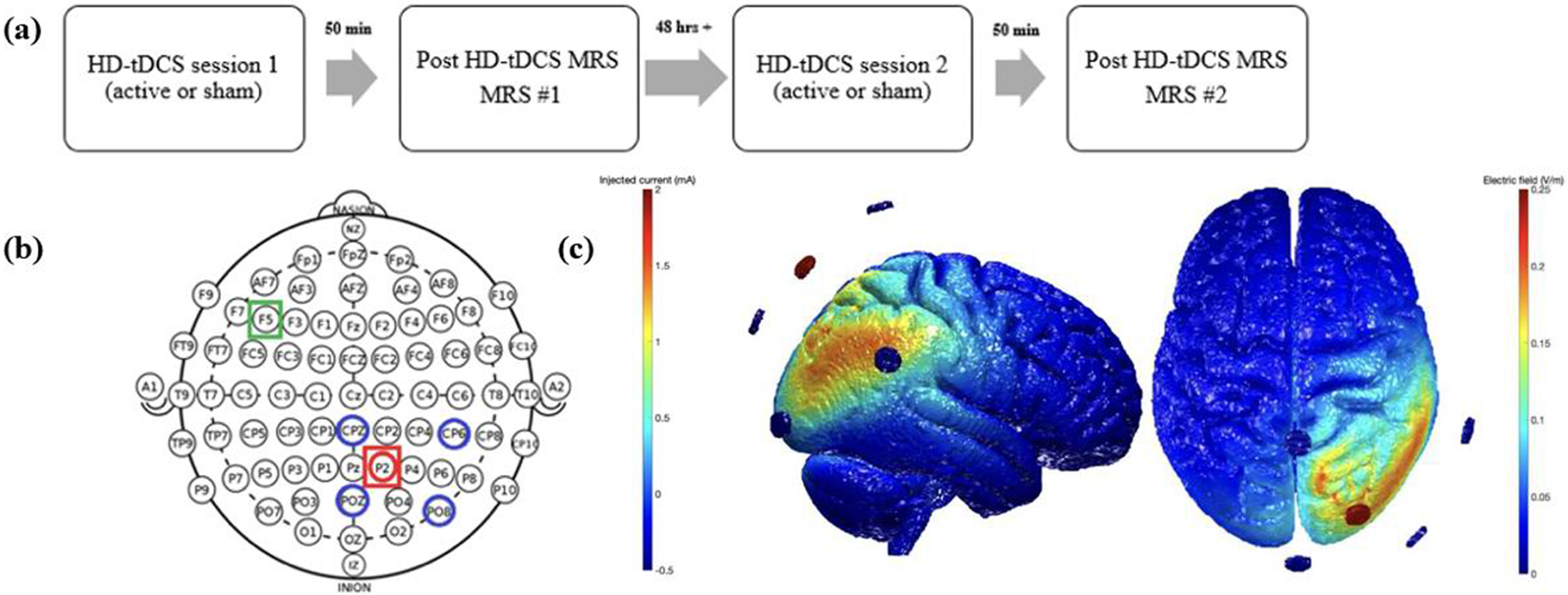
Illustration of (a) experimental design, (b) electrode locations based on international 10–10 electrode system, and (c) electric field arising from the montage via ROAST. Note: Intended location of MRS voxels are depicted in red (P2) and green (F5 – control voxel) boxes in Figure 1b.
HD-tDCS
HD-tDCS was performed in a quiet office approximately 30 feet from the MRI scanner. At the start of the session, study staff measured the participant’s head and identified the site for the center anode (P2) and surrounding cathodes (CPz, CP6, POz, and PO8; Figure 1b). Following our standard protocols, the team member created a cloth template using these measurements, which facilitated electrode placement during the second session. Once sites had been measured and marked, Surgilast head netting was placed over the participant’s head, HD electrode holders (Soterix Medical Inc.) were positioned through the holes at the target locations, and the team member ensured the scalp was visible through the holder (e.g., by moving hair). Each holder was filled with ~10ml conductive gel and checked to ensure there were no air bubbles. The silver/silver chloride HD electrode was then placed into the holder and additional gel added as necessary to ensure it was completely covered. The holder cap was then placed. Following our recently described methods [32], contact quality, as reflected by quality units (QUs), was measured at the start of the session as well as after a 10-minute phase, the latter of which was designed to allow the gel to saturate the scalp and reduce impedance. Additional modifications (e.g., moving hair, adding gel) were made as necessary following these measurements with the goal of achieving a QU≤2. After the 10-minute saturation phase, the team member entered the participant and session specific code into the Clinical Trial unit (Soterix Medical, Inc.), which powered the attached 4×1 HD-tDCS unit (Soterix Medical, Inc.). Active stimulation was delivered at 2mA for 20 minutes with a 30 second ramp up and ramp down period. Sham stimulation consisted of a 30 second ramp up to 2mA followed immediately by 30 second ramp down at both the start and end of the session; an approach that capitalized on both primacy and recency of sensory side effects. Participants completed a standard side effect questionnaire [33–34] and indicated whether they believed they received active or sham stimulation. Then, the electrodes and holders were removed, a vitamin E capsule placed at P2 and F5, and the participant immediately escorted to the MRI scanner.
MRI and H-MRS Acquisition
MRI and H-MRS spectra were acquired with a 3T General Electric Discovery MR750 MRI scanner and a 32-channel head coil made by Nova Medical. A whole-brain, high resolution, T1-weighted image using an SPGR sequence was collected with the following parameters: repetition time (TR) = 12.3ms, echo time (TE) = 5.2ms, inversion time (TI) = 500ms, flip angle = 15°, 156 sagittal slices of 1mm each, an in-plane resolution of 1mm x 1mm, and an in-plane matrix of 256 × 256. Structural scans were used to position a spectroscopic voxel of interest (20 × 20 × 20 mm) in the right superior parietal lobule using the vitamin E capsule placed at P2 as a general guide (see Figure 2 for example). A second 20mm3 voxel was placed over the left inferior frontal gyrus (F5) as a comparator, also marked with a vitamin E capsule. To control for possible confounding effects of atrophy in the region stimulated and/or measured, we acquired the raw brain volume for the right superior parietal lobe and left inferior frontal lobe for each participant via T1 scans using NeuroQuant (version 3.0.1, CorTechs Labs, La Jolla, California), which is an FDA-approved fully automated quantitative analysis tool that has documented reliability across scan sequences and scanner manufacturers.
Figure 2.

Examples of left frontal (F5; top) and right parietal (P2; bottom) voxel locations. Example spectral data shown for full spectrum with GLX identified (left) and GABA (right) (note: only GABA was acquired at the F5 voxel).
MRS scanning began approximately 50 minutes after completion of tDCS stimulation. The spectra data were collected using PRESS (Point RESolved Spectroscopy) and MEGA-PRESS (Mescher-Garwood PRESS) sequences, both of which were acquired in the right superior parietal lobule. Due to time constraints, only the PRESS sequence was collected in the left frontal lobe. Sequence parameters: PRESS (TE = 35ms, TR = 1500ms, 128 transients, 8 NEX, 5kHz spectral width, 4096 data points); MEGA-PRESS (TE = 68 ms, TR = 1800ms, 8 NEX, 5kHz spectral width, 4096 data points, 256 transients (128 ON interleaved with 128 OFF), frequency selective editing pulses (16ms duration, 77 Hz bandwidth) applied at 1.9 ppm (ON) and 7.46 ppm (OFF). Edited spectra were analyzed using the MATLAB-based GANNET v3.0 software [35] for GABA sequences and LCModel [36] for the PRESS sequence with the following simulated set: glutamate and GLX (glutamate and glutamine). Only GABA amplitudes with Cramer Rao lower bounds <20% were considered for analyses. Additionally, metabolites with standard deviations greater than 20 were excluded from analyses. Values extracted from the spectra data software were subsequently inspected for outliers using a cut-point of >3 standard deviations above or below the mean, a procedure which revealed 3 other outlier datapoints (2 at F5, 1 at P2) that were subsequently removed from analyses. Metabolite concentrations were quantified as the ratio of the metabolite to the total creatine (Cr) fitted from the unedited spectra. Normalizing values to Cr was chosen as it has been shown to reduce inter-subject variance due to differences in signal strength and tissue factors [37]. Also, in light of research suggesting the combination of glutamate and GABA may provide a more comprehensive picture of neurophysiology [7], two indices were calculated. We computed the ratio of glutamate to GABA, as well as the GLX/GABA ratio (glutamate and glutamine to GABA concentration ratio).
Although the highly experienced MRI technologist attempted to position the MRS voxels in the same location across sessions, this was often subjectively challenging, as has been described in the literature [23]. Unlike most prior research in this area, we addressed this challenge in several ways. First, the T1 scan acquired during the second scan session underwent a rigid body transformation to the first T1 scan via FSL [38] and the resulting transformation matrix was applied to the second MRS voxel coordinates to determine its location in the space of the first T1 scan. Second, we used the aligned voxel locations to develop 20mm3 cubes that represented the location of each MRS voxel, which allowed us to determine the between-session overlap. This process revealed poor agreement between the two sessions as evidenced by 43.53% (SD=27.76%) overlap at the right superior parietal lobule (SPL) location. Similarly, we found 48.85% (SD=20.75) overlap at the left inferior frontal location. Thus, percent overlap was used as a covariate in subsequent analyses, which to our knowledge has not previously been accounted for in (HD-) tDCS-MRS research.
Computational Modeling
We used a realistic volumetric-approach to simulate transcranial electric stimulation (ROAST) to estimate the amount of electrical current delivered to the targeted brain region in each participant [39]. ROAST is a fully automated, open-source software developed by Parra Labs which runs through MATLAB. The software segments each T1 MRI (via SPM), utilizes virtual electrodes at specified locations and the electrical current amplitude for each electrode, generates a finite element method (FEM) mesh, and solves for electric field and voltage values across the entire brain. For modeling purposes in this study, the anode was placed at P2 and provided 2mA and we assumed an equitable split (i.e., −0.5mA) across the four cathodes (see Figure 1c). ROAST’s tissue probability map (TPM) was replaced with SPM12’s default probability map as it led to improved tissue segmentation. ROAST’s default disc electrodes were used (6 mm radius, 2 mm height). ROAST’s internal tissue conductivities were used and are as follows: white matter = 0.126 S/m, gray matter = 0.276 S/m, cerebrospinal fluid = 1.65 S/m, bone = 0.01 S/m, skin = 0.465 S/m, air = 2.5e-14 S/m, gel = 0.3 S/m, and electrode = 5.9e7 S/m. We created a 10mm cube around the center point between the two MRS voxel locations for each participant and extracted the average EF from this region of interest (ROI).
Statistical Approach
All statistical analyses were performed using SPSS Statistics 27 (IBM, Armonk, NY, USA). We used repeated measures analysis of covariance (ANCOVA) to assess for changes in neurometabolite concentration values and ratios between HD-tDCS conditions and groups. These analyses were performed for each neurometabolite (i.e., P2 GABA, glutamate, GLX; F5 GABA) and the neurometabolites ratios (i.e., Glutamate/GABA, GLX/GABA at P2) with stimulation (sham vs. active) as the repeated measure (within subjects) factor, group (i.e., HOA vs. MCI groups) as the between-subjects factor; percent MRS voxel overlap and volume of the region measured (i.e., right superior parietal lobule (SPL) for P2 and left inferior frontal for F5). Partial eta-squared (η2p) was calculated within ANCOVA and Cohen’s d were used to quantify associated effect sizes as appropriate. Partial correlations (controlling for MRS voxel overlap and right SPL volume) were used to examine the role of electric field (EF) and change in excitatory to inhibitory neurotransmitter ratio levels (i.e., Glutamate/GABA and GLX/GABA). Given the lack of prior work in this area to guide a priori hypotheses and the relatively small sample size, we did not split our alpha, instead opting for an uncorrected p-value of < 0.05 and consideration of medium effect sizes or greater, the latter of which is emphasized in current statistical thought [40].
Results
Means and standard deviations of five neurometabolites and neurometabolites ratios between groups and stimulation conditions are shown in Table 2. We used ANCOVA to evaluate between session change at P2 after controlling for MRS voxel overlap and right SPL volume. Likewise, ACOVA was used to evaluate GABA change at F5 after controlling for MRS voxel overlap and left inferior frontal volume. As shown in Table 3, we found statistically significant effects of group, stimulation, stimulation by group, or a combination thereof, in four of the five analyses conducted (i.e., P2 GABA, P2 Glutamate, P2 Glutamate/GABA, and P2 GLX/GABA ratio). Importantly, there were no main or interaction effects on GABA at the left frontal (F5) control voxel.
Table 2.
Means and standard deviations for each neurometabolite and neurometabolite ratio across groups and stimulation conditions.
| Neurometabolite/Ratio | Sham Condition by Group | Active Condition by Group | Stimulation Condition for MCI and HOA Groups Combined | |||
|---|---|---|---|---|---|---|
| HOA | MCI | HOA | MCI | Sham | Active | |
| P2 GABA | .13 (.02) | .11 (.02) | .13 (.04) | .12 (.02) | .12 (.02) | .13(.04) |
| P2 Glutamate | 1.45 (.15) | 1.33 (.13) | 1.39 (.16) | 1.41 (.11) | 1.40 (.15) | 1.40 (.14) |
| P2 GLX | 1.83 (.14) | 1.73 (.16) | 1.80 (.19) | 1.77 (.14) | 1.79 (.15) | 1.79 (.17) |
| P2 Glutamate/GABA | 11.39 (3.78) | 12.04 (2.30) | 10.78 (1.09) | 12.67 (2.64) | 11.67 (3.17) | 11.62 (2.11) |
| P2 GLX/GABA | 13.73 (1.52) | 16.46 (2.96) | 14.73 (4.70) | 15.02 (2.68) | 14.93 (2.61) | 14.86 (3.87) |
| F5 GABA | .11 (.02) | .12 (.03) | .11(.03) | .12 (.02) | .12 (.02) | .11 (.03) |
Note: Values are not corrected for MRS overlap or P2 volume. MCI = mild cognitive impairment; HOA = healthy control; MRS = magnetic resonance spectroscopy; GLX = glutamate + glutamine.
Table 3.
Main and interaction effects of stimulation type (i.e., active vs. sham), group (i.e., MCI vs. HOA), and stimulation*group on metabolite concentrations controlling for % MRS voxel overlap and right SPL volume.
| Metabolite/Metabolic Ratio | Main/Interaction Effects | Mean Square | F | p-value | η2p |
|---|---|---|---|---|---|
| P2 GABA | Stimulation | 0.005 | 9.374 | 0.006 | 0.309 |
| Group | 0.006 | 8.259 | 0.009 | 0.282 | |
| Stimulation*Group | 0.000 | 0.036 | 0.852 | 0.002 | |
| P2 Glutamate | Stimulation | 0.015 | 0.987 | 0.330 | 0.039 |
| Group | 0.010 | 0.430 | 0.518 | 0.018 | |
| Stimulation*Group | 0.070 | 4.726 | 0.040 | 0.165 | |
| P2 GLX | Stimulation | 0.040 | 2.433 | 0.132 | 0.092 |
| Group | 0.062 | 1.786 | 0.194 | 0.069 | |
| Stimulation*Group | 0.018 | 1.126 | 0.299 | 0.045 | |
| P2 Glutamate/GABA | Stimulation | 42.538 | 10.032 | 0.005 | 0.323 |
| Group | 25.489 | 2.933 | 0.102 | 0.123 | |
| Stimulation*Group | 0.001 | 0.000 | 0.989 | 0.000 | |
| P2 GLX/GABA | Stimulation | 77.196 | 11.309 | 0.003 | 0.350 |
| Group | 29.606 | 2.475 | 0.131 | 0.105 | |
| Stimulation*Group | 1.700 | 0.249 | 0.623 | 0.012 | |
| F5 GABA | Stimulation | 0.001 | 1.045 | 0.320 | 0.055 |
| Group | 0.000 | 0.602 | 0.448 | 0.032 | |
| Stimulation*Group | 0.000 | 0.661 | 0.427 | 0.035 |
Note: Values in bold denote effects with p ≤ .05 and/or those associated with a medium or large effect size. MCI = mild cognitive impairment; HOA = healthy older adults; MRS = magnetic resonance spectroscopy; GLX = glutamate + glutamine. P2 GABA, P2 Glutamate/GABA, and P2 GLX/GABA represents F(1,21), P2 Glutamate and P2 GLX represent F(1,24), and F5 GABA represents F(1,18).
Effects of Group on Neurometabolites and Neurometabolite Ratios
Our analyses revealed a statistically significant effect of group on GABA levels at P2, such that the MCI group had lower levels than the HOA group (F1,21 = 8.26, p < 0.01, η2p = 0.28). By contrast, we did not find differences in GABA levels between MCI and HOA groups at the left frontal control voxel, F5. Although they fell short of the p <.05 threshold, medium effect sizes were found for effect of group on glutamate/GABA (F1,21 = 2.93, p = 0.10, η2p = 0.12) and GLX/GABA (F1,21 = 2.48, p = 0.13, η2p = 0.11) where values in the MCI group were relatively higher than in the HOA group.
Effects of Stimulation on Neurometabolites and Neurometabolite Ratios
There was a significant main effect of stimulation on right parietal (P2) GABA, which was about an 8% increase following the active compared to sham conditions and accompanied by a large effect size (F1,21 = 9.37, p <0.01, η2p = 0.31). Such stimulation effects on GABA were specific to the P2 location and were not found at the left frontal (F5) control voxel (F1,18 = 1.05, p = 0.32, η2p = 0.06). We also noted a significant main effect of stimulation on both ratios at P2 – Glutamate/GABA (F1,21 = 10.03, p < 0.01, η2p = 0.32) and GLX/ GABA (F1,21 = 11.31, p < 0.01, η2p = 0.35). Specifically, there were significant decreases in these ratios after active stimulation which were associated with large effect sizes. A significant group by stimulation interaction (with medium effect size) was evident for glutamate, where levels were relatively higher in the HOA than MCI group during the sham session but a cross-over occurred after active stimulation when the MCI group showed higher glutamate levels than did the HOA group (F1,24 = 4.73, p = 0.04, η2p = 0.17). However, post-hoc analyses with Fisher’s least significant difference adjustment for multiple comparisons revealed no significant differences between sham and active tDCS within either the HOA or MCI group (ps ranged .52 – .77).
Relationship between EF, Right SPL Volume, and Amount of Neurometabolic Change
Based on MRI computational modeling, participants experienced an average EF of only 0.13 V/m (SD = 0.041) within the 1 cm3 ROI. As seen in Figure 2a–c, there was significant variability in EF between participants, with individual values ranging from 0.06 to 0.25 V/m, a difference as high as ~ 410%. This finding is remarkable given that every individual received the exact same amount of electric current at the scalp (i.e., 2mA). Therefore, we assessed the relationship between mean EF at P2 and degree of change in GABA, glutamate, GLX, and corresponding ratios (i.e., glutamate/GABA and Glx/GABA), controlling for MRS overlap and right SPL volume. There were no significant correlations between EF and neurometabolite concentration changes in our entire sample or within either diagnostic group (Table 4); these correlations remained nonsignificant when controlling for right SPL volume (rs = −0.01 – −0.18, ps =.44 – .97; Figure 2d). However, as seen in Figure 2e, there was a statistically significant positive correlation between volume of the site of stimulation (the right SPL) and Glutamate/GABA and GLX/GABA change across the entire sample, such that larger volumes were associated with a larger increases in Glutamate/GABA and GLX/GABA (partial correlations = 0.57 and 0.60, ps < 0.01, R2s = 0.33 and 0.36, respectively); notably, these correlations remained robust even when controlling for EF strength (partial correlations = 0.57 and 0.62, ps < 0.01, respectively).
Table 4.
Partial correlations between change amount of neurometabolite ratio change, magnitude of EF, and right SPL volume, controlling for % MRS voxel overlap.
| Correlation with Mean EF | Correlation with Right SPL Volume | |||
|---|---|---|---|---|
| Partial Correlation (MRS Overlap) | Partial Correlation (MRS Overlap + right SPL volume) | Partial Correlation (MRS Overlap) | Partial Correlation (MRS Overlap + mean EF) | |
| ΔGlutamate/GABA | 0.04 (0.85) | −0.09 (0.68) | 0.57 (0.004) | 0.57 (0.005) |
| ΔGLX/GABA | −0.01 (0.97) | −0.18 (0.44) | 0.60 (0.002) | 0.62 (0.002) |
Note: Values in bold denote correlations associated with p ≤ .05 and/or those associated with a medium or large effect size.
Discussion
This is the first study, to our knowledge, to evaluate HD-tDCS effects using MRS in cognitively intact older adults and those with MCI. We addressed several key methodological weaknesses of prior work including using HD-tDCS to provide focal stimulation, including MRS voxels from both the targeted and a control region, controlling for MRS voxel overlap and volume of the targeted brain region, and by examining the effects of delivered EF. Two noteworthy findings emerged from our analyses.
First, we found a statistically significant increase in GABA and decrease in ratios of glutamate to GABA at the targeted right superior parietal lobe following active stimulation. Importantly, the change in GABA following stimulation was not apparent in the left frontal lobe control region, which reinforces the focality of HD-tDCS-induced neurometabolic effects. These findings are intriguing for several reasons. First, prior work using pad-based tDCS and young adults found reductions in GABA at the site of the anode [11–15]. This discrepancy may emerge from the delivery method as HD-tDCS has shown enhanced neurophysiologic effects relative to the traditional pad-based approach [31]. Alternatively, though not mutually exclusive, are the potential effects of age. Prior work revealed reduced GABA levels in both “normal” and pathological aging [41–43]. Such GABA reductions are presumed to disrupt neural excitation/inhibition balance, and thereby leading to hyperexcitability, neuronal damage, and cognitive decline in older adults [44–45]. These findings appear consistent with evidence of “hyper-”activity/connectivity seen in those with MCI [46, 47] and align with our finding of reduced right parietal GABA in the MCI group relative to the controls. The fact that there were no significant differences in GABA levels between groups within the left prefrontal region adds to the biological plausibility since AD typically accumulates in posterior brain regions early in the disease course [26]. If replicated in those with biologically confirmed AD, our findings strengthen the argument for targeting posterior regions rather than the typical prefrontal focus. Changes in glutamate were dependent on both stimulation and group as reflected by relative decline for the HOA group and relative increase for those with MCI; however, neither of these relative changes reached statistical significance. Regardless, replicating this phenotype specific pattern in a larger sample could also be informative in regards to effects of AD.
Second, consistent with prior research, our findings indicate significant variability in the magnitude of delivered current, on the order of ~410%. This variability highlights the need to move beyond the traditional one-size fits all dosing at the scalp by using individualized, prospective modeling to standardize the delivered dose. However, our correlational analyses did not reveal a significant relationship between strength of delivered current (i.e., EF at the midpoint between the MRS voxels) and level of neurometabolite change. While this preliminary finding may simply reflect that any amount of stimulation is sufficient for neuromodulatory purposes, we suspect two other factors contributed to the null relationship. First, the actual amount (<0.25 V/m) and the range (~0.19 V/m) of delivered current was very small and outside of the range where dose-response relationships have been identified [48]. Second, the EF values were extracted from the midpoint of the MRS voxels, which did not necessarily align with the targeted location/peak of stimulation. However, we felt the midpoint approach was most rigorous given our focus on MRS data. Interestingly, we identified a robust positive correlation between right superior parietal volume and degree of neurometabolite change, specifically the increase in glutamate and GLX relative to GABA, which could reflect the importance of availability of neurons/synapses within the region stimulated. This finding suggests that neurodegeneration that occurs with aging and neuropathological processes may dictate the window for possible neurophysiologic change/impact from tDCS.
Taken together, our findings highlight the importance of individualizing parameters of tDCS with considerations of age as well as variations in neuroanatomy (i.e., volume of the region targeted for stimulation). Our counterintuitive findings showing an increase (rather than a decrease) in GABA at the region below the anode, following active stimulation challenges the long-held and, perhaps, overly simplistic notion that stimulation under the anode is strictly excitatory, particularly for older and clinical populations with known neurostructural and functioning changes. The observed increase in GABA following tDCS may not be entirely surprising in the context of known disruption that occurs to both excitatory and inhibitory neurotransmission with aging [49, 50]. In fact, our study findings appear consistent with at least two known published studies investigating effects of tDCS on cortical excitability in older adults using other, non-MRS methodologies. Specifically, studies using transcranial magnetic stimulation (TMS) to measure cortical excitability (via event related intracranial inhibition vs. intracranial facilitation) have demonstrated increased inhibition in older adults under the anode, contrary to the reduced inhibition and increased facilitation seen in the younger adult counterparts [51, 52]. Thus, population-specific differences in baseline functioning of a stimulated brain region (i.e., too high/too low excitability/inhibition) could dictate the neuroplastic mechanisms, and may help explain the variability in tDCS response seen across studies and individuals [53].
Future work should replicate and extend our findings. While the first such report in this area, our findings are limited by the relatively small sample size, variability in voxel placement, lack of AD biomarkers for our MCI group, and lack of glutamate data at the left frontal control voxel location (which was omitted due to time constraints). We used the sham condition as our presumed baseline, though it should be noted that this may not equate to a true neurometabolic baseline. Additionally, the amount of delivered current (i.e., that which reached the brain) was quite low overall (EF range 0.06 – 0.25 V/m), which reinforces the need for improved dose-response information [47]. While addressing these limitations, follow-up studies should also incorporate other neuroimaging modalities, and well as concurrent cognitive / behavioral changes, to fully understand the HD-tDCS-induced MRS changes.
Conclusion
(HD-) tDCS is a promising rehabilitation tool for various clinical presentations, including older adults with cognitive impairment. Unfortunately, the underlying mechanisms through which tDCS elicits changes, are not well understood, especially for older adults. The present study is the first to explore the neuromodulatory mechanisms of HD-tDCS using MRS in a population of older adults with and without mild cognitive impairment. Contrary to extant findings in young, healthy adult samples, our older adults sample exhibited an unexpected increase in local GABA under the anode as well as a decrease in ratios of glutamate to GABA. Our findings suggest that older adult brains may differentially respond to HD-tDCS, potentially due to differences in brain structure and baseline neurometabolic functioning that occur with normal and pathological aging. Future research should replicate these preliminary findings and evaluate the associated functional implications. It remains to be seen whether increases in GABA are accompanied by decreases in neural activity and connectivity, increased modularity/efficiency, or positive cognitive or behavioral change. Additionally, clarifying how variations in baseline functional connectivity (i.e., hyper- versus hypo-connectivity) respond to HD-tDCS may help explain the intra-individual variability demonstrated across studies, and therefore help to validate it as a rehabilitation tool for appropriate populations.
Figure 3.
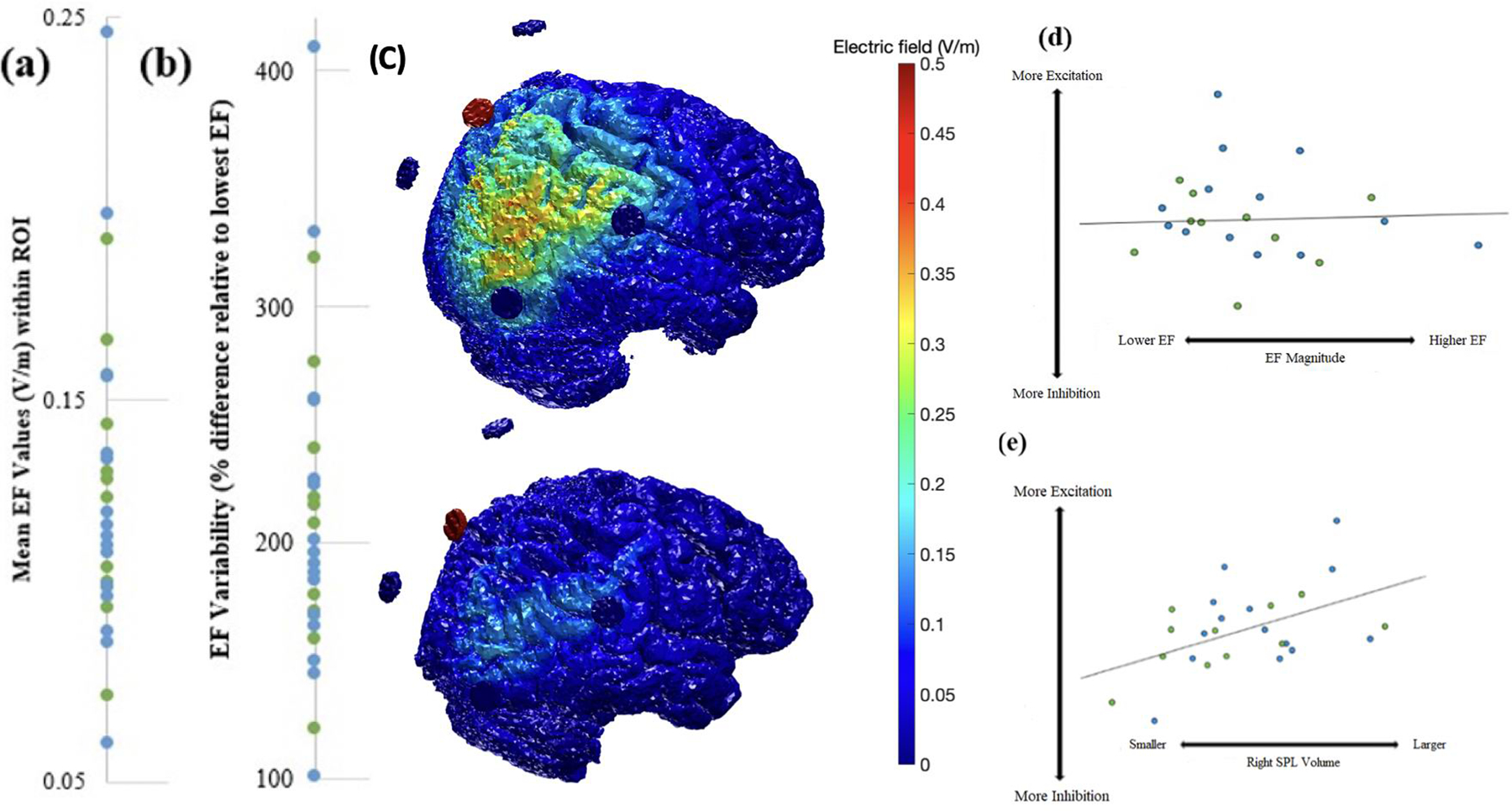
(a) Mean EF values within the P2 1 cm3 ROI across participants. (b) Percent differences in EF relative to participant with lowest EF. (c) ROAST EF models of two participants illustrating striking variability in EF values. (d) Relationships between EF and degree of change in excitatory to inhibitory neurotransmitters (i.e., glutamate/GABA ratio) for the Healthy Control and MCI groups, covarying for MRS overlap and P2 Volume. (e) Relationship between P2 Volume and degree of change in excitation (i.e., glutamate to GABA ratio) for the Healthy Older Adult (HOA) and MCI groups.
Acknowledgments
The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Funding:
Primary funding was provided by Small Projects in Rehabilitation Research (SPiRE) Award (IRX001381) grant awarded to BMH by Rehabilitation Research & Development, Office of Research and Development, Department of Veteran’s Affairs. Partial support from National Institute on Aging, National Institutes of Health via R35AG072262 (to BMH for analytic infrastructure and effort) and the Michigan Alzheimer’s Disease Research Center (P530AG053760-5 for participant characterization and recruitment) are also acknowledged.
Footnotes
Declaration of interest: None.
Ethics approval and consent to participate: The VA Ann Arbor Healthcare System’s Institutional Research Board approved this study. All participants provided written informed consent.
References
- [1].Coffman BA, Clark VP, Parasuraman R (2014) Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage 85, 895–908. [DOI] [PubMed] [Google Scholar]
- [2].Harty S, Sella F, Kadosh RC (2017) Transcranial electrical stimulation and behavior change: the intermediary influence of the brain. Front Hum Neurosci 11, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gonzalez PC, Kong KNK, Chung RCK, Ting K, Law LLF, Brown T (2018) Can transcranial direct current stimulation alone or combined with cognitive training be used as a clinical intervention to improve cognitive functioning in persons with mild cognitive impairment? A systematic review and meta-analysis. Front Hum Neurosci 12, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kuo M, Nitsche MA (2012) Effects of transcranial electrical stimulation on cognition. Clin EEG Neurosci 43, 192–199. [DOI] [PubMed] [Google Scholar]
- [5].Meinzer M, Lindenberg R, Darknow R, Ulm L, Copland D, Floel A. (2014) Transcranial direct current stimulation and simultaneous magnetic resonance imaging. J Visualized Exp 86, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nitsche MA, Paulus W (2001) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 15, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Krause B, Marquez-Ruiz J, Kadosh RC (2013) The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance. Front Hum Neurosci 7, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stagg CJ, O’Shea J, Kinsces T, Woolrich M, Mathews PM, Johansen-Berg H (2009) Modulation of movement associated cortical activation by transcranial direct current stimulation. Eur J Neurosci 30, 1412–1423. [DOI] [PubMed] [Google Scholar]
- [9].Stagg CJ, Nitsche MA (2011) Physiological basis of transcranial direct current stimulation. Neuroscientist 17, 37–53. [DOI] [PubMed] [Google Scholar]
- [10].Brunoni A, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F (2012) Clinical research with transcranial direct current stimulation (tDCS): Challenges and Future Directions. Brain Stimul 5, 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Tamas Kincses Z, Morris PG, Matthew PM, Johansen-Berg H (2009) Polarity sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29, 5202–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim S, Stephenson MC, Morris PG, Jackson SR (2014) tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7T magnetic resonance spectroscopy study. Neuroimage 99, 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bachtiar V, Near J, Johansen-Berg H, & Stagg CJ (2015) Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. eLife 4, e08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Antonenko D, Schubert F, Bohm F, Ittermann B, Aydin S, Hayek D, Grittner U, Floel A (2017) tDCS induced modulation of GABA levels and resting state functional connectivity in older adults. J Neurosci 37, 4065–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Antonenko D, Thielscher A, Saturnino GB, Aydin S, Ittermann B, Grittner U, Floel A (2019) Towards precise brain stimulation: is electric field simulation related to neuromodulation. Brain Stim 12, 1159–1168. [DOI] [PubMed] [Google Scholar]
- [16].Clark VP, Coffman BA, Trumbo MC, Gasparovic C (2011) Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a 1H magnetic resonance spectroscopy study. Neurosci Lett 500, 67–71. [DOI] [PubMed] [Google Scholar]
- [17].Dwyer GE, Craven AR, Hirnstein M, Kompus K, Assmus J, Ersland L, Hughahl K, Gruner R (2019) No effects of anodal tDCS on local GABA and Glx levels in the left posterior superior temporal gyrus. Front Neurol 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hone-Blanchet A, Edden RA, Fecteau S (2016) Online effects of transcranial direct current stimulation in real time on human prefrontal striatal metabolites. Biol Psychiatry 80, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H (2017). Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol 27, 2698–2705. [DOI] [PubMed] [Google Scholar]
- [20].Indahlastari A, Albizu A, O’Shea A, Forbes MA, Nissim NR, Kraft JN, Evangelista ND, Hausman HK, Woods AJ (2020) Modeling transcranial electrical stimulation in the aging brain. Brain Stimul 13, 664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Datta A, Truong D, Minhas P, Parra LC, Bikson M (2012) Interindividual variation during tdCS and normalization of dose using MRI derived computational models. Front Psychiatry 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M (2013) Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high definition tDCS. Neuroimage 74, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dou W, Speck O, Benner T, Kaufmann J, Li M, Zhong K, Walter M (2014) Automatic voxel positioning for MRS at 7 T. Magn Reson Mater Phy 28, 259–270. [DOI] [PubMed] [Google Scholar]
- [24].Laakson I, Mikkonen M, Koyama S, Hirata A, Tanaka S (2019) Can electric fields explain inter-individual variability in transcranial direct current stimulation of the motor cortex. Nature 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R (2008) Que PASA? The Posterior-Anterior Shift in Aging. Cerebral Cortex 5, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropath. Commun. 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [27].Steel A, Mikkelsen M, Edden RAE, Robertson CE (2020) Regional balance between glutamate+glutamine and GABA+ in the resting human brain. NeuroImage 220, 117112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies W, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 96–113. [DOI] [PubMed] [Google Scholar]
- [30].ACR Committee on MR Safety, Greenberg TD, Hoff MN, Arch TBGM, Jackson EF, Kanal E, McKinney AM, Och JG, Pedrosa I, Rampulla TL, Reeder SB, Rogg JM, Shellock FG, Watson RE, Weinreb JC, Gernandez D (2019) ACR guidance document on MR safe practices: updates and critical information 2019. J Magn Reson Imaging 51, 331–338. [DOI] [PubMed] [Google Scholar]
- [31].Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, Nitsche MA (2013) Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul 6, 644–648. [DOI] [PubMed] [Google Scholar]
- [32].Hampstead BM, Ehmann M, Rahman-Filipiak A (2020) Reliable use of silver chloride HD-tDCS electrodes. Brain Stimul 13, 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brunoni A, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F (2011) A systematic review of reporting and assessment of adverse effects associated with tDCS. International J Neuropsychopharmacol 14, 1133–1145. [DOI] [PubMed] [Google Scholar]
- [34].Reckow J, Rahman-Filipiak A, Garcia S, Schlaefflin S, Calhoun O, Dasilva AF, Bikson M, Hampstead BM (2018) Tolerability and blinding of 4×1 high-definition tDCS at two and three milliamps. Brain Stimul 11, 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ (2014) Gannet: A batch professing tool for quantitative analysis of gamma-aminobutyric acid-Edited MR Spectroscopy Spectra. J Magn Reson Imaging 40, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Provencher SW (2001) Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14, 260–264. [DOI] [PubMed] [Google Scholar]
- [37].Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, Trattnig S, Doerfler A, Stefan H, Hammen T (2010). In-vivo quantification of intracerebral GABA by single-voxel 1H-MRS-How reproducible are the results? Eur Radiol 73, 526–531. [DOI] [PubMed] [Google Scholar]
- [38].Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012) FSL. NeuroImage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- [39].Huang Y, Datta A, Bikson M, Parra LC (2019) Realistic volumetric-approach to simulate transcranial electric stimulation-ROAST- a fully automated open-source pipeline. J Neural Eng 16, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wasserstein RL, Schirm AL, Lazar NA (2019) Moving to a world beyond “p < 0.05.” J Am Stat Assoc 73, 1–19. [Google Scholar]
- [41].Cuypers K, Maes C, Swinnen SP (2018) Aging and GABA. Aging 10, 1186–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hermans L, Leunissen I, Maes C, Verstrawelen S, Cuypers K, Edden RA, Puts NA, Swinnen SP (2019) The aging brain and changes in GABA concentrations. Front Neurosci, Conference Abstract: 12th National Congress of the Belgian Society for Neuroscience. [Google Scholar]
- [43].Abdel-Aziz K, Solanky BS, Yiannakas MC, Altmann DR, Wheeler-Kingshott CA, Thompson AJ, Ciccarelli O (2014) Age related changes in metabolite concentrations in the normal spinal cord. PLoS One 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Marenco S, Meyer C, van der Veen JW, Zhang Y, Kelly R, Shen J, Weinberger DR, Dickinson D, Berman KF (2018) Role of gamma-amino-butyric acid in the dorsal anterior cingulate in age-associated changes in cognition. J Neuropsychopharmacol 43, 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nava-Mesa MO, Jimenez-Diaz L, Yajeya J, Navarro-Lopez JD (2014) GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer’s Disease. Front Cell Neurosci 8, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA (2005) Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. J Neurology 65, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE (2010) High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage 51, 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Esmaeilpour Z, Marangolo P, Hampstead BM, Bestman S, Galletta E, Knotkova H, Bikson M (2018). Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul 11, 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Varela EV, Etter G, Williams S (2019) Excitatory-inhibitory imbalance in Alzheimer’s disease and its therapeutic significance. Neurobiol Dis 127, 605–615. [DOI] [PubMed] [Google Scholar]
- [50].Duang D, Liu D, Yin J, Qian T, Shrestha S, Ni H (2017). Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiology 27, 2706–2707. [DOI] [PubMed] [Google Scholar]
- [51].Heise K, Niehoff M, Feldheim JF, Liuzzi G, Gerloff C, Hummel FC (2014) Differential behavioral and physiologic effects of transcranial direct current stimulation in healthy adults of younger and older age. Front Aging Neurosci 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vignaud P, Mondino M, Poulet E, Pal U, Brunelin J (2018) Duration but not intensity influences transcranial direct current stimulation after-effects on cortical excitability. Neurophysiol Clin 48, 89–92. [DOI] [PubMed] [Google Scholar]
- [53].Mongillo G, Rumpel S, Loewenstein Y (2018) Inhibitory connectivity defines the realm of excitatory plasticity. Nature 21, 1463–1470. [DOI] [PubMed] [Google Scholar]


