ABSTRACT
Within 8 weeks of primary Clostridioides difficile infection (CDI), as many as 30% of patients develop recurrent disease with the associated risks of multiple relapses, morbidity, and economic burden. There are no clear clinical correlates or validated biomarkers that can predict recurrence during primary infection. This study demonstrated the potential of a simple test for identifying hospitalized CDI patients at low risk for disease recurrence. Forty-six hospitalized CDI patients were enrolled at Emory University Hospitals. Samples of serum and a novel matrix from circulating plasmablasts called “medium-enriched for newly synthesized antibodies” (MENSA) were collected during weeks 1, 2, and 4. Antibodies specific for 10 C. difficile antigens were measured in each sample. Among the 46 C. difficile-infected patients, 9 (19.5%) experienced recurrence within 8 weeks of primary infection. Among the 37 nonrecurrent patients, 23 (62%; 23/37) had anti-C. difficile MENSA antibodies specific for any of the three toxin antigens: TcdB-CROP, TcdBvir-CROP, and/or CDTb. Positive MENSA responses occurred early (within the first 12 days post-symptom onset), including six patients who never seroconverted. A similar trend was observed in serum responses, but they peaked later and identified fewer patients (51%; 19/37). In contrast, none (0%; 0/9) of the patients who subsequently recurred after hospitalization produced antibodies specific for any of the three C. difficile toxin antigens. Thus, patients with a negative early MENSA response against all three C. difficile toxin antigens had a 19-fold greater relative risk of recurrence. MENSA and serum levels of immunoglobulin A (IgA) and/or IgG antibodies for three C. difficile toxins have prognostic potential. These immunoassays measure nascent immune responses that reduce the likelihood of recurrence thereby providing a biomarker of protection from recurrent CDI. Patients who are positive by this immunoassay are unlikely to suffer a recurrence. Early identification of patients at risk for recurrence by negative MENSA creates opportunities for targeted prophylactic strategies that can reduce the incidence, cost, and morbidity due to recurrent CDI.
KEYWORDS: Clostridioides difficile, Clostridium difficile, MENSA, antibodies, immunoassays, immunoglobulins, recurrence, serum, toxins
INTRODUCTION
Clostridioides difficile infection (CDI) is the most common cause of hospital-acquired infectious diarrhea with an estimated annual incidence greater than 300,000 cases in the United States (1). The primary challenge in treating CDI is recurrence (rCDI) which occurs in as many as 20 to 35% of patients within 60 days of completing treatment. Clinical risk factors for recurrence are weak predictors. For example, none of the most frequently cited risk factors, including previous hospitalization, underlying disease, age, and prior use of antibiotics has an associated relative risk greater than 2.0, not to mention that those factors apply to most relevant hospitalized patients (2–4). Furthermore, the risk of rCDI increases to 33 to 65% in patients with a history of prior recurrence. Remarkably, rCDI is responsible for as much as 50% of health care costs associated with CDI (5–7). This situation has been further compounded by the emergence of more virulent strains of Clostridioides difficile (CD) in the last 2 decades that have increased both the severity of primary infections and the frequency of recurrence (8–10).
Two approaches have been shown to reduce CDI recurrence rates. Bezlotoxumab (commercially, Zinplava), a monoclonal antibody therapy directed against C. difficile toxin B launched in 2017, provided 40% protection against recurrence in patients at high risk and did not appear to interfere with repopulation of the gut microbiome (5, 11–16). The second is fecal microbiota transplantation (FMT) wherein the antibiotic-depleted gut flora is repopulated with the microbiome harvested from healthy donors. Used primarily for patients who have suffered multiple recurrences, FMT has proven remarkably successful (17–27). However, these strategies are reserved for high-risk primary CDI patients and those with multiple recurrences. Cost precludes their broader use in most cases of primary CDI because most patients do not recur after the first episode. Therefore, a diagnostic test that can identify patients at risk for recurrent disease after the first episode would facilitate the prophylactic use of these therapies.
We have recently developed a novel MENSA (medium-enriched for newly synthesized antibodies)-based immunoassay, in which specific antibodies produced by recently activated antibody-secreting cells (ASC) are measured in response to primary CDI (28). During an acute infection, activated B cells in lymph nodes proliferate and differentiate into ASC that appear in the circulation for as long as the infection persists. In the case of CDI, most ASC produce C. difficile-specific antibodies before they undergo apoptosis (29). A small fraction of ASC migrates to secondary lymphoid organs and the bone marrow to become long-lived plasma cells (30). Although the role of C. difficile-specific serum antibodies has been debatable in some studies (31–34), we show ASC in MENSA offers immune correlates of protection. In all, C. difficile-specific antibodies made by ASC in the MENSA serve as biomarkers of active immune responses that identify patients at low risk for recurrence after primary CDI. Importantly, the timing of the MENSA response is crucial, as the appearance of ASCs can precede seroconversion by a few days and provides earlier identification of patients at low risk for recurrence (29).
In this study, we examined the MENSA and serum responses to 10 C. difficile antigens in 46 primary CDI patients and showed that IgA and IgG responses to three of those antigens provide a simple diagnostic tool for predicting nonrecurrence. Patients who test positive using our three-antigen MENSA immunoassay were less likely to suffer rCDI after their primary infections, whereas patients who tested negative were at a 19-fold greater risk for recurrence.
MATERIALS AND METHODS
Study approval.
The Emory and Dekalb Institutional Review Boards and the Grady Research Oversight Committee approved all protocols and procedures. Written informed consent was obtained from each patient before inclusion in the study.
Enrollment of CDI patients and controls.
CDI patients and control subjects were recruited at Emory University and Dekalb Medical Center (now Emory Decatur Hospital) from 2015 to 2017. A total of 46 patients with CDI, which was confirmed by PCR and/or enzyme-linked immunosorbent assay (ELISA) (Xpert C. difficile; C. DIFF QUIK CHEK COMPLETE, Alere), were enrolled. Whole blood samples were collected at one-to-three time points (draw 1:1 to 5 days postconfirmation [DPC], draw 2:6 to 12 DPC, draw 3:21 to 60 DPC). A patient was considered recurrent if he/she had follow-up diarrhea that was positive by PCR or QUIK CHEK within 60 days post symptom onset (DPSO). Demographic and clinical data were collected on multiple parameters, including those associated with an elevated risk of recurrence (Tables 1 and 2).
TABLE 1.
Demographics of control and C. difficile-infected subjects
| Subject group | Age |
Racea |
Sex |
|||
|---|---|---|---|---|---|---|
| <50 n (%) |
≥50 n (%) |
Black n (%) |
White n (%) |
Female n (%) |
Male n (%) |
|
| C0 controls | ||||||
| Healthy subjects (n = 38) | 31 (82) | 7 (18) | 19 (50) | 10 (26) | 26 (68) | 12 (32) |
| Healthcare workers (n = 26) | 22 (85) | 4 (15) | 19 (73) | 6 (23) | 21 (81) | 5 (19) |
| Total (n = 64) | 53 (83) | 11 (17) | 38 (59) | 16 (25) | 47 (73) | 17 (27) |
| CDI patients | ||||||
| Nonrecurrent (n = 37) | 14 (38) | 23 (62) | 23 (62) | 12 (32) | 22 (59) | 15 (41) |
| Recurrent (n = 9) | 2 (22) | 7 (78) | 5 (56) | 4 (44) | 6 (67) | 3 (33) |
| Total (n = 46) | 16 (35) | 30 (65) | 28 (61) | 16 (35) | 28 (61) | 18 (39) |
| C0 controls versus CDI patients | P < 0.0001 | P = 0.52 | P = 0.21 | |||
| Nonrecurrent versus recurrent | P = 0.46 | P = 0.70 | P = 1.0 | |||
Race percentages are <100% due to small subpopulation of other races not listed.
TABLE 2.
Relative risk measurements of risk factors for recurrence
| Characteristic | Measure | Nonrecurrent CDI (n = 37) | Recurrent CDI (n = 9) | Relative risk (P value) |
|---|---|---|---|---|
| Age (yrs) | avg ± SD | 53 ± 13 | 57 ± 13 | 2.06 (0.33) |
| >50 | 23 (62%) | 6 (78%) | ||
| <50 | 15 (38%) | 3 (22%) | ||
| Race | White/Other | 14 (32%) | 4 (44%) | 1.24 (0.72) |
| Black | 23 (62%) | 5 (56%) | ||
| Sex | Female | 22 (59%) | 6 (67%) | 1.30 (0.69) |
| Male | 15 (41%) | 3 (33%) | ||
| Peak WBC count (cells × 103/μL) | avg ± SD | 13.4 ± 7.2 | 17.0 ± 16.2 | 1.14 (0.83) |
| >13.4 | 15 (43%) | 4 (44%) | ||
| <13.4 | 22 (57%) | 5 (56%) | ||
| Peak creatinine (mg/dL) | avg ± SD | 3.0 ± 3.6 | 4.2 ± 5.6 | 1.14 (0.83) |
| >3.0 | 11 (30%) | 3 (33%) | ||
| 3.0 | 26 (70%) | 6 (67%) | ||
| Peak temp (°C) | avg ± SD | 37.6 ± 0.87 | 37.19 ± 1.24 | 1.14 (0.83) |
| >37.6 | 15 (41%) | 4 (44%) | ||
| <37.6 | 22 (59%) | 5 (56%) | ||
| PPI/H2A use 8 wks prior to admission | Yes | 14 (38%) | 5 (56%) | 1.78 (0.34) |
| No | 23 (62%) | 4 (44%) | ||
| Antibiotic use within 8 wks prior to admission | Yes | 18 (49%) | 7 (78%) | 2.94 (0.15) |
| No | 19 (51%) | 2 (22%) | ||
| Antibiotic use for non-CDI after CDI diagnosis | No | 22 (59%) | 7 (78%) | 2.05 (0.36) |
| Yes | 15 (41%) | 2 (22%) | ||
| Hospitalized 12 wks Prior to Admission | Yes | 17 (46%) | 6 (67%) | 2.00 (0.28) |
| No | 20 (54%) | 3 (33%) | ||
| History of prior CDI | Yes | 4 (11%) | 1 (11%) | 1.03 (0.98) |
| No | 33 (89%) | 8 (89%) | ||
| Antibiotic used for CDI treatment | Metronidazole | 16 (43%) | 5 (56%) | 1.50 (0.51) |
| Other | 21 (57%) | 4 (44%) | ||
| Serum for C. difficile toxins (MFI-B) | Negative (<C0) | 18 (49%) | 9 (100%) | 13.60 (0.07) |
| Positive (≥C0) | 19 (51%) | 0 (0%) | ||
| MENSA for C. difficile toxins (MFI-B) | Negative (<C0) | 14 (38%) | 9 (100%) | 19.00 (0.04) |
| Positive (≥C0) | 23 (62%) | 0 (0%) |
For control subjects, blood was collected at a single time point from 38 healthy subjects and 26 health care workers from Emory Hospitals. Samples were transported at room temperature (RT) to the MicroB-plex laboratory and processed within 24 h.
MENSA generation.
Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation (800 × g; 25 min) using a lymphocyte separation medium (Corning). Five washes with RPMI 1640 containing l-glutamine (Corning) were performed to remove serum immunoglobulins (800 × g; 5 min), with lysis of residual erythrocytes after the second wash and cell counting after the fourth. Harvested PBMC were cultured at 106 cells/mL in R10 Medium (RPMI 1640, 10% FBS [Sigma], 1% antibiotic/antimycotic [Gibco]) on a 12-well sterile tissue culture plate for 24 h. After incubation, the cell suspension was centrifuged (800 × g; 5 min) and the supernatant (MENSA) was separated from the PBMC pellet, aliquoted, and stored at −80°C.
Serum generation.
One clot activator tube containing 2 to 4 mL of whole blood was collected and incubated at RT for at least 30 min. The clot was discarded, and the remaining supernatant was centrifuged (800 × g; 10 min), removed from the pellet, aliquoted, and stored at −80°C.
Selection and preparation of recombinant antigens.
The selection and design of C. difficile recombinant antigens are presented in detail (28). Briefly, antigens, or domains thereof, were selected based on known immunogenicity and/or pathogenicity. The antigen repertoire included the combined repetitive oligopeptide (CROP) and glucosyltransferase (GTD) domains of the secretory toxins TcdA and TcdB, the CROP domain of TcdB associated with hypervirulent C. difficile strains (TcdBvir-CROP), the A (CDTa) and B (CDTb) subunits of the secreted binary toxin CDT, flagellin (FliC), a major cell wall protein (Cwp84), and the enzyme glutamate dehydrogenase (GDH). Published sequences were examined for homology across C. difficile strains and truncated to remove potentially problematic regions such as signal peptides and cross-reactive domains. His- or GST-tags were added to the N or C terminus of each protein to facilitate purification and/or expression. Each protein was characterized by size (SDS-PAGE), purity, and immuno-reactivity with available monoclonal antibodies or pooled positive sera from infected patients.
Multiplex immunoassays for quantification of anti-C. difficile antibodies.
All samples were tested using a multiplex immunoassay protocol previously described using the Luminex MAGPIX (28). All MENSA samples were tested undiluted; serum samples were diluted 1:1000 in Assay Buffer (PBS, 1% BSA, pH 7.5).
Data analysis.
Median fluorescent intensity (MFI) of individual and/or combined detection antibodies (anti-IgA+anti-IgG+anti-IgM) was analyzed using xPONENT 4.2 software. The background fluorescence of the assay buffer or R10 medium (∼10 to 30 MFI) was subtracted from each serum or MENSA result, respectively, to obtain MFI minus background (MFI-B). Positive cutoff values (C0) for each antigen were determined as the average plus five standard deviations (mean + 5 SD) of the 64 control subjects in MENSA samples and the mean + 4 SD for serum samples. For assay evaluation, patient samples collected within 2 to 12 DPSO were selected. In patients with two blood samples during this interval, the one with the higher sum of MFI-B values against nine antigens was selected (TcdBvir-CROP was excluded due to its similarity with TcdB-CROP to avoid duplication). Analysis and data representation was undertaken using Microsoft Excel, GraphPad Prism, and JMP statistical analysis packages.
Statistics.
Determination of the relative risk of recurrence between those patients positive in their MENSA for anti-TcdB-CROP, anti-TcdBvir-CROP, and/or anti-CDTb and those who were antibody-negative was calculated using online statistical software for Relative Risk (MedCalc.org) and Fisher’s Exact test (MedCalc.net).
RESULTS
Human subjects.
With approval from Emory University, Dekalb (now Emory Decatur Hospital), and Grady Institutional Review Boards, patients with primary CDI were enrolled and followed for recurrence. The primary CDI population (n = 46) was similar in size to the control population (n = 64) and in the proportion of races (P = 0.52) and sexes (P = 0.21; Table 1) (28). The control population was younger than the CDI population (P < 0.0001), but there was no significant difference between younger and older healthy controls in serum and MENSA levels of antibodies specific for C. difficile antigens (MENSA P = 0.71; Serum P = 0.07, older subjects were slightly lower). More importantly, the recurrent CDI population (n = 9) and the nonrecurrent population (n = 37) were similar in terms of age (over 50 years; P = 0.46), race (∼60% black; ∼40% white; P = 0.70), and sex (∼60% female; P = 1.0). The nine recurrent patients experienced recurrence onset 16 to 51 days post symptom onset (DPSO; median = 28 DPSO; mean = 30 DPSO); or 4 to 39 days following the end of initial antibiotic therapy.
Sample collection.
Whole blood samples were drawn during acute infection (2 to 12 DPSO) and recovery/recurrence periods (13 to 60 DPSO) to determine the timing of MENSA and serum responses. Because CDI is defined as watery diarrhea three or more times per day for 2 days, most patients began treatment 2 DPSO. Antibiotic treatment typically lasted 10 days, so the clinically relevant interval for prediction of nonrecurrence was within 12 DPSO.
C. difficile antigens in MENSA and serum assays.
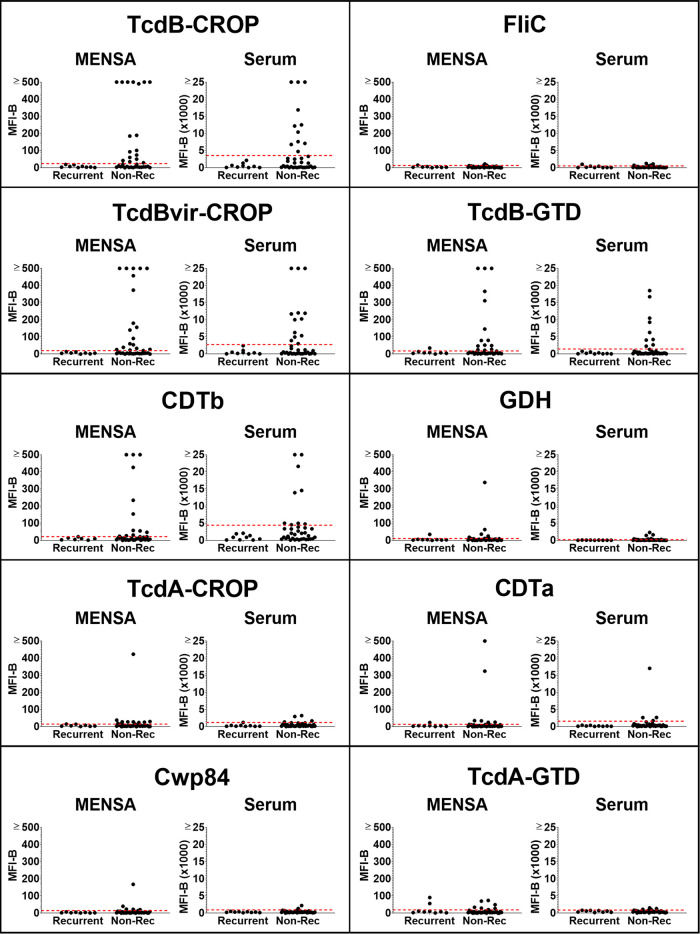
The combined antibody responses (IgA+IgG+IgM) specific for 10 C. difficile antigens were measured as previously described (28). Briefly, antigens or domains thereof were selected based on known immunogenicity and/or pathogenicity. The antigen repertoire included the combined repetitive oligopeptide (CROP) and glucosyltransferase (GTD) domains of the secretory toxins TcdA and TcdB, the CROP domain of TcdB associated with hypervirulent C. difficile strains (TcdBvir-CROP), the A (CDTa) and B (CDTb) subunits of the secreted binary toxin CDT, flagellin (FliC), a major cell wall protein (Cwp84), and the enzyme glutamate dehydrogenase (GDH). Among the 46 CDI patients who provided samples 2 to 12 DPSO, the nine recurrent patients made little or no anti-C. difficile antibody in their MENSA samples whereas most nonrecurrent patients generated new ASC indicated by their positive MENSA responses (Fig. 1). Twenty-six of the thirty-seven (70%) nonrecurrent patients demonstrated a positive MENSA response specific for at least one C. difficile antigen (Fig. 2). Among the 10 antigens, responses to four were prominent in terms of (i) magnitude (percentage of positive patients whose measured antibody levels were greater than five times the C0) and (ii) frequency of response (percentage of positive patients): TcdB-CROP, TcdBvir-CROP, CDTb, and TcdB-GTD. Specifically, among the 26 MENSA-positive, nonrecurrent patients, 17 were positive for TcdB-CROP, 19 for TcdBvir-CROP, 12 for CDTb, and 14 for TcdB-GTD.
FIG 1.
Antibody levels in MENSA and serum samples specific for 10 C. difficile antigens are found at greater frequencies and magnitudes in nonrecurrent CDI patients compared to those in patients who will experience recurrence. Levels of antibodies against each of the 10 C. difficile antigens in MENSA and serum samples 2 to 12 DPSO prepared from 37 nonrecurrent patients and 9 recurrent patients are compared. Measured values from each patient (black dots) against each antigen (listed at the top of each of the 10 segments in the figure) are presented separately with the MENSA responses on the left and serum responses on the right; measured responses on samples from recurrent patients are on the left in each panel and those from the nonrecurrent patients are on the right. C0 threshold values, based on control samples prepared from health care workers and healthy subjects, are represented by dashed red lines (28). MFI-B values represent the sum of IgA, IgG, and IgM antibodies reactive against each of the 10 antigens. Scale for MENSA samples ranges from 0 to 500 MFI with background subtracted (MFI-B; background MFI typically ∼10 for samples run undiluted); scale for serum samples runs from 0 to 25,000 MFI-B on samples diluted 1:1000.
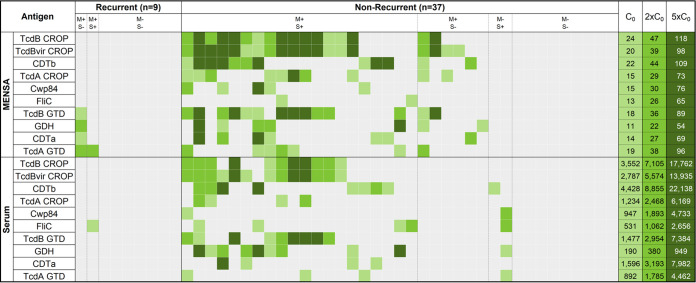
FIG 2.
Nonrecurrent patients had elevated levels of anti-C. difficile antibodies in MENSA and serum. Analysis of samples drawn 2 to 12 DPSO yielded this heat map showing the responses of each recurrent (n = 9) and nonrecurrent patient (n = 37). Each patient's responses to the 10 C. difficile antigens (listed on the left) are presented in a single vertical column, MENSA levels on the top and serum levels on the bottom. Recurrent and nonrecurrent patient groups are further subdivided into groups with antibodies present in (i) both MENSA and serum samples (M+S+); (ii) MENSA samples only (M+S−); (iii) serum samples only (M−S+); and (iv) neither MENSA nor serum (M−S−). MFI-B values less than C0 are presented in gray; the values for C0, 2×C0, 5×C0 are listed at the extreme right of the figure with their respective shade of green. C0 values were determined using samples prepared from the population of 64 healthy controls and health care workers in Table 1 (28).
Serum antibody responses from most patients were similar to those measured in MENSA samples collected on the same days (Fig. 1 and 2). Among the 37 nonrecurrent patients, serum antibody levels were elevated above the C0 in 59% (22/37) of those with ongoing C. difficile infections. Eleven were positive for TcdB-CROP, 13 for TcdBvir-CROP, 9 for CDTb, and 9 for TcdB-GTD. The other six antigens elicited weaker MENSA or serum antibody responses from smaller subsets of patients (Fig. 1 and 2).
Antigen selection.
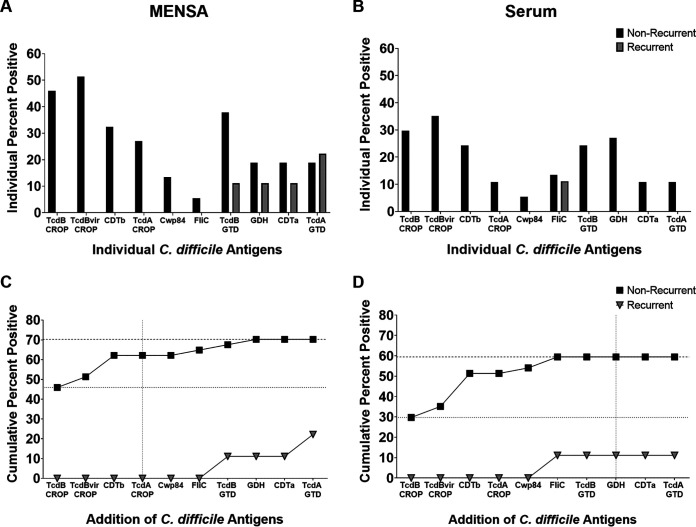
Three secreted toxin antigens (TcdB-CROP, TcdBvir-CROP, and CDTb) were the most effective for discriminating nonrecurrent from recurrent patients in both MENSA and serum (Fig. 3). Two of the recurrent patients had low antibody levels in their MENSA against other C. difficile antigens: TcdB-GTD, GDH, CDTa, and/or TcdA-GTD (Fig. 2 and 3A). One recurrent patient had a low serum response against FliC (Fig. 2 and 3B). With a single exception, nonrecurrent patients who secreted antibodies specific for TcdB-GTD also produced antibodies against the three toxin-derived antigens (TcdB-CROP, TcdBvir-CROP, and CDTb). In combination, MENSA responses to these three antigens positively identified the majority of MENSA-positive, nonrecurrent patients (23/26; 88%). Similarly, antibody responses in serum against the same three antigens identified the majority (19/22; 86%) of those positive for any C. difficile antigen. Therefore, we focused the analyses on the three secreted toxin antigens TcdB-CROP, TcdBvir-CROP, and CDTb in subsequent experiments.
FIG 3.
Selection of antigens by maximizing true positive responses and minimizing false-positive responses. (A) The percentage of nonrecurrent (black bars; n = 37) or recurrent (gray bars; n = 9) patients exhibiting a positive antibody response to each antigen in their MENSA sample. (B) The percentage of nonrecurrent (black bars; n = 37) or recurrent (gray bars; n = 9) patients exhibiting a positive antibody response to each antigen in their serum sample. (C) Combining MENSA antibody responses to certain antigens increases the prediction of true nonrecurrent positives while responses to other antigens increase the number of false-positives. Percent of patients positive for at least one antigen proceeding from left to right; nonrecurrent patients are presented in black squares; recurrent patients are presented in inverted gray triangles. (D) Similar analysis for the measurement of antibody levels in serum.
Positive MENSA was correlated with nonrecurrence and negative MENSA was correlated with an elevated risk of recurrence.
Fifty percent (n = 23) of the CDI patients had antibodies against at least one of the three toxin antigens. None of these patients experienced recurrence. Among the other 23 patients who were MENSA-negative for the three toxin antigens 2 to 12 DPSO, nine (9/23; 39%) suffered a recurrence in the following 6 weeks. Thus, patients with a negative MENSA response against all three C. difficile toxin antigens had a 19-fold greater relative risk of recurrence compared to MENSA-positive patients (P = 0.041; Fisher’s Exact Test P = 0.001). Comparable analysis of serum samples from the same patient population yielded similar but slightly less significant results with a relative risk of recurrence of 13.6 in the serum-negative population (P = 0.067; Fisher's Exact Test P = 0.006). In comparison, conventional clinical measures were weak predictors of recurrence with relative risk values in the range of 1.0 to 3.0 (Table 2).
Kinetics of MENSA and serum responses in CDI patients.
The kinetics of the humoral responses in infected patients is essential for MENSA or serum-based antibody levels to be used as clinical predictors of nonrecurrence. To assess the kinetics of antibody responses in MENSA compared to those in sera, percentages of nonrecurrent patients positive for the three selected toxin antigens in MENSA and serum samples were measured during four successive times intervals: 2 to 6, 7 to 12, 13 to 40, and 41 to 60 DPSO (Fig. 4A to D). Among the nonrecurrent patients, more were positive for the toxin antigens in their MENSA than in their serum between 2 and 6 DPSO (16/29, 55% in MENSA; 12/29, 41% in serum) and 7 to 12 DPSO (15/24, 63% in MENSA; 13/24, 54% in serum). After day 12, the number of patients with serum antibody levels match or surpass those who were positive in MENSA. At the later time points, the assays are not expected to be helpful because patients will already be experiencing recurrences. In addition, for two antigens (TcdB-CROP and TcdBvir-CROP), more patients remained MENSA-positive than seropositive as late as 40 DPSO. In contrast, the serum responses were better predictors of nonrecurrence after 40 DPSO although this result provides little clinical utility.
FIG 4.
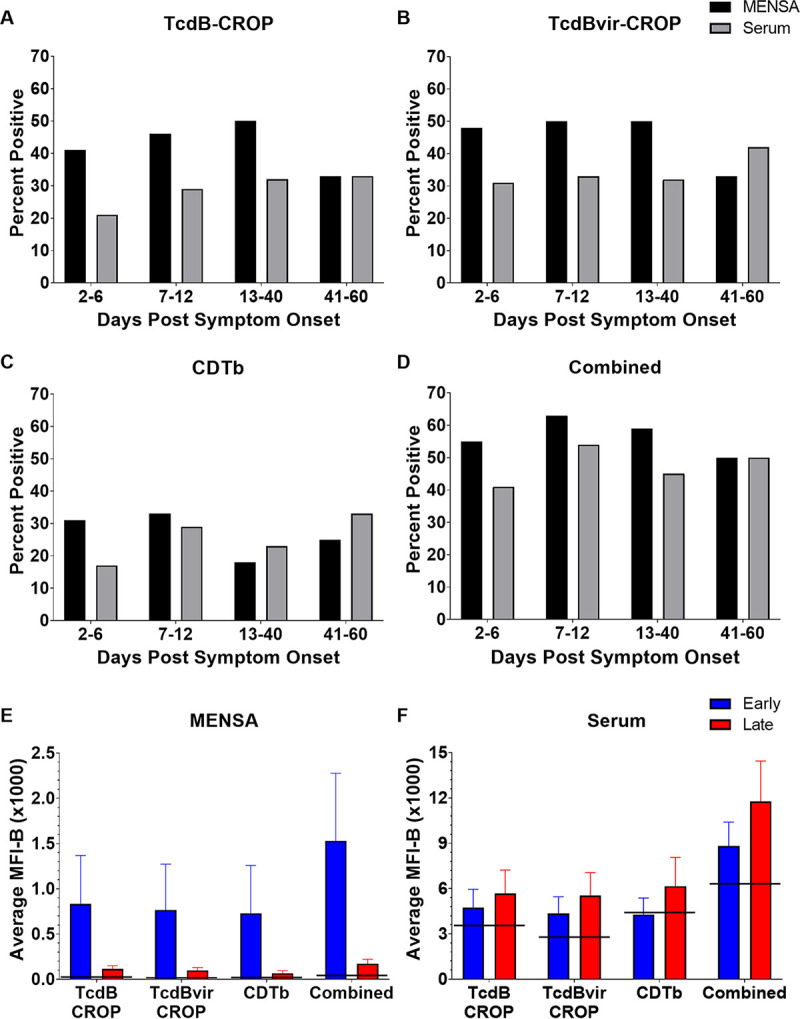
Kinetics of ASC-expressed anti-C. difficile antibodies reveal a peak MENSA response in nonrecurrent patients at 2–12 DPSO; serum responses are stronger after 12 DPSO. (A) The percentage of nonrecurrent patients who had positive anti-TcdB-CROP antibody levels in their MENSA (black) or serum (gray) during different time intervals post-symptom onset: days 2 to 6 (n = 29); days 7 to 12 (n = 24); days 13 to 40 (n = 22); and days 41 to 60 (n = 12). Please note the number of patients in each time interval varies due to the timing of sample acquisition. (B and C) The same analysis on the same population for antibody levels in MENSA and serum against the other toxin antigens: TcdBvir-CROP (B) and CDTb (C). (D) The percentage of nonrecurrent patients who had positive antibody levels for any of the three toxin antigens during each time interval. (E) Average levels (MFI-B) of antibodies specific for TcdB-CROP, TcdBvir-CROP, and CDTb in MENSA samples from nonrecurrent patients drawn during days 2 to 12 (blue) and days 13 to 60 (red). The combined MENSA value was calculated from CDTb plus the average of TcdB-CROP and TcdBvir-CROP values from the same nonrecurrent patients. (F) Similar analysis of measurements from serum samples. Horizontal black lines indicate the C0 values for each antigen in MENSA or serum.
The magnitude (MFI-B) of the MENSA and serum responses were compared early (2 to 12 DPSO) and late (13 to 60 DPSO) (Fig. 4E and F). C. difficile-specific antibody levels declined in the MENSA dramatically at the later time points (Fig. 4E). The trend in serum was that antibody levels rose in the first 12 days and remained elevated throughout the 60-day observation period (Fig. 4F).
High MENSA levels at early time points correlate with later serum antibody changes.
A critical element of MENSA-based diagnostics is that the cells responsible for the secreted antibodies (ASC) emerge into the circulation earlier than the effective rise in serum antibody levels. To examine this hypothesis, the late (13 to 60 DPSO) increases in serum anti-C. difficile toxin levels were plotted against the early (2 to 12 DPSO) MENSA antibody responses (Fig. 5). Among the 13 patients with positive MENSA and serum titers ((Nonrecurrent [NR]) M+S+; green dots), nine had an early MENSA response and significant elevation in serum levels later. Two MENSA-positive patients had high serum titers at the early time point and did not demonstrate a rise in serum antibody level (green dots, lower right quadrant). In contrast, the lower left quadrant is populated by patients who had a negative MENSA response and no subsequent increase in serum titers (M-S-). Significantly, this population included the recurrent patients for whom samples were available (Rec M-S-; inverted gray triangles).
FIG 5.
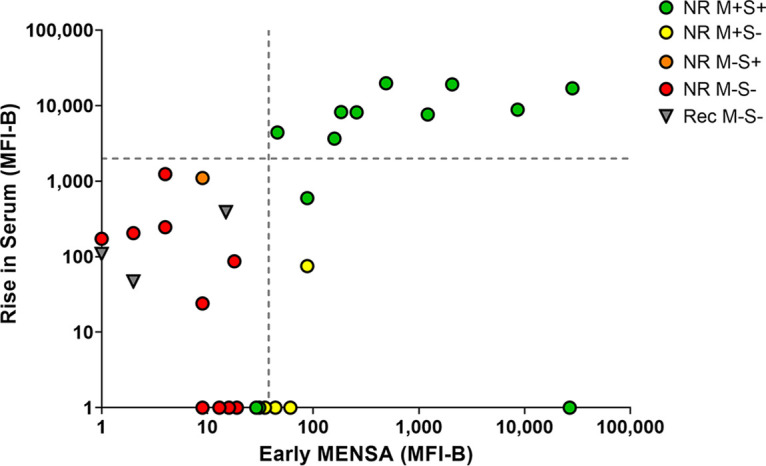
Early MENSA positivity predicts later serum antibody increases. The sum of the MFI-B values for CDTb plus the average of those for TcdB-CROP and TcdBvir-CROP was calculated for each patient at each time point. The rise in serum antibodies specific for the three toxin antigens between the first blood draw (2 to 12 DPSO) and a later blood draw (13 to 60 DPSO) is presented as a function of the MENSA value at the earlier time point. Patients were identified as MENSA and/or serum positive according to Fig. 2. Green circles indicate nonrecurrent patients who were positive in their MENSA and serum (NR M+S+); red circles indicate nonrecurrent patients who were negative early in their MENSA and never seroconverted (NR M−S−); inverted gray triangles represent recurrent patients (rec M−S−). The single orange circle represents the only patient in this cohort who was marginally seropositive and MENSA negative (NR M−S+); the yellow circles represent patients who were MENSA positive early but never seroconverted (NR M+S-). The sums of CDTb plus the average of TcdB-CROP and TcdBvir-CROP MFI-B values were calculated for each of the 64 control subjects; the vertical dashed line represents the average plus five standard deviations of the control population. The horizontal dashed line indicates a 2,000 MFI increase in serum levels between the early (2 to 12 DPSO) and the late (13 to 60 DPSO) draws. Due to the log scale of this graph, patients with negative or zero values were placed directly on the x-axis or y-axis for data inclusion.
Anti-C. difficile antibodies in MENSA are predominantly IgA and IgG.
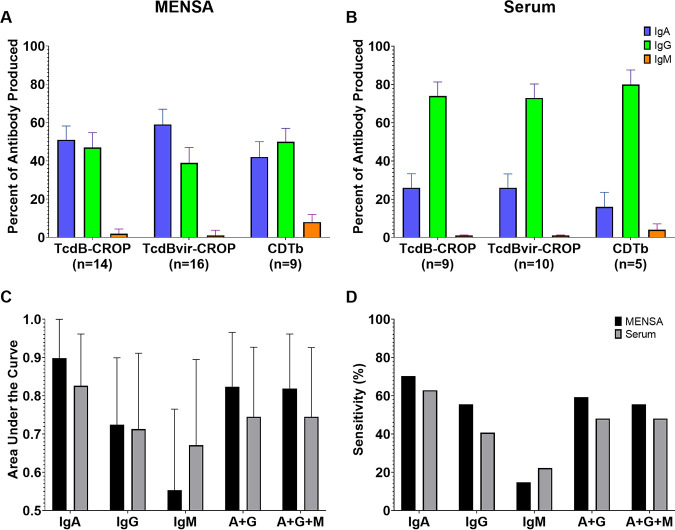
To examine the isotypes of antibodies produced among CDI responses, the levels of Ig against each of the three C. difficile antigens were detected with secondary antibodies specific for IgA, IgG, or IgM. In serum, IgG predominates (>70% of total Ig). In contrast, IgA and IgG were similarly abundant for the three toxin antigens in MENSA (39 to 59%), with IgM contributing less than 10% (Fig. 6A and B).
FIG 6.
Elevated MENSA and serum IgA, IgG, or IgM levels of anti-TcdB-CROP, anti-TcdBvir-CROP, and anti-CDTb. (A) Bar graph showing the relative abundance of the different immunoglobulin isotypes in the MENSA response to each antigen. Each antigen is indicated on the x-axis; the number beneath the antigen indicates the number of patients who were positive for that antigen in total Ig and for whom sufficient samples remained for analysis. The y-axis (percentage of antibody produced) indicates the average percentage of the total specific antibody response in each positive patient sample that was of the indicated Ig isotype: IgA (blue); IgG (green); IgM (orange). (B) The same analysis was applied to the serum samples. (C) Area under the curve (AUC) values determined by ROC curve analysis of CDTb plus the average of TcdB-CROP and TcdBvir-CROP from the 35 (n = 27 for nonrecurrent; n = 8 for recurrent) patients in this study for whom sufficient sample remained for separate isotype analysis. AUC values range from 0.5 (no predictive value) to 1.0 (perfect predictive value); Black bars indicate AUC values for MENSA samples; Gray bars indicate AUC values for serum samples. Error bars indicate 95% confidence interval upper limit. (D) Sensitivity values from the same ROC analysis in C.
IgA responses in MENSA correlated with recurrence-free recovery in the first 2 months.
MENSA and serum samples from nonrecurrent (n = 27) and recurrent (n = 8) patients were reexamined with separate detection for anti-IgA, anti-IgG, and anti-IgM for the three C. difficile toxins and analyzed using receiver operating characteristic curves (ROC). Bar graphs showing AUC and y-axis intercept values (as a minimal measure of analytic sensitivity) for both MENSA and serum are presented in Fig. 6C and D. In MENSA, the AUC values for the resulting ROC curves varied from 0.55 for IgM to 0.90 for IgA. MENSA yielded higher AUC values and sensitivities than serum for all isotypes and combinations except for IgM. The correlation with patients unlikely to suffer recurrence ranged from 15% sensitivity for IgM to 70% for IgA. IgG alone or IgG+IgA yielded AUC values of 0.72 and 0.82 and y-axis intercepts of 56% and 59%, respectively. Analysis of serum samples demonstrated similar trends: IgA alone yielded the highest AUC value (0.83) and sensitivity (63%), followed by combinations of IgA+IgG and IgA+IgG+IgM (AUC = 0.75; 48% sensitivity for each), IgG alone (AUC = 0.71; 41% sensitivity), and IgM (AUC = 0.67; 22% sensitivity). By using MENSA IgA alone or a combination of IgA+IgG, a substantial subpopulation unlikely to experience recurrence was identified.
DISCUSSION
Summary.
Despite medical advances in CDI treatment, the high incidence of recurrence remains a significant unmet need. To address this problem, we demonstrate the diagnostic utility of measuring anti-C. difficile antibodies in serum and MENSA. MENSA is a novel analytic fluid containing antibodies produced in vitro by circulating ASC. Unlike serum antibodies, MENSA antibodies reflect only the new humoral response. We found that circulating ASC are present in the blood 2 to 12 DPSO, and that MENSA antibodies can serve as early biomarkers for protection against recurrence, before seroconversion. From a repertoire of 10 C. difficile antigens, we identified three against which MENSA Ig responses were predictive of nonrecurrence. Antibodies specific for these three C. difficile antigens in the MENSA identified 62% (23/37) of primary CDI patients who would not suffer a recurrence. Conversely, among negative patients, there was a 39% (9/23) probability of recurrence. Remarkably, our three-antigen MENSA assay outperforms widely used recurrence risk factors (19.0 versus <3.0; Table 2). Measurement of MENSA IgA specific for the C. difficile toxins may be the single strongest predictor of protection against rCDI.
MENSA anti-TcdB-CROP and anti-CDTb are early biomarkers of protection against CDI recurrence.
Typically, stool samples are tested for C. difficile DNA or antigens and then antibiotics are administered for 10 to 14 days (1). The mean interval between CDI symptom onset and treatment initiation was <2 days for hospital-acquired infection (35). The optimal window for measuring the CDI MENSA response is 2 to 12 DPSO when most hospitalized CDI patients undergo treatment. Although seroconversion of anti-C. difficile IgG occurs in many patients by 12 DPSO (36), our results show that serum antibody measurement detects only 50% of C. difficile-infected patients within 12 days. In contrast, measurement of anti-toxin Ig in MENSA can identify 62% of nonrecurrent patients by that time. Overall, measurement of anti-toxin antibodies in MENSA can reveal a low risk of recurrence in a higher percentage of patients at an earlier time point than in serum, possibly before hospital discharge (29). Most patients with early positive anti-TcdB-CROP, anti-TcdBvir-CROP, and/or anti-CDTb MENSA responses developed increased protective serum levels in the weeks following (Fig. 5).
Antibodies in MENSA are better for the identification of nonrecurrent patients than antibodies in serum.
The improved sensitivity in MENSA samples is primarily due to MENSA-positive patients who remained seronegative (Fig. 2, M+S-), an unexpected subpopulation. None of the six patients in this group experienced recurrence, a group too small to assess clinical significance (P = 0.27). Exactly what defines this subpopulation is not known; however, MENSA responses may arise without seroconversion if the immune response is aborted early or if the circulating IgA-ASC migrates to mucosal sites and do not contribute to the serologic response. Thus, MENSA antibodies alone could be effective biomarkers for protection from recurrence. A secondary issue concerns healthy subjects who have anti-C. difficile antibodies in their serum, a phenomenon already well documented (32, 37). In our previous study, we observed that 7% (5/71) of control subjects had antibody responses to one or more of the three toxin antigens in their serum; of those five seropositive controls, only two had responses in their MENSA (3%; 2/71) and the other 66 controls were MENSA negative (28). Thus, advantages of MENSA analysis include: (i) earlier rise than serum levels (29); (ii) positivity in at least some patients who fail to seroconvert; and 3) less interference by preexisting antibody levels.
The secreted toxins are the best antigens for the diagnosis of nonrecurrence.
In this study, the secreted toxins TcdB-CROP, TcdBvir-CROP, and CDTb provided the best results among the C. difficile antigens. C. difficile displays an array of cell surfaces and secreted antigens. Extensively described in our previous studies (28), these include defensive enzymes (GDH (38)), flagellar components (FliC (39–41)), and cell wall proteins associated with biofilm building (Cwp84 (42–45)). Overall, these added little or no value for MENSA or serum-based diagnostics as they produced false positives or were redundant with the three secreted toxin antigens. Antibody levels specific for the TcdA domains, CROP, and GTD, were also redundant with the larger responses to the TcdB and CDTb toxins. Likewise, the TcdB-GTD and CDTa antigens added little or nothing. Thus, these antigens were eliminated from further consideration.
IgA is a strong predictor in both MENSA and serum.
In serum, most measured antibodies were IgG (73 to 80%), while in MENSA, IgG and IgA were equally abundant (39 to 50% and 42 to 59% of total specific antibodies, respectively). In both MENSA and serum samples, IgA yielded higher AUC values and sensitivities than the other isotypes, alone or in combination, suggesting the focus of the MENSA measurement to IgA alone. As a pathogen of the gastrointestinal tract, C. difficile is likely to elicit a strong IgA response. However, IgG had greater magnitude in serum and MENSA responses demonstrating its importance. Further development of this assay will explore the roles of both IgA and IgG, alone and in combination.
Limitations of this study.
Fundamental limitations of this study were the population size and enrollment of patients from a tertiary referral center with a substantial number of immunocompromised patients due to transplantation and malignancies. Enrollment from community hospitals that reflect the primary CDI burden in the United States health care settings would improve future studies.
Another concern was the utility of the TcdB nucleic acid amplification test (NAAT) in stool samples for primary diagnosis in this study. Recent reports suggest that screening for C. difficile genes by NAAT may overestimate the frequency of infections (46, 47), due to its inability to distinguish true infections from colonization (48, 49). Since colonization does not elicit a MENSA response, some of our MENSA-negative patients may not have had true CDI.
Finally, two of the selected antigens are the secreted toxin TcdB-CROP domains produced by the historical strain VPI 10463 and the hypervirulent epidemic strain, NAP1/B1/027 R20291 (50). Results for MENSA or serum antibodies specific for each antigen are similar but the selection of a single or combination antigen will require additional clinical evaluation.
MENSA-based diagnostics in primary CDI targets preventative rCDI therapies.
In practice, we imagine a physician administering this test to a patient currently undergoing antibiotic treatment once or twice within 2 to 12 DPSO. Positive patients would be regarded as low risk for recurrence while negative patients would be monitored more closely.
Recently, Bezlotoxumab (tradename: Zinplava) (16) and FMT have been shown to prevent rCDI. The favorable safety profiles of these therapies make a reasonable case for administration after primary CDI in the subset of patients who are at risk for recurrence. However, if given to all patients with primary CDI, only 30% could benefit. The utilization of our C. difficile MENSA immunoassay at the time of primary CDI could identify patients at the greatest risk of recurrence.
In conclusion, we propose a new strategy for stratifying patients during primary CDI into those at low versus higher risk for recurrence. The model measures the host immune response as a clinical prediction tool for recognizing CDI patients who are not at risk for recurrence, thereby enabling preventative measures to be directed to patients who would derive maximal benefit. Ultimately, this C. difficile MENSA immunoassay could lead to substantial health care cost reductions while decreasing recurrence.
ACKNOWLEDGMENTS
We give special thanks to the clinical coordinators who assisted in this project. Coordinators at Emory Hospitals were Hinel Patel, Aja Bowser, and Maya Lindsay. Our thanks also to the clinical coordinators at Dekalb Medical Center and Infectious Disease Specialists of Atlanta, Travis Stewart, and Julia Norton. Finally, special thanks to our sponsor on this effort, L. Clifford McDonald at the Centers for Disease Control and Prevention for his continued advice and support.
This work was supported by phase I and phase II research contracts number 200-21-88233 titled “Antibody Secreting Cells (ASC) in Human Clostridium difficile Infection, Colonization, and Recurrence” from the Centers for Disease Control and Prevention.
F.E.L. and J.L.D. designed and supervised the study. N.S.H., S.N., S.O., F.E.L., and J.L.D. wrote the manuscript with support/input/feedback from all authors. N.S.H., S.N., G.K., and S.O. contributed to sample preparation, carried out the experiments, and analyzed the data. C.S.K, A.B., and P.A.R. acquired the patient samples. Y.W. confirmed CDI diagnosis in patient samples. H.W. provided statistical guidance. S.N.T.L. oversaw patient recruitment. L.E.C. reviewed the initial study design and data. R.R., M.C.R., and A.B. provided clinical data to calculate the recurrence risk factors. MK provided detailed editing and a thoughtful review of the manuscript. Each author contributed to the manuscript revision, read, and approved the submitted version.
Contributor Information
John L. Daiss, Email: jdaiss52@gmail.com.
Yi-Wei Tang, Cepheid.
REFERENCES
- 1.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. 2018. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 66:e1–e48. 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. 2016. Clostridium difficile infection. Nat Rev Dis Primers 2:16020. 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura T, Snijder R, Sugitani T. 2019. Characterization and risk factors for recurrence of Clostridioides (Clostridium) difficile infection in Japan: a nationwide real-world analysis using a large hospital-based administrative dataset. J Infect Chemother 25:615–620. 10.1016/j.jiac.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 4.De Roo AC, Regenbogen SE. 2020. Clostridium difficile Infection: an Epidemiology Update. Clin Colon Rectal Surg 33:49–57. 10.1055/s-0040-1701229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieterle MG, Young VB. 2017. Reducing Recurrence of C. difficile Infection. Cell 169:375. 10.1016/j.cell.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Lofgren ET, Cole SR, Weber DJ, Anderson DJ, Moehring RW. 2014. Hospital-acquired Clostridium difficile infections: estimating all-cause mortality and length of stay. Epidemiology 25:570–575. 10.1097/EDE.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanwa N, Kendzerska T, Krahn M, Kwong JC, Daneman N, Witteman W, Mittmann N, Cadarette SM, Rosella L, Sander B. 2015. The economic impact of Clostridium difficile infection: a systematic review. Am J Gastroenterol 110:511–519. 10.1038/ajg.2015.48. [DOI] [PubMed] [Google Scholar]
- 8.Ma GK, Brensinger CM, Wu Q, Lewis JD. 2017. Increasing Incidence of Multiply Recurrent Clostridium difficile Infection in the United States: a Cohort Study. Ann Intern Med 167:152–158. 10.7326/M16-2733. [DOI] [PubMed] [Google Scholar]
- 9.Magee G, Strauss ME, Thomas SM, Brown H, Baumer D, Broderick KC. 2015. Impact of Clostridium difficile-associated diarrhea on acute care length of stay, hospital costs, and readmission: a multicenter retrospective study of inpatients, 2009–2011. Am J Infect Control 43:1148–1153. 10.1016/j.ajic.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Olson DC, Scobey MW. 2016. The Challenge of Clostridium difficile Infection. N C Med J 77:206–210. 10.18043/ncm.77.3.206. [DOI] [PubMed] [Google Scholar]
- 11.Couture-Cossette A, Carignan A, Ilangumaran S, Valiquette L. 2017. Bezlotoxumab for the prevention of Clostridium difficile recurrence. Expert Opin Biol Ther 17:1439–1445. 10.1080/14712598.2017.1363886. [DOI] [PubMed] [Google Scholar]
- 12.Deeks ED. 2017. Bezlotoxumab: a Review in Preventing Clostridium difficile Infection Recurrence. Drugs 77:1657–1663. 10.1007/s40265-017-0809-y. [DOI] [PubMed] [Google Scholar]
- 13.Dzunkova M, D'Auria G, Xu H, Huang J, Duan Y, Moya A, Kelly CP, Chen X. 2016. The Monoclonal Antitoxin Antibodies (Actoxumab-Bezlotoxumab) Treatment Facilitates Normalization of the Gut Microbiota of Mice with Clostridium difficile Infection. Front Cell Infect Microbiol 6:119. 10.3389/fcimb.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, Gerding DN, Kelly CP, Katchar K, Baxter R, Ambrosino D, Molrine D. 2010. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine 28:965–969. 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox M, Dorr MB, Pedley A. 2017. Bezlotoxumab and Recurrent Clostridium difficile Infection. N Engl J Med 376:1594–1596. 10.1056/NEJMc1702531. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, Cornely OA, Rahav G, Bouza E, Lee C, Jenkin G, Jensen W, Kim YS, Yoshida J, Gabryelski L, Pedley A, Eves K, Tipping R, Guris D, Kartsonis N, Dorr MB, Modify I, Investigators MI. 2017. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med 376:305–317. 10.1056/NEJMoa1602615. [DOI] [PubMed] [Google Scholar]
- 17.Arbel LT, Hsu E, McNally K. 2017. Cost-Effectiveness of Fecal Microbiota Transplantation in the Treatment of Recurrent Clostridium Difficile Infection: a Literature Review. Cureus 9:e1599. 10.7759/cureus.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bafeta A, Yavchitz A, Riveros C, Batista R, Ravaud P. 2017. Methods and Reporting Studies Assessing Fecal Microbiota Transplantation: a Systematic Review. Ann Intern Med 167:34–39. 10.7326/M16-2810. [DOI] [PubMed] [Google Scholar]
- 19.Chen B, Avinashi V, Dobson S. 2017. Fecal microbiota transplantation for recurrent clostridium difficile infection in children. J Infect 74 Suppl 1:S120–S7. 10.1016/S0163-4453(17)30202-5. [DOI] [PubMed] [Google Scholar]
- 20.Fischer M, Kao D, Kelly C, Kuchipudi A, Jafri SM, Blumenkehl M, Rex D, Mellow M, Kaur N, Sokol H, Cook G, Hamilton MJ, Phelps E, Sipe B, Xu H, Allegretti JR. 2016. Fecal Microbiota Transplantation is Safe and Efficacious for Recurrent or Refractory Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 22:2402–2409. 10.1097/MIB.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 21.Fischer M, Sipe B, Cheng YW, Phelps E, Rogers N, Sagi S, Bohm M, Xu H, Kassam Z. 2017. Fecal microbiota transplant in severe and severe-complicated Clostridium difficile: a promising treatment approach. Gut Microbes 8:289–302. 10.1080/19490976.2016.1273998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianotti RJ, Moss AC. 2017. Fecal Microbiota Transplantation: from Clostridium difficile to Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 13:209–213. [PMC free article] [PubMed] [Google Scholar]
- 23.Hagel S, Fischer A, Ehlermann P, Frank T, Tueffers K, Sturm A, Link A, Demir M, Siebenhaar A, Storr M, Glueck T, Siegel E, Solbach P, Goeser F, Koelbel CB, Lohse A, Luebbert C, Kandzi U, Maier M, Schuerle S, Lerch MM, Tacke D, Cornely OA, Stallmach A, Vehreschild M, German Clinical Microbiome Study Group (GCMSG) . 2016. German Clinical Microbiome Study G. Fecal Microbiota Transplant in Patients With Recurrent Clostridium Difficile Infection. Dtsch Arztebl Int 113:583–589. 10.3238/arztebl.2016.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juul FE, Garborg K, Bretthauer M, Skudal H, Oines MN, Wiig H, Rose O, Seip B, Lamont JT, Midtvedt T, Valeur J, Kalager M, Holme O, Helsingen L, Loberg M, Adami HO. 2018. Fecal Microbiota Transplantation for Primary Clostridium difficile Infection. N Engl J Med 378:2535–2536. 10.1056/NEJMc1803103. [DOI] [PubMed] [Google Scholar]
- 25.Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, Reinert SE, Machan JT, Brandt LJ. 2016. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: a Randomized Trial. Ann Intern Med 165:609–616. 10.7326/M16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oprita R, Bratu M, Oprita B, Diaconescu B. 2016. Fecal transplantation - the new, inexpensive, safe, and rapidly effective approach in the treatment of gastrointestinal tract diseases. J Med Life 9:160–162. [PMC free article] [PubMed] [Google Scholar]
- 27.Varier RU, Biltaji E, Smith KJ, Roberts MS, Kyle Jensen M, LaFleur J, Nelson RE. 2015. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol 36:438–444. 10.1017/ice.2014.80. [DOI] [PubMed] [Google Scholar]
- 28.Haddad NS, Nozick S, Kim G, Ohanian S, Kraft C, Rebolledo PA, Wang Y, Wu H, Bressler A, Le SNT, Kuruvilla M, Cannon LE, Lee FE, Daiss JL. 2021. Novel immunoassay for diagnosis of ongoing Clostridioides difficile infections using serum and medium enriched for newly synthesized antibodies (MENSA). J Immunol Methods 492:112932. 10.1016/j.jim.2020.112932. [DOI] [PubMed] [Google Scholar]
- 29.Carter MJ, Mitchell RM, Meyer Sauteur PM, Kelly DF, Truck J. 2017. The Antibody-Secreting Cell Response to Infection: kinetics and Clinical Applications. Front Immunol 8:630. 10.3389/fimmu.2017.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L, Kaminiski D, Fucile CF, Albizua I, Kyu S, Chiang KY, Bradley KT, Burack R, Slifka M, Hammarlund E, Wu H, Zhao L, Walsh EE, Falsey AR, Randall TD, Cheung WC, Sanz I, Lee FE. 2015. Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 43:132–145. 10.1016/j.immuni.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta SB, Mehta V, Dubberke ER, Zhao X, Dorr MB, Guris D, Molrine D, Leney M, Miller M, Dupin M, Mast TC. 2016. Antibodies to Toxin B Are Protective Against Clostridium difficile Infection Recurrence. Clin Infect Dis 63:730–734. 10.1093/cid/ciw364. [DOI] [PubMed] [Google Scholar]
- 32.Kelly CP, Kyne L. 2011. The host immune response to Clostridium difficile. J Med Microbiol 60:1070–1079. 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 33.Kyne L, Warny M, Qamar A, Kelly CP. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189–193. 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 34.Bauer MP, Nibbering PH, Poxton IR, Kuijper EJ, van Dissel JT. 2014. Humoral immune response as predictor of recurrence in Clostridium difficile infection. Clin Microbiol Infect 20:1323–1328. 10.1111/1469-0691.12769. [DOI] [PubMed] [Google Scholar]
- 35.Champredon D, Zhang K, Smieja M, Moghadas SM. 2019. Clostridium difficile intervention timelines for diagnosis, isolation, and treatment. Am J Infect Control 47:1370–1374. 10.1016/j.ajic.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Kyne L, Warny M, Qamar A, Kelly CP. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 342:390–397. 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 37.Mulligan ME, Miller SD, McFarland LV, Fung HC, Kwok RY. 1993. Elevated levels of serum immunoglobulins in asymptomatic carriers of Clostridium difficile. Clin Infect Dis 16:S239–44. 10.1093/clinids/16.Supplement_4.S239. [DOI] [PubMed] [Google Scholar]
- 38.Girinathan BP, Braun SE, Govind R. 2014. Clostridium difficile glutamate dehydrogenase is a secreted enzyme that confers resistance to H2O2. Microbiology (Reading) 160:47–55. 10.1099/mic.0.071365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghose C, Eugenis I, Sun X, Edwards AN, McBride SM, Pride DT, Kelly CP, Ho DD. 2016. Immunogenicity and protective efficacy of recombinant Clostridium difficile flagellar protein FliC. Emerg Microbes Infect 5:e8. 10.1038/emi.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pechine S, Gleizes A, Janoir C, Gorges-Kergot R, Barc MC, Delmee M, Collignon A. 2005. Immunological properties of surface proteins of Clostridium difficile. J Med Microbiol 54:193–196. 10.1099/jmm.0.45800-0. [DOI] [PubMed] [Google Scholar]
- 41.Tasteyre A, Barc MC, Collignon A, Boureau H, Karjalainen T. 2001. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect Immun 69:7937–7940. 10.1128/IAI.69.12.7937-7940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradshaw WJ, Kirby JM, Thiyagarajan N, Chambers CJ, Davies AH, Roberts AK, Shone CC, Acharya KR. 2014. The structure of the cysteine protease and lectin-like domains of Cwp84, a surface layer-associated protein from Clostridium difficile. Acta Crystallogr D Biol Crystallogr 70:1983–1993. 10.1107/S1399004714009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirby JM, Ahern H, Roberts AK, Kumar V, Freeman Z, Acharya KR, Shone CC. 2009. Cwp84, a surface-associated cysteine protease, plays a role in the maturation of the surface layer of Clostridium difficile. J Biol Chem 284:34666–34673. 10.1074/jbc.M109.051177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantaleon V, Soavelomandroso AP, Bouttier S, Briandet R, Roxas B, Chu M, Collignon A, Janoir C, Vedantam G, Candela T. 2015. The Clostridium difficile Protease Cwp84 Modulates both Biofilm Formation and Cell-Surface Properties. PLoS One 10:e0124971. 10.1371/journal.pone.0124971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pechine S, Deneve C, Le Monnier A, Hoys S, Janoir C, Collignon A. 2011. Immunization of hamsters against Clostridium difficile infection using the Cwp84 protease as an antigen. FEMS Immunol Med Microbiol 63:73–81. 10.1111/j.1574-695X.2011.00832.x. [DOI] [PubMed] [Google Scholar]
- 46.Chapman TP, Parks T, Culver EL, Scarborough M. 2011. Over-diagnosis of Clostridium difficile. BMJ 343:d4919. 10.1136/bmj.d4919. [DOI] [PubMed] [Google Scholar]
- 47.Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, Nguyen HH, Huang B, Tang YW, Lee LW, Kim K, Taylor S, Romano PS, Panacek EA, Goodell PB, Solnick JV, Cohen SH. 2015. Overdiagnosis of Clostridium difficile Infection in the Molecular Test Era. JAMA Intern Med 175:1792–1801. 10.1001/jamainternmed.2015.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong LY, Davies K, Wilcox MH. 2019. The perils of PCR-based diagnosis of Clostridioides difficile infections: painful lessons from clinical trials. Anaerobe 60:102048. 10.1016/j.anaerobe.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Kraft CS, Parrott JS, Cornish NE, Rubinstein ML, Weissfeld AS, McNult P, Nachamkin I, Humphries RM, Kirn TJ, Dien Bard J, Lutgring JD, Gullett JC, Bittencourt CE, Benson S, Bobenchik AM, Sautter RL, Baselski V, Atlas MC, Marlowe EM, Miller NS, Fischer M, Richter SS, Gilligan P, Snyder JW. 2019. A Laboratory Medicine Best Practices Systematic Review and Meta-analysis of Nucleic Acid Amplification Tests (NAATs) and Algorithms Including NAATs for the Diagnosis of Clostridioides (Clostridium) difficile in Adults. Clin Microbiol Rev 32:e00032-18. 10.1128/CMR.00032-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanis JM, Heinlen LD, James JA, Ballard JD. 2013. Clostridium difficile 027/BI/NAP1 encodes a hypertoxic and antigenically variable form of TcdB. PLoS Pathog 9:e1003523. 10.1371/journal.ppat.1003523. [DOI] [PMC free article] [PubMed] [Google Scholar]