Abstract
Background/Aims
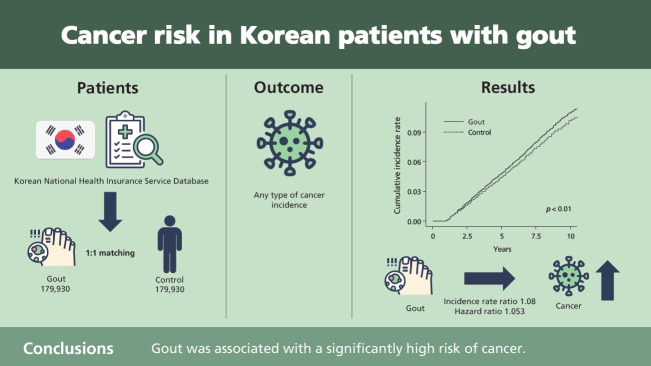
Using a nationwide cohort, we investigated the cancer risk in Korean patients with gout.
Methods
Data were obtained from the Korean National Health Insurance Service Database. Patients with gout were defined as those aged ≥ 20 years who were diagnosed with gout and received anti-gout medication (allopurinol, colchicine, and benzbromarone) between 2008 and 2010. Patients with nail disorders were randomly assigned to a control group (1:1 ratio) after frequency matching for age and sex. Cancer incidence was then investigated between 2012 and 2018. Cox proportional hazard regression analysis was used to investigate the association between gout and cancer after adjusting for concomitant diseases.
Results
This study included 179,930 patients with gout and an equal number of matched controls. The incidence of overall cancer was higher in patients with gout than in controls (incidence rate ratio, 1.08). Cox proportional hazards regression analysis showed that gout was associated with a hazard ratio of 1.053 (95% confidence interval, 1.031 to 1.077) after adjusting for concomitant diseases.
Conclusions
Gout was associated with a significantly high risk of cancer, especially esophageal, stomach, colon, liver, pancreatic, lung, ovarian, renal, and bladder cancers.
Keywords: Gout, Neoplasms, Incidence
INTRODUCTION
Gout is a disorder characterized by monosodium urate crystal deposition in various tissues in patients with hyperuricemia. Notably, the hyperuricemic state precedes the first gout attack in most patients. Uric acid plays a dual role in the body as an anti- and pro-oxidant. Uric acid is a potent scavenger of free radicals, which clinically reduces cell death and protects against neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis [1]. However, uric acid is a known pro-oxidant that contributes to free radical formation, resulting in oxidative cell damage, low-grade inflammation, insulin resistance, and negative cardiovascular effects [2]. To date, studies in the available literature have reported conflicting data regarding the association between gout and cancer. A study performed by Kuo et al. [3] showed that low serum uric acid (SUA) levels were associated with a high risk of cancer-related mortality, thereby lending credence to the antioxidant action of uric acid that protects against cancer. However, epidemiological data in this regard remain controversial. A Swedish study based on hospital admission records reported that the incidence of various cancers was higher in patients with gout [4]. A study performed in Taiwan reported a high risk of cancers, particularly urological cancer, in patients with gout [5]. Using data from a nationwide population-based longitudinal database, we investigated the association between gout and cancer risk in this retrospective cohort study.
METHODS
Study design and database
This nationwide, population-based, retrospective cohort study was based on data obtained between January 2007 and December 2018 from the Korean National Health Insurance (NIH) claims database. This database includes all claims documented by the Korean NIH system and the Korean Medical Aid program during the study period. The International Classification of Diseases, 10th Revision (ICD-10), was used to record the diagnosis in the database.
Study population
This study included 179,930 patients aged ≥ 20 years whose records were available in the NIH database. Patients with gout were defined as those who were diagnosed with gout (ICD-10 code M10) and received medical treatment between 2008 and 2010. Medical treatment was defined as the prescription of any of the following drugs: allopurinol, colchicine, or benzbromarone. To ensure that only patients newly diagnosed with gout were enrolled, all patients diagnosed with gout in 2007 were excluded from the study. Patients diagnosed with nail disorders (ICD10 code L60) during the same period were assigned to a control group. Patients with gout or cancer were excluded from the control group. Patients randomly assigned to the control group were matched with the gout group (1:1 ratio) after frequency matching for age and sex. We set 1 year as the wash-out period, and newly diagnosed cancers were recorded during follow-up visits between 2012 and 2018. All cancers were defined using ICD-10 codes C00 to C97. The cancer types were categorized based on anatomical sites as follows: head and neck cancer (C00 to C14), esophageal cancer (C15), stomach cancer (C16), colon cancer (C18 to C20), liver cancer (C22), pancreatic cancer (C25), lung cancer (C33, C34), skin cancer (C43), breast cancer (C50), cervical cancer (C53), endometrial cancer (C54), ovarian cancer (C56), renal cancer (C64), prostate cancer (C61), bladder cancer (C64), thyroid cancer (C73), lymphoma (C82 to C86), and leukemia (C91 to 95).
Confounding variables
We selected concomitant diseases as confounding variables, and these concomitant diseases were then defined using the specific ICD-10 code corresponding to the main diagnosis or the sub-diagnosis between 2008 and 2010. Confounding variables in the form of concomitant diseases included hypertension (I10 to I15), diabetes (E10 to E14), dyslipidemia (E78), renal failure (N17 to N19), ischemic heart diseases (IHDs, I20 to I25), and cerebrovascular diseases (CVDs, I60 to I69).
Statistical analysis
The incidence and incidence rate ratios were calculated in patients with gout as well as in the control group. Multivariate Cox proportional hazards regression analysis was used to determine the hazard ratio (HR) for the development of cancer after adjusting for confounding variables. Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). A p < 0.05 was considered statistically significant. This study was approved by the Institutional Review Board of the Kangwon National University Hospital (IRB no. KNUH-2019-06-007). Written informed consent by the patients was waived due to a retrospective nature of our study.
RESULTS
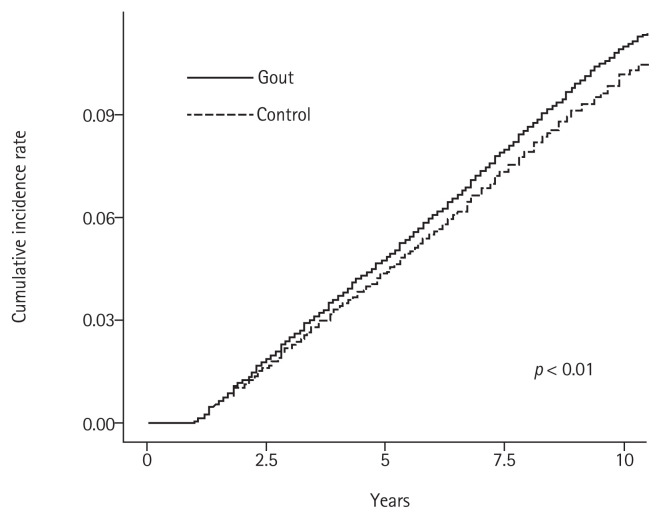
This study included 179,930 patients with gout and an equal number of age- and sex-matched controls without gout. The baseline characteristics of both study groups are shown in Table 1. The mean ± standard deviation age of patients with and without gout (controls) was 51.85 ± 14.55 years and 51.57 ± 14.59 years, respectively, and 78.86% of the patients in each group were men. The prevalence of concomitant diseases was significantly higher in patients with gout than in controls (Table 1). Most patients with gout (85.49%) received allopurinol, and only 11.74% patients received colchicine. The incidence rates of all cancers in patients with gout and in controls are shown in Table 2. The incidence rate ratio of overall cancers in patients with gout and in controls was 1.08. Notably, the incidence rates were significantly higher in patients with gout than in controls; however, the incidence rates of skin, cervical, and prostate cancer were lower in patients with gout. Fig. 1 shows the cumulative hazard risks of overall cancer in patients with gout and controls. Table 3 shows the results of Cox proportional hazards regression analysis of cancer risk in patients with gout and controls. Cox proportional hazard regression analysis showed that the unadjusted HR for the association between gout and cancer was 1.082 (95% confidence interval [CI], 1.059 to 1.105). Following adjustment for concomitant diseases, gout was significantly associated with cancer (HR, 1.053; 95% CI, 1.031 to 1.077). Subgroup analysis of each type of cancer revealed a significantly positive association between gout and esophageal, stomach, colon, liver, pancreatic, lung, ovarian, renal, and bladder cancers. Interestingly, only prostate cancer was negatively associated with gout (adjusted HR, 0.882; 95% CI, 0.838 to 0.929).
Table 1.
Baseline characteristics of patients with and without gout (controls)
| Characteristic | Control group (n = 179,930) | Patients with gout (n = 179,930) | p value |
|---|---|---|---|
| Age, yr | 51.57 ± 14.59 | 51.85 ± 14.55 | 0.21 |
| Male sex | 141,575 (78.68) | 141,575 (78.68) | 1.00 |
| Comorbidities | |||
| Hypertension | 2,445 (0.68) | 15,371 (4.27) | < 0.01 |
| Diabetes mellitus | 2,501 (0.69) | 6,353 (1.77) | < 0.01 |
| Dyslipidemia | 766 (0.21) | 10,892 (3.01) | < 0.01 |
| Renal failure | 79 (0.02) | 3,704 (1.03) | < 0.01 |
| Ischemic heart disorder | 189 (0.05) | 1,823 (0.51) | < 0.01 |
| Cerebrovascular disorder | 283 (0.08) | 983 (0.27) | < 0.01 |
Values are presented as mean ± standard deviation or number (%).
Table 2.
Incidence rate of all cancers in patients with and without gout (controls)
| Variable | Control group | Patients with gout | IRR | ||
|---|---|---|---|---|---|
|
|
|
||||
| IR | 95% CI | IR | 95% CI | ||
| Overall cancers | 1,001.7 | 986.5–1,017.2 | 1,082.8 | 1,067.0–1,098.9 | 1.08 |
|
| |||||
| Head and neck cancer | 15.0 | 13.3–17.0 | 15.9 | 14.1–17.9 | 1.06 |
|
| |||||
| Esophageal cancer | 7.4 | 6.2–8.9 | 10.5 | 9.0–12.2 | 1.41 |
|
| |||||
| Stomach cancer | 106.9 | 102.0–112.0 | 119.6 | 114.4–125.0 | 1.12 |
|
| |||||
| Colon cancer | 106.3 | 101.4–111.4 | 121.0 | 115.8–126.5 | 1.14 |
|
| |||||
| Liver cancer | 108.0 | 103.1–113.2 | 122.7 | 117.4–128.2 | 1.14 |
|
| |||||
| Pancreatic cancer | 43.7 | 40.6–47.0 | 51.1 | 47.8–54.7 | 1.17 |
|
| |||||
| Lung cancer | 89.8 | 85.3–94.5 | 98.6 | 93.9–103.5 | 1.10 |
|
| |||||
| Skin cancer | 4.2 | 3.3–5.3 | 3.5 | 2.7–4.5 | 0.84 |
|
| |||||
| Breast cancer | 21.2 | 19.1–23.6 | 22.2 | 20.0–24.6 | 1.05 |
|
| |||||
| Cervical cancer | 5.4 | 4.4–6.6 | 4.5 | 3.6–5.6 | 0.83 |
|
| |||||
| Endometrial cancer | 2.9 | 2.2–3.8 | 4.0 | 3.2–5.1 | 1.40 |
|
| |||||
| Ovarian cancer | 6.2 | 5.1–7.5 | 9.5 | 8.1–11.1 | 1.53 |
|
| |||||
| Renal cancer | 21.6 | 19.4–24.0 | 30.5 | 27.9–33.3 | 1.41 |
|
| |||||
| Prostate cancer | 198.5 | 191.7–205.4 | 180.7 | 174.3–187.4 | 0.91 |
|
| |||||
| Bladder cancer | 29.1 | 26.6–31.8 | 34.3 | 31.6–37.3 | 1.18 |
|
| |||||
| Thyroid cancer | 67.1 | 63.2–71.2 | 72.7 | 68.7–76.9 | 1.08 |
|
| |||||
| Lymphoma | 12.3 | 10.7–14.1 | 13.1 | 11.5–15.0 | 1.07 |
|
| |||||
| Leukemia | 11.0 | 9.5–12.8 | 10.9 | 9.4–12.6 | 0.99 |
IR, incidence rate; CI, confidence interval; IRR, incidence rate ratio.
Figure 1.
Cumulative incidence rate of overall cancers in patients with and without gout (controls).
Table 3.
HR for cancers in patients with and without gout (controls) after adjusting for covariates
| Variable | Crude HR | 95% CI | p value | Adjusted HRa | 95% CI | p value |
|---|---|---|---|---|---|---|
| Overall cancers | 1.082 | 1.059–1.105 | < 0.01 | 1.053 | 1.031–1.077 | < 0.01b |
| Head and neck cancer | 1.056 | 0.887–1.258 | 0.54 | 1.022 | 0.854–1.223 | 0.81 |
| Esophageal cancer | 1.415 | 1.121–1.786 | < 0.01 | 1.387 | 1.093–1.759 | < 0.01b |
| Stomach cancer | 1.121 | 1.051–1.195 | < 0.01 | 1.103 | 1.032–1.178 | < 0.01b |
| Colon cancer | 1.139 | 1.068–1.215 | < 0.01 | 1.113 | 1.042–1.189 | < 0.01b |
| Liver cancer | 1.137 | 1.067–1.212 | < 0.01 | 1.110 | 1.040–1.185 | < 0.01b |
| Pancreatic cancer | 1.170 | 1.058–1.292 | < 0.01 | 1.172 | 1.058–1.298 | < 0.01b |
| Lung cancer | 1.099 | 1.024–1.179 | < 0.01 | 1.075 | 1.000–1.156 | < 0.05b |
| Skin cancer | 0.838 | 0.589–1.191 | 0.32 | 0.756 | 0.524–1.093 | 0.14 |
| Breast cancer | 1.047 | 0.903–1.213 | 0.54 | 1.035 | 0.890–1.203 | 0.66 |
| Cervical cancer | 0.830 | 0.609–1.132 | 0.24 | 0.742 | 0.535–1.028 | 0.07 |
| Endometrial cancer | 1.398 | 0.961–2.032 | 0.08 | 1.380 | 0.941–2.024 | 0.10 |
| Ovarian cancer | 1.534 | 1.194–1.971 | < 0.01 | 1.601 | 1.242–2.064 | < 0.01b |
| Renal cancer | 1.413 | 1.233–1.620 | < 0.01 | 1.276 | 1.108–1.470 | < 0.01b |
| Prostate cancer | 0.911 | 0.867–0.957 | < 0.01 | 0.882 | 0.838–0.929 | < 0.01b |
| Bladder cancer | 1.180 | 1.044–1.334 | < 0.01 | 1.147 | 1.012–1.300 | < 0.05b |
| Thyroid cancer | 1.084 | 0.999–1.177 | 0.05 | 1.074 | 0.987–1.168 | 0.09 |
| Lymphoma | 1.070 | 0.883–1.298 | 0.49 | 1.029 | 0.844–1.255 | 0.78 |
| Leukemia | 0.989 | 0.804–1.217 | 0.92 | 0.960 | 0.776–1.188 | 0.71 |
HR, hazard ratio; CI, confidence interval.
Adjusted for concomitant hypertension, diabetes, dyslipidemia, renal failure, ischemic heart disorder, and cerebrovascular disorder.
p < 0.05.
DISCUSSION
This study investigated the association between gout and cancer. Compared to the control group, patients with gout showed a higher incidence of cancer. Cancer risk remained high even after adjusting for concomitant disorders including hypertension, diabetes, dyslipidemia, renal failure, IHD, and CVD. Previous studies have investigated the association between gout and cancer; Boffetta et al. [4] investigated the incidence of cancer among patients with gout admitted to a hospital in Sweden and observed that the incidence of oral cavity, pharynx, colon, liver, biliary tract, pancreas, lung, skin, endometrial, and kidney cancers in gout patients was higher than that in the general population. A study performed by Chen et al. [5] in Taiwan reported that patients with gout are more likely to develop prostate, bladder, colon, kidney, liver, stomach, and lung cancers. Kuo et al. [6] reported a significantly higher overall incidence of cancer in patients with gout than in controls. However, all these studies analyzed the data only after adjusting for age, sex, and socioeconomic status. Gout is a complex phenotype mixed with various concomitant disorders including hypertension, renal impairment, dyslipidemia, diabetes, IHD, and CVD. Therefore, appropriate screening and optimal management of concomitant disorders are essential in routine clinical practice [7].
Chronic disorders are known contributors to cancer. Accumulating evidence in the available literature suggests that chronic disorders including diabetes, renal failure, hypertension, dyslipidemia, and CVD could predispose to the development of cancer [8]. Therefore, chronic diseases should be considered as confounding variables when evaluating cancer risk. In this study, patients with gout were compared with age- and sex-matched controls to calculate the HR adjusted for hypertension, diabetes, renal failure, dyslipidemia, IHD, and CVD. After adjusting for these chronic diseases, the HR for overall cancer incidence in patients with gout was 1.053 (95% CI, 1.031 to 1.077). With regard to specific cancers, gout was significantly associated with the risk of esophageal, stomach, colon, liver, pancreatic, lung, ovarian, renal, and bladder cancers. In contrast to our results, a recently published study from Taiwan reported that, after adjusting for concomitant disorders including hypertension, diabetes, and dyslipidemia, patients with gout did not show a high risk of colorectal cancer. This observation could be attributed to the fact that urate-lowering agents and anti-inflammatory drugs might lower the risk of colorectal cancer [9]. A previous study from Taiwan reported that the risk of colon cancer was not significantly high in patients with gout [6]. The risk of colon cancer in Taiwanese patients with gout might differ from that in a similar population of Korean patients, thus providing a potential direction for future research. Our results concur with the results of a meta-analysis reported by Wang et al. [10], who investigated the association between gout and cancer. Their results showed that the risk of cancer, particularly urological, gastrointestinal tract, and lung cancer, was significantly high in patients with gout. With regard to prostate cancer, our results differed from those reported by other studies. Reportedly, gout is associated with an increased risk of prostate cancer; however, we observed a negative association between gout and prostate cancer in our study. This finding could be attributed to the following factors. (1) Most patients with gout (85.49%) received allopurinol. The antioxidant and anti-inflammatory actions of allopurinol protect against carcinogenesis [11], and therefore, allopurinol use lowers the risk of prostate cancer in patients with gout. Xanthine oxidase is known to produce reactive oxygen species and subsequently cause inflammation and tissue injury [12]. We assumed that the main effect of allopurinol for cancer prevention is an anti-inflammatory one rather than one of lowering uric acid levels. (2) Regional differences could play a contributory role in this context. The results reported by the aforementioned studies from Taiwan and Sweden showed that gout was associated with a significantly high cancer risk in both countries; however, the cancer risk varied across different cancer types. The risk of oral cavity and pharynx, colon, liver, biliary tract, pancreas, lung, skin, endometrial, and kidney cancer was increased in patients with gout in Sweden. However, studies from Taiwan reported that the risk of liver, colon, lung, stomach, and bladder cancer was not high and that the HR of breast cancer was significantly decreased in patients with gout [6]. Therefore, it appears that regional and ethnic differences affect the cancer risk of specific cancer types in patients with gout.
Some studies have reported high cancer-related mortality in patients with hyperuricemia and gout. Strasak et al. [13] investigated the association between uric acid and cancer mortality in an Austrian cohort of women and reported that hyperuricemia was independently associated with an increased overall cancer mortality risk. Similar results were observed in an Austrian cohort comprising men; hyperuricemia was significantly associated with mortality secondary to gastrointestinal and respiratory system cancers [14]. A recently published meta-analysis reported a linear association between SUA levels and overall cancer mortality [15]. However, the results of a prospective cohort study of Korean men reported by Jee at al. [16] differed from those reported by the aforementioned meta-analysis. These authors proposed that SUA was not independently associated with cancer-related mortality; however, a significant interactive effect of uric acid and diabetes was observed in the study. Interestingly, some authors have described a complex association between uric acid and cancer and deny a simple linear correlation. Strasak et al. [17] proposed a J-shaped model to depict the association between uric acid and cancer risk. The cancer incidence was higher in patients showing low and high SUA levels compared with those showing a medium SUA level (4.5 mg/dL). Another study that investigated the association between uric acid and mortality reported that patients with SUA levels at either extreme were at a higher risk for cancer-related mortality [3]. The increased cancer risk in patients with gout could be attributed to the following factors: hyperuricemia contributes to tumorigenesis by promoting inflammatory stress on reactive oxygen/nitrogen species synthesis and cyclo-oxygenase activation. Following neoplastic transformation, the free radical scavenging property of extracellular uric acid protects cancer cells against oxidative stress-induced apoptosis, thereby facilitating tumor cell proliferation, migration, and survival [18]. Additionally, uric acid can induce chemotaxis of mesenchymal stromal human cells, which are associated with tumor progression and metastasis, and can therefore reduce tumor surveillance by promoting differentiation of immunosuppressive lymphocytes [19].
The following are the limitations of our study. (1) The Control Group in this study included patients with nail disorders and not healthy individuals. We intended to include healthy individuals as the control group; however, the Korean NIH service could not provide data for healthy individuals in this study. Therefore, patients with nail disorders were assigned to the control group after excluding patients with both gout and cancer. The population of nail disorders seems to be similar to that of healthy controls because nail disorder is a very heterogeneous and relatively benign disorder. In our view, this control group was appropriate for the data analysis performed in this study. (2) The prevalence of concomitant diseases in this study was lower than that observed in other studies, which could be attributed to the fact that we defined concomitant diseases using the main and sub-diagnosis codes, and not the entire diagnosis code. Notably, definitions used to categorize disease were the same in patients with gout and in controls; therefore, it is unlikely that our results were significantly affected. (3) We did not reflect the severity of disease in the analysis because of limited availability of claim data. Our results would have been more meaningful if clinical data such as the presence of tophi, bone erosion, smoking habits, alcohol consumption, physical activity, familial history, or dietary habits in cancer patients were available.
To summarize, we investigated the risk of various cancers after adjusting for concomitant disorders over a relatively long-term follow-up period in patients with gout, which, in our opinion, serves as a strength of this study. We propose regular and close monitoring of patients to identify occult malignancy to improve the quality of care delivered to patients with gout.
KEY MESSAGE
1. The incidence of overall cancer was higher in gout patients than in control patients.
2. The risks for cancer of esophageal, stomach, colon, liver, pancreatic, lung, ovarian, renal, and bladder remained higher in gout patients after adjusting for concomitant diseases.
3. The incidence of overall cancer was higher in gout patients than in control patients.
4. In contrast to the reports from other countries that the risk for prostate cancer is increased in gout patients, the risk of prostate cancer is decreased in Korean gout patients than control patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008;324:1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 2.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo CF, See LC, Yu KH, Chou IJ, Chiou MJ, Luo SF. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology (Oxford) 2013;52:127–134. doi: 10.1093/rheumatology/kes223. [DOI] [PubMed] [Google Scholar]
- 4.Boffetta P, Nordenvall C, Nyren O, Ye W. A prospective study of gout and cancer. Eur J Cancer Prev. 2009;18:127–132. doi: 10.1097/CEJ.0b013e328313631a. [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Yen JH, Chang SJ. Gout patients have an increased risk of developing most cancers, especially urological cancers. Scand J Rheumatol. 2014;43:385–390. doi: 10.3109/03009742.2013.878387. [DOI] [PubMed] [Google Scholar]
- 6.Kuo CF, Luo SF, See LC, Chou IJ, Fang YF, Yu KH. Increased risk of cancer among gout patients: a nationwide population study. Joint Bone Spine. 2012;79:375–378. doi: 10.1016/j.jbspin.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Richette P, Doherty M, Pascual E, et al. 2016 Updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 8.Tu H, Wen CP, Tsai SP, et al. Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ. 2018;360:k134. doi: 10.1136/bmj.k134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang JP, Lee JC, Leu TH, Hidajah AC, Chang YH, Li CY. Association of gout and colorectal cancer in Taiwan: a nationwide population-based cohort study. BMJ Open. 2019;9:e028892. doi: 10.1136/bmjopen-2019-028892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Xu D, Wang B, et al. Increased risk of cancer in relation to gout: a review of three prospective cohort studies with 50,358 subjects. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/680853. 680853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shih HJ, Kao MC, Tsai PS, Fan YC, Huang CJ. Long-term allopurinol use decreases the risk of prostate cancer in patients with gout: a population-based study. Prostate Cancer Prostatic Dis. 2017;20:328–333. doi: 10.1038/pcan.2017.14. [DOI] [PubMed] [Google Scholar]
- 12.Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase in cancer: more than a differentiation marker. Cancer Med. 2016;5:546–557. doi: 10.1002/cam4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strasak AM, Rapp K, Hilbe W, et al. The role of serum uric acid as an antioxidant protecting against cancer: prospective study in more than 28000 older Austrian women. Ann Oncol. 2007;18:1893–1897. doi: 10.1093/annonc/mdm338. [DOI] [PubMed] [Google Scholar]
- 14.Strasak AM, Rapp K, Hilbe W, et al. Serum uric acid and risk of cancer mortality in a large prospective male cohort. Cancer Causes Control. 2007;18:1021–1029. doi: 10.1007/s10552-007-9043-3. [DOI] [PubMed] [Google Scholar]
- 15.Xie Y, Xu P, Liu K, et al. Hyperuricemia and gout are associated with cancer incidence and mortality: a meta-analysis based on cohort studies. J Cell Physiol. 2019;234:14364–14376. doi: 10.1002/jcp.28138. [DOI] [PubMed] [Google Scholar]
- 16.Jee SH, Lee SY, Kim MT. Serum uric acid and risk of death from cancer, cardiovascular disease or all causes in men. Eur J Cardiovasc Prev Rehabil. 2004;11:185–191. doi: 10.1097/01.hjr.0000130222.50258.22. [DOI] [PubMed] [Google Scholar]
- 17.Strasak AM, Lang S, Kneib T, et al. Use of penalized splines in extended Cox-type additive hazard regression to flexibly estimate the effect of time-varying serum uric acid on risk of cancer incidence: a prospective, population-based study in 78,850 men. Ann Epidemiol. 2009;19:15–24. doi: 10.1016/j.annepidem.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fini MA, Elias A, Johnson RJ, Wright RM. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 2012;1:16. doi: 10.1186/2001-1326-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenbacher JL, Schrezenmeier H, Jahrsdorfer B, et al. S100A4 and uric acid promote mesenchymal stromal cell induction of IL-10+/IDO+ lymphocytes. J Immunol. 2014;192:6102–6110. doi: 10.4049/jimmunol.1303144. [DOI] [PubMed] [Google Scholar]