ABSTRACT
Diagnosis of coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) remains unclear especially in nonimmunocompromised patients. The aim of this study was to evaluate seven mycological criteria and their combination in a large homogenous cohort of patients. All successive patients (n = 176) hospitalized for COVID-19 requiring mechanical ventilation and who clinically worsened despite appropriate standard of care were included over a 1-year period. Direct examination, culture, Aspergillus quantitative PCR (Af-qPCR), and galactomannan testing were performed on all respiratory samples (n = 350). Serum galactomannan, β-d-glucan, and plasma Af-qPCR were also assessed. The criteria were analyzed alone or in combination in relation to mortality rate. Mortality was significantly different in patients with 0, ≤2, and ≥3 positive criteria (log rank test, P = 0.04) with death rate of 43.1, 58.1, and 76.4%, respectively. Direct examination, plasma qPCR, and serum galactomannan were associated with a 100% mortality rate. Bronchoalveolar lavage (BAL) galactomannan and positive respiratory sample culture were often found as isolated markers (28.1 and 34.1%) and poorly repeatable when a second sample was obtained. Aspergillus DNA was detected in 13.1% of samples (46 of 350) with significantly lower quantitative cycle (Cq) when associated with at least one other criterion (30.2 versus 35.8) (P < 0.001). A combination of markers and/or blood biomarkers and/or direct respiratory sample examination seems more likely to identify patients with CAPA. Af-qPCR may help identifying false-positive results of BAL galactomannan testing and culture on respiratory samples while quantifying fungal burden accurately.
KEYWORDS: Aspergillus, COVID-19, critical care, diagnosis, COVID-19-associated pulmonary aspergillosis
INTRODUCTION
Reports on coronavirus disease 2019 (COVID-19)-associated aspergillosis (CAPA) are increasing but incidence, diagnosis, and management remains unclear. Because no host factor meeting European Organization for Research and Treatment of Cancer and Mycosis Study Group Education and Research Consortium (EORTC/MSGERC) definitions is present in most patients, alternative classifications were needed (1). Despite several attempts to clarify CAPA definition by expert panels (2–4), diagnosis remains arguable (5–7). The lack of impact of both antifungal treatment and prophylaxis in many large cohort studies and the impact of prophylaxis on the incidence of CAPA cases lead to the hypothesis that many of these cases may only be colonization rather than infection (8, 9). Therefore, incidence and prognosis may be overestimated, while the impact of treatment may be underestimated in real aspergillosis cases. The minimum requirement for CAPA diagnosis according the European Confederation for Medical Mycology and International Society for Human and Animal Mycology (ECMM/ISHAM) in a COVID-19 patient presenting with acute respiratory distress syndrome includes at least one imaging characteristic (i.e., pulmonary infiltrates), one clinical characteristic (i.e., refractory fever, chest pain), and one mycological criterion (3). The first two requirements (imaging and clinical) are poorly specific and quite frequent in severe COVID-19 patients; therefore CAPA diagnosis mainly relies on mycological criteria, with one criterion alone being sufficient. The performances of such mycological criteria lack proper evaluation in this context mostly because the gold standard depends on histopathological data evidencing invasive hyphae in lung tissue. Lung biopsies would be difficult to justify in severe COVID-19 patients given the number of patients, infection control risks, and the lack of identified infected site in most cases on imaging. In parallel, rare studies, including autopsies, have been published. The few autopsy data available rarely include routinely performed fungal investigations before the death of the patients, and mycological tests were mostly not evaluated (10, 11). Despite the absence of accessible gold standards, more data are needed on routinely performed individual mycological criteria, regarding their advantages and shortcomings in the context of critically ill COVID-19 patients. Mortality is known to be high in invasive aspergillosis, and the high mortality rate may better reflect infection over colonization in CAPA-suspected patients. Therefore, the aim of our study was to evaluate the performances of seven mycological criteria and their combination, analyzed in terms of clinical outcome in mechanically ventilated COVID-19 patients.
MATERIALS AND METHODS
Patients and study design.
A retrospective observational cohort study was conducted. All successive COVID-19 patients admitted to the four intensive care units (ICUs) of our two university hospitals between 15 March 2020 and 1 March 2021 with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR test (Cobas SARS-CoV-2 test, Roche, Meylan, France) and ≥1 respiratory sample (bronchoalveolar lavage [BAL], tracheal aspirate, or sputum) sent to the mycology department were included. Of note, the 105 first patients included were partially analyzed to assess risk factors associated with CAPA (12). Culture and Aspergillus quantitative PCR (qPCR) were systematically performed on all types of respiratory samples (BAL, sputum, tracheal, or bronchial aspirates). Direct examination of those specimens was only performed in samples received after 26 March 2020 because of previous lack of evidence regarding the risk of contamination of lab staff members. After this date, direct examinations in 0.1 M KOH were performed again as SARS-CoV-2 was found to be inactivated at pH >10. Galactomannan (GM) detection was performed in the supernatant of every BAL fluid (after centrifugation) following strict recommendations of the manufacturer. If a blood sample was concomitantly received, GM and β-d-glucan (BDG) detection were performed on serum, and Aspergillus qPCR was performed on plasma. The patients were classified as having probable invasive pulmonary aspergillosis (IPA) according to the EORTC/MSGERC updated consensus criteria (1) if a host factor was present and according to the ECMM/ISHAM consensus criteria (3) otherwise. The following clinical data were collected, including age, gender, length of ICU stay, prescription of antifungals, and mortality.
Mycology criteria testing.
(i) Direct examination and culture of respiratory samples. All mucous respiratory samples were diluted to 1:10, transferred to sterile 50-mL Falcon tubes, incubated at 37°C for 15 min, and centrifuged 10 min at 3,000 × g. BAL supernatant was reserved for GM detection. A direct examination was performed by mixing 50 μl of the pellet, 10 μl of Calcofluor White, and 10 μl of KOH and was read at 475 nm with a Nikon Eclipse E600 fluorescence microscope. The other part of the pellet was seeded in parallel on BBL CHROMagar Candida medium (BD, Grenoble, France), 2% MALT extract and chloramphenicol agar (VWR Chemical, Leuven, Belgium), and Sabouraud agar supplemented with chloramphenicol/gentamicin (Bio-Rad, Marnes-la-Coquette, France). Candida chromogenic media and one Sabouraud were incubated at 37°C. MALT plates and one Sabouraud slant was incubated at 30°C for a maximum of 3 weeks.
(ii) Aspergillus fumigatus qPCR (Af-qPCR). Whole nucleic acids together with the internal control DiaControlDNA (Diagenode, Seraing, Belgium) were extracted from 1 mL of plasma or from half of the pellet of the respiratory sample (other half was used for culture), which was submitted to bead-beating in 1,000 μl DNA-free water using the QIAsymphony DSP virus/pathogen kit (Qiagen, Hilden, Germany) and a QIAsymphony apparatus (Qiagen). All extracts were tested in duplicate using the 28S rRNA gene PCR assay previously reported (13, 14). PCR assays were performed on a LightCycler 480 instrument (Roche Diagnostics, Meylan, France).
(iii) GM testing.
GM detection was performed using a Platelia Bio-Rad kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer. Each positive result was retested the following day with the same sample, as recommended (15). Only samples found positive twice were considered positive. Optical density indexes (ODI) of >0.5 and >1 were considered positive in serum and BAL fluid, respectively (3, 4).
(iv) BDG testing.
BDG testing was performed using Fungitell assay (Cape Cod Diagnostics) according to the manufacturer’s instructions. BDG was considered positive when >80 pg/mL.
Statistics.
The data were reported in percentage, mean and standard deviation (±SD) if variable, followed a Gaussian distribution or median and interquartile ranges (IQRs) [Q1 to Q3] otherwise. Univariate analyses were performed using Fisher’s exact, chi-square, and Wilcoxon tests as appropriate. P < 0.05 (two-tailed) was considered statistically significant. Mortality analysis was performed using Kaplan-Meier survival curves and log rank test. No statistical adjustments were made for multiple testing or confounders. All analyses were performed using Prism, version 8.0.
Ethical statements.
Our institutional ethics committee approved the study (IDRCB, 2020-A00256-33; CPP, 11-20-20.02.04.68737). Written information was given to the patient or the next of kin.
RESULTS
Population characteristics.
The patient characteristics are described in Table 1. Briefly, 176 patients for whom 350 respiratory samples were received by the mycology department were included (Fig. 1). Patients were mainly males (male/female sex ratio = 3.6) with a median age of 64 years [IQR, 58 to 72]. A median of 2 [IQR, 1 to 3; range, 1 to 9] respiratory samples were received per patient. Three (1.7%) patients had EORTC/MSGERC host factor (kidney transplant, n = 2; hematological malignancy, n = 1). Totals of 42 (23.9%) and 11 (6.3%) patients could be classified as probable and possible CAPA, respectively, according to ECMM/ISHAM consensus criteria (3) (Table S1). The incidence of probable CAPA in our cohort, was estimated to be 7.1, 12.8, and 23.9% in patients admitted to the ICU, those mechanically ventilated, and those who clinically worsened after initiation of mechanical ventilation, respectively. The mean delay between intubation and CAPA diagnosis was 8 (5 to 17) days. A total of 13 patients with possible (n = 2) or probable (n = 11) CAPA received more than 3 days of antifungals (voriconazole, n = 12; liposomal amphotericin B, n = 1). Mortality in patients who received antifungals for probable CAPA was not significantly different from patients with probable CAPA who did not (76.5% versus 52.0%, P = 0.11). Differences between the first and second COVID-19 waves were compared (Table S2).
TABLE 1.
Patient characteristicsa
| Characteristics | Patients (n = 176) | Samples (n = 350) |
|---|---|---|
| Age (yrs), median [Q1 to Q3] | 64 [58 to 72] | |
| Male, n (%) | 139 (78.1%) | |
| Sample per patient, median [Q1 to Q3] | 2 [1 to 3] | |
| Type of samples, n (%) | ||
| BAL, n (%) | 269 (76.9%) | |
| Tracheal aspirate n (%) | 74 (21.1%) | |
| Bronchial aspirate n (%) | 7 (2.0%) | |
| Patients with sufficient diagnostic mycological criteria, n (%) | ||
| For IPAb | 3 (1.7%) | |
| For probable CAPAc | 42 (23.9%) | |
| For possible CAPAc | 11 (6.3%) | |
| Patients with positive BDG only, n (%) | 13 (7.3%) |
aBAL, bronchoalveolar lavage; BDG, β-1,3-d-glucan; CAPA, COVID-19-associated pulmonary aspergillosis; IPA, invasive pulmonary aspergillosis.
bAccording to European Organization for Research and Treatment of Cancer and Mycosis Study Group Education and Research Consortium (EORTC/MSGERC) guidelines.
cAccording to European Confederation for Medical Mycology and International Society for Human and Animal Mycology (ECMM/ISHAM) guidelines.
FIG 1.

Study flowchart. *, as recommended by the manufacturer; BAL, bronchoalveolar lavage; BDG, β-1,3-d-glucans; GM, galactomannan; ICU, intensive care unit; qPCR, quantitative PCR; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Evaluation of mycological criteria in relation with patient mortality.
Mortality rate tended to increase in patients with probable CAPA (61.9%) compared to patients who were not classified as CAPA (46.3%, P = 0.08), with no difference with possible CAPA (54.5%, P = 0.60). Survival curves are shown in Fig. 2A (log rank test, P = 0.06). Mortality was significantly different in patients with 0, ≤2, and ≥3 mycological criteria (log rank test, P = 0.04) with death rates of 43.1, 58.1, and 76.4%, respectively (Fig. 2B).
FIG 2.
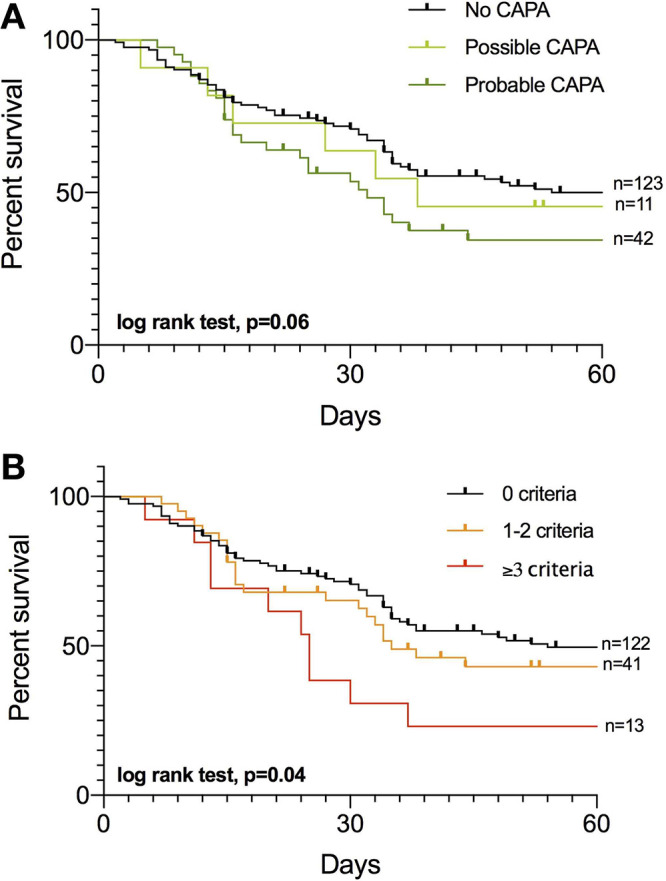
Kaplan-Meier survival curves for 60-day mortality of patients with no COVID-19-associated pulmonary aspergillosis (CAPA) or possible or probable CAPA according to European Confederation for Medical Mycology and International Society for Human and Animal Mycology (ECMM/ISHAM) consensus criteria (log rank test, P = 0.06) (A) and with 0, 1 or 2, and ≥3 positive mycological criteria (B) (log rank test, P = 0.008). The following were considered Aspergillus criteria: from respiratory specimens: (i) direct respiratory sample examination with branched hyphae suggestive of Aspergillus-type hyphae, (ii) respiratory sample culture growing Aspergillus sp., (iii) galactomannan in bronchoalveolar lavage (index of >1.0), and (iv) Aspergillus qPCR in respiratory sample with quantitative cycle (Cq) of <36; and from serum (i) galactomannan in blood (index of >0.5), (ii) positive Aspergillus qPCR in blood with Cq of <40, and (iii) blood BDG of >80 pg/mL.
All mycological criteria were studied separately and analyzed in the light of repeated samples: (i) Direct examination evidenced Aspergillus-type hyphae in 2.5% (8 of 318) of samples (BAL, n = 5 and tracheal aspirate, n = 3) from 8 of 160 patients and was systematically associated with a median of 3 other mycological criteria [IQR 2-4] and a mortality rate of 100%. (ii) A proportion of 11.7% (41 of 350) respiratory samples (BAL, n = 29; tracheal aspirate, n = 12) from 32 of 176 patients grew Aspergillus spp. (A. fumigatus, n = 35; Aspergillus niger, n = 6). Overall mortality in patients with a positive Aspergillus culture was 68.8%. Isolated positive cultures (i.e., no other positive mycological criteria) were found in 14 patients, of which 4 had a second respiratory sample. Only 50.0% (2 of 4) remained positive despite the absence of antifungal prescription between both samples. Mortality in this subgroup was 71.4% (10 of 14). (iii) GM was measured in 255 BAL samples from 144 patients, and 8.2% (21 of 255) had an index level of >1.0. A total of 6 samples from 5 patients showed isolated increased GM levels ranging from 1.6 to 4.3 with a median of 3.1 [IQR, 2.3 to 3.9]. All these patients had a second BAL performed in the next 7 days or less, and none was positive, while only one patient received voriconazole between both samples. A proportion of 20% (1 of 5) of these patients died, while 62.5% (5 of 8) died if BAL GM was associated with at least one other positive criterion (P = 0.26). (iv) Aspergillus DNA was detected in 13.1% (46 of 350 from 32 of 176 patients) of respiratory samples with a median Cq value of 30.7 [IQR, 29.6 to 32.3]. Cq values were significantly lower when positive Af-qPCR was associated with other mycological criteria with a median of 30.2 [IQR, 29.4 to 31.8] versus 35.8 [IQR, 31.5 to 36.9] when found as the sole mycological criterion (P < 0.001). A receiver operating characteristic (ROC) curve was computed to identify the best Af-qPCR Cq value to predict mortality (Fig. S1). In our settings, including 46 positive values, a cutoff value of Cq = 32 was associated with the best sensitivity (88.9% [95% inhibitory concentration (IC95), 67.2 to 98.0]) and specificity (57.1% [IC95, 32.6 to 78.6]) to predict mortality in case of positive Af-qPCR. The area under the curve was 0.73 (P = 0.03). Mortality associated with Cq cutoff values of 36 and 32 is shown in Fig. 3A and B, respectively. While a Cq of <32 was significantly associated with mortality (log rank test, P = 0.007), a Cq of <36 was not (log rank test, P = 0.19). Of note, 7 patients had Cq values between 32 and 36, but only 14.3% (1 of 7) died. qPCR data acquired from BAL and non-BAL samples were compared (Fig. 3C). Cq values were lower in non-BAL samples compared to BAL samples in nonsurvivors (P = 0.009) but not significantly different in survivors (P = 0.30). For 12 patients with positive qPCR, a second sampling was performed, of which 75% (9 of 12) were still positive, confirming the first sample result. In the 3 patients with negative second sample, 1 received voriconazole between both samples. A total of 29 positive Af-qPCR tests were performed on BAL with concomitant GM tests. As expected, a significant inverse correlation was observed between GM index and Cq values (slope = −0.60 ± 0.20; R2 = 0.26; P = 0.005) (Fig. 3D). Of note, 22 respiratory samples presented positive Af-qPCR and negative culture despite intermediate/high fungal load with a median Cq of 31.8 [30.7 to 35.2]. A proportion of 81.8% (18 of 22) of positive Af-qPCR/negative Af culture grew Candida spp. in culture, in comparison to 29.1% (7 of 24) in positive Af-qPCR/positive Af culture samples (P < 0.001). (v) At least one specific positive blood marker, i.e., serum GM tested in 73.3% (129 of 176) and/or Af-qPCR in plasma available in 50.0% (88 of 176) of our patients, was positive in 8 patients, systematically associated with additional mycological criteria (median = 3.5 [IQR, 3 to 5]) and a 100% mortality rate, except for 1 patient with GM serum level of 0.6, for whom no other samples were obtained and who survived (Table S3). (vi) BDG was performed in 74.4% (131 of 176) of our patients. Positive BDG was associated with death in all patients (75.8 versus 47.9%; P = 0.006).
FIG 3.
Characteristics of A. fumigatus qPCR (Af-PCR) in respiratory sample. (A, B) Kaplan-Meier survival curves for 60-day mortality associated with a qPCR cutoff. (A) 36 Cq. (B) 32 Cq. (C) Scatterplot of qPCR Cq values if performed in BAL and non-BAL samples depending on the patient’s outcome. (D) Correlation between GM index (ODI) and quantification cycles (Cq value) in qPCR-positive BAL samples.
DISCUSSION
In this large homogeneous 1-year retrospective cohort of 176 mechanically ventilated COVID-19 patients (n = 350 respiratory samples), we evaluated the diagnostic and prognostic values of seven mycological criteria in CAPA. A positive examination of respiratory samples (i.e., Aspergillus-type hyphae), a serum GM index of >0.5, and a positive plasma Af-qPCR were systematically associated with other mycological criteria and to a mortality of 100%. Conversely, a BAL GM index of >1.0 and positive respiratory sample culture were frequently found as isolated mycological criteria (28.5 and 34.1% of positive samples, respectively) but were mostly not confirmed on a second sample if performed in the absence of treatment. This raises doubts on the ability of these criteria to accurately diagnose CAPA, taking into account the possibility of false-positive results of culture (sample, culture contamination) and galactomannan (GM cross-reaction).
Evaluation of single criteria in critically ill COVID-19 patients.
According to ECMM/ISHAM consensus criteria (3), BAL direct examination identifying Aspergillus-type hyphae, positive Aspergillus sp. culture of BAL, BAL GM of >1.0, BAL Af-qPCR of <36 Cq, and serum GM of >0.5 are each sufficient criterion to diagnose CAPA, without any superiority between them. Here, we found positive direct examination to be associated with 100% mortality rate and at least two other mycological criteria highlighting the importance of this criteria, most likely reflecting high fungal burden and therefore poor prognosis. The algorithm of Blot et al. that uses more stringent criteria to diagnose pulmonary aspergillosis in the critically ill patients included positive cytological smear showing branching hyphae as mandatory criteria (16).
Surprisingly, 8.4% (14 of 176) of patients had a single positive culture with a negative qPCR expected to be similarly or more sensitive than culture. Discrepancies can be attributed to extremely low fungal load or contamination of the sample by ubiquitous Aspergillus spores during sampling or handling of the sample. Therefore, cautious interpretation of an isolated positive culture may be recommended even in BAL and semiquantification or quantification of CFU obtained from agar plate culture should be interesting to notify and evaluate.
GM >1.0 in BAL was found as an isolated fungal criterion in six samples but not confirmed on a second BAL. GM is widely used to diagnose invasive aspergillosis but is hampered by variable occurrence of unreproducible positive results (15), and its concentration was not associated with a higher mortality rate in a recent multicentric study (17). Aigner et al. suggested that heavy colonization of the respiratory tract by Candida spp. may trigger galactomannan positivity (18). In our study, five of six isolated BAL GM were associated with a positive culture for Candida albicans. Overall, BAL GM seems to be a poor marker to distinguish infection and colonization and can be affected by technical issues. Positive BAL GM could be confirmed by a second positive biomarker such as fungal DNA to initiate antifungal treatment.
In our study, we confirmed the results of a previously published multicentric study using mortality to analyze mycological criteria characteristics (17). This study, like ours, showed an association between serum GM detection (>0.5) and an increased mortality rate in proven/probable CAPA cases (87.5% in serum GM-positive CAPA patients versus 41.7% in serum GM-negative CAPA patients).
Many interesting features of Af-qPCR are highlighted by our study. Af-qPCR could serve as a confirmation tool to identify BAL GM and false-positive culture results, while quantifying fungal burden. Its performance appears similar in BAL and non-BAL samples, supporting the use of endotracheal samples to help diagnose patients with CAPA as already proposed (5). Af-qPCR may also help detect A. fumigatus when unable to grow due to heavy Candida spp. load in the respiratory tract preventing Aspergillus growth, supported by the high significant rate of Candida colonization in positive Af-qPCR/negative Af culture samples in our study. However, pitfalls remain, including the potential variability in the quantification due to heterogeneity of the specimen and the variable performances of the different Af-qPCR assays among centers (19). PCR performances depends on many factors, including DNA extraction method, primers, and probe, as well as DNA target amplified, reagents, and thermocycler used (20, 21). A Cq value of 36, as defined by ECMM/ISHAM consensus criteria, does not appear to be the ideal threshold, as our study reveals that this threshold does not reflect the prognosis of the patient. We suggest that the threshold of 32 (i.e., fungal load increased by 20), as significantly associated with an increased mortality rate, may be considered a more relevant mycological criterion to diagnose CAPA. More studies are needed as any specific cutoff will have to be optimized for each specific qPCR assay.
Does sample type really matter?
Tracheal aspirates are reported to be more often positive than BAL fluid in critically ill COVID-19 patients but represent upper airway colonization (3). This was the trend in our study, although the results were not significant with 16.2 and 10.8% of tracheal aspirates and BAL fluid being positive in culture, respectively (P = 0.20). Furthermore, mortality in patients with a positive culture in tracheal aspirate was similar to that in BAL fluid (75.0% versus 72.4%; P = 0.99) raising doubts about the colonization-only hypothesis. Fungal load evaluated by median Af-qPCR was also similar in both sample types. Bronchial aspirates were also previously suggested to be more sensitive than BAL fluids for the diagnosis of invasive pulmonary aspergillosis in allogeneic hematopoietic stem cell transplant recipients associated with a more bronchial tropism during this type of invasive disease (22). Overall, a positive endotracheal sample should be sufficient to delineate probable aspergillosis for patient management, as suggested by others, especially if it is supported by one or more other criteria (5, 16).
Combination of criteria for the diagnosis of CAPA.
According to the significant difference in the survival of patients depending on the number of positive criteria and initially proposed by White et al. to diagnose CAPA when clear recommendations were lacking (23), we propose to use a combination of criteria to diagnose CAPA, but our suggestion needs further discussion. In White et al., ≥2 positive criteria were needed when much more stringent radiological evidence than ECMM/ISHAM guidelines was lacking (i.e., nodules, halo, or consolidation instead of infiltrates being sufficient as currently recommended). Of 135 screened patients, 16 had multiple Aspergillus criteria, while 23 had a single criterion, but mortality was not mentioned for either group. Although the number of positive criteria would reflect fungal burden, which was already suggested in immunocompromised IPA patients (24) and recently also observed in CAPA patient with no known risk factors (25), combining criteria could help overcome false-positive issues and overdiagnosis of CAPA. Reflecting most probably higher fungal loads, positive direct examination, positive serum GM and Af-qPCR should be included in a future algorithm as criteria with higher weighting coefficient (Fig. 4). Isolated BAL GM and an isolated positive culture may be given less weight especially if not coupled and confirmed with Af-qPCR on the same sample or associated with another criteria. This could also harmonize the diagnosis regardless of the type of respiratory sample, which makes classifying patients compelling.
FIG 4.
Combination and weight of mycological criteria for the diagnosis of COVID-19-associated pulmonary aspergillosis according to the impact on prognosis. Created with BioRender.com.
Limits of this study.
Diagnosis of CAPA has been a hurdle in patients with no EORTC/MSGERC host factors, preventing the identification of patients who might benefit from antifungal prophylaxis or treatment. Similarly to every study evaluating mycological criteria characteristics for the diagnosis of CAPA, our design lacks an adequate gold standard (i.e., histopathological data showing tissue-invasive hyphae). Evaluating these criteria in relationship to mortality has many biases in this population, in which death is most likely multifactorial resulting from bacterial/fungal superinfection (26), refractoriness of COVID-19, and underlying disease decompensation (27). However, only a few patients received an effective and early antifungal treatment, which may be the reason why there is no difference in mortality between treated and untreated patients. Therefore, using mortality may help identify significant criteria or combinations of criteria reflecting the invasiveness of the Aspergillus disease known to be associated with a high mortality rate.
Conclusion.
Despite the lack of gold standard, evaluating available mycological criteria for CAPA diagnosis is a priority as the pandemic is likely to continue, and more data are needed. One isolated positive criterion is most likely insufficient to diagnose CAPA, especially for respiratory sample criteria, or should at least be reevaluated in a second sample. Af-qPCR to address false positives and avoid false negatives in cases of intense concomitant Candida colonization should be considered systematically. However, standardized prospective studies are needed to compare autopsy results and mycological data to confirm these findings and better target patients that could benefit from antifungals.
ACKNOWLEDGMENT
We thank all the staff, nurses, and lab technicians who were essential to patient care in the intensive care units and medical mycology departments of Saint-Louis Hospital and Lariboisière Hospital.
No external funding was received for the present study, as part of the routine testing in our lab.
Writing original draft, S.D. and A.A.; Writing review and editing, all authors; Conceptualization, S.D., A.A.; Investigation, S.D., E.D., S.F., M.C.; Data curation, S.D., E.D., S.F., S.V., M.C.; Formal analysis, S.D.; Visualization, S.D., A.A.; Supervision, A.A.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Sarah Dellière, Email: sarah.delliere@gmail.com.
Alexandre Alanio, Email: alexandre.alanio@pasteur.fr.
Kimberly E. Hanson, University of Utah
REFERENCES
- 1.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, Clancy CJ, Wingard JR, Lockhart SR, Groll AH, Sorrell TC, Bassetti M, Akan H, Alexander BD, Andes D, Azoulay E, Bialek R, Bradsher RW, Bretagne S, Calandra T, Caliendo AM, Castagnola E, Cruciani M, Cuenca-Estrella M, Decker CF, Desai SR, Fisher B, Harrison T, Heussel CP, Jensen HE, Kibbler CC, Kontoyiannis DP, Kullberg BJ, Lagrou K, Lamoth F, Lehrnbecher T, Loeffler J, Lortholary O, Maertens J, Marchetti O, Marr KA, Masur H, Meis JF, Morrisey CO, Nucci M, Ostrosky-Zeichner L, Pagano L, Patterson TF, Perfect JR, Racil Z, et al. 2020. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 71:1367–1376. 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, Calandra T, Clancy CJ, Cornely OA, Chiller T, Depuydt P, Giacobbe DR, Janssen NAF, Kullberg BJ, Lagrou K, Lass-Flörl C, Lewis RE, Liu PW-L, Lortholary O, Maertens J, Martin-Loeches I, Nguyen MH, Patterson TF, Rogers TR, Schouten JA, Spriet I, Vanderbeke L, Wauters J, van de Veerdonk FL. 2020. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 46:1524–1535. 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, Klimko N, Lass-Flörl C, Oladele RO, Vinh DC, Zhu L-P, Böll B, Brüggemann R, Gangneux J-P, Perfect JR, Patterson TF, Persigehl T, Meis JF, Ostrosky-Zeichner L, White PL, Verweij PE, Cornely OA, European Confederation of Medical Mycology, International Society for Human Animal Mycology, Asia Fungal Working Group, INFOCUS LATAM/ISHAM Working Group, ISHAM Pan Africa Mycology Working Group, European Society for Clinical Microbiology, Infectious Diseases Fungal Infection Study Group, ESCMID Study Group for Infections in Critically Ill Patients, Interregional Association of Clinical Microbiology and Antimicrobial Chemotherapy, Medical Mycology Society of Nigeria, Medical Mycology Society of China Medicine Education Association, Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology, Association of Medical Microbiology, Infectious Disease Canada. 2021. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 21:e149–e162. 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verweij PE, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, Buil JB, Calandra T, Chiller T, Clancy CJ, Cornely OA, Depuydt P, Koehler P, Lagrou K, de Lange D, Lass-Flörl C, Lewis RE, Lortholary O, Liu PW-L, Maertens J, Nguyen MH, Patterson TF, Rijnders BJA, Rodriguez A, Rogers TR, Schouten JA, Wauters J, van de Veerdonk FL, Martin-Loeches I. 2021. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med 47:819–816. 10.1007/s00134-021-06449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson GR III, Cornely OA, Pappas PG, Patterson TF, Hoenigl M, Jenks JD, Clancy CJ, Nguyen MH. 2020. Invasive aspergillosis as an under-recognized superinfection in COVID-19. Open Forum Infect Dis 7:ofaa242. 10.1093/ofid/ofaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fekkar A, Neofytos D, Nguyen MH, Clancy CJ, Kontoyiannis DP, Lamoth F. 2021. COVID-19 associated pulmonary aspergillosis (CAPA): how big a problem is it? Clin Microbiol Infect 27:1376–1378. 10.1016/j.cmi.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Permpalung N, Maertens J, Marr KA. 2021. Diagnostic dilemma in COVID-19-associated pulmonary aspergillosis. Lancet Infect Dis 21:766–767. 10.1016/S1473-3099(21)00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzl S, Reisinger AC, Posch F, Prattes J, Stradner M, Pilz S, Eller P, Schoerghuber M, Toller W, Gorkiewicz G, Metnitz P, Rief M, Prüller F, Rosenkranz AR, Valentin T, Krause R, Hoenigl M, Schilcher G. 2021. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: an observational study. Crit Care 25:335. 10.1186/s13054-021-03753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangneux J-P, Dannaoui E, Fekkar A, Luyt C-E, Botterel F, de Prost N, Tadié J-M, Reizine F, Houzé S, Timsit J-F, Iriart X, Riu-Poulenc B, Sendid B, Nseir S, Persat F, Wallet F, Le Pape P, Canet E, Novara A, Manai M, Cateau E, Thille Aw Brun S, Cohen Y, Alanio A, Mégarbane B, Cornet M, Terzi N, Lamhaut L, Sabourin E, Desoubeaux G, Ehrmann S, Hennequin C, Voiriot G, Nevez G, Aubron C, Letscher-Bru V, Meziani F, Blaize M, Mayaux J, Monsel A, Boquel F, Robert-Gangneux F, Le Tulzo Y, Seguin P, Guegan H, Autier B, Lesouhaitier M, Pelletier R, Belaz S, Bonnal C, et al. 2021. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med S2213-2600(21)00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kula BE, Clancy CJ, Hong NM, Schwartz IS. 2021. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. Lancet Microbe 2:e405–e414. 10.1016/S2666-5247(21)00091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortarezza F, Boscolo A, Pezzuto F, Lunardi F, Jesús Acosta M, Giraudo C, Del Vecchio C, Sella N, Tiberio I, Godi I, Cattelan A, Vedovelli L, Gregori D, Vettor R, Viale P, Navalesi P, Calabrese F. 2021. Proven COVID-19-associated pulmonary aspergillosis in patients with severe respiratory failure. Mycoses 64:1223–1229. 10.1111/myc.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic P-A, Salmona M, Depret F, Ghelfenstein-Ferreira T, Plaud B, Chousterman B, Bretagne S, Azoulay E, Mebazaa A, Mégarbane B, Alanio A. 2020. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect 27:790.e1–790.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Challier S, Boyer S, Abachin E, Berche P. 2004. Development of a serum-based TaqMan real-time PCR assay for diagnosis of invasive aspergillosis. J Clin Microbiol 42:844–846. 10.1128/JCM.42.2.844-846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alanio A, Menotti J, Gits-Muselli M, Hamane S, Denis B, Rafoux E, Peffault de la Tour R, Touratier S, Bergeron A, Guigue N, Bretagne S. 2017. Circulating Aspergillus fumigatus DNA is quantitatively correlated to galactomannan in serum. Front Microbiol 8:405–408. 10.3389/fmicb.2017.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guigue N, Lardeux S, Alanio A, Hamane S, Tabouret M, Bretagne S. 2015. Importance of operational factors in the reproducibility of Aspergillus galactomannan enzyme immune assay. PLoS One 10:e0124044. 10.1371/journal.pone.0124044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blot SI, Taccone FS, Van den Abeele A-M, Bulpa P, Meersseman W, Brusselaers N, Dimopoulos G, Paiva JA, Misset B, Rello J, Vandewoude K, Vogelaers D, AspICU Study Investigators. 2012. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 186:56–64. 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 17.Ergün M, Brüggemann RJM, Alanio A, Dellière S, van Arkel A, Bentvelsen RG, Rijpstra T, van der Sar-van der Brugge S, Lagrou K, Janssen NAF, Buil JB, van Dijk K, Melchers WJG, Reijers MHE, Schouten JA, Wauters J, Cordey A, Soni S, White PL, van de Veerdonk FL, Verweij PE. 2021. Aspergillus test profiles and mortality in critically-ill COVID-19 patients. J Clin Microbiol 59:e0122921. 10.1128/JCM.01229-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aigner M, Wanner M, Kreidl P, Lass-Flörl C, Lackner M. 2019. Candida in the respiratory tract potentially triggers galactomannan positivity in nonhematological patients. Antimicrob Agents Chemother 63:e00138-19. 10.1128/AAC.00138-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White PL, Mengoli C, Bretagne S, Cuenca-Estrella M, Finnstrom N, Klingspor L, Melchers WJG, McCulloch E, Barnes RA, Donnelly JP, Loeffler J, European Aspergillus PCR Initiative. 2011. Evaluation of Aspergillus PCR protocols for testing serum specimens. J Clin Microbiol 49:3842–3848. 10.1128/JCM.05316-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocchi S, Scherer E, Mengoli C, Alanio A, Botterel F, Bougnoux ME, Bretagne S, Cogliati M, Cornu M, Dalle F, Damiani C, Denis J, Fuchs S, Gits-Muselli M, Hagen F, Halliday C, Hare R, Iriart X, Klaassen C, Lackner M, Lengerova M, Letscher-Bru V, Morio F, Nourrisson C, Posch W, Sendid B, Springer J, Willinger B, White PL, Barnes RA, Cruciani M, Donnelly JP, Loeffler J, Millon L. 2021. Interlaboratory evaluation of Mucorales PCR assays for testing serum specimens: a study by the Fungal PCR Initiative and the Modimucor study group. Med Mycol 59:126–138. 10.1093/mmy/myaa036. [DOI] [PubMed] [Google Scholar]
- 21.Dellière S, Gits-Muselli M, White PL, Mengoli C, Bretagne S, Alanio A. 2019. Quantification of Pneumocystis jirovecii: cross-platform comparison of one qPCR assay with leading platforms and six master mixes. J Fungi 6:9. 10.3390/jof6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergeron A, Porcher R, Sulahian A, de Bazelaire C, Chagnon K, Raffoux E, Vekhoff A, Cornet M, Isnard F, Brethon B, Lacroix C, Poirot JL, Bouges C, Derouin F, Tazi A, Ribaud P. 2012. The strategy for the diagnosis of invasive pulmonary aspergillosis should depend on both the underlying condition and the leukocyte count of patients with hematologic malignancies. Blood 119:1831–1837. 10.1182/blood-2011-04-351601. [DOI] [PubMed] [Google Scholar]
- 23.White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, Pandey M, Whitaker H, May A, Morgan M, Wise MP, Healy B, Blyth I, Price JS, Vale L, Posso R, Kronda J, Blackwood A, Rafferty H, Moffitt A, Tsitsopoulou A, Gaur S, Holmes T, Backx M. 2021. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis 73:e1634–e1644. 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lortholary O, Gangneux JP, Sitbon K, Lebeau B, Monbrison F, Le Strat Y, Coignard B, Dromer F, Bretagne S, French Mycosis Study Group. 2011. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005–2007). Clin Microbiol Infect 17:1882–1889. 10.1111/j.1469-0691.2011.03548.x. [DOI] [PubMed] [Google Scholar]
- 25.Bretagne S, Sitbon K, Botterel F, Dellière S, Letscher-Bru V, Chouaki T, Bellanger A-P, Bonnal C, Fekkar A, Persat F, Alanio A, Dromer F. 2021. COVID-19 associated pulmonary aspergillosis, fungemia, and pneumocystosis in ICU: a retrospective multicenter observational cohort during the first French pandemic wave. Microbiol Spectr 9:e0113821. 10.1128/Spectrum.01138-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, Fernandez-Pittol M, Pitart C, Inciarte A, Bodro M, Morata L, Ambrosioni J, Grafia I, Meira F, Macaya I, Cardozo C, Casals C, Téllez A, Castro P, Marco F, García F, Mensa J, Martínez JA, Soriano A, COVID-19 Researchers Group. 2021. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 27:83–88. 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.RECOVERY Collaborative Group. 2021. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384:693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3 and Fig. S1. Download jcm.02169-21-s0001.pdf, PDF file, 0.2 MB (242KB, pdf)




