Background:
Genetics have a strong influence on calcified atherosclerotic plaques; however, data regarding the heritability of noncalcified plaque volume are scarce. We aimed to evaluate genetic versus environmental influences on calcium (coronary artery calcification) score, noncalcified and calcified plaque volumes by coronary computed tomography angiography in adult twin pairs without known coronary artery disease.
Methods:
In the prospective BUDAPEST-GLOBAL (Burden of Atherosclerotic Plaques Study in Twins—Genetic Loci and the Burden of Atherosclerotic Lesions) classical twin study, we analyzed twin pairs without known coronary artery disease. All twins underwent coronary computed tomography angiography to assess coronary atherosclerotic plaque volumes. Structural equation models were used to quantify the contribution of additive genetic, common environmental, and unique environmental components to plaque volumes adjusted for age, gender, or atherosclerotic cardiovascular disease risk estimate and statin use.
Results:
We included 196 twins (mean age±SD, 56±9 years, 63.3% females), 120 monozygotic and 76 same-gender dizygotic pairs. Using structural equation models, noncalcified plaque volume was predominantly determined by environmental factors (common environment, 63% [95% CI, 56%–67%], unique environment, 37% [95% CI, 33%–44%]), while coronary artery calcification score and calcified plaque volumes had a relatively strong genetic heritability (additive genetic, 58% [95% CI, 50%–66%]; unique environmental, 42% [95% CI, 34%–50%] and additive genetic, 78% [95% CI, 73%–80%]; unique environmental, 22% [95% CI, 20%–27%]), respectively.
Conclusions:
Noncalcified plaque volume is mainly influenced by shared environmental factors, whereas coronary artery calcification score and calcified plaque volume are more determined by genetics. These findings emphasize the importance of early lifestyle interventions in preventing coronary plaque formation.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01738828.
Keywords: atherosclerosis, computed tomography angiography, coronary angiography, coronary artery disease, prevalence
Clinical Perspective.
In a prospective twin study of 196 subjects, coronary computed tomography angiography was performed. Using structural equation models, the heritability of coronary artery calcification score, calcified and noncalcified plaque volumes were estimated. Noncalcified plaque volume was predominantly determined by environmental factors (common environment: 63%, unique environment: 37%), while coronary artery calcification score and calcified plaque volumes had a relatively strong genetic heritability (additive genetic: 58%, unique environmental: 42% and additive genetic: 78%, unique environmental: 22%, respectively). Based on our findings, the genetic background of noncalcified plaque is smaller than that of coronary artery calcification score and calcified plaque. Our results are hypothesis generating but could also imply that socioeconomic status and lifestyle parameters have bigger impact on noncalcified plaque volumes as compared with coronary artery calcification score and calcified plaque volumes. Based on these, there is an urgent need to start primary prevention of coronary artery disease as early in life as possible.
Coronary artery disease (CAD) is a multifactorial disease, influenced by the interplay of environmental and genetic factors.1 Heritability of CAD has been estimated to be 40% to 60%, suggesting that genetics play an important role in its development.2 Coronary artery calcification (CAC) as assessed by noncontrast computed tomography (CT) has a substantial genetic component, ranging from 30% to 45% in the literature.3–7 However, the heritability of noncalcified plaque (NCP) volume has only been addressed in a small number of family studies.8–10 Healthy individuals with a family history of early onset CAD have a higher prevalence of NCP.8 NCPs are also more prevalent in younger patients with a family history of CAD, compared with patients with no family history9 or even to symptomatic patients.10 It has been suggested that NCPs represent an earlier stage in atherosclerotic plaque development.11 On the contrary, calcification of atherosclerotic plaque may occur in later stages, and calcified plaques (CPs) are less prone to cause events as compared with NCPs.11,12
Investigating familial aggregation of complex traits is limited. Shared environment confounds efforts to characterize genetic heritability of complex traits.13 However, twins with their precisely matching age represent a unique cohort among family studies,13 and they also share a wide range of environmental and socioeconomic variables that may influence the expression of complex traits, such as CAD.14 Twin studies by modeling the shared environment, provide a powerful tool to investigate the genetic and environmental factors contributing to a multifactorial disease.14,15
Therefore, our aim was to assess the relative contribution of genetic and environmental factors on NCP, CAC score, and CP volumes using coronary CT angiography (CTA) in adult twin pairs without known CAD.
Methods
The data will be made available from the corresponding author on reasonable request after institutional approval.
Study Population
This prospective, single-center, classical twin study was conducted under the name of BUDAPEST-GLOBAL (Burden of Atherosclerotic Plaques Study in Twins—Genetic Loci and the Burden of Atherosclerotic Lesions) study.16 Participants had been co-enrolled within an international, multicenter clinical study; Genetic Loci and the Burden of Atherosclerotic Lesions (http://www.ClinicalTrials.gov: NCT01738828).16,17
Detailed study description and enrollment criteria were published previously.17 Monozygotic and same-gender dizygotic twins were recruited from the Hungarian Twin Registry,18 all participants provided informed consent. We assessed zygosity using a multiple self-reported questionnaire.19,20 The study was approved by the National Scientific and Ethics Committee (institutional review board number: ETT TUKEB 58401/2012/EKU [828/PI/12]) and was performed according to the principles stated in the Declaration of Helsinki.21 We excluded 3 twin pairs due to insufficient image quality, resulting in 196 twin subjects as our final cohort.
Anthropometric Data, Medical History, and Laboratory Analysis
Information regarding past medical history and current medical therapy were recorded by self-reported questionnaires. Fasting peripheral blood draw and brachial blood pressure measurements as well as anthropometric data were recorded before the coronary CTA examination. Fasting blood samples were analyzed by standard methods in a certified laboratory.
Coronary CTA Image Acquisition and Assessment
Coronary CTA was performed with a 256-slice multidetector CT (Brilliance iCT; Philips HealthTech, Best, the Netherlands).17 Prospective ECG triggered noncontrast and contrast-enhanced scans were acquired. Beta-blockers were administered as needed to reach a target heart rate below 60/min. Sublingual nitroglycerin (0.8 mg) was administered on the table. Four-phasic contrast injection protocol was used (Iomeprol 400 g/cm3; Iomeron, Bracco Imaging S.p.A., Milan, Italy).22 All image analyses were performed offline on a dedicated workstation (Intellispace portal, Philips Healthcare). The coronary CTA data sets were analyzed quantitatively to determine coronary plaque volumes. CAC score was quantified on the noncontrast-enhanced images using the Agatston method.
Semiautomated Plaque Quantification
Quantitative CT analysis was performed by an experienced reader (Dr Drobni) using a dedicated software tool for plaque assessment (QAngio CT; Medis BV, Leiden, the Netherlands; Figure 1). Coronary atherosclerotic plaques were defined as follows: any visible structure that could be assigned to the coronary artery wall in at least 2 independent planes and had a density below the contrast-enhanced coronary lumen but above the surrounding connective tissue. Furthermore, NCP components were defined as a CT density between (−100) HU and 350 HU and CP components as a density between 351 and 2000 HU.23 Detailed plaque analysis method is reported in the Supplemental Material.
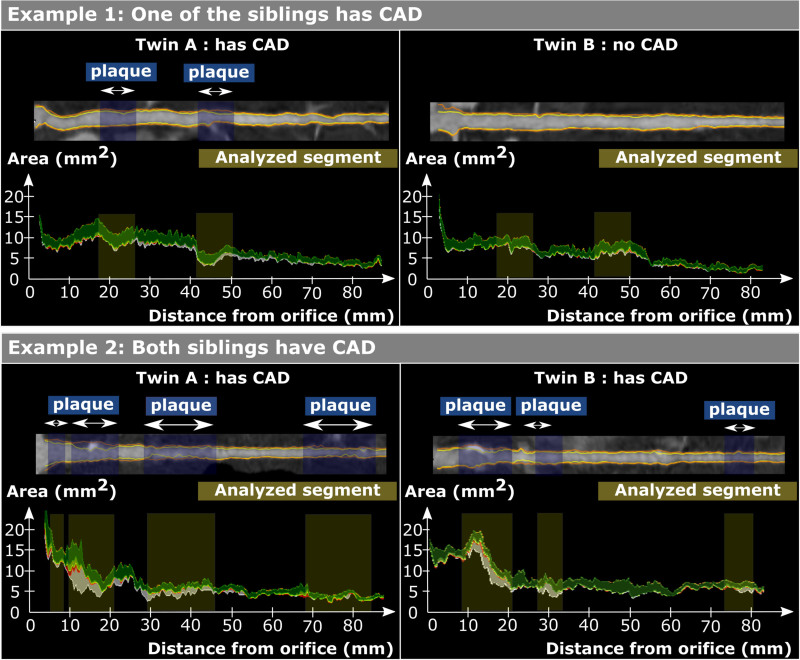
Figure 1.
Demonstration of the plaque analysis method. Stretched multiplanar reconstruction of the left anterior descending (LAD) artery and quantification graph of the LAD. Yellow shaded regions correspond to analyzed segments and blue shaded regions correspond to plaques. Components of coronary plaque are shown as follows: lipid-rich (low-computed tomographic attenuation) plaque components are shown in red; fibro-fatty tissue is shown in light green; fibrous tissue is shown in dark green; calcium is shown in white. Example 1: One of the siblings had plaques; plaques were analyzed, and for the other sibling, segments in the same location and same length were analyzed. Example 2: Both siblings had plaques; all the segments were analyzed which included any plaque in either of the siblings. In case neither of the siblings had coronary artery disease (CAD), a plaque volume of 0-0 was used.
Statistical Analysis
Continuous variables are presented as means and SD, while categorical parameters are shown as numbers and percentages. Continuous variables were compared using Student t test, whereas binary variables were compared using Fisher exact-test and categorical parameters using the χ2 test between monozygotic and dizygotic individuals. Reproducibility of plaque quantification was tested by 2 independent observers (Dr Drobni: 5 years of experience, Dr Simon: 2 years of experience) based on 10 randomly selected monozygotic twin pair and 10 randomly selected dizygotic twin pair images using the intraclass correlation (ICC) coefficient. Coefficient values are interpreted as 1.00 to 0.81: excellent; 0.80 to 0.61: good; 0.60 to 0.41: moderate; 0.40 to 0.21: fair; 0.20 to 0.00: poor. Descriptive statistics, correlations and reproducibility measurements were calculated using IBM SPSS Statistics version 25 (IBM, Armonk, NY).
Genetic structural equation models were built to quantify the proportion of genetic and environmental factors contributing to CAC score, CP, and NCP. These models are based on variation between the twins, which can be broken down to additive genetic factors (A), common environmental factors (C) and unique environmental factors (E), therefore the acronym ACE model.24
Additive genetic factors describe the effect of multiple genetic alleles that exert influence additively. Common environmental factors are shared by the twin pairs during their lifetime, such as the same early childhood, education in the same school, living in the same town, sharing similar socioeconomic status even in adulthood, etc.24 Unique environmental factors are conditions to which only one of the siblings was exposed.25
Sub models of the full models (full ACE model) were calculated, and the more parsimonious model with the best fit was selected. All calculations were adjusted for age and sex or atherosclerotic cardiovascular disease (ASCVD) risk estimate and statin use.26 Analysis was performed on the full cohort for CAC and plaque volume (NCP and CP) analysis and on subsets, where siblings with no disease were excluded. Siblings with no disease are referenced as 0-0 pairs. For the 2 different analysis, 2 0-0 subsets were created. For the CAC analysis one subset with those who had a CAC >0 was created, and for the plaque volume analysis another subset, with those who had coronary plaque (either NCP or CP) on the CTA images.
Our raw data on CAC score, NCP and CP were transformed to a log scale, and an inverse-normal transformation on age-sex categories was also performed for the full cohort and for the subsets. Log likelihood-based 95% CI were calculated for all estimated parameters. All analyses were performed using R version 3.5.2.27 Twin modeling was performed using OpenMx version 2.12.2. A P value of <0.05 was considered statistically significant.
Results
Study Population
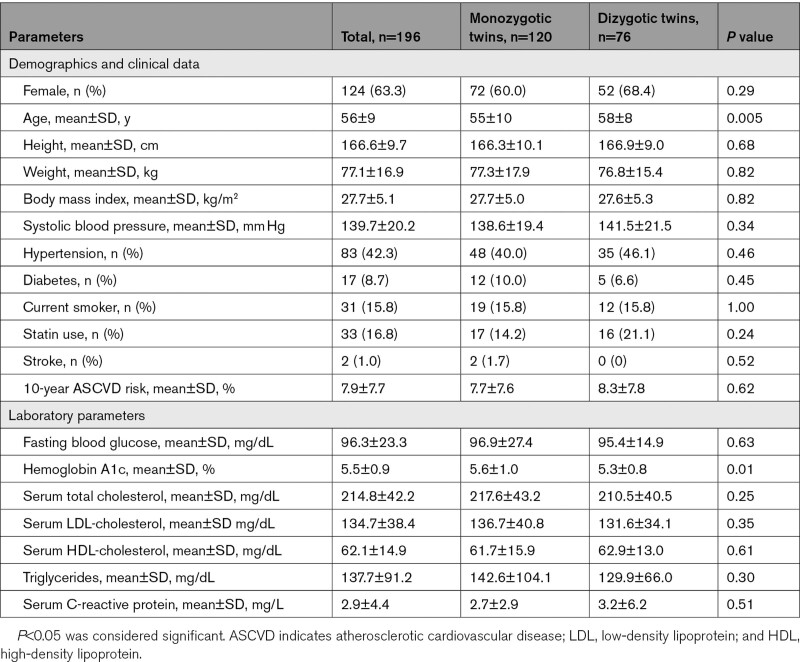
Baseline demographics and clinical characteristics of the overall population (n=196) are summarized in Table 1. The mean±SD age of the cohort was 56±9 years (63.3% female), and dizygotic subjects were older than the monozygotic subjects (58±8 versus 55±10 years, P=0.005). The most prevalent cardiovascular risk factor in the total cohort was hypertension (83/196, 42.3%), and 16.8% (33/196) of the participants were on primary preventive statin therapy (Table 1). Both total cholesterol (214.8±42.2 mg/dL) and LDL (low-density lipoprotein)-cholesterol levels (134.7±38.4 mg/dL) were slightly elevated with no difference between monozygotic and dizygotic groups (P=0.25 and P=0.35, respectively). The 10-year ASCVD risk estimate was 7.9±7.7% for the total cohort, with 83 subjects as low-risk (<5.0%), 34 subjects as borderline risk (5.0%–7.4%), 63 subjects as intermediate risk (7.5%–19.9%), and 16 high risk (>20.0%) subjects. Significant difference was observed in the HbA1c levels between the monozygotic and dizygotic group (5.6±1.0% versus 5.3±0.8%, P=0.01). Otherwise, there were no significant differences between the groups (Table 1).
Table 1.
Basic Demographics and Clinical-Laboratory Characteristics of Twin Subjects
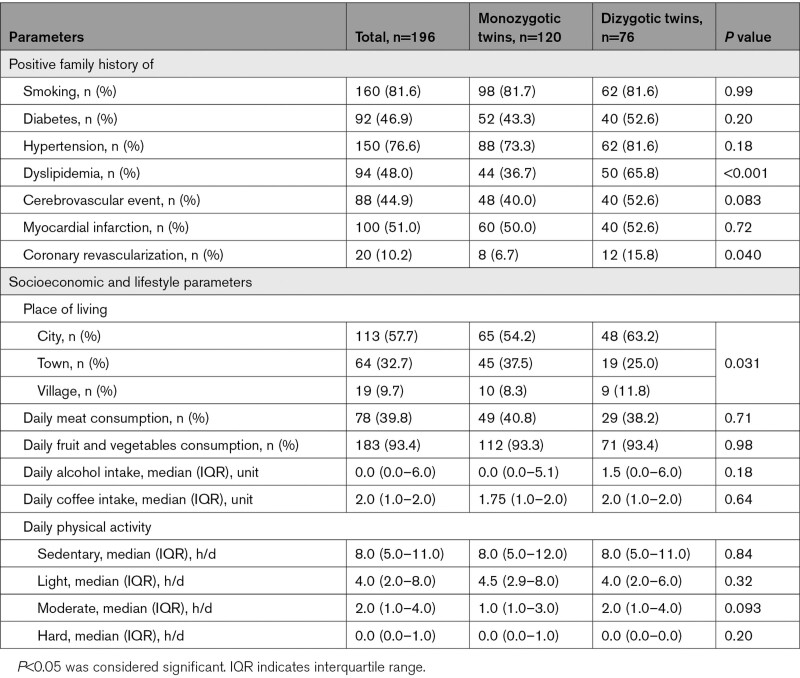
Data regarding family history, socioeconomic status, and lifestyle parameters were available in all subjects, and these results are summarized in Table 2. Dizygotic twins had a stronger family history for dyslipidemia (65.8% [50/76] versus 36.7% [44/120], P<0.001) and for coronary revascularization (15.8% [12/76] versus 6.7% [8/120], P=0.04) as compared with monozygotic twins.
Table 2.
Data on Family History and Lifestyle Parameters
CAD Characteristics
Intrareader and interreader agreement showed excellent reproducibility for all plaque volume measurements (ICCintra-reader for NCP and CP was 0.99 and 0.98, and ICCinter-reader for NCP and CP was 0.97 and 0.99, respectively).
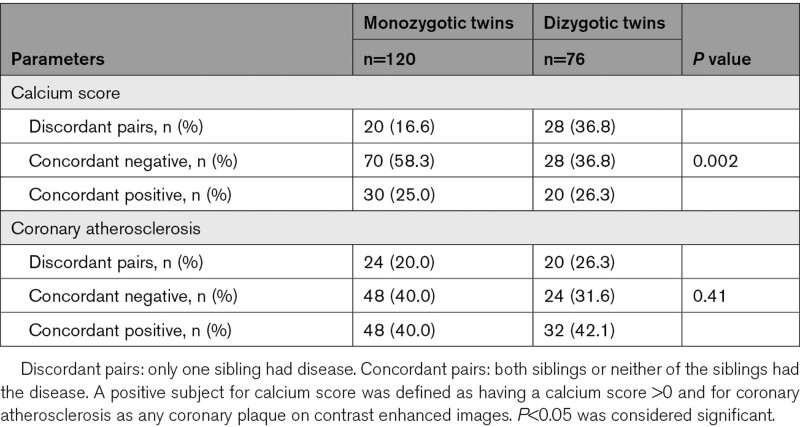
Out of the 196 twins, 74 (37.8 %) had coronary calcification on noncontrast enhanced images, 34 were dizygotic and 40 were monozygotic. The prevalence of a CAC score higher than zero was not different among the monozygotic and dizygotic twins (44.7% [34/76] versus 33.3% [40/120] P=0.13). The prevalence of discordant twin pairs, meaning one sibling had a CAC score higher than zero, whereas the other did not, was significantly higher in the dizygotic group (Table 3). The prevalence of concordant twin pairs with a CAC score of 0 was higher in the monozygotic group (Table 3). Comparing monozygotic and dizygotic twins, we found no differences regarding the median CAC score values in those who had a CAC score higher than 0 (115 [8–336] for the monozygotic group versus 97 [47–208] for the dizygotic group; P>0.9).
Table 3.
Data on Coronary Artery Disease Characteristics
Out of the 196 twins, 102 (52.0%) had coronary plaques, 42 were dizygotic and 60 were monozygotic. The prevalence of any CAD was not different among the groups. (dizygotic, 55.3% [42/76] versus monozygotic, 50.0% [60/120] group P=0.56). In those, who had CAD, the median segment involvement score was 3 (2–5), and the median segment stenosis score was 4 (2–8), with no significant difference among the dizygotic and monozygotic groups (P=0.2 for both).
Comparing monozygotic and dizygotic twins with CAD, we found no differences regarding NCP volume (dizygotic, 107 [52–178] mm3 versus monozygotic, 79 [36–175] mm3; P=0.5) and CP volume (dizygotic, 43 [7–65] mm3 versus monozygotic, 18 [5–84] mm3, P=0.4).
Heritability of CAC Score, CP, and NCP
Using genetic structural equation models, adjusted for age and gender, ASCVD risk estimate and statin use, the best fitting models were selected both for the full cohort and for the 2 subsets (Figure 2). Twin statistics were performed on the raw data, log transformed and on the inverse normal transformed data. Here, we report the results from the inverse normal transformed data. All the other results can be found in the Supplemental Material.
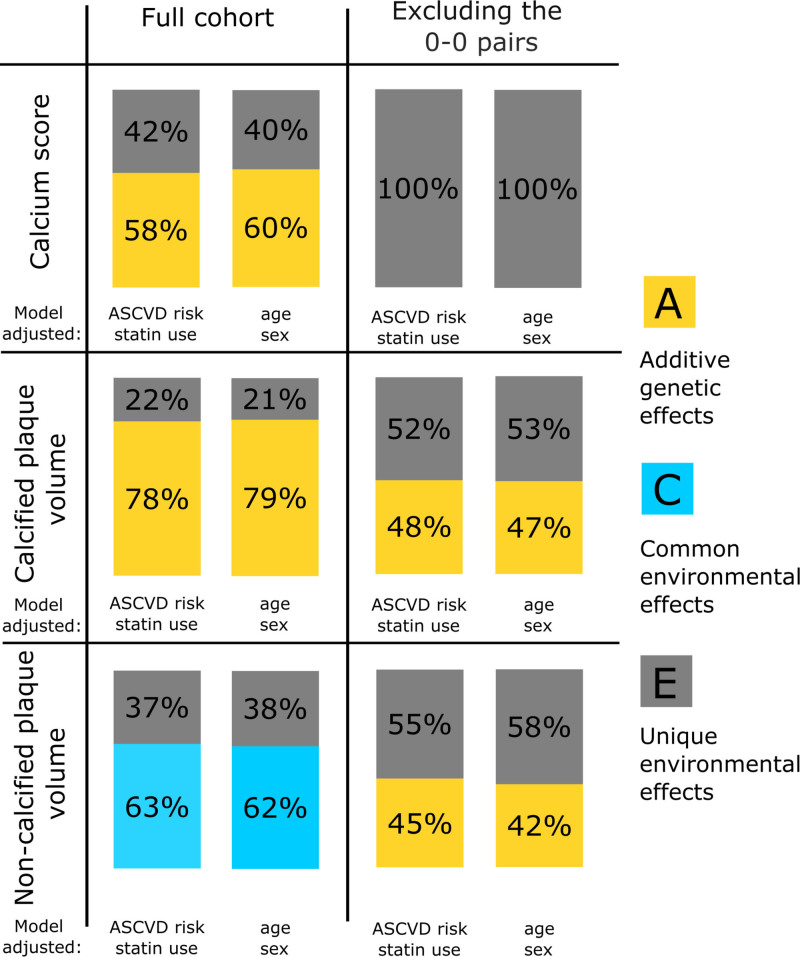
Figure 2.
Twin statistics results. Bar graphs demonstrate the results of the genetic structural equation model of calcium score, calcified, and noncalcified plaque volumes in both the total cohort and in the 2 subsets of siblings with 0-0 pairs excluded. ASCVD indicates atherosclerotic cardiovascular disease.
In the full cohort, CAC score had a strong genetic heritability (adjusted for ASCVD risk and statin use; A: 58% [95% CI, 50%–66%], E: 42% [95% CI, 34%–50%], Table S1 and S2) similarly to CP (adjusted for ASCVD risk and statin use; A: 78% [95% CI, 73%–80%], E: 22% [95% CI, 20%–27%], Tables S3 and S4; Figure 2). On the contrary, NCP quantities were influenced predominantly by common, shared environmental factors (adjusted for ASCVD risk and statin use; C: 63% [95% CI, 56%–67%], E: 37% [95% CI, 33%–44%], Table S5 and S6; Figure 2). Similar results were found when the genetic structural equation models were adjusted for age and gender (Tables S1 through S6).
We repeated the analysis in the subsets of siblings, where both twins without disease were excluded. This resulted in 98 subjects for CAC score, and in 124 subjects for plaque volume (CP and NCP) analysis. Adjusting for ASCVD risk and statin use, the best fitting model was model E, for CAC score showing that 100% of the phenotypic variance in CAC score was attributable to unique environmental factors (E: 100% [95% CI, 100%–100%]). In the subset analysis, the heritability of CP and NCP was similar. In both cases the best fitting model was AE with a modest genetic influence (CP; A: 48% [95% CI, 41%–55%], E: 52% [95% CI, 44%–59%], Table S4 and NCP; 45% [95% CI, 36%–52%], E: 55% [95% CI, 48%–64%], Table S6).
Discussion
Using coronary CTA in adult twins without known CAD, we found that NCP volume was mostly influenced by environmental, rather than genetic factors, while CAC score and CP volume had a relatively strong genetic heritability. Our results remained similar, irrespective of the adjusted covariates. We repeated our analysis in 2 cohorts where the 0-0 pairs were excluded. The contribution of genetic factors to phenotypic variance of CAC score disappeared and diminished for CP from 78% to 48%. The contribution of common, shared environmental factors to the phenotypic variance of NCP disappeared, and a moderate genetic background of 45% was found when the 0-0 pairs were excluded. The loss of a genetic contribution in the subgroup analysis for CAC score could also suggest that the overwhelming majority of genetic contributions are protective against coronary calcification.
Positive family history of CAD is considered to be an independent risk factor for future cardiovascular events.28 In the Framingham Heart Study, a positive family history of CAD was a strong predictor of CAD29,30 and was the strongest clinical predictor in younger patients of future myocardial infarction.9 Healthy first-degree relatives of patients with early onset CAD have an ≈5-fold increase of total coronary plaque volume compared with symptomatic patients.10 It has been demonstrated that CAC has a substantial genetic component.3–7 In line with these findings, the investigators of a community-based study from Rochester reported that >40% of the interindividual variation in CAC quantity is attributed to genetic factors.3 These findings suggest a strong hereditary component of coronary atherosclerosis. In our study, we found a slightly stronger genetic background both for CAC score (58%) and CP (78%) as previously reported in the above-mentioned studies.
The Swedish twin trial enrolled over 20 000 twins, and the genetic basis of CAD-related mortality was evaluated. An increased risk for CAD was found among close relatives.1,2 If a twin sibling had died from early onset CAD, the sibling’s relative hazard of death due to CAD was roughly double in male monozygotic twins as compared with male dizygotic twins, and nearly 6× higher in females.1 Among our twin subjects, there were more discordant pairs for CAC score among the dizygotic group and more concordant pairs among the monozygotic groups. These results also imply a strong genetic background of coronary calcification as also shown in more detail with the genetic structural models in our main analysis.
In our classical twin study, we found a relatively strong genetic dependence of CAC score and CP volumes, corroborating former observations.3,7 On the contrary, we observed that environment, especially common environmental factors play a more important role than genetic effects in determining NCP volumes in individuals without known CAD. These findings are seemingly in contradiction to former suggestions, which describe the importance of genetics in CAD development.8,10 However, the fact that a trait runs in family does not mean that it has a strong genetic background, since families may share not only genes but common environmental factors, for example socioeconomic status, dietary, and exercise habits.13,24 In a family study, distinction between genetic and common environmental effects may be difficult,13,31,32 but twin studies can overcome this limitation, since twin studies can estimate the magnitude of genetic and common environmental components to a specific trait, by modeling the shared environment.13,15,33 This may be a reason why we only found a strong genetic component behind NCP volumes after adjusting for age and gender or ASCVD risk estimate and statin use and only in a subset of twins when we excluded the 0-0 pairs. Importantly, twin studies, compared with family studies, can be more powerful to examine heritability of a complex trait because they can take advantage of a unique characteristics of twins: their shared genetics and matching age.15 Furthermore, a twin design naturally accounts for maternal factors and a range of early environmental factors, which might potentially bias the associations.14 Twins are exposed to higher degree of shared family environment compared with nontwin siblings, and they also share a range of environmental variables and socioeconomic status even in adulthood,32 which do contribute to the expression of complex traits.33 Therefore, we believe that our findings are complimentary and additive to previous observations regarding the importance of genetic and environmental factors influencing plaque volumes.
Our results did change when we analyzed CAD heritability in a subgroup, excluding the 0-0 plaque pairs. Since this study enrolled asymptomatic subjects with no history of CAD, excluding the 0-0 pairs did significantly reduce our sample size. Performing twin statistics on a phenotype which can be 0 is extremely challenging, and we approached it from several angles. In cases where one sibling had CAD and the other did not, we co-registered the plaque localization and used that instead of 0 for the nondiseased sibling. Moreover, we performed a rank based inverse normal transformation, to balance out the 0 tail in the data distribution caused by the nondiseased subjects. The inverse normal transformation removed the heavy 0 tail, and we further adjusted it for age, sex, and ASCVD risk estimate and statin use.
It is important to note that our findings do not explain the underlying pathophysiological background of CAD and plaque formation. Nevertheless, it is generally accepted that during coronary plaque formation, NCPs are present at early stage while CPs represent a later stage of plaque development.11 Accordingly, our results indicate that early development of coronary plaques is mainly influenced by common environmental factors, such as dietary and exercise habits and socioeconomic status, in contrast to plaque calcification which is more dependent on genetics.
An important clinical implication of our findings is within primary prevention of CAD, especially of NCP. Based on our findings, the genetic background of NCP is smaller than the CAC score and CP, and our data also imply that socioeconomic status and lifestyle parameters have bigger impact on NCP as compared with CAC score and CP. Based on these, there is an urgent need to start primary prevention of CAD as early in life as possible. Starting with lifestyle changes could be the foundation of preventative measures for NCP. The data and analysis presented in this paper should be considered hypothesis-generating and may need further confirmation.
Limitations
Our sample size is relatively modest and comparable with other twin studies.34 The aim was to balance the overall population for 50% females and ≥50% dizygotic twins; however, 63% of the twins are female and 39% are dizygotic twins. This might be due to the fact that females and monozygotic twins are more willing to participate in research than males.35 In the present study, statins were used in 16.8% of the patients, which is relatively low; however, it can still influence the presence and phenotype of CAD.36 Measurement error may appear as part of the unique environmental component as it is uncorrelated across measurements. Due to the cross-sectional nature of our study, we had no data about the plaque development over time. The study was a single-center investigation with twins from a White population, which may limit the generalizability of our findings. A potential bias could be the age difference between the monozygotic and dizygotic groups, which could inflate the correlation among the dizygotic group, especially for CP volumes. However, calcified and NCP volumes were not different among the monozygotic and dizygotic groups. We believe that by performing the heritability analysis on the inverse-normal transformed data on age-sex categories and adjusting for age and gender and for ASCVD risk estimate, we were able to address this issue.
Conclusions
We have observed that NCP volume is predominantly determined by common environmental factors while CAC score and CP volume are influenced mainly by genetics. These findings suggest that lifestyle may have an important role in the initiation of CAD (NCP), and genetics may have a strong effect on CP formation. These results underline the importance of optimal risk factor management early in life.
Article Information
Acknowledgments
We thank the team at the Heart and Vascular Center for their help in performing the study.
Sources of Funding
This study was supported by a New Horizons Grant from the EASD to Dr Jermendy. Dr Drobni was supported by the ÚNKP-21-4-I-SE new national excellence program of the ministry for innovation and technology from the source of the national research, development, and innovation fund. Dr Merkely was funded by Project no. NVKP_16-1–2016-0017 (National Heart Program) with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the NVKP_16 funding scheme. The research was financed by the Thematic Excellence Programme (2020-4.1.1.-TKP2020) of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapeutic Development and Bioimaging thematic programmes of the Semmelweis University.
Disclosures
Dr Voros is a shareholder in Global Genomics Group, LLC, and receives salary from Global Genomics Group, LLC. The other authors report no conflicts.
Supplemental Materials
Supplemental Document
Tables S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- A
- additive genetic factors
- ASCVD
- atherosclerotic cardiovascular disease
- BUDAPEST-GLOBAL
- Burden of Atherosclerotic Plaques Study in Twins—Genetic Loci and the Burden of Atherosclerotic Lesions
- C
- common environmental factors
- CAC
- coronary artery calcification
- CAD
- coronary artery disease
- CP
- calcified plaque
- CT
- computed tomography
- CTA
- computed tomography angiography
- E
- Unique environmental factors
- NCP
- noncalcified plaque
For Sources of Funding and Disclosures, see page 140.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.121.013348.
Contributor Information
Zsofia D. Drobni, Email: zdrobni@mgh.harvard.edu.
Adam L. Jermendy, Email: gyjermendy@gmail.com.
Adam D. Tarnoki, Email: tarnoki2@gmail.com.
David L. Tarnoki, Email: tarnoki4@gmail.com.
Judit Simon, Email: juditsimon21@gmail.com.
Balint Szilveszter, Email: szilveszter.balint@gmail.com.
Levente Littvay, Email: littvayl@ceu.edu.
Szilard Voros, Email: szilard.voros@globalgenomicsgroup.com.
Gyorgy Jermendy, Email: gyjermendy@gmail.com.
Bela Merkely, Email: merkely.study@gmail.com.
References
- 1.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994; 330:1041–1046. doi: 10.1056/NEJM199404143301503 [DOI] [PubMed] [Google Scholar]
- 2.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, De Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. J Intern Med. 2002; 252:247–254. doi: 10.1046/j.1365-2796.2002.01029.x [DOI] [PubMed] [Google Scholar]
- 3.Peyser PA, Bielak LF, Chu JS, Turner ST, Ellsworth DL, Boerwinkle E, Sheedy PF, 2nd. Heritability of coronary artery calcium quantity measured by electron beam computed tomography in asymptomatic adults. Circulation. 2002; 106:304–308. doi: 10.1161/01.cir.0000022664.21832.5d [DOI] [PubMed] [Google Scholar]
- 4.Paixao AR, Berry JD, Neeland IJ, Ayers CR, Rohatgi A, de Lemos JA, Khera A. Coronary artery calcification and family history of myocardial infarction in the Dallas heart study. JACC Cardiovasc Imaging. 2014; 7:679–686. doi: 10.1016/j.jcmg.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Khera A, Joshi P. What’s a Malignant Family History?: you’ll know it when you see it. JACC Cardiovasc Imaging. 2017; 10(10 Pt A):1136–1138. doi: 10.1016/j.jcmg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 6.Rampersaud E, Bielak LF, Parsa A, Shen H, Post W, Ryan KA, Donnelly P, Rumberger JA, Sheedy PF, 2nd, Peyser PA, et al. The association of coronary artery calcification and carotid artery intima-media thickness with distinct, traditional coronary artery disease risk factors in asymptomatic adults. Am J Epidemiol. 2008; 168:1016–1023. doi: 10.1093/aje/kwn211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagenknecht LE, Bowden DW, Carr JJ, Langefeld CD, Freedman BI, Rich SS. Familial aggregation of coronary artery calcium in families with type 2 diabetes. Diabetes. 2001; 50:861–866. doi: 10.2337/diabetes.50.4.861 [DOI] [PubMed] [Google Scholar]
- 8.Kral BG, Becker LC, Vaidya D, Yanek LR, Qayyum R, Zimmerman SL, Dey D, Berman DS, Moy TF, Fishman EK, et al. Noncalcified coronary plaque volumes in healthy people with a family history of early onset coronary artery disease. Circ Cardiovasc Imaging. 2014; 7:446–453. doi: 10.1161/CIRCIMAGING.113.000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otaki Y, Gransar H, Berman DS, Cheng VY, Dey D, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, et al. Impact of family history of coronary artery disease in young individuals (from the CONFIRM registry). Am J Cardiol. 2013; 111:1081–1086. doi: 10.1016/j.amjcard.2012.12.042 [DOI] [PubMed] [Google Scholar]
- 10.Christiansen MK, Jensen JM, Nørgaard BL, Dey D, Bøtker HE, Jensen HK. Coronary plaque burden and adverse plaque characteristics are increased in healthy relatives of patients with early onset coronary artery disease. JACC Cardiovasc Imaging. 2017; 10(10 Pt A):1128–1135. doi: 10.1016/j.jcmg.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 11.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014; 11:390–402. doi: 10.1038/nrcardio.2014.60 [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006; 47:1655–1662. doi: 10.1016/j.jacc.2006.01.041 [DOI] [PubMed] [Google Scholar]
- 13.Martin N, Boomsma D, Machin G. A twin-pronged attack on complex traits. Nat Genet. 1997; 17:387–392. doi: 10.1038/ng1297-387 [DOI] [PubMed] [Google Scholar]
- 14.Mangino M, Spector T. Understanding coronary artery disease using twin studies. Heart. 2013; 99:373–375. doi: 10.1136/heartjnl-2012-303001 [DOI] [PubMed] [Google Scholar]
- 15.Craig JM, Calais-Ferreira L, Umstad MP, Buchwald D. The value of twins for health and medical research: a third of a century of progress. Twin Res Hum Genet. 2020; 23:8–15. doi: 10.1017/thg.2020.4 [DOI] [PubMed] [Google Scholar]
- 16.Voros S, Maurovich-Horvat P, Marvasty IB, Bansal AT, Barnes MR, Vazquez G, Murray SS, Voros V, Merkely B, Brown BO, et al. Precision phenotyping, panomics, and system-level bioinformatics to delineate complex biologies of atherosclerosis: rationale and design of the “Genetic Loci and the Burden of Atherosclerotic Lesions” study. J Cardiovasc Comput Tomogr. 2014; 8:442–451. doi: 10.1016/j.jcct.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 17.Maurovich-Horvat P, Tárnoki DL, Tárnoki ÁD, Horváth T, Jermendy ÁL, Kolossváry M, Szilveszter B, Voros V, Kovács A, Molnár AÁ, et al. Rationale, design, and methodological aspects of the BUDAPEST-GLOBAL Study (Burden of Atherosclerotic Plaques Study in Twins-Genetic Loci and the Burden of Atherosclerotic Lesions). Clin Cardiol. 2015; 38:699–707. doi: 10.1002/clc.22482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Littvay L, Métneki J, Tárnoki AD, Tárnoki DL. The hungarian twin registry. Twin Res Hum Genet. 2013; 16:185–189. doi: 10.1017/thg.2012.76 [DOI] [PubMed] [Google Scholar]
- 19.Heath AC, Nyholt DR, Neuman R, Madden PA, Bucholz KK, Todd RD, Nelson EC, Montgomery GW, Martin NG. Zygosity diagnosis in the absence of genotypic data: an approach using latent class analysis. Twin Res. 2003; 6:22–26. doi: 10.1375/136905203762687861 [DOI] [PubMed] [Google Scholar]
- 20.Christiansen L, Frederiksen H, Schousboe K, Skytthe A, von Wurmb-Schwark N, Christensen K, Kyvik K. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res. 2003; 6:275–278. doi: 10.1375/136905203322296610 [DOI] [PubMed] [Google Scholar]
- 21.Rickham PP. Human experimentation. Code of ethics of the world medical association. Declaration of helsinki. Br Med J. 1964; 2:177. doi: 10.1136/bmj.2.5402.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karády J, Panajotu A, Kolossváry M, Szilveszter B, Jermendy ÁL, Bartykowszki A, Károlyi M, Celeng C, Merkely B, Maurovich-Horvat P. The effect of four-phasic versus three-phasic contrast media injection protocols on extravasation rate in coronary CT angiography: a randomized controlled trial. Eur Radiol. 2017; 27:4538–4543. doi: 10.1007/s00330-017-4866-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, Pohle K, Baum U, Anders K, Jang IK, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004; 109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F [DOI] [PubMed] [Google Scholar]
- 24.Zyphur MJ, Zhang Z, Barsky AP, Li W-D. An ACE in the hole: twin family models for applied behavioral genetics research. Leadersh Q. 2013; 24:572–594 [Google Scholar]
- 25.Franić S, Dolan CV, Borsboom D, Boomsma DI. Structural Equation Modeling in Genetics. 2012, Guilford Press; 617–635 [Google Scholar]
- 26.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 27.Team RC. 2017, R: A language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 28.Colditz GA, Stampfer MJ, Willett WC, Rosner B, Speizer FE, Hennekens CH. A prospective study of parental history of myocardial infarction and coronary heart disease in women. Am J Epidemiol. 1986; 123:48–58. doi: 10.1093/oxfordjournals.aje.a114223 [DOI] [PubMed] [Google Scholar]
- 29.Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004; 291:2204–2211. doi: 10.1001/jama.291.18.2204 [DOI] [PubMed] [Google Scholar]
- 30.Murabito JM, Pencina MJ, Nam BH, D’Agostino RB, Sr, Wang TJ, Lloyd-Jones D, Wilson PW, O’Donnell CJ. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005; 294:3117–3123. doi: 10.1001/jama.294.24.3117 [DOI] [PubMed] [Google Scholar]
- 31.MacGregor AJ, Snieder H, Schork NJ, Spector TD. Twins. Novel uses to study complex traits and genetic diseases. Trends Genet. 2000; 16:131–134. doi: 10.1016/s0168-9525(99)01946-0 [DOI] [PubMed] [Google Scholar]
- 32.Krieger N, Chen JT, Coull BA, Selby JV. Lifetime socioeconomic position and twins’ health: an analysis of 308 pairs of United States women twins. PLoS Med. 2005; 2:e162. doi: 10.1371/journal.pmed.0020162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018; 137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elder SJ, Lichtenstein AH, Pittas AG, Roberts SB, Fuss PJ, Greenberg AS, McCrory MA, Bouchard TJ, Jr, Saltzman E, Neale MC. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J Lipid Res. 2009; 50:1917–1926. doi: 10.1194/jlr.P900033-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coccaro EF, Jacobson KC. PennTwins: a population-based cohort for twin studies. Twin Res Hum Genet. 2006; 9:998–1005. doi: 10.1375/183242706779462633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achenbach S, Ropers D, Pohle K, Leber A, Thilo C, Knez A, Menendez T, Maeffert R, Kusus M, Regenfus M, et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002; 106:1077–1082. doi: 10.1161/01.cir.0000027567.49283.ff [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.