Abstract
Objectives:
To explore the value of contrast-enhanced ultrasound (CEUS) in predicting early lymph node metastasis in clinically node-negative oral cancer patients.
Methods:
We recruited 42 patients (a total of 70 lymph nodes) with clinically node-negative oral cancer in the study. All of them received both conventional ultrasound (B-mode and power Doppler) and CEUS before operation and then they were taken for pathological examination to analyze the diagnostic accuracy. And their CEUS data were analyzed qualitatively and quantitatively.
Results:
The sensitivity and specificity of CEUS in the diagnosis of cervical lymph node metastasis of oral cancer were 82.7 and 82.9%, respectively. The accuracy of conventional ultrasound was only 67.1% while that of CEUS was up to 82.9%. The area under the cure (AUC) of CEUS in detecting lymph node metastasis was 0.828, which was higher than the 0.614 by conventional ultrasound, with statistically significant differences observed (p < 0.05). Most of the metastatic lymph nodes were characterized by inhomogeneous enhancement and the peak intensity (PI) of the metastatic group was lower than that of the non-metastatic group (p < 0.05).
Conclusions:
Compared with conventional ultrasound, CEUS may have higher clinical value for predicting early lymph node metastasis in clinically node-negative oral cancer patients. And quantitative parameters obtained from CEUS may provide valuable information in the diagnosis of cervical lymph node metastasis.
Keywords: Ultrasonography, Oral cancer, Lymph nodes
Introduction
Oral cancer is regarded as one of the most common cancers of head and neck carcinoma, and more than 90% of oral cancers are squamous cell carcinoma.1 Oral cancer occurs in correlation of some bad oral habits such as smoking, drinking alcohol, and HPV infections.2
Lymph node metastasis is a common event in oral cancer. About 50% of oral cancer patients have metastatic lymph nodes and 20–30% patients have occult lymph node metastases in early-stage (cT1-2N0) oral squamous cell carcinoma.3,4 Studies have shown that the prognosis of the oral cancer was closely related to the cervical lymph node status. The presence of metastatic lymph nodes commits patients to an advanced-stage disease category and can reduce the survival rate.5 Treatment of oral cancer is surgical resection of the primary tumor with or without postoperative adjuvant therapy (radiation or chemoradiation therapy).6 But there are no standard treatments of neck lymph nodes for early-stage oral cancer. Unnecessary prophylactic neck dissection does harm to patients without metastasis, while the selection of observation may result in the metastasis of lymph node.7 Therefore, evaluation of lymph node metastasis is extremely essential for the treatment and prognostication of oral cancer.
Fine needle aspiration is the most accurate method for preoperative evaluation of suspicious lymph node. However, fine needle aspiration is an invasive method and not every node is suitable for biopsy.8 And the information provided by biopsy is not enough to achieve accurate stage, which is critical for determining treatment strategy and improving the prognosis of the disease.9 Therefore, imaging examination plays an important role in the diagnosis of lymph node metastasis. Some imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) can detect large lymph node metastases by morphologic changes and size increase; however, small or early lymph node metastases might be misdiagnosed with these imaging techniques.10 Therefore, a noninvasive tool with good diagnostic value is highly demanded. Ultrasonography has been shown to be a useful tool to differentiate benign from malignant cervical lymph nodes. It has better resolution than CT and MRI.11 But CEUS displays the lymphatic microvessels better than conventional ultrasonography. Some studies have shown that the diagnostic accuracy of CEUS was higher than that of ultrasonography of superficial lymph nodes.12 Sato et al13 used CEUS to demonstrate that the increase of lymph node vascular density preceded any changes in lymph node volume through animal models. Thus, CEUS may facilitate early diagnosis of lymph node metastasis by monitoring blood vessel density. In addition, CEUS is noninvasive, repeatable and cost effective, achieved by injecting microbubbles into blood vessels.14 These microbubbles can be used safely in patients, with no concern for cardiotoxicity or nephrotoxicity.15,16 Furthermore, compared with conventional ultrasound-guided fine needle aspiration, the CEUS-guided fine needle aspiration may also provide a more efficient method in the diagnosis of lymph node metastasis.17
As a new noninvasive ultrasonic imaging technology, CEUS can evaluate tissue perfusion in real time.18 It has a higher sensitivity to the microvessels in lymph node that cannot be identified by Doppler techniques, thus leading to a better evaluation in distinguishing benign from malignant superficial lymph nodes. Several studies have reported that CEUS might be a potential modality for the evaluation of superficial lymph node metastasis, but the quantity of studies on oral cancer is limited. The aim of our study was to evaluate the clinical value of CEUS in predicting early lymph node metastasis in clinically node-negative oral cancer.
Methods and materials
Patients
This retrospective study was approved by institutional review board. From January 2015 to June 2018, patients with newly diagnosed oral cancer were recruited into the study. Informed consent for the examination was obtained from all patients. The inclusion criteria were oral cancer diagnosed by preoperative biopsy or pathology examination, and diagnosed with no lymph node metastasis by the CT, MRI (preoperative clinical stage of Tis, T1N0M0, or T2N0M0), and could not be clearly judged by conventional ultrasound (B-mode and power Doppler). Finally, 42 patients were enrolled in this study, including 22 males and 20 females, mean age of 54.57 ± 14.41 years old (range 23–82). In these patients, there were 29 cases of tongue cancer, 5 cases of buccal cancer, 1 case of parotid cancer, 4 cases of gingival cancer, 1 case of lip cancer, 1 case of palate cance and 1 case of mouth floor cancer.
Methods
Ultrasound examinations were performed using an ultrasound system (Esaote MyLab Twice, Genoa, Italy) equipped with high-frequency linear array probes with frequency of 7–12 MHz, which allows fundamental B-mode and power Doppler mode. In the first step, patients were examined using conventional ultrasonography (B-mode and power Doppler). All lymph nodes in our study were superficial lymph nodes, which can be detected by ultrasound. The features of lymph node metastasis were as follows: rounded shape with increased anteroposterior diameter; loss of hilum; blurred margins and presence of microcalcification. Then, the suspicious lymph nodes were examined with CEUS. The contrast agent was Sonovue (Bracco SpA, Milan, Italy). Sonovue dry powder of each vial was mixed with 5-ml physiological saline to make microbubble suspension. Then, a bolus of 2.4 ml of microbubble suspension was injected via the antecubital vein, immediately followed by a wash with 5 ml of saline. In the meanwhile, the timer of the ultrasonic instrument was turned on and areas of contrast accumulation were then imaged dynamically with live dual images and collected as a video of DICOM data.
The lymph nodes enhancement patterns were as follows: (1) homogeneous enhancement; (2) heterogeneous enhancement; (3) peripheral high enhancement and (4) no enhancement. The first pattern was considered as benign signs and the other three were considered as malignant signs. According to the lesion’s enhancement degrees compared with adjacent tissues at peak enhancement, the perfusion intensity of lymph nodes was divided into three modes: Hypo-enhancement, Equal-enhancement, and Hyper-enhancement. The parametric analysis of this images was performed with the software QontraXt (Bracco, Milan, Italy). The region of interest (ROI) was placed in the whole lymph node to measure the average intensity of ROI. After selecting the ROI, the QontraXt software processed the perfusion image automatically to generate the time-intensity curve (TIC) of the node. Resulted from the TIC, the quantitative parameters included the following: (1) rise time (RT), defined as the time from the beginning of lymph node enhancement to reach the maximum signal intensity; (2) time to peak (TTP), defined as the time it takes to reach the maximum signal intensity from the beginning of contrast agent injection; (3) peak intensity (PI), defined as maximum signal intensity value in TIC.
After being detected by CEUS, the targeted lymph nodes were made marks on corresponding areas vertically to the skin. During the operation, the clinician injected methylene blue into the target lymph node and then sent it for pathological examination. The CEUS results were compared to the histological results.
Statistical analysis
The analysis was performed with SPSS Statistics v.26.0 (IBM Corp., Armonk, NY, USA). The results of CEUS and conventional ultrasound (B-mode and power Doppler) were compared with pathological results, and presented in the form of fourfold table (Table 1). According to Table 1, the chi-square was adopted to calculate the sensitivity, specificity, positive-predictive value, negative-predictive value, and diagnostic accuracy of CEUS and conventional ultrasound, respectively (Table 2). Means of the numerical results were compared by the independent samples t-test, and chi-square was used to compare the categorical parameters. The receiver operating characteristic (ROC) curve (Figure 1) was made according to the comparison of conventional ultrasound results and CEUS results with pathological results. A p-value less than 0.05 was considered to be statistically significant difference.
Table 1.
Pathological results of CEUS and US
| Inspection method | Pathology | Total | |
|---|---|---|---|
| Positive LN | Negative LN | ||
| CEUSa | |||
| Positive LNb | 24 | 7 | 31 |
| Negative LN | 5 | 34 | 39 |
| USc | |||
| Positive LN | 8 | 2 | 10 |
| Negative LN | 21 | 39 | 60 |
| Sub total | 29 | 41 | 70 |
CEUS: contrast-enhanced ultrasound.
LN: lymph node.
US: B-mode and power Doppler ultrasound.
Table 2.
Diagnostic values of CEUS and US
| Sensitivity | Specificity | Positive-predictive value | Negative-predictive value | Diagnostic accuracy | |
|---|---|---|---|---|---|
| CEUSa | 82.7% | 82.9% | 77% | 87.2% | 82.9% |
| USb | 27.6% | 95.1% | 80% | 65% | 67.1% |
| χ2 | 17.846 | 1.997 | 0.001 | 6.004 | 4.610 |
| P-value | <0.01 | 0.158 | 1.000 | 0.014 | 0.032 |
CEUS: contrast-enhanced ultrasound.
US: B-mode and power Doppler ultrasound.
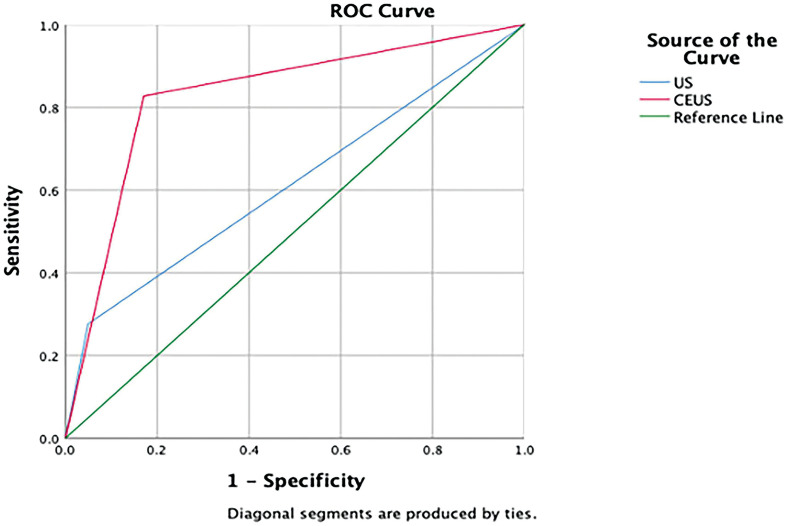
Figure 1.
The ROC curve. A curve for the capability of CEUS and conventional ultrasound in distinguishing benign and malignant lymph nodes in oral cancer patients.
Results
Diagnostic values of CEUS and conventional ultrasound (B-mode and power Doppler)
There were 42 patients with 70 lymph nodes in this study altogether. All of them underwent both CEUS examination and pathological diagnosis. In these cervical lymph nodes, pathological diagnoses were negative for 29 cases and positive for 41 cases. The pathological results of conventional ultrasound and CEUS were summarized in Table 1. The sensitivity (82.7 %) and accuracy (82.9 %) of CEUS were significantly higher than conventional ultrasound (27.6% and 67.1%, respectively), but the specificity between the two groups has no significant difference (Table 2). The ROC analysis (Figure 1) was used to compare CEUS and conventional ultrasound in the diagnosis of lymph node metastasis by calculating the area under the curve. The AUC of CEUS (0.828, 95% CI: 0.724–0.933) was also higher than that of conventional ultrasound (0.614, 95% CI: 0.475–0.752) and the difference was statistically significant (Z = 2.404, p < 0.05).
Characteristics of CEUS image
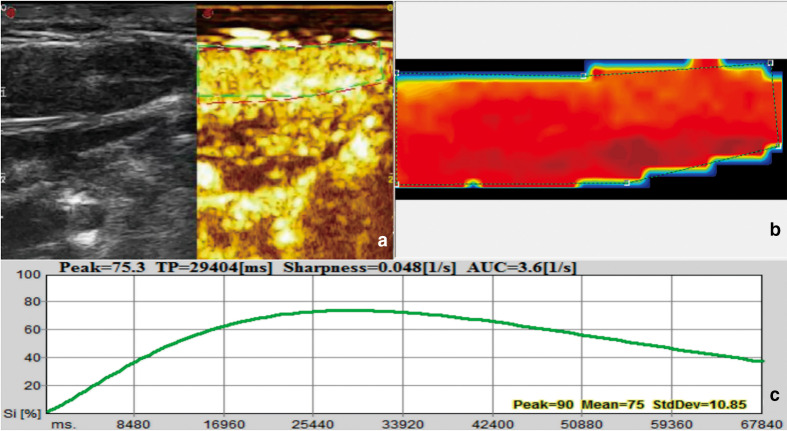
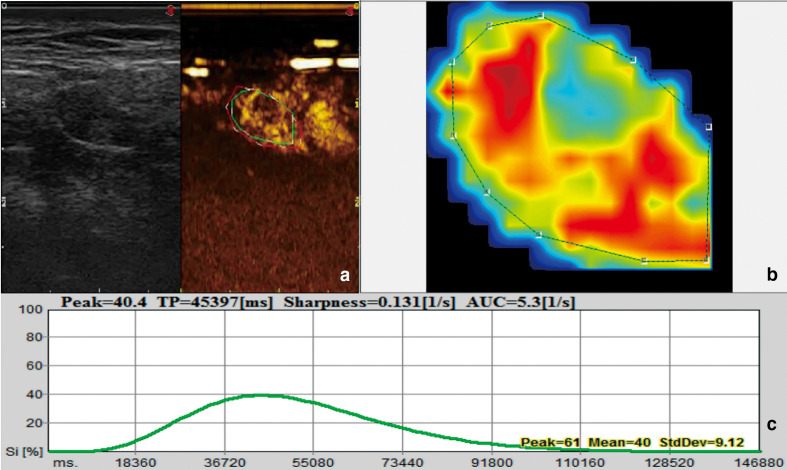
The CEUS enhancement patterns of 70 cases of lymph nodes could be divided into four types: type I: homogeneous enhancement (37 cases); type II, heterogeneous enhancement (18 cases); type III, peripheral high enhancement (5 cases); and type IV, no enhancement (10 cases). Most benign lymph nodes (80.49%, 33/41) showed homogeneous enhancement patterns (Figure 2), while most metastatic lymph nodes (82.76%, 24/29) showed inhomogeneous enhancement (Figure 3) or peripheral high enhancement or no enhancement, and the difference was statistically significant (p < 0.05). But there was no significant difference in perfusion intensity between the two groups (Table 3). PI of benign lymph nodes was considerably higher than that of malignant nodes (p = 0.001, Table 3), while no statistical difference was found in the rise time and time to peak between the two groups (p = 0.768 and 0.660, respectively, Table 3).
Figure 2.
US image and quantitative analysis of benign lymph node. Image of a 32-year-old female. (a) B-mode ultrasound and CEUS image. (b) Signal intensity image: red means large perfusion; green means small perfusion. (c) Time-intensity curve. Si: Signal intensity. TP: Time to peak.
Figure 3.
US image and quantitative analysis of metastatic lymph node. Image of a 49-year-old male. (a) B-mode ultrasound and CEUS image. (b) Signal intensity image: red means large perfusion; green means small perfusion. (c) Time-intensity curve. Si: Signal intensity. TP: Time to peak.
Table 3.
Comparison of CEUS image qualitative and quantitative characteristics between metastatic and benign LNs
| Metastatic LNa | Benign LN | χ2/t | p value | |
|---|---|---|---|---|
| Perfusion defects | 27.379 | <0.001 | ||
| Yes | 24 | 8 | ||
| No | 5 | 33 | ||
| Enhancement degree | 0.353 | 0.838 | ||
| Hypo-enhancement | 19 | 24 | ||
| Equal-enhancement | 4 | 7 | ||
| Hyper-enhancement | 6 | 10 | ||
| Rise time (s) | 22.86±12.62 | 23.92±16.21 | 0.296 | 0.768 |
| Time to peak (s) | 39.79±15.90 | 42.12±25.04 | 0.441 | 0.660 |
| Peak intensity (dB) | 45.41±22.82 | 61.90±18.33 | 3.348 | 0.001 |
LN: lymph node.
Discussion
In our results, most of malignant nodes (82.8%) in our study were inhomogeneous on CEUS, while the majority of benign nodes (80.5%) were found to be homogeneously enhancing. Previous studies showed that benign lymph nodes had homogeneous enhancement, while most metastatic lymph nodes had nonuniform enhancement and perfusion defects,8,19 which was consistent with the results of this study, indicating that there were some similarities in the angiographic features of metastatic lymph nodes. The reason can be explained that in metastatic lymph nodes, tumor cells grew fast and invaded blood vessels easily, leading to insufficient blood supply in lymph nodes, resulting in local ischemic necrosis. Compared with benign lymph nodes, the PI of metastatic lymph nodes was significantly lower. The PI value reflected the local tissue perfusion. The tumor metastasis could destroy the normal structure of the lymph nodes and the central vessels of lymph nodes were compressed, resulting in inadequate blood supply compared with normal lymph nodes.20
However, 17.2% of the metastatic lymph nodes showed homogeneous enhancement, and 19.5% of the benign lymph nodes showed inhomogeneous enhancement. It suggests that CEUS has some limitations in the diagnosis of lymph nodes. Necrosis was usually recognized as a malignant feature, but in fact necrosis is not equal to lymph node metastasis. Necrosis could also be caused by inflammation, tuberculosis, and other phenomena. These atypical phenomena might reduce the specificity of CEUS in evaluating lymph node metastasis.
The sensitivity and specificity of CEUS for the diagnosis of cervical metastatic lymph nodes in this study were 82.7 and 82.9%, respectively. According to the ROC curve analysis, the parameters of CEUS were highly sensitive in the diagnosis of metastatic lymph nodes in oral cancer. This indicates that parameters of CEUS have high clinical value in the differential diagnosis of benign and malignant lymph nodes. Ding et al21 examined 48 patients with oral cancer by CEUS. The results showed that the sensitivity and specificity of CEUS in the diagnosis of lymph node metastasis were 69.39 and 94.71%, respectively. Dudau et al22 performed CEUS in 17 patients with head and neck cancer. The results showed that the sensitivity and specificity of CEUS for lymph node involvement were 100 and 85.7%, respectively. A meta-analysis showed that CEUS had high sensitivity (88%) and reasonable specificity (80%) in the diagnosis of superficial metastatic lymph nodes.23 The diagnostic criteria of different studies were different, so the results were heterogeneous, but in general, the sensitivity and specificity of CEUS in the diagnosis of lymph node metastasis were at a high level.
In this study, our subjects were patients who could not be clearly diagnosed by conventional ultrasound (B-mode and power Doppler). Therefore, the sensitivity and accuracy of conventional ultrasound were low, which could not represent the diagnostic value of conventional ultrasound in differentiating benign and malignant lymph nodes. Chaukar et al studied 75 patients with the clinically node-negative neck in oral cancer.24 The results showed that the sensitivity and accuracy of ultrasound were 78.9 and 73.25%, respectively. Stuckensen et al investigated 2196 neck lymph nodes of 106 patients diagnosed with squamous cell carcinoma of the oral cavity and concluded that the sensitivity and accuracy of ultrasound were 84 and 76%, respectively.25 These previous studies showed that the conventional ultrasound had certain diagnostic value for occult metastasis of cervical lymph nodes in patients with oral cancer. In our study, for those who could not be clearly judged by conventional ultrasound, the diagnostic accuracy of CEUS was higher than that of conventional ultrasound, indicating that CEUS can improve the diagnostic accuracy of lymph node metastasis.
There were some limitations of this study. For example, it was a retrospective study. In addition, the number of cases included in this study was relatively limited. Future studies will enroll more patients.
In conclusion, CEUS may have higher clinical value than conventional ultrasound for predicting early lymph node metastasis in clinically node-negative oral cancer patients. CEUS would result in an acceptable diagnostic value and need further study to provide more valuable information.
Contributor Information
Shiwen Ding, Email: dsw549815064@163.com.
Ping Xiong, Email: xiongpxp@163.com.
Jiaxin Zuo, Email: 820145@sh9hospital.org.cn.
REFERENCES
- 1.Shibahara T. Oral cancer -diagnosis and therapy. Clin Calcium 2017; 27: 1427–33. doi: https://doi.org/CliCa171014271433 [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3.Arun I, Maity N, Hameed S, Jain PV, Manikantan K, Sharan R, et al. Lymph node characteristics and their prognostic significance in oral squamous cell carcinoma. Head Neck 2021; 43: 520–33. doi: 10.1002/hed.26499 [DOI] [PubMed] [Google Scholar]
- 4.Mahieu R, de Maar JS, Nieuwenhuis ER, Deckers R, Moonen C, Alic L, et al. New developments in imaging for sentinel lymph node biopsy in early-stage oral cavity squamous cell carcinoma. Cancers 2020; 12: 305520 10 2020. doi: 10.3390/cancers12103055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, et al. Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol 2017; 35: 3601–9. doi: 10.1200/JCO.2016.71.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am 2015; 24: 491–508. doi: 10.1016/j.soc.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanai N, Asakage T, Kiyota N, Homma A, Hayashi R. Controversies in relation to neck management in N0 early oral tongue cancer. Jpn J Clin Oncol 2019; 49: 297–305. doi: 10.1093/jjco/hyy196 [DOI] [PubMed] [Google Scholar]
- 8.Xiang D, Hong Y, Zhang B, Huang P, Li G, Wang P, et al. Contrast-Enhanced ultrasound (CEUS) facilitated us in detecting lateral neck lymph node metastasis of thyroid cancer patients: diagnosis value and enhancement patterns of malignant lymph nodes. Eur Radiol 2014; 24: 2513–9. doi: 10.1007/s00330-014-3288-5 [DOI] [PubMed] [Google Scholar]
- 9.Omura K. Current status of oral cancer treatment strategies: surgical treatments for oral squamous cell carcinoma. Int J Clin Oncol 2014; 19: 423–30. doi: 10.1007/s10147-014-0689-z [DOI] [PubMed] [Google Scholar]
- 10.Mori N, Mugikura S, Miyashita M, Kudo Y, Suzuki M, Li L, et al. Perfusion contrast-enhanced ultrasound to predict early lymph-node metastasis in breast cancer. Jpn J Radiol 2019; 37: 145–53. doi: 10.1007/s11604-018-0792-6 [DOI] [PubMed] [Google Scholar]
- 11.Ling W, Nie J, Zhang D, Yang Q, Jin H, Ou X, et al. Role of contrast-enhanced ultrasound (CEUS) in the diagnosis of cervical lymph node metastasis in nasopharyngeal carcinoma (NPC) patients. Front Oncol 2020; 10: 972. doi: 10.3389/fonc.2020.00972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu M, Liu Q, Song H-P, Han Z-H, Su H-L, He G-B, , GB H, et al. Clinical application of contrast-enhanced ultrasonography in diagnosis of superficial lymphadenopathy. J Ultrasound Med 2010; 29: 735–40. doi: 10.7863/jum.2010.29.5.735 [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Takemura T, Ouchi T, Mori S, Sakamoto M, Arai Y, et al. Monitoring of blood vessel density using contrast-enhanced high frequency ultrasound may facilitate early diagnosis of lymph node metastasis. J Cancer 2017; 8: 704–15. doi: 10.7150/jca.18027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Liu X, He J, Gou B, Luo Y, Deng S, et al. Percutaneous contrast-enhanced ultrasound for localization and diagnosis of sentinel lymph node in early breast cancer. Sci Rep 2019; 9: 13545. doi: 10.1038/s41598-019-49736-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ter Haar G. Safety and bio-effects of ultrasound contrast agents. Med Biol Eng Comput 2009; 47: 893–900. doi: 10.1007/s11517-009-0507-3 [DOI] [PubMed] [Google Scholar]
- 16.Tang C, Fang K, Guo Y, Li R, Fan X, Chen P, et al. Safety of sulfur hexafluoride microbubbles in sonography of abdominal and superficial organs: retrospective analysis of 30,222 cases. J Ultrasound Med 2017; 36: 531–8. doi: 10.7863/ultra.15.11075 [DOI] [PubMed] [Google Scholar]
- 17.Cui Z, Gao Y, Wang W, Zhu Z, Zhang Y, Ma Z. Evaluation of neck lymph node metastasis on contrast-enhanced ultrasound: an animal study. Clin Exp Otorhinolaryngol 2017; 10: 109–14. doi: 10.21053/ceo.2015.01284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poanta L, Serban O, Pascu I, Pop S, Cosgarea M, Fodor D. The place of CEUS in distinguishing benign from malignant cervical lymph nodes: a prospective study. Med Ultrason 2014; 16: 7–14. doi: 10.11152/mu.2014.2066.161.lp1os2 [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Zhang J, Zhu Q-L, Jiang Y-X, Sun Q, Zhou Y-D, et al. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: a prospective study. Eur Radiol 2018; 28: 1654–61. doi: 10.1007/s00330-017-5089-0 [DOI] [PubMed] [Google Scholar]
- 20.Cui Q-L, Yin S-S, Fan Z-H, Yang W, Wang S, Yan K. Diagnostic value of contrast-enhanced ultrasonography and Time-Intensity curve in differential diagnosis of cervical metastatic and tuberculous lymph nodes. J Ultrasound Med 2018; 37: 83–92. doi: 10.1002/jum.14311 [DOI] [PubMed] [Google Scholar]
- 21.Ding Z, Deng C, Wang Z, Liu L, Ma X, Huang J, et al. Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography for the diagnosis of cervical lymph node metastasis in squamous cell carcinoma of the oral cavity. Int J Oral Maxillofac Surg 2021; 50: 294–301. doi: 10.1016/j.ijom.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 22.Dudau C, Hameed S, Gibson D, Muthu S, Sandison A, Eckersley RJ, et al. Can contrast-enhanced ultrasound distinguish malignant from reactive lymph nodes in patients with head and neck cancers? Ultrasound Med Biol 2014; 40: 747–54. doi: 10.1016/j.ultrasmedbio.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 23.Mei M, Ye L, Quan J, Huang P. Contrast-Enhanced ultrasound for the differential diagnosis between benign and metastatic superficial lymph nodes: a meta-analysis. Cancer Manag Res 2018; 10: 4987–97. doi: 10.2147/CMAR.S174751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaukar D, Dandekar M, Kane S, Arya S, Purandare N, Rangarajan V, et al. Relative value of ultrasound, computed tomography and positron emission tomography imaging in the clinically node-negative neck in oral cancer. Asia Pac J Clin Oncol 2016; 12: e332–8. doi: 10.1111/ajco.12255 [DOI] [PubMed] [Google Scholar]
- 25.Stuckensen T, Kovács AF, Adams S, Baum RP. Staging of the neck in patients with oral cavity squamous cell carcinomas: a prospective comparison of PET, ultrasound, CT and MRI. J Craniomaxillofac Surg 2000; 28: 319–24. doi: 10.1054/jcms.2000.0172 [DOI] [PubMed] [Google Scholar]