ABSTRACT
The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is an international susceptibility testing committee, organized by the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) and functioning as the breakpoint advisory committee of the European Medicines Agency (EMA). The original remit of EUCAST was to harmonize European clinical breakpoints, but very soon, the activities expanded beyond the borders of Europe and included newly licensed agents in Europe. Among the milestones were the aggregating of large numbers of MIC distributions, creating software to display these distributions, the EUCAST concept of identifying epidemiological cutoff values (ECOFF), and the development of a EUCAST disk diffusion method. The EUCAST Development Laboratory has played a critical role in the development of antimicrobial susceptibility testing (AST) methodology, including development work for novel antimicrobial agents and for rapid AST directly from blood culture bottles. EUCAST has several standing subcommittees, including for AST in fungi (AFST) and mycobacteria (AMST) and for microorganisms of veterinary interest (VetCAST), and ad hoc subcommittees on subjects such as anaerobic bacteria, MIC and zone diameter distributions and epidemiological cutoff values, the relationship between phenotypic and genotypic resistance, and expert rules and methods for the detection of resistance mechanisms. All EUCAST decisions are subjected to the EUCAST public consultation process, the only exception being breakpoints of novel antimicrobial agents where confidentiality agreements during the licensing process prevent public participation. EUCAST has recently revised the definitions of clinical susceptibility interpretive categories S, I, and R, acknowledging the intimate relationship between drug exposure and susceptibility reporting.
KEYWORDS: antifungal, antimicrobial susceptibility testing, breakpoint, drug development, minimal inhibitory concentration, mycobacteria, pharmacokinetics and pharmacodynamics, resistance mechanisms, veterinary
EUCAST: THE ORIGIN AND HISTORY
In 1997, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) formed the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (Table 1) with the intention that it acts as a European breakpoint committee. At that time, there were six identifiable national breakpoint committees in Europe, and many countries used the NCCLS (now CLSI) guidelines. In effect, there were seven different guidelines and a plethora of methods and technical recommendations (1). The original EUCAST had no relationship with the European national breakpoint committees, and there was no collaboration or coordination among the national committees. Harmonization remained elusive.
TABLE 1.
Milestones in EUCAST
| Yr (range) | Activity |
|---|---|
| 1997–2001 | Chair: Ian Phillips |
| 2001–2012 | Chair: Gunnar Kahlmeter |
| 2012–2016 | Chair: Rafael Cantón |
| 2016–present | Chair: Christian Giske |
| 1997–2016 | Scientific secretary: Derek Brown |
| 2016–present | Scientific secretary: John Turnidge |
| 2001–present | Webmaster: Gunnar Kahlmeter |
| 2016–present | Clinical Data Coordinator: Rafael Cantón |
| 2012–present | Technical Data Coordinator: Gunnar Kahlmeter |
| 2002 | 2002: First publication in PubMed using EUCAST acronyms |
| 2002 | 2002: The first harmonized clinical breakpoints: aminoglycosides |
| 2003 | 2003: A first EUCAST website is created. |
| 2003 | 2003: The idea to aggregate MIC distributions from many international sources, with software to accomplish this and display distributions and ECOFFs, was created by the chairman. Distributions have been systematically collected and aggregated since then and are publicly available at https://mic.eucast.org/. |
| 2003 | The term epidemiological cutoff value (ECOFF) was coined in 2003. It is defined as the highest MIC for organisms devoid of phenotypically detectable acquired resistance mechanisms. It defines the upper end of the wild-type MIC distribution. |
| 2003–2009 | Breakpoints for existing individual agents and classes harmonized. |
| 2003–2004 | First European Union contract for funding as part of the DG Sanco call for “proposals for public health,” later followed by contractual tender-based agreements with ECDC, the first in 2008. |
| 2003 | EUCAST process for iterated review and revision of breakpoints in the light of new scientific information |
| 2003–present | International uptake of EUCAST guidelines |
| 2005 | Agreement between EUCAST and the European Medicines Agency (EMA), SOP/H/3043, released. |
| 2005 | The first clinical breakpoint on a new agent (daptomycin) generated for and accepted by EMA. |
| 2009 | A decision to develop a EUCAST disk diffusion test to match EUCAST clinical breakpoints and ECOFFs and the setting up of EUCAST Development Laboratories. |
| 2010 | The complete EUCAST breakpoint table encompassing all agents (existing and new) was published. |
| 2010 | EUCAST proposed that all countries (irrespective of whether on EUCAST or other AST systems) form National AST Committees (NACs). |
| 2011 | Expert Rules and Intrinsic Resistance in susceptibility testing |
| 2013 | Formation of Antifungal Susceptibility Testing Subcommittee |
| 2013 | Guidelines for the detection of resistance mechanisms. |
| 2015 | The public consultation process was inaugurated |
| 2015 | Subcommittee on the role of whole-genome sequencing in antimicrobial susceptibility testing |
| 2015–2019 | Review and revision of definitions of susceptibility categories commenced; from Susceptible, Intermediate, and Resistant to Susceptible at standard exposure, Susceptible at increased exposure, and Resistant. The new definitions are |
| S, Susceptible, standard dosing regimen. A microorganism is categorized as “susceptible, standard dosing regimen” when there is a high likelihood of therapeutic success using a standard dosing regimen of the agent. | |
| I, Susceptible, increased exposure. A microorganism is categorized as “susceptible, increased exposure” when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or by its concentration at the site of infection. | |
| R, Resistant. A microorganism is categorized as “resistant” when there is a high likelihood of therapeutic failure even when there is increased exposure. | |
| 2015 | Formation of Veterinary Susceptibility Testing Subcommittee |
| 2018 | Formation of Antimycobacterial Susceptibility Testing Subcommittee |
| 2020 | Formation of Anaerobe Susceptibility Testing Subcommittee |
| 2020–2021 | New software for displaying MIC and inhibition zone diameters was launched. |
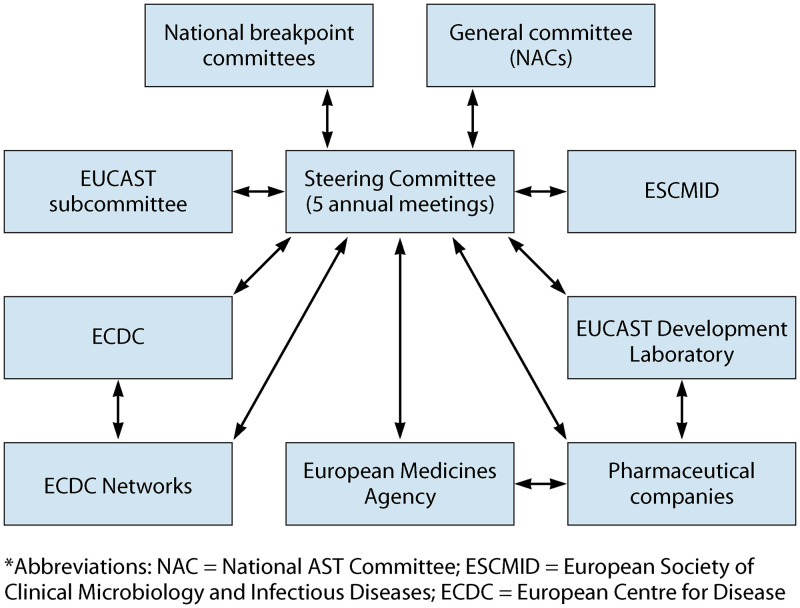
On the advice of the new chair, ESCMID, in 2001 and 2002, reorganized the EUCAST with national committees being given a major role and with a new task, namely, to harmonize breakpoints and methods in Europe (1–3). A General Committee (GC), with representatives from almost all European countries, and a Steering Committee (SC) were formed. National breakpoint committees appointed a representative each to the SC, and the GC appointed two representatives. The organization is detailed on the EUCAST website (4). A decision-making process was established. This involved the EUCAST General Committee and national committees and, from 2015, also the general medical community (5). The final decisions are in the hands of the Steering Committee.
EUCAST was initially financed only by ESCMID. However, in answer to a call for proposals issued by European Commission Directorate General for Health and Consumer Affairs in 2003, several EUCAST activities were financed by the European Union. This arrangement was later replaced by a succession of contracts with the European Centre for Disease Prevention and Control (ECDC) and has been ongoing.
EUCAST was tasked with harmonizing MIC breakpoints across Europe and recommending common reference methodologies. At the time, there was no accepted reference method for determination of MIC values. Under the auspices of the International Organization for Standardization (ISO) and in collaboration with NCCLS (now CLSI), there was general agreement to build on the two existing descriptions of how to perform the broth microdilution technique in bacteria and, through ISO, create a common international reference method. For disk diffusion, EUCAST decided to develop a method based on Mueller-Hinton agar and with zone diameter breakpoints to match EUCAST clinical breakpoints (6) and ECOFFs.
Initially, there was no relationship with regulatory authorities such as the European Medicines Agency (EMA). In 2005, EMA agreed to an informal collaboration governed by a standard operating procedure (SOP) whereby EUCAST was tasked with proposing breakpoints for existing and new agents (7). The ECDC was yet to be formed.
ORGANIZATION OF EUCAST AND ITS MANDATE
EUCAST statutes.
The original statutes of EUCAST were published in 1999 and most recently amended in 2016 (8). EUCAST’s remit is to (i) determine, review, and revise European clinical breakpoints and ECOFFs for surveillance of antimicrobial resistance in close collaboration with EMA and ECDC; (ii) promote the development and standardization of in vitro AST methods used in Europe; (iii) promote quality assurance of in vitro AST; (iv) promote education and training in AST; (v) advise ECDC and other European Union health agencies on issues related to AST and detection of resistance determinants relevant to public health; (vi) collaborate with international groups, ECDC, and other European Union health agencies involved in AST and/or the epidemiology of antimicrobial resistance in human pathogens; and (vii) work toward international consensus and harmonization of clinical breakpoints and AST.
EUCAST is led by a Steering Committee of 10 to 12 experts in the field of antimicrobial agents and breakpoints. The original national breakpoint committees (one representative each) and the EUCAST General Committee (two representatives) are represented. ESCMID appoints the chairperson, the scientific secretary, the clinical data coordinator, and the technical coordinator; the latter is also responsible for the work of the EUCAST Development Laboratory (see below). All countries with an interest in EUCAST can appoint a member to the General Committee. The Steering Committee has 5 meetings per year, and the General Committee has 1 meeting per year. EUCAST interacts via EMA with pharmaceutical companies in the process of setting breakpoints for new antimicrobial agents.
The interaction between EUCAST, national breakpoint committees, and European Union agencies is shown in Fig. 1.
FIG 1.
Interaction between EUCAST, national breakpoint committees, and European Union agencies.
National susceptibility testing committees.
In 2010, EUCAST decided to encourage all countries to form a national AST committee (NAC). In brief, the proposed remits of the NAC were to appoint a EUCAST General Committee member, support the EUCAST decision process, and introduce EUCAST guidelines nationally. Several have also formed websites where translations of EUCAST documents are posted. There is more information in the guidance, “How to organize and form a NAC” (9).
EUCAST subcommittees.
EUCAST has a series of standing and ad hoc subcommittees, including subcommittees on antifungal (AFST), antimycobacterial (AMST), and veterinarian susceptibility testing (VetCAST). The activities of these subcommittees are described in further detail on the EUCAST website (https://www.eucast.org/ast_of_fungi/; https://www.eucast.org/mycobacteria/; https://www.eucast.org/ast_of_veterinary_pathogens/).
THE EUCAST DECISION PROCESS
Decisions are formally taken by the EUCAST Steering Committee in collaboration with the national breakpoint committees. As more countries adopted EUCAST guidelines and formed NAC, a wider and more formal public consultation process was needed. This was instituted in 2015. Prior to final decisions, proposals are published on the EUCAST website (5). A period of 6 to 12 weeks is allowed for comments and counterproposals. These are discussed by the Steering Committee and will influence the final decision. All comments and counterproposals are published on the website with comments and rebuttals from the Steering Committee. Based on the discussion and feedback, a final decision is taken, or on occasion, a final decision is preceded by yet another period of consultation. Decisions are always based on consensus agreement between all Steering Committee members.
Breakpoint changes are always preceded by a period of consultation. The reason for the proposed change is outlined in a EUCAST Rationale Document (10), and EMA, companies, and the medical community are informed of the proposed change, directed to the consultation page, and encouraged to express an opinion. Since 2015, when the consultation process was introduced, 41 separate consultations have been undertaken.
EUCAST decisions are based on information on clinical results related to antimicrobial dosing and exposure, MIC values, and, when relevant, specific resistance mechanisms. Decisions will, furthermore, be based on pharmacokinetic and pharmacodynamic data, MIC distributions for target species with and without acquired resistance to the relevant agent, and the calibration of MIC versus inhibition zone diameters (11). Breakpoints are always set to avoid splitting distributions of wild-type organisms. This strategy minimizes the risk of miscategorization of AST results that can arise naturally through assay variation.
MIC DISTRIBUTIONS AND ECOFFS
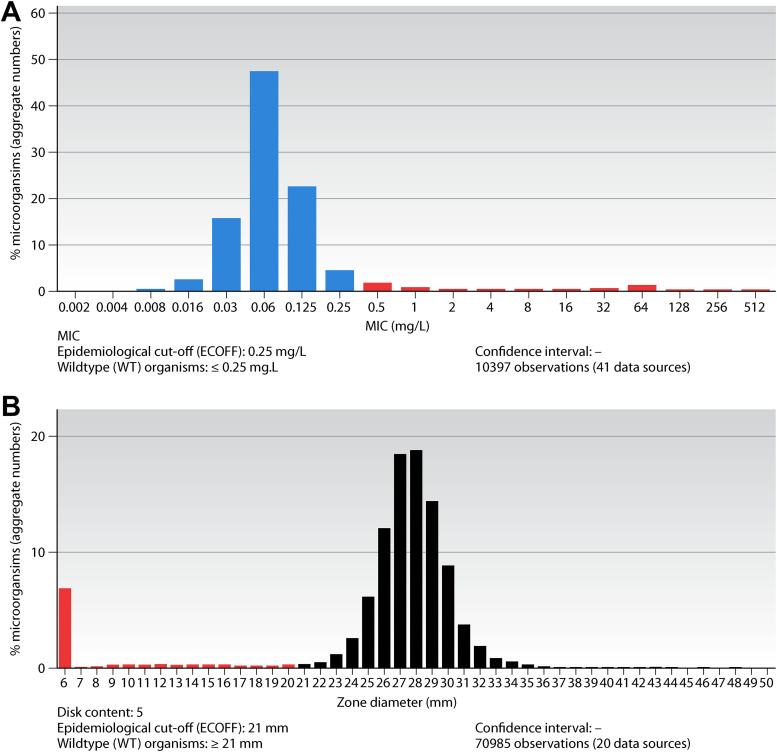
Aggregating MIC distributions for bacteria and fungi from many international investigators to form a reference wild-type MIC distribution is a unique feature for EUCAST and a key tool in breakpoint setting. It was invented and organized by EUCAST in 2001 and has, since then, grown with the contributions of scientists around the world. Today, there are more than 30,000 distributions in the database, and many aggregated distributions will consist of more than 50,000 MIC values. The database is curated by the EUCAST Subcommittee on MIC distributions and ECOFFs (12, 13). Currently, all distributions are systematically curated using the tools described in SOP10.1 (14). A typical example is shown in Fig. 2A and B. To define MIC distributions is important for determining clinical breakpoints: the ECOFF will distinguish between isolates with and without acquired resistance mechanisms, irrespective of whether these are clinically important or not.
FIG 2.
(A) MIC distributions and ECOFFs for Escherichia coli and cefotaxime. (B) Inhibition zone diameter distributions and ECOFFs for Escherichia coli and cefotaxime.
RECENT AND CURRENT BREAKPOINT REVIEWS
Having finalized the review and harmonization of national breakpoints for all existing agents, and having come to an agreement with EMA, the EUCAST has determined breakpoints for 16 new agents as part of the EMA registration process. Several more were handled by EUCAST but failed to obtain a final license. In recent years, EUCAST has dealt with several new beta-lactam-beta-lactamase inhibitor (BLBLI) combinations (ceftolozane-tazobactam, ceftazidime-avibactam, meropenem-vaborbactam, imipenem-relebactam) and cefiderocol. For many novel agents, the indication is very specific and often limited to “the treatment of infections with multiresistant microorganisms where no other therapies are available.” The emergence of the new agents has revolutionized the potential treatment of multidrug- and extensively drug-resistant Gram-negative bacilli, for which previous therapies usually consisted of combination therapy with agents such as polymyxins, carbapenems (despite low-level resistance), glycylcyclines, and even rifampin. More BLBLI combinations are in the pipeline, and some of them have been given a preliminary assessment by EUCAST, although they are still in development (15). For all BLBLI combinations, EUCAST advocates MIC testing with the inhibitor in a fixed concentration. For cefiderocol, the testing conditions are of critical importance and include iron depletion of the broth, followed by addition of a standardized amount of iron (16).
With regard to agents particularly active against Gram-positive pathogens, EUCAST has, in recent years, set breakpoints for tedizolid, lefamulin, and delafloxacin. Tedizolid is closely related to linezolid and is indicated for skin and skin structure infections, with breakpoints being set for staphylococci and viridans group streptococci. Lefamulin is the first systemic pleuromutilin for human use and is indicated for community-acquired pneumonia, with breakpoints being set for Streptococcus pneumoniae and Staphylococcus aureus. Finally, delafloxacin is licensed for skin and skin structure infections and community-acquired pneumonia, with breakpoints being set for staphylococci, pneumococci, beta-hemolytic streptococci, Streptococcus anginosus group, Haemophilus influenzae, and Escherichia coli.
Several important breakpoint revisions have been carried out over the last 3 years. One of the most substantial changes was the revision of fluoroquinolones and aminoglycoside breakpoints. The introduction of breakpoints in brackets for systemic aminoglycoside breakpoints, i.e., breakpoints with a caveat, was introduced following a long consultation process. It was decided that aminoglycosides can still be reported as susceptible if the focus of infection is in the urinary tract, but in other foci, it should be reported as susceptible only if another active therapy is given in combination. The change was based on a long process and was driven largely by lack of clinical evidence for using aminoglycosides in monotherapy of infections not emanating from the urinary tract. Moreover, pharmacokinetic/pharmacodynamic (PK/PD) data suggested that drug exposure is insufficient even with the highest dosing regimens in current use (17).
Tigecycline breakpoints were also recently revised. The new breakpoints were generally lowered due to insufficient drug exposure to reach the previously set breakpoints. Also, the fact that the I group in the EUCAST system now means “susceptible, increased exposure” led to a need to revisit the R breakpoint, as there is no high-exposure dosing regimen. This breakpoint change led to the removal of tigecycline breakpoints for other Enterobacterales than E. coli and Citrobacter koseri since these would require clinicians to use off-label dosing regimens. This has been explained in a guidance document for use of tigecycline in Enterobacterales beyond E. coli. During the same time, piperacillin-tazobactam breakpoints for Enterobacterales were revised. Based on largely clinical outcome data from the MERINO trial (18), it was decided that the R breakpoint was too high and that 4 g ×4 is an appropriate dosing regimen for many systemic infections, corresponding to an S breakpoint of 8 mg/L. The exceptions are urinary tract infections and intra-abdominal infections, where it seems as if 4 g ×3 can be sufficient to treat the entire S group. Another old agent receiving EUCAST breakpoints for the first time was temocillin, which is licensed in some European countries. Temocillin is a ticarcillin derivative and has activity against Enterobacterales. The breakpoint process led to the wild type of some Enterobacterales species being reported in the I group, signaling that the drug can be used in high exposure (2 g ×3) to treat Enterobacterales. Finally, EUCAST also revised breakpoints for oral fosfomycin, resulting in E. coli becoming the only target species for therapy and decreasing the breakpoint from 32 to 8 mg/L. This revision was driven by both clinical data and PK/PD data, which were not previously available (19).
Many species that have not previously had breakpoints have received breakpoints over the last few years. Most recently, Achromobacter xylosoxidans and Bacillus species were introduced in the breakpoint tables in 2021 and, prior to that, Burkholderia pseudomallei in 2020. Major work was undertaken in the EUCAST Development Laboratory to generate MIC and disk diffusion correlates, and additionally, EUCAST scrutinized the literature for clinical evidence supporting agents to be included in the breakpoint tables. Currently, work is ongoing with Nocardia species, Corynebacterium diphtheriae, and Vibrio species.
Another important task in recent years was the revision of meningitis breakpoints. It was deemed necessary to revise these breakpoints because an I group is not logical for meningitis, given the fact that meningitis dosing is already the highest possible. Finally, EUCAST set breakpoints for oral dosing of amoxicillin and amoxicillin-clavulanic acid in S. pneumoniae and H. influenzae and also defined breakpoints for H. influenzae versus piperacillin-tazobactam.
THE REVISION OF THE DEFINITIONS OF SUSCEPTIBILITY CATEGORIES
A major change has been the introduction of new EUCAST definitions of the S, I, and R categories. This change, following several consultations (5), has, in effect, brought us from having two categories indicating resistance (I and R), often lumped together as “nonsusceptible,” and one susceptible category (S) to having two susceptible categories (S and I) and one resistant category (R). These are the new definitions where the relationship between exposure and effect (exposure being a function of the mode of administration, the dose, dosing interval, infusion time, as well as distribution and excretion of the antimicrobial agent at the site of infection) is emphasized.
-
•
S, Susceptible, standard dosing regimen. A microorganism is categorized as “susceptible, standard dosing regimen” when there is a high likelihood of therapeutic success using a standard dosing regimen of the agent.
-
•
I, Susceptible, increased exposure. A microorganism is categorized as “susceptible, increased exposure” when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or by its concentration at the site of infection.
-
•
R, Resistant. A microorganism is categorized as “resistant” when there is a high likelihood of therapeutic failure even when there is increased exposure.
With this change, the part of the old definition indicating uncertainty was removed from the definition. Instead, a number of difficult situations were identified. EUCAST systematically avoids setting breakpoints that divide wild-type distributions of target organisms since this would invite poor reproducibility of results. For other forms of uncertainty, EUCAST introduced the concept “an area of technical uncertainty (ATU).” This provides information to the laboratory about especially difficult areas, mostly where there is overlap between wild-type and non-wild-type isolates. It is defined by species (or group of species) and agent, and it simply warns staff about a predictable difficulty. The laboratory takes responsibility for resolving ATU results prior to release of the results to the clinician.
THE EUCAST DEVELOPMENT LABORATORY
The original intention of EUCAST was that harmonized and new breakpoints would be integrated into the existing national guidelines for AST. However, in 2008, it was realized that for European harmonization to occur, it was necessary to create a common method to host the EUCAST MIC breakpoints. The task fell on the public clinical laboratory of the chairman at the time, situated in Växjö, Sweden. The regional public health administration agreed to host and help administer a EUCAST Development Laboratory (EDL). Following staff appointments and in agreement with the EUCAST Steering Committee, a Mueller-Hinton agar-based disk diffusion method was created. The first complete EUCAST breakpoint table, made available January 2010, contained both MIC and disk diffusion correlates. For transparency, it was decided to always make calibrations between MIC values and disk diffusion results publicly available on the EUCAST website (11). Since then, the EDL has developed and/or administered many projects designed to facilitate the use of EUCAST clinical breakpoints, including developing methodology and calibrating disk diffusion criteria against broth microdilution MIC values, developing and validating methods for screening for and excluding resistance, defining background material needed for decisions on new agents and species not yet in the breakpoint tables, developing methods for determining disk contents for new agents, developing and maintaining EUCAST methodology for rapid AST directly from positive blood cultures in 4 to 8 hours, developing quality control (QC) criteria, educational activities within AST (including observerships and webinars), and investigating complaints related to AST materials and issuing warnings (20) against poorly functioning materials and tests.
Projects in 2021 and 2022 are organizing aggregated MIC and zone diameter distributions for C. diphtheriae, Corynebacterium ulcerans, Vibrio cholerae, and 4 other Vibrio species pathogenic to humans, describing and field testing the EUCAST disk diffusion methodology recently developed for anaerobic bacteria, and extending the range of agents for rapid AST directly from blood culture bottles. The EDL is financed by the ESCMID Förderverein and from research grants applied for by the staff of EDL.
EUCAST INTERNATIONAL UPTAKE
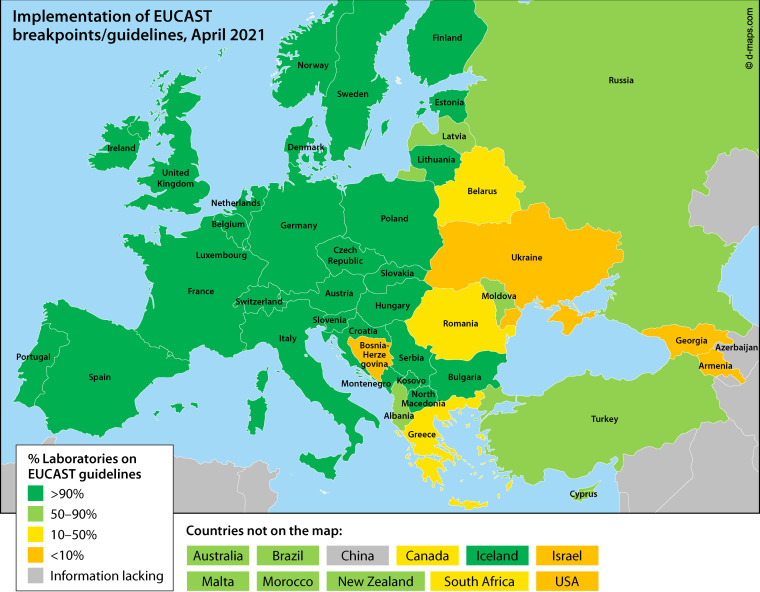
European countries gradually adopted the harmonized European guidelines. The recognition of EUCAST afforded by EMA and ECDC, and the adoption of ECOFFs for AMR surveillance by the European Food Safety Agency (EFSA), stimulated laboratories to make the transition to EUCAST methods and breakpoints. Gradually, also, countries outside Europe (Australia, New Zealand, South Africa, Brazil, Tunisia, China, and others) asked to join, and opportunities for discussion and influence were created by offering seats on both the EUCAST General Committee and on the Steering Committee. The current situation is described in Fig. 3.
FIG 3.
International uptake of EUCAST guidelines.
THE EUCAST WEBSITES
Both EUCAST websites, the general website (4) and the website for aggregating and displaying MIC and zone diameter distributions (13), are publicly and freely available. All EUCAST guidelines, methods in AST, including rapid AST directly from positive blood culture bottles, educational material, MIC and zone diameter distributions, raw data generated as part of EUCAST development programs, scientific publications, expert rules, intrinsic resistances, detection of resistance mechanisms, and much more, are available and updated when relevant. By leaving a valid email address for the newsletter function, a monthly summary of news is distributed (21).
The MIC and zone diameter distribution website, organized and cross-tabulated according to agent and species, presents MIC data and inhibition zone diameter data as shown for cefotaxime and E. coli in Fig. 2A and B.
COLLABORATION WITH OTHER ORGANIZATIONS
The close historical connection to ESCMID is fundamental to EUCAST. ESCMID organizes colleagues in clinical microbiology and infectious diseases across Europe and many other parts of the world. The scientific basis of ESCMID is important to EUCAST. ESCMID organizes thematic study groups, and EUCAST routinely interacts with several of them, both as part of the EUCAST consultation process but also in the development of guidelines and the arrangement of postgraduate educational courses and proposing sessions for scientific conferences, most notably the European Congress on Clinical Microbiology and Infectious Diseases (ECCMID). EUCAST also collaborates with other scientific societies in Europe. Together with the European Society of Pediatric Infectious Diseases (ESPID), we currently strive to clarify how dosing in adults, intimately related to EUCAST breakpoints since 2020, may be aligned to dosing and administration in children. There is also a long tradition for collaboration between EUCAST and CLSI on a range of activities.
CONCLUSIONS AND FUTURE PERSPECTIVES
EUCAST has established itself firmly in international clinical microbiology and infectious diseases. All countries in Europe and many countries in other parts of the world have adopted EUCAST and implemented EUCAST guidelines in everyday microbiology and patient care. The decision process allows colleagues and organizations to influence final decisions. Because of the relationship between EUCAST and European agencies such as EMA, ECDC, and EFSA, and between EUCAST and ESCMID, the professional and scientific integrity of EUCAST is fully accepted. All output from EUCAST, including raw data used in nonconfidential processes, is made freely available, and EUCAST invites public discussions in real life and online. Many colleagues have taken the opportunity offered to be observers during EUCAST Steering Committee meetings or for many days in the EUCAST Development Laboratory. All clinical breakpoints need iterative review and sometimes revision. New resistance mechanisms impact clinical outcome. New species need to be included in the system, and new agents need breakpoints as part of the licensing process.
TRANSPARENCY DECLARATION
On occasion, Steering Committee members have had reason to recuse themselves from a decision; this is minuted by the secretary. It is most often related to the early development phase of a new agent whereby the expertise of a Steering Committee member has been sought by those responsible.
ACKNOWLEDGMENTS
Other EUCAST Steering Committee members 2021 include Sören Gatermann (Germany), Gérard Lina (France), Christoffer Lindemann (Norway), Alasdair MacGowan (United Kingdom), Joseph Meletiadis (Greece), Shampa Das (United Kingdom), Gian Maria Rossolini (Italy), and Jorge Sampaio (Brazil).
Contributor Information
Christian G. Giske, Email: christian.giske@sll.se.
Romney M. Humphries, Vanderbilt University Medical Center
REFERENCES
- 1.Kahlmeter G, Brown DFJ, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, Rodloff A, Steinbakk M, Urbaskova P, Vatopoulos A. 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J Antimicrob Chemother 52:145–148. 10.1093/jac/dkg312. [DOI] [PubMed] [Google Scholar]
- 2.Kahlmeter G. 2015. The 2014 Garrod Lecture: EUCAST – are we heading towards international agreement? J Antimicrob Chemother 70:2427–2439. 10.1093/jac/dkv145. [DOI] [PubMed] [Google Scholar]
- 3.Brown D, Cantón R, Dubreuil L, Gatermann S, Giske C, MacGowan A, Martínez-Martínez L, Mouton J, Skov R, Steinbakk M, Walton C, Heuer O, Struelens MJ, Diaz Högberg L, Kahlmeter G. 2015. Widespread implementation of EUCAST breakpoints for antibacterial susceptibility testing in Europe. Eurosurveillance 20:21008. 10.2807/1560-7917.ES2015.20.2.21008. [DOI] [PubMed] [Google Scholar]
- 4.European Committee on Antimicrobial Susceptibility Testing. EUCAST general website. www.eucast.org.
- 5.European Committee on Antimicrobial Susceptibility Testing. EUCAST public consultations. https://www.eucast.org/publications_and_documents/consultations/.
- 6.European Committee on Antimicrobial Susceptibility Testing. EUCAST clinical breakpoint table. https://www.eucast.org/clinical_breakpoints/.
- 7.European Committee on Antimicrobial Susceptibility Testing. 2007. SOP between EMA and EUCAST describing the role of EUCAST in determining breakpoints. https://www.eucast.org/fileadmin/src/media/PDFs/4ESCMID_Library/3Publications/EUCAST_Documents/Other_Documents/EMEA_CHMP_EUCAST_SOP_on_Harmonising_European_Breakpoints_2007.pdf.
- 8.European Committee on Antimicrobial Susceptibility Testing. 2016. EUCAST statutes. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_Statutes_20160411_approved_by_General_Committee.pdf.
- 9.European Committee on Antimicrobial Susceptibility Testing. 2019. The organization of national antimicrobial susceptibility testing committees (NAC). https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Organisation_and_NACs/NACs_2019__1_.docx.
- 10.European Committee on Antimicrobial Susceptibility Testing. 2021. EUCAST Rationale Documents. https://www.eucast.org/publications_and_documents/rd/.
- 11.European Committee on Antimicrobial Susceptibility Testing. 2021. Calibration of zone diameter breakpoints against MIC values. https://www.eucast.org/ast_of_bacteria/calibration_and_validation/.
- 12.European Committee on Antimicrobial Susceptibility Testing. 2021. The EUCAST Subcommittee on MIC distributions and ECOFFs. https://www.eucast.org/organization/subcommittees/mic_distributions_and_ecoffs/.
- 13.European Committee on Antimicrobial Susceptibility Testing. 2021. Display of MIC and inhibition zone diameter distributions and ECOFFs. https://mic.eucast.org. Accessed 2 May 2021.
- 14.European Committee on Antimicrobial Susceptibility Testing. 2019. MIC distributions and the setting of epidemiological cutoff (ECOFF) values. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/2021/EUCAST_SOP_10.2_MIC_distributions_and_epidemiological_cut-off_value__ECOFF__setting_20211202.pdf.
- 15.Yahav D, Giske CG, Grāmatniece A, Abodakpi H, Tam VH, Leibovici L. 2020. New β-lactam-β-lactamase inhibitor combinations. Clin Microbiol Rev 34:e00115-20. 10.1128/CMR.00115-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Committee on Antimicrobial Susceptibility Testing. 2020. Guidance document on broth microdilution testing of cefiderocol. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Guidance_documents/Cefiderocol_MIC_testing_EUCAST_guidance_document_201217.pdf.
- 17.Bhavnani SM, Onufrak NJ, Hammel JP, Andes DR, Bradley JS, Flamm RK, Ambrose PG, Jones RN. 2018. Re-appraisal of aminoglycoside (AG) susceptibility testing breakpoints based on the application of pharmacokinetics-pharmacodynamics (PK-PD) and contemporary microbiology surveillance data. Poster No. 2562. IDWeek, San Francisco, CA, 3 to 7 October 2018. [Google Scholar]
- 18.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). 2018. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 320:984–994. 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Committee on Antimicrobial Susceptibility Testing. 2020. EUCAST fosfomycin consultation document. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/2020/EUCAST_General_Consultation_on_Fosfomycon_trometamol_2020.pdf.
- 20.European Committee on Antimicrobial Susceptibility Testing. 2021. EUCAST warnings. https://www.eucast.org/ast_of_bacteria/warnings/.
- 21.European Committee on Antimicrobial Susceptibility Testing. 2021. EUCAST newsletter signup. https://www.eucast.org/eucast_news/.