Abstract
Exposure to radiation during the treatment of CNS tumors leads to detrimental damage of the blood brain barrier (BBB) in normal tissue. Effects are characterized by leakage of the vasculature which exposes the brain to a host of neurotoxic agents potentially leading to white matter necrosis, parenchymal calcification, and an increased chance of stroke. Vasculature of the blood tumor barrier (BTB) is irregular leading to poorly perfused and hypoxic tissue throughout the tumor that becomes resistant to radiation. While current clinical applications of cranial radiotherapy use dose fractionation to reduce normal tissue damage, these treatments still cause significant alterations to the cells that make up the neurovascular unit of the BBB and BTB. Damage to the vasculature manifests as reduction in tight junction proteins, alterations to membrane transporters, impaired cell signaling, apoptosis, and cellular senescence. While radiotherapy treatments are detrimental to normal tissue, adapting combined strategies with radiation targeted to damage the BTB could aid in drug delivery. Understanding differences between the BBB and the BTB may provide valuable insight allowing clinicians to improve treatment outcomes. Leveraging this information should allow advances in the development of therapeutic modalities that will protect the normal tissue while simultaneously improving CNS tumor treatments.
Keywords: Blood brain barrier, Blood tumor barrier, Radiation, Neurovascular unit
1. Introduction
The role of the blood brain barrier (BBB) is to maintain homeostasis within the central nervous system (CNS). This is achieved through a unique microvasculature network that physically blocks and selectively transports specific molecules across the barrier via combined efforts of endothelial cells, tight junction (TJ) proteins, pericytes, basal lamina, and astrocytes that make up the neurovascular unit (NVU). When functioning properly, the NVU creates a tight seal around blood vessel walls that requires adsorptive and carrier mediated transportation of non-lipid soluble agents [1]. In addition, endothelial cells contain ATP dependent efflux transporters that actively push foreign material back into the lumen [1,2]. Conversely, CNS tumor vasculature, commonly referred to as the blood tumor barrier (BTB), is prone to irregularities when unregulated and disorganized vasculature growth causes disruptions in the barrier within glioblastomas (GBM) and secondary metastases [3–5]. The importance of a well-organized BBB is highlighted when this system fails to filter foreign material from the blood and it is allowed to enter the brain. We can observe such failures in the BBB and BTB directly after radiotherapeutic interventions by measuring leakage into the parenchymal space [6–9]. While preserving the BBB is critical to maintaining homeostasis within the healthy CNS, exploration into new physical and chemical treatment options endeavor to therapeutically disrupt the BTB for drug delivery [10–13].
Current treatment plans for CNS tumors involve surgical resection, irradiation, and chemotherapeutic regimens. An estimated 86,000 new cases of CNS cancer occurred in 2019. Even with the latest treatment options available, the five-year survival rate for patients with all types of malignant tumors was 35.8%, while GBMs specifically had a five-year survival rate of only 6.8% [14]. This low rate of survival is in part attributed to insufficient drug intervention options due to the relative impenetrability of the BBB and even within the barrier compromised BTB. In GBM, the integrity of the BTB is not uniform, leaving regions of the tumor variably exposed to drugs that are readily blocked by the BBB [15,16]. However, even with the compromised vasculature of the BTB, uptake of chemotherapeutic drugs paclitaxel and doxorubicin exhibit relatively low penetrance within metastatic tumors [17]. This indicates that while the BTB may be more susceptible to toxic leakage, other interventions are required to open the vasculature further within the tumor. This is likely the reason that many chemotherapeutic drugs fail during clinical trials as the variance in BBB and BTB permeability is not fully understood [18]. Clearly, failure to treat the entire tumor parenchyma compromises the efficacy of any chemotherapeutic treatment regimen leading to tumor recurrence, a routine and confounding complication.
To circumvent these limitations, new methods must be implemented to permeabilize the vasculature of targeted regions to facilitate drug delivery, or to actively assist in denaturing the semi-intact BTB within the CNS while protecting the surrounding normal tissue. Others have highlighted the benefits of creating a porous BBB and/or BTB with the use of radiation [19,20], ultrasound [21,22] and receptor mediated transport [23] to improve drug delivery. The caveat to these strategies is that permeabilizing the BBB has non-targeted effects in normal tissues that may lead to indiscriminate damage to the brain. Further studies that define key differences between the BTB and BBB may provide new insight, allowing for targeted breakdown of tumor vasculature and improved chemotherapeutic treatment within the CNS.
This review describes the foundational differences between the BBB and BTB and the dichotomy between the deleterious effects and therapeutic opportunities that radiotherapy presents, emphasizing the current efforts to create leakage across the BBB/BTB and its implications for the penetrance of therapeutic interventions and neurotoxicity.
2. Neurovascular unit (NVU)
The NVU is a collection of all cell types that form and regulate the capillary network within the brain. Primarily the NVU defines a physical barrier, formed by the binding of endothelial cells via TJ proteins to create a non-fenestrated capillary wall. Endothelial cells play a primary role in controlling entry into the brain parenchyma through expression of transporter proteins. These cells are supported and encased by pericyte cells, the basement membrane, and astrocytic endfeet that provide additional regulation and feedback from signaling within the brain. Local neurons also play a regulatory role by controlling nutrient demand and feedback signals among these cell types to modify molecular, ion and aqueous transport in a process called neurovascular coupling (NVC) (Fig. 1).
Fig. 1. Comparison of the neurovascular unit of the blood brain barrier and blood tumor barrier.
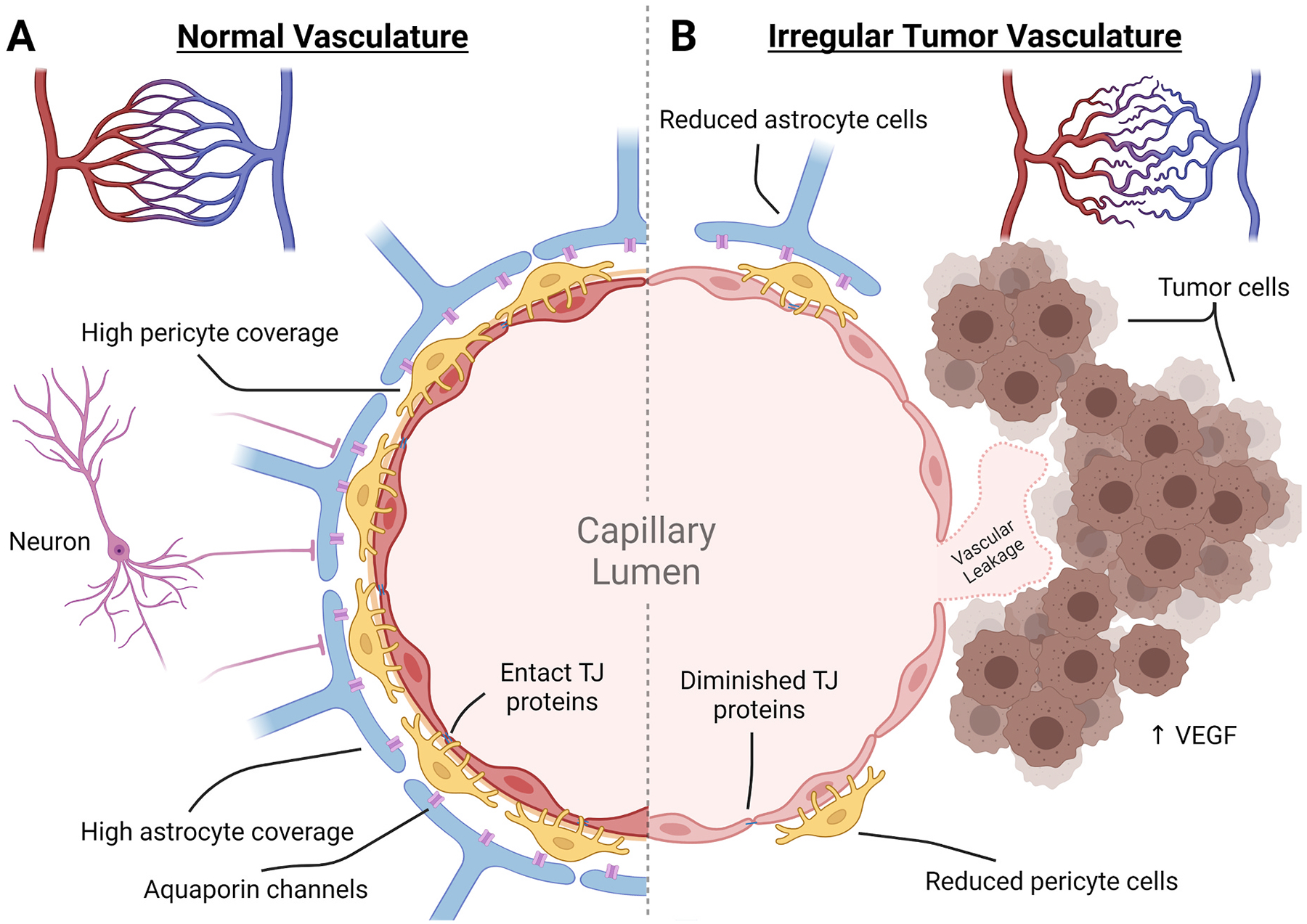
Normal vasculature of the brain develops from pial arteries to arterioles to capillaries where transfer of nutrients and oxygen can take place. In the normal tissue, capillaries are spread out somewhat uniformly to ensure equal distribution. In the BTB, there is a reduced presence of capillary endings and an irregular overlay creating areas of hypoxic tumor tissue. (A) In the normal tissue capillaries, the neurovascular unit controls the influx of materials by forming a non-fenestrated endothelial cell layer that is tightly bound together with TJ proteins. These are enveloped by pericyte cells and astrocytic endfeet that aid in maintaining the endothelial cell transport proteins, TJ proteins, induction of angiogenesis, and influx of ions and fluids. This is controlled by local input as well as neuronal demand that can interact directly or through astrocytic channels. (B) Within the BTB, integrity is compromised. Tumor cells attach to the outer vascular wall and induce vascular growth through VEGF expression. The new capillary bed is irregular and leaves areas of the tumor hypoxic. The new vasculature becomes the foundation of the BTB and has been found to have reductions in astrocytic endfeet coverage, pericyte cells, TJ proteins, and neuron attachment. The resulting capillary wall of the BTB is susceptible to neurotoxic leakage.
2.1. Endothelial cells and tight junction proteins
The microvasculature of the CNS relies primarily on the layer of endothelial cells bound together by TJ proteins encased within the basement membrane. This endothelial monolayer creates the interface via which molecules enter the brain. These cells harness carrier and receptor mediated efflux, organic anion and cation, nucleoside, and large amino acid transporter systems to maintain gradients and push unwanted waste and metabolites into the glymphatic waste clearance system. Of particular importance are ATP-driven efflux pump transporters including the ATP-Binding Cassette (ABC), P-glycoprotein, breast cancer resistance protein, and multidrug resistance related proteins, as these work to actively remove chemotherapeutics and xenobiotics [24,25]. Facilitated transporters Glut1 and major facilitator superfamily domain-containing protein 2A (Mfsd2a) are also considered critical to proper BBB function and overall health. Glut1 is responsible for influx of glucose, the primary fuel for neurons, and Glut1 deficiency is known to cause mitochondrial disfunction and epilepsy in children [26]. Mfsd2a transporters are in part responsible for transport of essential omega-3 fatty acids in CNS endothelial cells. In transgenic knockout mouse models of Mfsd2a, levels of docosahexaenoic acid (DHA, an essential omega-3 fatty acid) are diminished along with leakage of 10 kDa dextran observed from embryonic through adult stages. Further electron microscopy imaging has indicated that cells and TJ proteins were structurally intact, indicating a role for Mfsd2a in preventing foreign material from crossing the BBB [27]. Additionally, loss of Mfsd2a and concomitant reductions in DHA diminish mitochondrial activity, likely responsible for impaired cognition and ataxia in mice [28,29].
TJ proteins are essential to creating a sealed barrier between endothelial cells, forcing molecules to enter the CNS through active transporter mediated mechanisms as opposed to passive diffusion. This is achieved by a series of proteins that function together to create a non-fenestrated vasculature. Junctional adhesion molecule 1 (JAM-1) proteins help connect cells while simultaneously recruiting TJ proteins, including claudin family proteins, ZO-1, and Occludin, that further tighten the seal between endothelial cells and facilitate cell-cell signaling [30–32]. Damage to these TJ protein populations can allow larger molecules to bypass endothelial cell regulatory mechanisms. While cells can lose TJ proteins, repair mechanisms are in place to maintain structural integrity of the BBB. An in vitro study of TJ proteins performed under real-time confocal microscopy demonstrated that EGTA and laser insults reduced TJ integrity and increased permeability that was actively repaired by transient localized Rho activation [33]. The dynamic degradation and rapid repair of TJ proteins provides a brief window of permeability to the BBB, a feature that could be exploited clinically for drug delivery to the CNS.
2.2. Pericytes
Pericytes play a crucial part of the NVU as they regulate cerebral blood flow, stabilizing vessels, initiating angiogenesis, and maintaining the BBB. These cells make up a large portion of the BBB, almost matching the number of endothelial cells [34]. The versatility of pericytes is due to specialized subpopulations that support and regulate the BBB and assist angiogenesis within the BBB. Ensheathing pericytes physically control cerebral blood flow by contracting or relaxing within regions based on metabolic demand [35,36]. This is achieved through expression of α-smooth muscle actin (α-SMA), tropomyosin, and myosin [36–38] that actuate contractile processes.
Defining subpopulations of pericytes can be complicated as there is no defined marker for each type, rather they are referred to by protein expression that determines functionality of the particular cell. Further complicating the categorization of pericytes, expression of these defining proteins can be dependent on tissue-specific cell type, developmental stage of angiogenesis, or maturity of the cell. Pericytes with the platelet-derived growth factor-β (PDGFβ) receptor are recruited by endothelial cells via PDGFβ and transforming growth factor-β (TGF- β), where they implant within the basement membrane of the blood vessel to bolster the BBB [34,39]. These pericytes help to stabilize the vasculature [40], control the cell cycle of endothelial cells [39], control regulation of TJ proteins [34], and assist in angiogenesis by secreting notch homolog protein 3 (NOTCH3) and vascular endothelial growth factor (VEGF) [36,40]. Pericyte cells express desmin and α-SMA that form contractile filaments and allow cells to exhibit control of blood flow by constriction on the blood vessels. Desmin and α-SMA expression in pericytes are found on venular and arterioles, while only desmin+ cells are found on capillaries [41,42]. This likely indicates that these cells have unique abilities that larger blood vessels require, while capillaries may specifically require desmin+ pericytes only to regulate blood flow. Pericytes have also been shown to exhibit immuno-regulatory functions through their capability to phagocytose other cells, respond to inflammatory markers, and present antigens to T cells [43]. In response to inflammatory cytokines interleukin-1b and tumor necrotic factor-alpha (TNF-α), pericytes can express matrix metalloprotease 9 which leads directly to BBB breakdown [44,45]. Since these cells are an integral part of the BBB, damage to pericytes can have detrimental effects on the BBB and the homeostasis of the CNS. In that light, loss of pericytes likely exacerbates several adverse neurological conditions including Alzheimer’s Disease and ischemia [35,46].
2.3. Astrocytes and the gliovascular unit
Glial cells are an integral part of the NVU, and are intermediaries between neurons and the vasculature, defining a smaller subset of the NVU called the gliovascular unit (GVU). The GVU is primarily responsible for linking the needs of the neuronal population in which they are embeded and the vasculature system that delivers the required oxygen and nutrients into the perivascular space. In vitro studies of astrocytes cocultured with endothelial cells have suggested that a complex relationship exists between the two cell types. Endothelial cells have shown increased expression of ABC transporters and TJ proteins when they are co-cultured with either astrocytes or astrocyte conditioned media [47, 48], suggesting that cytokine signals from astrocytes create a stronger BBB. This could be due to astrocytic expression of glucagon-like-peptide-1 (GLP-1R) which has been shown to reduce the permeability of the BBB [49]. Co-culture studies have also shown the importance of TGFB1, as reduction of this astrocytic-derived signal reduces Msfd2a in endothelial cells, causing increased permeability [50]. However, astrocytes are also known to secrete vascular endothelial growth factor VEGF and NFkB that act as pro-inflammatory effectors as well as metalloproteinases (MMPs), chemokines and cytokines that can directly or indirectly damage the BBB. Angiogenesis induced by VEGF causes a breakdown of the BBB by downregulating CLDN5 and OCLN allowing vascular modifications [51,52]. Leakage of the BBB has been detected through measurements of FITC labeled dextran crossing into regions of the CNS undergoing angiogenesis expressing elevated VEGF [53,54]. It has also been found that over expression of MMP-9 causes the degradation of CLDN5 and OCLN in vitro [55] and reductions of other TJ proteins in a mouse model of ischemia [56]. Furthermore, blocking MMP-9 prevents the degradation of TJ proteins and preserves the functional integrity of the BBB in rat models of ischemia [57], suggesting the importance of this class of proteins for BBB integrity.
2.4. Neurovascular coupling
Being highly specialized cells that require constant metabolic input, neurons act as overseers of the NVU and regulate blood flow through a process called neurovascular coupling or cerebral hyperemia. When neurons are activated, metabolic demand is increased to restore ionic gradients, recycle neurotransmitters, or produce ATP. Increase in this metabolic demand is largely driven by aerobic metabolism, requiring copious amounts of glucose and oxygen. Neurovascular coupling is thought to induce vascular dilation via release of many different vasoactive factors from regions of synaptically active neurons, increasing access to glucose and oxygen to fuel high metabolic demands. These factors include neurotransmitters, ions, and metabolic by-products released from neuron projections and interneurons extending to the vasculature that act directly on the BBB or through distal paracrine signaling extending to reach upstream arteriole regions of the vasculature (>100 mm) [58,59]. Of special note are the 3 isoforms of nitric oxide synthase (NOS) enzymes that catalyze nitric oxide (NO), a potent vasodilator, from l-arginine; neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). All three isoforms of NOS produce NO and are expressed within the brain. Conflicting reports of NOS1-knockout mice (nNOS) have shown a lack of dilation during stimulation [60] as well as normal NVC dilation function [61]. This may indicate that other isoforms of NOS may help stabilize the loss of neuronal NOS production, but also that neuronal activity is not the sole triggering event to activate dilation [62].
Control of dilation in the microenvironment of the brain is hypothesized to rely on retrograde neuronal signaling transmitted to the pial arteries and penetrating arterioles which are wrapped in contractile smooth muscle cells. Early studies in rabbits showed that activation of NMDA receptors led to NO-mediated dilation of upstream pial arterioles [63]. Since then, a host of other neurotransmitters have been linked to mediation of vascular changes, including dopamine, substance P, serotonin, γ-aminobutyric acid, noradrenaline, neuropeptide Y, somatostatin and acetylcholine [64,65]. While no consensus signaling pathway within the neurovascular unit has emerged, it is likely that these signals can travel a certain distance (>1 mm) via endothelial hyperpolarization to upstream pial arteries to modify blood flow [66].
2.5. Glymphatics
Until recently, it was generally believed that the brain was responsible for recycling its own waste while only a small amount of protein was shed back through the BBB due to the lack of or of an ill-defined lymphatic system [67]. This idea changed when Iliff et al. demonstrated clearance of cerebral spinal fluid (CSF) and Aβ protein along perivenous drainage pathways and reported it to be astrocytic protein aquaporin-4 (AQP4) dependent [68,69]. Since then, it has been largely accepted that the glymphatic system is responsible for maintaining and regulating cerebral fluid dynamics, hydrostatic pressure, waste product removal and ion concentrations.
The glymphatic system facilitates waste clearance in the brain through the several different mechanisms. Influx of CSF travels through the perivascular space surrounding penetrating arteries, crossing into the interstitial space through AQP4 channels. Here it mixes with interstitial fluids (ISF), releasing CSF waste consisting of metabolic byproducts, ions, and fluids into the parenchyma. The resulting ISF are then drawn from arteries, through the parenchyma and towards the veins of the paravenous space through a process called convective flow [70,71]. This exchange of ions and flush of interstitial fluid waste is regulated by arterial pulsations during deep sleep [69,70,72,73].
Proper waste clearance from the brain appears to be vital as neurodegeneration is being attributed to glymphatic disfunction in the elderly. One hypothesis attributes age related reductions of deep REM sleep with decreased arterial pulsations that drive clearance, creating favorable conditions for Aβ aggregation [70,72,74,75]. Additionally, cardiac health and systemic blood pressure are the drivers of arterial pulsations, directly controlling convective flow. This has been observed in murine models of both hypertension and diabetes where direct reductions in glymphatic function were found [76,77].
3. Blood tumor barrier
The vast majority of brain cancers form due to metastasis of primary tumors of the lungs, breast and skin [78]. This occurs when cells of the existing primary tumor release and enter the circulatory system (lymphatics and blood), traverse, infiltrate and implant themselves within the brain. As newly formed tumor cells grow and proliferate, nutrients are leeched from the existing blood supply in a process called cooption [52,79,80] while simultaneously new vasculature is created through the increased expression of VEGF by astrocytes and pericytes (Fig. 1). Clinically, anti-angiogenic agents fail to stop tumor growth because tumors have evolved multiple strategies to compensate for hypoxia and localized nutrient deprivation [81]. However, some studies have shown the benefit of using anti-angiogenic agents alongside radiotherapy. It has been shown that as tumors grow past 1–2 mm in diameter within the parenchyma of the brain, the protective nature of the BBB begins to diminish, coinciding with the onset of tumor hypoxia [82,83]. Hypoxia triggers hypoxia-inducible factor (HIF) stabilization that drives VEGF expression, known to promote vascular permeability along-side its pro-angiogenesis functions, that contribute to irregularities in the BTB as the tumor vasculature responds to dynamic changes in the microenvironment [84–86]. However, use of anti-angiogenesis treatments that target VEGF/VEGF2 have led to mixed results. According to Liu et al. this is likely due to GBM endothelial cell transformation and genetic reprogramming that allows proliferation and migration even under conditions of down regulated VEGF [87,88]. This indicates that other unknown factors are involved in developing irregular, chemo-resistant vasculature of the BTB. Efforts have also been focused on normalizing the tumor vasculature through the use of angiogenic stimulators, (for reviews see Refs. [89,90]), with the overarching goal to reduce leakage, increase oxygenation, and to facilitate immune cell infiltration to enhance sensitivity to therapeutic intervention.
Tumor-derived angiogenesis plays only one part of BTB permeability as formation of the tumor vasculature is structurally compromised at many levels. Brain tumors exhibit altered expression of TJ proteins, contributing to vascular leakage via paracellular diffusion [91,92]. This is likely due to reductions in Kruppel-like transcription factor 4 (KLF4) expression, resulting in decreased mRNA production of TJ proteins Claudin5, ZO-1 and Occludin [92]. Further, measurements of GBM in patients indicate down regulation of the critical TJ proteins Claudin1, 3, and 5 [91]. While these changes in TJ proteins are wide spread, resultant vascular permeability is heterogeneous across the BTB, evident from unequal distribution of low molecular weight compounds within brain tumors [93]. Despite research focused on manipulating the BTB in efforts to aid drug delivery, realizing bona-fide improvements in the therapeutic index have been difficult to obtain.
While endothelial cells within the BBB are the first line of defense against intrusion of foreign materials, their presence in the BTB can be compromised during tumor progression leading to enhanced leakage. Tumor growth can alter expression and integrity of TJ proteins while inducing angiogenesis and adversely impact the expression of transporter proteins that help maintain homeostasis. Mfsd2a, a DHA transporter, downregulated within the endothelial cells of the BTB, has been found to be attributed to widespread leakage [50]. Significant loss of perivascular astrocytes in the developing BTB and a subsequent decrease in TGFβ1 was speculated to decrease Mfsd2a expression, thereby reducing the protective properties of endothelial cells in the BTB [50]. Liquid chromatography with tandem mass spectrometry measurements of GBM vasculature samples show a significant decrease of facilitator transporter Glut1 and efflux transporters ABCB1 And ABCG2 [94]. Disruption of these transport markers likely play a large part in BTB permeability, as shown in knockout models showing increased influx of fluorescent labeled 10 kDa dextran into the normal tissue parenchyma [27].
Reduction in pericyte numbers correlates with increased permeability within the BTB. In a clinical study following GBM patients, pericyte coverage was inversely correlated to the prognosis of patients treated with chemotherapeutic drugs [95]. The authors attribute the extended life span of these patients to the increase in BTB permeability, allowing enhanced chemotherapeutic access to the tumors. In breast cancer metastasis of the brain, it was found that PDGFRβ+ pericytes decreased by 75%, while Desmin+ pericyte cell numbers more than doubled along the BTB [93]. Desmin+ pericytes are less involved with protection of the BBB than their PDGFRβ+ counterparts and are found to migrate and accumulate in newly forming BTB to promote angiogenesis [96]. While further work is required to determine the individual roles of these different classes of pericytes, it is interesting to note that the BTB can alter subpopulations of these cells to actively modulate vascularization.
The transition from a normal to a cancerous cell is a complex process that is incompletely understood. The brain is particularly susceptible to oxidative damage due to its high metabolic demands, vascularization, oxygen utilization and lipid content. The collective generation of reactive oxygen and nitrogen species (ROS and RNS) leads to macromolecule damage, lipid peroxidation, and reactive aldehydes which promote carcinogenesis and continue throughout tumorigenic growth. Several studies have revealed that a biproduct of oxidative stress, 4-hydroxynonenal (4-HNE), is increased in regions of brain tumors and correlates with poorer prognosis [97–99]. In addition, the GBM stem cell marker CD133 has been found to co-label with 4-HNE, suggesting that 4-HNE may play a role in cancer stem cell transitions [100]. Oxidative injury caused by lipid peroxidation and reactive aldehydes lead to upregulation of matrix metalloproteinases (MMPs), ultimately leading to breakdown of the extracellular matrix and increases in vascular permeability [101], a pattern that has been observed within GBM [98]. An in vitro study of rat brain endothelial cells exposed to 4-HNE exhibited increases in sodium fluorescein permeability by 10 fold within 20 min, highlighting its ability to modify the extracellular matrix that maintains the vasculature [102]. Furthermore, 4-HNE has been equated to a growth regulating factor as it is highly expressed during conditions of sepsis and ischemia within the brain [103]. Drawing on these studies, it seems likely that 4-HNE is involved in remodeling the stromal and tumor vasculature, allowing tumors to hijack vessels and nutrients for their growth.
As gliomas grow and co-opt surrounding blood vessels, astrocytic endfeet are displaced from the vasculature, thereby disrupting homeostasis of the surrounding neurons [80,104–106]. As gliomas increasingly co-opt the NVU, they produce VEGF to elicit angiogenesis, promoting blood vessel dilation and regional NVU permeability along the BTB [4]. Astrocytic endfeet also play a role in brain metastasis, as activated astrocytes physically surround the growing lesion to trigger a neuroinflammatory response. Activated astrocytes have been found to upregulate astrocytic sphigosine-1 phosphate receptor 3 (S1P3) which was found to inhibit constriction of the BTB. Additionally, by knocking down S1P3 in an in vitro model of activated or reactive astrocytes, reduced permeability was found within both the BBB and BTB as well as decreases in secreted chemokines, growth factors and interleukins [107]. While astrocytes are not as directly involved in forming a physical barrier as pericytes and endothelial cells, they play key regulatory roles in the secretion of multiple factors that maintain equilibrium among the collective components of the NVU.
4. Radiation induced changed within the BBB and BTB
Radiotherapy is frequently prescribed for the treatment of CNS tumors and vascular malformations, inducing damage to the target region as well as to any normal tissue along the path of the beam. Damage produced by irradiation of tissue is caused by both direct and indirect pathways. Direct ionization of cellular macromolecules including DNA, RNA, lipids and proteins can induce lethal or sublethal damage to a cell [108]. Ionizing radiation also indirectly damages cells through the generation of reactive oxygen species that damage intracellular target molecules [108]. Resultant damage can lead to mitotic catastrophe, necrosis, apoptosis, autophagy, or senescence (Fig. 2). As a potent radiosensitizer, oxygen levels play a large role in dictating therapeutic outcomes, and vascular irregularities common to tumors routinely lead to hypoxia that compromises curative intent. Dose fractionation, beam modalities, and stereotaxic approaches are used to maximize damage to the tumor while minimizing exposure to the surrounding normal tissue. However, unavoidable vascular injury to normal tissues can still impact both acute and late responses to radiotherapy. Of particular concern to clinicians is the development of edema and the increased risk of stroke, late effects that transpire in the irradiated brain alongside white matter necrosis, lacunar infarcts, and parenchymal calcification, although each transpires with temporally distinct onsets [109].
Fig. 2.
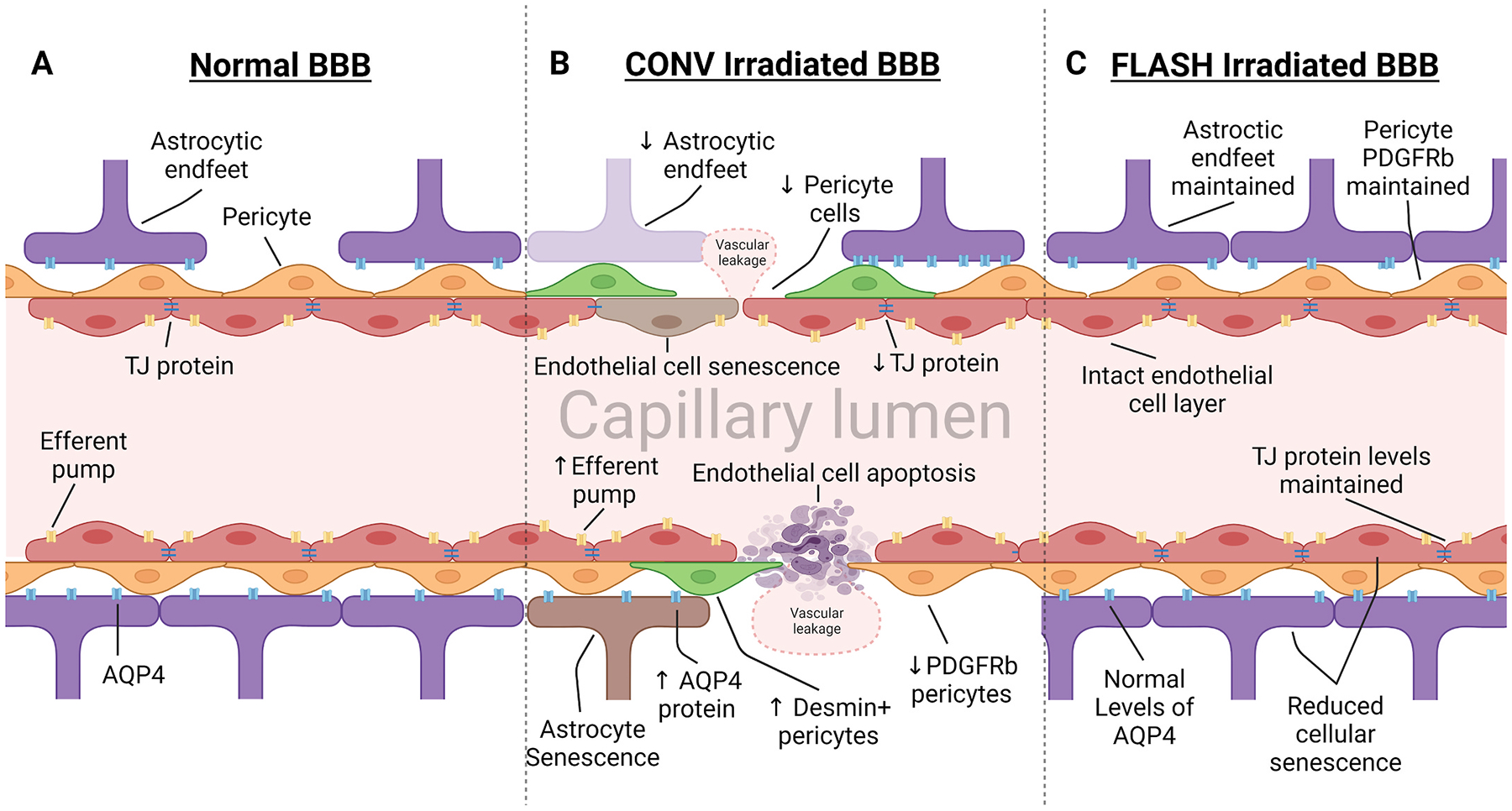
Irradiation of the blood brain barrier leads to damage of the neurovascular unit. Vasculature of the BBB is susceptible to radiotherapy treatment, inducing modifications to protein expression and apoptosis and senescence of cells that alter homeostasis. (A) Normal BBB expresses high levels of TJ proteins, sealing gaps between endothelial cells, preventing paracellular crossing. Expression of efferent pumps on the lumen side of endothelial cells actively work to remove foreign materials and chemotherapeutic drugs from the brain. Pericytes embedded within the basement membrane provide support and regulation over dilation and TJ expression. Astrocytic endfeet express aquaporin protein (AQP4), regulating fluid transfer across the membrane. (B) Radiotherapy induces leakage of the BBB through apoptosis and senescence in cells of the NVU. Additionally, declines in TJ protein expression lead to vascular leakage through paracellular channels. Pericyte subpopulations respond to BBB damage by reducing expression of PDGFRβ and replacing it with desmin. Astrocytes produce irregular expression of AQP4, contributing directly to dysregulated fluid transfer into the CNS, causing edema. (C) Ultra-high dose rate FLASH irradiation protects the vasculature through reduced induction of cellular apoptosis and conservation of TJ proteins. Due to the normal tissue sparing effect observed in previous FLASH irradiation studies, we hypothesize that protection of the BBB is due to reduced toxicity to endothelial cells, pericytes, and astrocytic endfeet.
Shortly after CNS irradiation, injection of radioactive tracers or fluorescent dyes indicate leakage of the BBB in human clinical trials, and in in vitro and in vivo models [7,110–116]. Within the first 24 h after irradiation, vascular leakage is attributed to radiation-induced cell death. In one study, a 25 Gy dose resulted in a 15% reduction of endothelial cells within the CNS 24 h later [117]. Direct irradiation, or subsequent ROS production can induce apoptosis, typically starting at 4 h post-irradiation and peaking at approximately 12 h, leaving gaps within the barrier [118–120]. An in vitro model of H2O2-induced oxidative stress indicated that induction of lipid peroxidation in cerebral endothelial cells led to membrane dysfunction [122]. Furthermore, an in vitro BBB model using 4-HNE was shown to increase permeability of fluorescein, likely due to a reduction in glutathione, indicating that oxidative stress was the primary cause [102]. These effects are also observed in tumors as oxidative stress and subsequent lipid peroxidation of the vasculature can modify VEGF expression leading to vascular irregularities and leakage in irradiated GBMs [121]. Functional alterations persisting in irradiated endothelial cells that survive exposure can contribute further to permeability. Interestingly, endothelial cell expression of P-glycoprotein as well as modified glutathione, which upregulates efflux transporters, has been shown to increase after radiation exposure [123,124]. Such an effect can compromise treatment efficacy, as radiation induced overexpression of efflux transporters reduces the half-life of chemotherapeutics in the tumor.
Radiation exposure has also been shown to induce delayed vascular dilation within the brain, likely due to an increase in eNOS production [113,114,115]. Over expression of eNOS has, in part been linked to induction of vasogenic edema [125,126] which can elevate intracranial pressure leading to white matter necrosis. Breakdown of the BBB allows for intravascular proteins and fluid exchange into the extracellular space. Areas of white matter are particularly susceptible due to lowered tissue density, increased number of parallel axonal tracts and the ability of fluids to enter and travel along the white matter tracts [127]. Furthermore, vasogenic edema contributes to only a portion of intracranial pressure build up, as the glymphatic system should work to reduce pressure and remove waste. It is likely that the glymphatic system is compromised after radiotherapy, however, to date few studies have directly interrogated glymphatic system function after clinical radiotherapy regimens.
As detailed above, TJ proteins between endothelial cells dictate the integrity of the BBB. While cranial irradiation leads to minimal changes in TJ protein levels within 24 h, modifications are found within the subsequent weeks [8,115], where reduced levels of TJ proteins were observed. Protracted changes in TJ proteins may portend longer-term cerebrovascular complications such as stroke and edema [128–130]. Of particular interest is the expression of VEGF, as it has been shown to be upregulated after irradiation and is known to alter expression and phosphorylation of TJ proteins [52,131,132]. While anti-VEGF treatments have proved to be relatively ineffective in clinical efforts to forestall tumor vascularization and growth [133], they may reduce fenestrations and damage to TJs [134,135].
Pericytes of the BBB are particularly susceptible to radiation induced cell death and could contribute regions of necrosis in the brain through reduced vasculature homeostasis. In a post-mortem analysis of normal tissue after GBM treatment, Lee et al. found that radionecrotic tissues of the BBB were devoid of pericyte support cells even though no morphological changes to the vasculature were found [136]. The authors posit that the lack of pericyte support within the cerebral vasculature could be responsible for fibrinoid necrosis and telangiectasis; However, it could be possible that cell death of these pericytes is caused by some unknown factor in the regions of necrosis. While this study provides the first evidence linking pericyte loss to radiation necrosis, it has also been found that loss of these cells leads to increased neuroinflammation in pericyte deficient mouse models [137] and permanent vascular constriction [35]. It is therefore pertinent to the field of radiobiology to further investigate the radio-response of pericyte cells as they may play a vital role in regulating the neuroinflammatory response post-irradiation. Indeed, strategies able to augment or recruit pericytes to the BTB could improve GBM therapeutic outcome by improving vascularization, reducing hypoxia and enhancing radiosensitivity [90, 138].
In addition to cells of the NVU being susceptible to radiation induced apoptosis, senescence remains a great concern as it initiates persistent injury signatures within the normal tissue. Endothelial cells that survive radiation exposure may enter senescence which leads to further breakdown of the vasculature [139–141]. Studies in senescence accelerated mice (SAMP8), increases in BBB leakage were found from the initial screening at 3 months of age until end of life at 12–15 months [142, 143]. Such data suggest that BBB damage from senescent endothelial cells is not repaired and/or contributes to a senescence associated secretory phenotype (SASP) and may persist chronically. SASP modified cell expression alters interleukins, chemokines, growth factors, and proteases that create a protumorigenic environment by enabling tumor growth and metastasis [144]. In addition to endothelial cells, radiation-induced senescence of astrocytes is of particular concern as these cells escape apoptosis and linger within the parenchyma to foster chronic inflammation [145]. Senescent astrocytes have been reported to secrete proinflammatory cytokines IL-6, IL-1β, TNF-α [146,147] which can mediate persistent inflammatory signaling in the irradiated brain [148,149].
5. Clinical manipulation of the BBB and BTB
The idea that radiation alone or in combination with chemotherapy might provide an avenue for increasing the efficacy of brain tumor treatments has been a topic explored extensively [15,90,150]. Under normal, healthy conditions, endothelial cells of the BBB are known to actively remove anticancer agents by way of ATP dependent efflux transporters, reducing cytotoxicity [11–13,151]. By using tumor targeted therapies, clinicians could capitalize on the inherent leakiness of the BTB, while the intact BBB protects the normal tissue from treatments. While tumor morphology is generally thought to play a role in permeability, as masses of larger size exhibit more leakage than smaller tumors [83,152,153], some observations contradict this assertion [114]. To further complicate matters, tumor lineage must be considered, as phenotypic differences between GBM and metastatic tumors of various other origins influence success of induced permeabilization. Measurements of tumors indicated that morphometrically GBMs are far more engrafted into the normal tissue of the CNS while metastases are generally ovoidal in shape with a clearly defined border. Both tumor types have similar levels of cerebral blood flow, however GBMs are more prone to hemorrhaging, indicating potential predisposition to leakage on a greater scale than secondary tumors [154,155]. One factor to consider is that alterations to metastatic permeability are likely due to the genetic background of the founding tumor along with patient specific factors such as sex and age and origin of the metastasis. Differences in cytokine pattern expression from metastatic tumor origins could potentially lead to different vasculature phenotypes. One particular observation has shown that edema around secondary metastatic tumors is far higher when they originated in the lung as compared to the breast [156], likely due to increased aggressiveness and inflammation in lung cancer. Unfortunately, current literature lacks critical reviews of vasculature phenotypes of different metastatic tumors, as it may become clear that bypassing the BTB will require therapeutic treatment tailored to metastasis origins. Numerous studies have been performed to bypass the BBB or BTB by use of both physical and chemical interventions to improve efficacy of drug delivery. While many of these techniques have proven to increase permeability of the BBB, few have been shown to improve clinical treatment efficacy.
5.1. -. Radiation
As described, radiation alters the microenvironment of both tumor and normal tissues though a variety of mechanisms. The effects of radiation exposure on the BBB have been studied for decades [157–159], and while numerous animal studies have reported an increase in BBB permeability, these effects have been difficult to leverage for therapeutic gain when applied to the BTB. In part this may be due to difficulties in defining a reliable window of time following irradiation to deliver chemotherapeutic interventions. In studies of radiation induced BBB openings in mice, a single acute 20 Gy dose induced openings in the BBB that can manifest anywhere between 24 h and 90 days post irradiation [139,160]. One particular study suggests that to induce permeability of the BBB, total doses of 20–30 Gy delivered at 3 Gy/fraction are required [161]; however, to reduce long-term neurotoxicity it also been suggested that fraction sizes of under 2 Gy are required [162]. Late responding tissues (i.e., brain, spinal cord, lung, esophagus, kidney) to radiation toxicities have long been known to be sensitive to the dose/-fraction. Additionally, the dynamics of radiation induced BTB permeability follow very different kinetics within a given tumor as they are heterogeneous, fluctuating with density and distance from sources of oxygen. These considerations are clearly impacted by tumor size as Teng et al. has found that while small metastatic tumors exhibit less leakage than larger, they display higher levels of radiation induced leakage in human patients [6]. Such data suggests limitations in the amount of radiation induced vascular leakage that can be induced in tumors, prompting the search for alternative treatments to enhance the potential of this therapeutic strategy.
Radiotherapy fractionation schedules must also account for total dose, number of fractions, tissue organization, and target volumes to minimize early and late toxicities. While late toxicities in the brain and other tissues are largely attributed to functional changes in parenchymal cells involving secondary cascades of oxidative stress and inflammation, stromal compartments cannot be discounted. Indeed, precisely how different primary and secondary malignancies in the brain recruit, organize and re-program host stromal cells and the microenvironment will likely dictate the success of any strategy designed to transiently permeabilize the BTB. In support of this, other methods of permeabilizing the BTB are likely required to open the vasculature up enough to support therapeutic gains.
5.2. -. Mechanical disruption of the BBB
Hyperosmotic agents and focused ultrasound (FUS) are two primary methods of mechanical BBB disruptions that increase permeability in the vasculature by exerting pressure on endothelial cells and TJ proteins. For several decades, osmotic disruption of the BBB has been studied with mixed results. Initially early studies discovered that injecting hyperosmotic solution of urea led to shrinkage of the endothelial cells, breaking TJ bonds and allowing agents within the blood to pass through paracellular pathways [163,164]. These principal findings led to the experimental use of mannitol, another hyperosmotic agent, just prior to chemotherapeutic delivery to prime the BBB and BTB for uptake. Limited clinical studies of mannitol, delivered via the carotid artery just prior to chemotherapy, have shown improved delivery of chemotherapeutic agents in both normal and tumor tissue [165,166]. However, use of hyperosmotic agents lack the specificity to open only the BTB, ultimately leading to difficulty in leveraging therapeutic gain as tumor and normal tissue damage from chemotherapeutics will have to be balanced. Additionally, this treatment is particularly difficult to perform as a surgically implanted intra-arterial cranial catheter is required, involving an extended hospital stay and the use of general anesthesia and is not without risk.
One of the more promising and far less invasive methods of mechanical BBB disruption is the combined use of circulating microbubbles (MB) and FUS. Having first been investigated to induce thermal lesions within the brain (Lynn et al., 1942), increased permeability of the BBB was found as a side effect for a brief period of ~24 h [167]. Currently accepted methods utilize an injection of lipid, albumin, or polymer MB which are then vibrated using FUS, causing a physical disruption of the vasculature for anywhere between 6 and 24 h after treatment [167–169]. Though the exact mechanism of opening the BBB is unknown, it is hypothesized that rapid expansion and contraction of the microbubbles exerts pressure against the vessel walls to break the TJs of non-fenestrated capillaries.
While FUS holds promise as a novel technique to aid in drug delivery past the BBB, the overall safety is still being evaluated. FUS has been used within numerous regions of the human brain with only mild to moderate side effects [170]; however one of those side effects, a short lived inflammatory response, has received attention as of late. Upregulation of gene transcription associated with inflammation has been observed 6 h post-FUS + MB that returned to baseline within 24 h [171]. Additionally, numerous proinflammatory markers including in IBA-1, GFAP, CD68, and apoptosis (Tunel+) were found to increase within the first 24 h [172]. Genomic analysis corroborated these findings as upregulation of inflammatory genes were correlated to an increase in BBB permeability after receiving FUS + MB [171]. While FUS + MB induced inflammation is of concern and requires further interrogation, human clinical studies have continued showing minimal side effects.
5.3. -. Manipulation of receptor-mediated transporters
Receptor mediated transporters embedded within endothelial cell of the BBB and BTB actively work to pull molecules across the vasculature. It has been hypothesized that manipulation of these transporters using chemical interventions and antigen masking could allow chemicals to cross into the parenchyma; however, these studies have found limited success. In one approach, strategies were developed to expose transferrin and insulin receptors by masking drugs with antibodies that would facilitate transport across the BTB [173]. Of particular interest to researchers was the manipulation of transferrin receptor as it has been found that they are over expressed within tumors [174]. Early clinical trials presented great success in a variety of cancer types [175], however phase-III trials indicated high level of toxicity for the transferrin conjugates [176]. Despite tumor overexpression of transferrin, limitations of this treatment are due to ubiquitous expression of this receptor throughout the body, allowing for dispersal of toxic drugs in a not specific manner.
Others have experimented using a class of antibodies called “angiopeps” that target lipoprotein receptor-related protein (LRP-1) [10,177]. Development of a new drug combining paclitaxel and the angiopep2 protein called ANG1005 was hypothesized to cross the BBB via LRP-1 mediated transcytosis. One study implementing ANG1005 using tumor-bearing and tumor-free mice found that it was able to cross both the BBB and BTB, but uptake was only 30–40% of the expected value in either [178]. The authors speculated that this was due to either multiple unknown uptake mechanisms or transport played a much smaller role in transiting these conjugates across the BBB/BTB than anticipated. Phase-II clinical trial in patients with breast cancer metastasis underwent ANG1005 treatment leading to an increased treatment effect [179]. Phase-III trials are underway and are expected to be completed in 2024. This drug, similar to many other treatments previously discussed is nonspecifically distributed and requires dose limitations due to normal tissue toxicity. The lack of tumor specificity in many of these treatments highlights the need to further understand differences between the BBB and BTB to hopefully lead to the development of targeted therapeutic treatments.
5.4. Manipulation of cellular pathways
Upregulation of NOS after radiotherapy leads to secondary side effects of BBB leakage and edema, as previously mentioned. Reducing the deleterious effects of radiotherapy through protection of the BBB may well ameliorate radiation-induced neurotoxic leakage and improve outcomes for survivors of GBM and brain metastases. Pre-clinical studies have shown the benefits of NOS inhibitors on the rat blood retinal barrier (BRB) by reducing NO formation and downstream reactive species (i.e. peroxynitrite), as well as preserving occludin expression after electromagnetic pulse radiation exposure [180]. Use of NOS inhibitors have also proven beneficial in protecting the BBB in rodent models of acute ischemia [181–183]. Further, given the wealth of preclinical data on the benefits of NOS inhibition after traumatic brain injury, the drug Ronopterin was developed for human use to combat vascular disturbances. Recent clinical trials of Ronopterin (VAS203) in patients with moderate and severe traumatic brain injury showed improved recovery as indicated by the Glasgow Outcome Scale [184]. However, phase III clinical trials have recently been completed for Ronopterin and have failed to find statistical significance between the placebo and drug (NCT02794168). On a more positive note, pre-clinical studies of squamous carcinoma xenografts in mice found that use of a NOS inhibitor, L-NNA, decreased in tumor blood flow and increased tumor growth control when given alone or when combined with 4 Gy irradiation [185]. Thus, while previous clinical trials failed to find significant benefits using NOS inhibitors for traumatic brain injury, their potential utility to minimize vascular damage and control tumor growth suggest an opportunity for the management of cancer.
Oxidative stress is an early and key initiator of radiation-induced damage within the CNS, as antioxidant mechanisms can succumb when faced with a heavy burden of reactive species. Under normal conditions, cells use glutathione, catalases, and a family of superoxide dismutase (SOD) enzymes to detoxify reactive species and prevent them from damaging cellular components, including the CNS vasculature [186]. Given the active metabolic state of the brain, these protective systems can be compromised after irradiation [187]. Redox-active and lipophilic cationic compounds that act as SOD mimetics provide one strategy for minimizing oxidative stress and injury in the CNS, especially given their capability to cross the BBB and accumulate in the mitochondria [188,189]. Reducing oxidative stress by increasing SOD activity has proven beneficial to the maintenance of BBB integrity in both in vivo and in vitro models [190,191]. Manganese porphyrin compounds are a particular class of SOD mimetics that exert anti-inflammatory effects through the blockade of transcription factors NF-κB and HIF-1α [192–194]. The manganese porphyrin compound BMX-001, is currently in a phase 2 trial in combination with temozolomide and radiotherapy for the treatment of high-grade gliomas or multiple brain metastases, in efforts to ameliorate the deterioration of cognitive outcomes and quality of life [[188,195], NCT02655601, NCT03608020]. While these clinical studies focus on quality of life outcomes in brain tumor survivors, SOD mimetics may also prove helpful in minimizing related vasculature side effects associated with edema and ischemia.
6. Conclusions and future perspectives
Radiotherapy is a double-edged sword when treating CNS tumors, while effective at forestalling tumor growth it invariably damages the delicate normal tissue structures that are critical to neurocognitive functionality. Despite ever improving radiotherapeutic modalities, overall survival of GBM patients remains dismal, and normal tissue complications resulting from cranial irradiation of primary and secondary CNS malignancies still confound quality of life. The latter is particularly true for childhood brain cancer, where despite success in eradicating tumors, longer term survival is plagued by neurocognitive and cerebrovascular complications. Fractionation schedules and stereotactic approaches have been adapted to reduce the amount of normal tissue damage, however, unintended consequences of treatment that manifest as cognitive dysfunction, edema, white matter necrosis, lacunar infarcts, and parenchymal calcification remain unmet medical needs.
This review highlights the limitations of using radiotherapy as a tool to permeabilize the BTB, where transient and incomplete opening of the tumor vasculature preclude optimal penetrance of drugs to the tumor parenchyma. While more focused research efforts have been placed on opening the BTB with radiation, the promise of alternative approaches involving focused ultrasound and angiopeps, have yet to be optimized. Given the foregoing, and the limitations of treating cerebral tumors with drugs, radiotherapy will likely remain a frontline treatment for controlling malignant growth in the brain. Thus, oncologists must balance curative intent that is largely dependent on delivering higher total doses, against the cost of elevating normal tissue damage, which becomes increasingly difficult with larger treatment volumes that may include critical structures.
Modern day radiotherapy reduces normal tissue injury by implementing select fractionation schemes tailored to tumors residing in early (e.g. GI) or late (e.g. CNS) responding normal tissue beds. Fractionation along with advancements in conformal beam delivery have resulted in significant benefits, but collateral damage remains problematic for normal tissues, and dictates dose limiting toxicities that define the maximum tolerated dose that can be prescribed. In this light, evidence does not support the hypothesis that the vascular compartment is the dominant factor dictating neurocognitive outcomes following cranial radiotherapy, although for cerebrovascular complications, vascular and stromal compartments are likely to play prominent roles. Regardless of the target, recent developments in ultra-high dose rate “FLASH” radiotherapy using instantaneous dose rates ≥ 106 Gy/s may provide novel capability to resolve many long-standing issues related to dose limiting normal tissue toxicities. Central to this emerging radiation modality is that dose rate modulation can be used for therapeutic gain. Evidence in support of this comes from multiple preclinical models, but perhaps most convincingly in the CNS. FLASH radiotherapy has been found to elicit isoefficient tumor kill, but in the relative absence of normal tissue complications that confound standard-of-care conventional irradiation protocols using low dose rates of ≥0.01 Gy/s [196–199]. While recent interest in this new technique has been robust, to date, only one study has focused on the protective impact of FLASH radiotherapy on the BBB. In that work, FLASH irradiation was found to preserve TJ proteins and reduce eNOS dependent vasculature dilation, a beneficial effect not observed after conventional irradiation [8].
Given the widespread normal tissue benefits of FLASH radiotherapy, within and peripheral to the CNS, it is plausible that cells within the NVU exposed to ultra-high dose rate radiation will undergo reduced apoptosis and/or senescence, thereby aiding in the maintenance of BBB integrity (Fig. 2-C.). Beyond the CNS, Velalopoulou et al. (2021) has shown peripheral vascular benefits using proton irradiation delivered at 69 Gy/s (i.e. proton FLASH) to the hind limb in rodents. Irradiation of the highly vascularized hind limb by either proton FLASH or conventional proton dose rates produced an equal amount of low-grade lymphedema approximately 7 weeks later in rats. Conversely, standard dose rate irradiation, but not proton FLASH, led to severe late onset lymphedema, hypothesized to have developed from late forming fibrosis [200]. While the extent to which radiation induced disruptions to glymphatic clearance in the CNS remain uncertain, the study by Velalopoulou et al. does suggest that protection of lymphatic clearance can be realized by FLASH. As the mechanism of action behind the protective effects of FLASH on normal tissue remain incompletely understood, additional studies are warranted to elucidate the extent of BBB protection and its potential differential impact on the BTB. This underscores the need for more definitive comparisons between radiation dose rates on the cerebral and tumor vasculature in efforts to translate this promising technology from the preclinical to clinical setting.
This review has focused on differences that distinguish the radiobiological effects on the BBB from those observed for the BTB, highlighting the dysregulated development and leaky nature of the tumor vasculature that is primarily driven by hypoxia, cell senescence, and loss of proteins and transporters. While the current state of technology has not produced robust interventions to improve chemotherapeutic delivery to the tumor parenchyma in the brain, it remains a task worthy of continued investigation, especially given the adverse clinical outcomes facing brain tumor patients. Understanding key distinctions between the BBB and BTB will be critically important to address the efficacy of future interventions targeted to the vasculature. Ultimately this will lead to a more comprehensive understanding of the fundamental biological variations between normal and tumor tissues and how they can be exploited more effectively for therapeutic gain.
Acknowledgments
This work was supported by NIH grants PO1CA244091 and R01CA254892 (C.L.L.)
References
- [1].Patel MM, Patel BM, Crossing the blood–brain barrier: recent advances in drug delivery to the brain, CNS Drugs 31 (2017) 109–133, 10.1007/s40263-016-0405-9. [DOI] [PubMed] [Google Scholar]
- [2].Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Davis TP, Ronaldson PT, Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr. Pharmaceut. Des 20 (2014) 1422. /pmc/articles/PMC3913737/. (Accessed 8 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Quail DF, Joyce JA, The microenvironmental landscape of brain tumors,, Cancer Cell 31 (2017) 326–341, 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jain RK, Di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT, Angiogenesis in brain tumours, Nat. Rev. Neurosci 8 (2007) 610–622, 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- [5].Arvanitis CD, Ferraro GB, Jain RK, The blood–brain barrier and blood–tumour barrier in brain tumours and metastases,, Nat. Rev. Cancer 20 (2020) 26–41, 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Teng F, Tsien CI, Lawrence TS, Cao Y, Blood–tumor barrier opening changes in brain metastases from pre to one-month post radiation therapy, Radiother. Oncol 125 (2017) 89–93, 10.1016/j.radonc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- [7].Rubin P, Gash DM, Hansen JT, Nelson DF, Williams JP, Disruption of the blood-brain barrier as the primary effect of CNS irradiation, Radiother. Oncol 31 (1994) 51–60, 10.1016/0167-8140(94)90413-8. [DOI] [PubMed] [Google Scholar]
- [8].Allen BD, Acharya MM, Montay-Gruel P, Jorge PG, Bailat C, Petit B, Vozenin MC, Limoli C, Maintenance of tight junction integrity in the absence of vascular dilation in the brain of mice exposed to ultra-high-dose-rate FLASH irradiation, Radiat. Res 194 (2020) 625–635, 10.1667/RADE-20-00060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nordal RA, Wong CS, Molecular targets in radiation-induced blood-brain barrier disruption, in: int. J. Radiat. Oncol. Biol. Phys, Elsevier Inc., 2005, pp. 279–287, 10.1016/j.ijrobp.2005.01.039. [DOI] [PubMed] [Google Scholar]
- [10].Demeule M, Régina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, Moghrabi A, Béliveau R, Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier, Vasc. Pharmacol 38 (2002) 339–348, 10.1016/S1537-1891(02)00201-X. [DOI] [PubMed] [Google Scholar]
- [11].Stamatovic S, Keep R, Andjelkovic A, Brain endothelial cell-cell junctions: how to “open” the blood brain barrier, curr, Neuropharmacology 6 (2008) 179–192, 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ayloo S, Gu C, Transcytosis at the blood–brain barrier, Curr. Opin. Neurobiol 57 (2019) 32–38, 10.1016/j.conb.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pardridge WM, Drug transport across the blood-brain barrier, J. Cerebr. Blood Flow Metabol 32 (2012) 1959–1972, 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS, CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016, neuro, Oncol 21 (2019), 10.1093/neuonc/noz150. V1–V100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fortin D, The blood-brain barrier: its influence in the treatment of brain tumors metastases, curr, Cancer Drug Targets 12 (2012) 247–259, 10.2174/156800912799277511. [DOI] [PubMed] [Google Scholar]
- [16].Arvanitis CD, Ferraro GB, The blood – brain barrier and blood – tumour barrier in brain tumours and metastases,, Nat. Rev. Cancer 20 (2020), 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR, Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer, Clin. Cancer Res 16 (2010) 5664–5678, 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Groeneveld GJ, Hay JL, Van Gerven JM, Measuring blood–brain barrier penetration using the NeuroCart, a CNS test battery, Drug Discov, Today Technol 20 (2016) 27–34, 10.1016/j.ddtec.2016.07.004. [DOI] [PubMed] [Google Scholar]
- [19].Appelboom G, Detappe A, LoPresti M, Kunjachan S, Mitrasinovic S, Goldman S, Chang SD, Tillement O, Stereotactic modulation of blood-brain barrier permeability to enhance drug delivery, Neuro, Oncol. 18 (2016) 1601–1609, 10.1093/neuonc/now137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fauquette W, Amourette C, Dehouck MP, Diserbo M, Radiation-induced blood-brain barrier damages: an in vitro study, Brain Res. 1433 (2012) 114–126, 10.1016/j.brainres.2011.11.022. [DOI] [PubMed] [Google Scholar]
- [21].Wang JB, Di Ianni T, Vyas DB, Huang Z, Park S, Hosseini-Nassab N, Aryal M, Airan RD, Focused ultrasound for noninvasive, focal pharmacologic neurointervention, front, Neurosci 14 (2020), 10.3389/fnins.2020.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, Aubert I, Heyn C, Black SE, Hynynen K, Lipsman N, Zinman L, First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound, Nat. Commun 10 (2019) 1–9, 10.1038/s41467-019-12426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lajoie JM, V Shusta E, B. Engineering, HHS Public Access 10.1146/annurev-pharmtox-010814-124852.Targeting, 2016, 613–631. [DOI]
- [24].S S, W Z, ABC transporters and drug efflux at the blood-brain barrier, Rev. Neurosci 21 (2010) 29–53, 10.1515/REVNEURO.2010.21.1.29. [DOI] [PubMed] [Google Scholar]
- [25].Miller DS, Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier, Trends Pharmacol. Sci 31 (2010) 246, 10.1016/J.TIPS.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lankford J, Butler IJ, Koenig MK, Glucose transporter type I deficiency causing mitochondrial dysfunction. 10.1177/0883073811426503, 2011. 10.1177/0883073811426503, 27,796–798. [DOI] [PubMed] [Google Scholar]
- [27].Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C, Mfsd2a is critical for the formation and function of the blood-brain barrier, Nature 509 (2014) 507–511, 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alakbarzade V, Hameed A, Quek DQY, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, Crosby AH, A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome, Nat. Genet 477 (47) (2015) 814–817, 10.1038/ng.3313, 2015. [DOI] [PubMed] [Google Scholar]
- [29].Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, Schroth J, Copeland B, Vaux KK, Cazenave-Gassiot A, Quek DQY, Wong BH, Tan BC, Wenk MR, Gunel M, Gabriel S, Chi NC, Silver DL, Gleeson JG, Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome, Nat. Genet. 2015 477 (47) (2015) 809–813, 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martínez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G, Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells, J. Biol. Chem 276 (2001) 9291–9296, 10.1074/jbc.M006991200. [DOI] [PubMed] [Google Scholar]
- [31].Bazzoni G, Martínez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E, Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin, J. Biol. Chem 275 (2000) 20520–20526, 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- [32].Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ, Structure and function of the blood-brain barrier, Neurobiol. Dis 37 (2010) 13–25, 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- [33].Stephenson RE, Higashi T, Erofeev IS, Arnold TR, Leda M, Goryachev AB, Miller AL, Rho flares repair local tight junction leaks, Dev. Cell 48 (2019) 445–459, 10.1016/j.devcel.2019.01.016, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Armulik A, Genové G, Betsholtz C, Pericytes: developmental, physiological, and pathological perspectives, problems, and promises, Dev. Cell 21 (2011) 193–215, 10.1016/J.DEVCEL.2011.07.001. [DOI] [PubMed] [Google Scholar]
- [35].Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, Oâ Farrell FM, Buchan AM, Lauritzen M, Attwell D, Capillary pericytes regulate cerebral blood flow in health and disease, Nature 508 (2014) 55–60, 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brown LS, Foster CG, Courtney J, King NE, Howells DW, Sutherland BA, Jackson J, Sutherland BA, Pericytes, Neurovascular, Function in the Healthy and Diseased Brain 13 (2019) 1–9, 10.3389/fncel.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Alarcon-Martinez L, Yilmaz-Ozcan S, Yemisci M, Schallek J, Kılıç K, Can A, Di Polo A, Dalkara T, Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection, Elife 7 (2018), 10.7554/eLife.34861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rucker HK, Wynder HJ, Thomas WE, Cellular mechanisms of CNS pericytes, brain res, Bull. (Arch. Am. Art) 51 (2000) 363–369, 10.1016/S0361-9230(99)00260-9. [DOI] [PubMed] [Google Scholar]
- [39].Bergers G, Song S, The role of pericytes in blood-vessel formation and maintenance, Neuro, Oncol. 7 (2005) 452–464, 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ribatti D, Nico B, Crivellato E, The role of pericytes in angiogenesis, Int, J. Dev. Biol 55 (2011) 261–268, 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- [41].Nehls V, Zeitler-Zapf P, Drenckhahn D, Different sequences of expression of band 3, spectrin, and ankyrin during normal erythropoiesis and erythroleukemia., Am, J. Pathol 142 (1993) 1565. /pmc/articles/PMC1886907/?report=abstract. (Accessed 6 July 2021). [PMC free article] [PubMed] [Google Scholar]
- [42].Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM, Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors, Am. J. Pathol 160 (2002) 985–1000, 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rustenhoven J, Jansson D, Smyth LC, Dragunow M, Brain pericytes as mediators of neuroinflammation, Trends Pharmacol. Sci 38 (2017) 291–304, 10.1016/j.tips.2016.12.001. [DOI] [PubMed] [Google Scholar]
- [44].Underly RG, Levy M, Hartmann DA, Grant RI, Watson AN, Shih AY, Pericytes as inducers of rapid, matrix metalloproteinase-9-dependent capillary damage during ischemia, J. Neurosci 37 (2017) 129–140, 10.1523/JNEUROSCI.2891-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen F, Ohashi N, Li W, Eckman C, Nguyen JH, Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure, Hepatology 50 (2009) 1914–1923, 10.1002/hep.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV, Pericyte loss influences Alzheimer-like neurodegeneration in mice, Nat. Commun 4 (2013), 10.1038/NCOMMS3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [47].Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel Á, Tanaka K, Niwa M, A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes, Neurochem, Bar Int. 54 (2009) 253–263, 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- [48].Puech C, Hodin S, Forest V, He Z, Mismetti P, Delavenne X, Perek N, Assessment of HBEC-5i endothelial cell line cultivated in astrocyte conditioned medium as a human blood-brain barrier model for ABC drug transport studies, Int, J. Pharm. (Lahore) 551 (2018) 281–289, 10.1016/j.ijpharm.2018.09.040. [DOI] [PubMed] [Google Scholar]
- [49].Shan Y, Tan S, Lin Y, Liao S, Zhang B, Chen X, Wang J, Deng Z, Zeng Q, Zhang L, Wang Y, Hu X, Qiu W, Peng L, Lu Z, The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke,, J. Neuroinflammation 16 (2019), 10.1186/s12974-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tiwary S, Morales JE, Kwiatkowski SC, Lang FF, Rao G, McCarty JH, Metastatic brain tumors disrupt the blood-brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter Mfsd2a, Sci. Rep 8 (2018) 1–13, 10.1038/s41598-018-26636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR, Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease, J. Clin. Invest 122 (2012) 2454–2468, 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR, VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown, Proc. Natl. Acad. Sci. U, S. A 106 (2009) 1977–1982, 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Van Bruggen N, Chopp M, VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain, J. Clin. Invest 106 (2000) 829–838, 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chapouly C, Argaw AT, Horng S, Castro K, Zhang J, Asp L, Loo H, Laitman BM, Mariani JN, Farber RS, Zaslavsky E, Nudelman G, Raine CS, John GR, Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions, Brain 138 (2015) 1548–1567, 10.1093/brain/awv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen F, Ohashi N, Li W, Eckman C, Nguyen JH, Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure, Hepatology 50 (2009) 1914–1923, 10.1002/hep.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Y Y, Ey E, Jf T, W L, Ga R, Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat, J. Cereb, Blood Flow Metab 27 (2007) 697–709, 10.1038/SJ.JCBFM.9600375. [DOI] [PubMed] [Google Scholar]
- [57].Yang Y, Rosenberg GA, MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia, Methods Mol. Biol 762 (2011) 333–345, 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Girouard H, Iadecola C, HIGHLIGHTED TOPIC Regulation of the Cerebral Circulation Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease, J. Appl. Physiol 100 (2006) 328–335, 10.1152/japplphysiol.00966.2005.-The. [DOI] [PubMed] [Google Scholar]
- [59].Herron PO, Chhatbar PY, Levy M, Shen Z, Schramm AE, Lu Z, Kara P, Neural correlates of single-vessel haemodynamic responses in vivo, Nature 534 (2016) 378–382, 10.1038/nature17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].G Y, Y Z, ME R, C I, Attenuation of activity-induced increases in cerebellar blood flow in mice lacking neuronal nitric oxide synthase, Am. J. Physiol. Heart Circ, Physiol 285 (2003), 10.1152/AJPHEART.00043.2003. [DOI] [PubMed] [Google Scholar]
- [61].Ma J, Ayata C, Huang PL, Fishman MC, Moskowitz MA, Regional cerebral blood flow response to vibrissal stimulation in mice lacking type I NOS gene expression. 10.1152/Ajpheart.1996.270.3.H1085, 1996. , 270. [DOI] [PubMed] [Google Scholar]
- [62].Lindauer U, Megow D, Matsuda H, Dirnagl U, Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex, Am. J. Physiol. - hear. Circ, Physiol 277 (1999) 799–811, 10.1152/ajpheart.1999.277.2.h799. [DOI] [PubMed] [Google Scholar]
- [63].Faraci FM, Breese KR, Nitric oxide mediates vasodilatation in response to activation of N- methyl-D-aspartate receptors in brain, Circ. Res 72 (1993) 476–480, 10.1161/01.RES.72.2.476. [DOI] [PubMed] [Google Scholar]
- [64].Hillman EMC, Coupling Mechanism and Significance of the BOLD Signal: A Status Report, (n.d.). 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed]
- [65].Iadecola C, Neurovascular regulation in the normal brain and in Alzheimer’s disease, Nat. Rev. Neurosci 5 (2004) 347–360, 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- [66].Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EMC, A critical role for the vascular endothelium in functional neurovascular coupling in the brain,, J. Am. Heart Assoc 3 (2014) 1–14, 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dc R, The roles of intracellular protein-degradation pathways in neurodegeneration,, Nature 443 (2006) 780–786, 10.1038/NATURE05291. [DOI] [PubMed] [Google Scholar]
- [68].Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M, CEREBROSPINAL fluid circulation A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, Including Amyloid b 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M, Cerebral Arterial Pulsation Drives Paravascular CSF – Interstitial Fluid Exchange in the Murine Brain 33 (2013) 18190–18199, 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nedergaard M, Goldman SA, Glymphatic failure as a final common pathway to dementia, Science (80-.) 370 (2020) 50–56, 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rasmussen Martin Kaag, Mestre Humberto, Nedergaard Maiken, Fluid transport in the brain, Rev (585) (2021), 10.1152/physrev.00031.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, Donnell JO, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M, Sleep drives metabolite clearance from the adult brain, 2013, pp. 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, Levan P, Keilholz S, Zang YF, Hennig J, Nedergaard M, Ultra-fast magnetic resonance encephalography of physiological brain activity-Glymphatic pulsation mechanisms? J. Cerebr. Blood Flow Metabol 36 (2016) 1033–1045, 10.1177/0271678X15622047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jessen NA, Munk ASF, Lundgaard I, Nedergaard M, The glymphatic system: a beginner’s guide,, Neurochem. Res 40 (2015) 2583–2599, 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M, Circadian control of brain glymphatic and lymphatic fluid flow, Nat. Commun. 2020 111 (11) (2020) 1–11, 10.1038/s41467-020-18115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier ALR, Gjedde A, Benveniste H, Nedergaard M, Impaired glymphatic transport in spontaneously hypertensive rats, J. Neurosci 39 (2019) 6365–6377, 10.1523/JNEUROSCI.1974-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kim Y-K, Il Nam K, Song J, The glymphatic system in diabetes-induced dementia, front, Neurol 9 (2018) 867, 10.3389/FNEUR.2018.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nayak L, Lee EQ, Wen PY, Epidemiology of brain metastases, Curr. Oncol. Rep 14 (2012) 48–54, 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- [79].Voutouri C, Kirkpatrick ND, Chung E, Mpekris F, Baish JW, Munn LL, Fukumura D, Stylianopoulos T, Jain RK, Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies, (n.d.). 10.1073/pnas.1818322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zagzag D, Amirnovin R, Greco MA, Yee H, Holash J, Wiegand SJ, Zabski S, Yancopoulos GD, Grumet M, Vascular apoptosis and involution in gliomas precede neovascularization: a novel concept for glioma growth and angiogenesis, Lab, Investig 80 (2000) 837–849, 10.1038/labinvest.3780088. [DOI] [PubMed] [Google Scholar]
- [81].Bergers G, Hanahan D, Modes of resistance to anti-angiogenic therapy, Nat. Rev. Cancer 8 (8) (2008) 592–603, 10.1038/nrc2442.Modes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Neuwelt EA, Greig NH, Raffel C, Amar AP, Apuzzo MLJ, Antel JP, Rosenberg GA, Mechanisms of disease: the blood-brain barrier,, Neurosurgery 54 (2004) 131–142, 10.1227/01.NEU.0000097715.11966.8E. [DOI] [PubMed] [Google Scholar]
- [83].Zhang RD, Price JE, Fujimaki T, Bucana CD, Fidler IJ, Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice, Am. J. Pathol. 141 (1992) 1115–1124. /pmc/articles/PMC1886664/?report=abstract (accessed September 15, 2020). [PMC free article] [PubMed] [Google Scholar]
- [84].Jv L, Eg A, B M, VEGFR-2 expression in brain injury: its distribution related to brain-blood barrier markers, J. Neural. Transm 113 (2006) 487–496, 10.1007/S00702-005-0407-0. [DOI] [PubMed] [Google Scholar]
- [85].Wen L, Tan Y, Dai S, Zhu Y, Meng T, Yang X, Liu Y, Liu X, Yuan H, Hu F, Drug Delivery VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. 10.1080/10717544.2017.1386731, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dvorak HF, Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy,, J. Clin. Oncol 20 (2002) 4368–4380, 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- [87].Liu T, Ma W, Xu H, Huang M, Zhang D, He Z, Zhang L, Brem S, O’Rourke DM, Gong Y, Mou Y, Zhang Z, Fan Y, PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma, Nat. Commun 9 (2018), 10.1038/s41467-018-05982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang Q, He Z, Huang M, Liu T, Wang Y, Xu H, Duan H, Ma P, Zhang L, Zamvil SS, Hidalgo J, Zhang Z, O’Rourke DM, Dahmane N, Brem S, Mou Y, Gong Y, Fan Y, Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α, Nat. Commun 9 (2018) 10.1038/s41467-018-03050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK, Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges, Nat. Rev. Clin. Oncol 15 (2018) 325–340, 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK, Normalization of the vasculature for treatment of cancer and other diseases, Physiol. Rev 91 (2011) 1071–1121, 10.1152/physrev.00038.2010.-New. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H, Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme, Acta Neuropathol. 100 (2000) 323–331, 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- [92].Ma J, Wang P, Liu Y, Zhao L, Li Z, Xue Y, Krüppel-like factor 4 regulates blood-tumor barrier permeability via ZO-1, occludin and claudin-5, J. Cell. Physiol 229 (2014) 916–926, 10.1002/jcp.24523. [DOI] [PubMed] [Google Scholar]
- [93].Lyle LT, Lockman PR, Adkins CE, Mohammad AS, Sechrest E, Hua E, Palmieri D, Liewehr DJ, Steinberg SM, Kloc W, Izycka-Swieszewska E, Duchnowska R, Nayyar N, Brastianos PK, Steeg PS, Gril B, Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer, Clin. Cancer Res 22 (2016) 5287–5299, 10.1158/1078-0432.CCR-15-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bao X, Wu J, Xie Y, Kim S, Michelhaugh S, Jiang J, Mittal S, Sanai N, Li J, Protein Expression, Functional Relevance, Of efflux and uptake drug transporters at the blood–brain barrier of human brain and glioblastoma, Clin. Pharmacol. Ther 107 (2020) 1116–1127, 10.1002/cpt.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhou W, Chen C, Shi Y, Wu Q, Gimple RC, Fang X, Huang Z, Zhai K, Ke SQ, Ping YF, Feng H, Rich JN, Yu JS, Bao S, Bian XW, Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy, Cell Stem Cell. 21 (2017) 591–603, 10.1016/j.stem.2017.10.002, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Verhoeven D, Buyssens N, Desmin-positive stellate cells associated with angiogenesis in a tumour and non-tumour system, Virchows Arch. B Cell Pathol, Incl. Mol. Pathol 54 (1987) 263–272, 10.1007/BF02899222. [DOI] [PubMed] [Google Scholar]
- [97].Zarkovic K, Juric G, Waeg G, Kolenc D, Zarkovic N, Immunohistochemical appearance of HNE-protein conjugates in human astrocytomas, Biofactors 24 (2005) 33–40, 10.1002/biof.5520240104. [DOI] [PubMed] [Google Scholar]
- [98].Juric-Sekhar G, Zarkovic K, Waeg G, Cipak A, Zarkovic N, Distribution of 4-hydroxynonenal-protein conjugates as a marker of lipid peroxidation and parameter of malignancy in astrocytic and ependymal tumors of the brain, Tumori 95 (2009) 762–768, 10.1177/030089160909500620. [DOI] [PubMed] [Google Scholar]
- [99].Zajdel A, Wilczok A, Slowinski J, Orchel J, Mazurek U, Aldehydic lipid peroxidation products in human brain astrocytomas, J. Neuro Oncol 84 (2007) 167–173, 10.1007/s11060-007-9367-6. [DOI] [PubMed] [Google Scholar]
- [100].Kolenc D, Jakovčevíc A, MacAn M, Žarković K, The co-expression of 4-hydroxynonenal and prominin-1 in glioblastomas, Transl, Neurosci 2 (2011) 163–167, 10.2478/s13380-011-0012-7. [DOI] [Google Scholar]
- [101].Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y, Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction, J. Neurochem 101 (2007) 566–576, 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- [102].Mertsch K, Blasig I, Grune T, 4-Hydroxynonenal impairs the permeability of an in vitro rat blood-brain barrier, Neurosci. Lett 314 (2001) 135–138, 10.1016/S0304-3940(01)02299-6. [DOI] [PubMed] [Google Scholar]
- [103].Žarković N, Žarković K, Schaur RJ, Štolc S, Schlag G, Redl H, Waeg G, Borović S, Loňcarić I, Jurić G, Hlavka V, 4-Hydroxynonenal as a second messenger of free radicals and growth modifying factor, Life Sci. 65 (1999) 1901–1904, 10.1016/S0024-3205(99)00444-0. [DOI] [PubMed] [Google Scholar]
- [104].Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H, Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells, Nat. Commun 5 (2014) 4196, 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P, Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis,, Glia 53 (2006) 799–808, 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- [106].Nagano N, Sasaki H, Aoyagi M, Hirakawa K, Invasion of experimental rat brain tumor: early morphological changes following microinjection of C6 glioma cells, Acta Neuropathol. 86 (1993) 117–125, 10.1007/BF00334878. [DOI] [PubMed] [Google Scholar]
- [107].Gril B, Paranjape AN, Woditschka S, Hua E, Dolan EL, Hanson J, Wu X, Kloc W, Izycka-Swieszewska E, Duchnowska R, Pe¸ksa R, Biernat W, Jassem J, Nayyar N, Brastianos PK, Hall OM, Peer CJ, Figg WD, Pauly GT, Robinson C, Difilippantonio S, Bialecki E, Metellus P, Schneider JP, Steeg PS, Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases, Nat. Commun 9 (2018) 1–18, 10.1038/s41467-018-05030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hall EJ, Giaccia AJ, Radiobiology for the radiologist. 10.1016/j.ijrobp.2006.06.027, 2006. sixth ed. [DOI] [Google Scholar]
- [109].Walker AJ, Ruzevick J, Malayeri AA, Rigamonti D, Lim M, Redmond KJ, Kleinberg L, Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol. 10 (2014) 1277–1297, 10.2217/fon.13.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS, Hypoxia in radiation-induced blood-spinal cord barrier breakdown, Cancer Res. 61 (2001) 3348–3354. [PubMed] [Google Scholar]
- [111].Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE, Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes, Int. J. Radiat. Oncol. Biol. Phys 66 (2006) 860–866, 10.1016/j.ijrobp.2006.06.043. [DOI] [PubMed] [Google Scholar]
- [112].Wilson CM, Gaber MW, Sabek OM, Zawaski JA, Merchant TE, Radiation-Induced Astrogliosis, Blood-Brain Barrier, Damage can Be abrogated using anti-TNF treatment, int, J. Radiat. Oncol. Biol. Phys 74 (2009) 934–941, 10.1016/j.ijrobp.2009.02.035. [DOI] [PubMed] [Google Scholar]
- [113].Lim WH, Choi SH, Yoo RE, Kang KM, Yun TJ, Kim JH, Sohn CH, Does radiation therapy increase gadolinium accumulation in the brain?: quantitative analysis of T1 shortening using R1 relaxometry in glioblastoma multiforme patients, PLoS One 13 (2018) 1–14, 10.1371/journal.pone.0192838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Murrell DH, Zarghami N, Jensen MD, Chambers AF, Wong E, Foster PJ, Evaluating changes to blood-brain barrier integrity in brain metastasis over time and after radiation treatment, Transl. Oncol 9 (2016) 219–227, 10.1016/j.tranon.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fauquette W, Amourette C, Dehouck MP, Diserbo M, Radiation-induced blood-brain barrier damages: an in vitro study, Brain Res. 1433 (2012) 114–126, 10.1016/j.brainres.2011.11.022. [DOI] [PubMed] [Google Scholar]
- [116].Diserbo M, Agin A, Lamproglou I, Mauris J, Staali F, Multon E, Amourette C, Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study, Can, J. Physiol. Pharmacol 80 (2002) 670–678, 10.1139/y02-070. [DOI] [PubMed] [Google Scholar]
- [117].Nv L, Mk L, Ed P, LKh E, Endothelial cell population dynamics in rat brain after local irradiation, Br. J. Radiol 64 (1991) 934–940, 10.1259/0007-1285-64-766-934. [DOI] [PubMed] [Google Scholar]
- [118].Rubin DB, Drab EA, Ward WF, Physiological and biochemical markers of the endothelial cell response to irradiation, Int. J. Radiat. Biol 60 (1991) 29–32, 10.1080/09553009114551461. [DOI] [PubMed] [Google Scholar]
- [119].Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS, Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation, Cancer Res. 63 (2003) 5950–5956. [PubMed] [Google Scholar]
- [120].Peña LA, Fuks Z, Kolesnick RN, Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency, Cancer Res. 60 (2000) 321–327. [PubMed] [Google Scholar]
- [121].Hovinga KE, Stalpers LJA, van Bree C, Donker M, Verhoeff JJC, Rodermond HM, Bosch DA, van Furth WR, Radiation-enhanced vascular endothelial growth factor (VEGF) secretion in glioblastoma multiforme cell lines - a clue to radioresistance? J. Neuro Oncol 74 (2005) 99–103, 10.1007/s11060-004-4204-7. [DOI] [PubMed] [Google Scholar]
- [122].Blasig IE, Mertsch K, Haseloff RF, Nitronyl nitroxides, a novel group of protective agents against oxidative stress in endothelial cells forming the blood-brain barrier, Neuropharmacology 43 (2002) 1006–1014, 10.1016/S0028-3908(02)00180-6. [DOI] [PubMed] [Google Scholar]
- [123].Kim WK, Kim JH, Yoon K, Kim S, Ro J, Kang HS, Yoon S, Salinomycin, a p-glycoprotein inhibitor, sensitizes radiation-treated cancer cells by increasing DNA damage and inducing G2 arrest, Invest. N. Drugs 30 (2012) 1311–1318, 10.1007/s10637-011-9685-6. [DOI] [PubMed] [Google Scholar]
- [124].Wang Y, Chen Q, Jin S, Deng W, Li S, Tong Q, Chen Y, Up-regulation of P-glycoprotein is involved in the increased paclitaxel resistance in human esophageal cancer radioresistant cells, Scand. J. Gastroenterol 47 (2012) 802–808, 10.3109/00365521.2012.683042. [DOI] [PubMed] [Google Scholar]
- [125].Nagane M, Yasui H, Sakai Y, Yamamori T, Niwa K, Hattori Y, Kondo T, Inanami O, Activation of eNOS in endothelial cells exposed to ionizing radiation involves components of the DNA damage response pathway, Biochem. Biophys. Res. Commun 456 (2015) 541–546, 10.1016/j.bbrc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- [126].Kim JE, Ryu HJ, Kang TC, Status epilepticus induces vasogenic edema via tumor necrosis factor-α/endothelin-1-mediated two different pathways, PLoS One 8 (2013) 1–13, 10.1371/journal.pone.0074458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ho ML, Rojas R, Eisenberg RL, Cerebral edema, Am. J. Roentgenol 199 (2012) 258–273, 10.2214/AJR.11.8081. [DOI] [PubMed] [Google Scholar]
- [128].Kazmierski R, Michalak S, Wencel-Warot A, Nowinski WL, Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients, Neurology 79 (2012) 1677–1685, 10.1212/WNL.0b013e31826e9a83. [DOI] [PubMed] [Google Scholar]
- [129].Rosenberg GA, Yang Y, Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia, Neurosurg. Focus 22 (2007) 1–9, 10.3171/FOC.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- [130].Winkler L, Blasig R, Breitkreuz-Korff O, Berndt P, Dithmer S, Helms HC, Puchkov D, Devraj K, Kaya M, Qin Z, Liebner S, Wolburg H, V Andjelkovic A, Rex A, Blasig IE, Haseloff RF, Tight junctions in the blood–brain barrier promote edema formation and infarct size in stroke – ambivalent effects of sealing proteins. 10.1177/0271678X20904687, 2020. 10.1177/0271678X20904687, 41, 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kevil CG, Keith Payne D, Mire E, Alexander JS, Vascular Permeability Factor/Vascular Endothelial Cell Growth Factor-mediated Permeability Occurs through Disorganization of Endothelial Junctional Proteins* 1998 (2020). http://www.jbc.org/. (Accessed 20 December 2020). [DOI] [PubMed]
- [132].Murakami T, Felinski EA, Antonetti DA, Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability, J. Biol. Chem 284 (2009) 21036–21046, 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sitohy B, Nagy JA, Dvorak HF, Anti-VEGF/VEGFR therapy for cancer: reassessing the target, Cancer Res. 72 (2012) 1909–1914, 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM, Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts, Am. J. Pathol (2004) 63273–63277, 10.1016/S0002-9440(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Deissler HL, Deissler H, Lang GE, Inhibition of vascular endothelial growth factor (VEGF) is sufficient to completely restore barrier malfunction induced by growth factors in microvascular retinal endothelial cells, Br. J. Ophthalmol 95 (2011) 1151–1156, 10.1136/bjo.2010.192229. [DOI] [PubMed] [Google Scholar]
- [136].Lee ST, Seo Y, Bae JY, Chu K, Kim JW, Choi SH, Kim TM, Kim IH, Park SH, Park CK, Loss of pericytes in radiation necrosis after glioblastoma treatments, Mol. Neurobiol 55 (2018) 4918–4926, 10.1007/s12035-017-0695-z. [DOI] [PubMed] [Google Scholar]
- [137].Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV, Pericytes Control Key Neurovascular Functions, Neuronal, Phenotype in the adult brain and during brain aging, Neuron 68 (2010) 409–427, 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sato Y, Persistent vascular normalization as an alternative goal of anti-angiogenic cancer therapy, Cancer Sci. 102 (2011) 1253–1256, 10.1111/j.1349-7006.2011.01929.x. [DOI] [PubMed] [Google Scholar]
- [139].Sándor N, Walter FR, Bocsik A, Sántha P, Schilling-Tóth B, Léner V, Varga Z, Kahán Z, Deli MA, Sáfrány G, Hegyesi H, Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice, PLoS One 9 (2014), 10.1371/JOURNAL.PONE.0112397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Y Y, Dj B, M T, Cc L, van D, Jm B, Tg, G B, T K, Vascular cell senescence contributes to blood-brain barrier breakdown, Stroke 47 (2016) 1068–1077, 10.1161/STROKEAHA.115.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].O A, W S, H S, J M-P, R Y, Mv B, D J, M U, Mj A, G M, S T, Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction, J. Proteome Res 14 (2015) 1203–1219, 10.1021/PR501141B. [DOI] [PubMed] [Google Scholar]
- [142].C P, Am C, del VJ, G C, Ma S, A C, M P, J V, Increased permeability of blood-brain barrier on the hippocampus of a murine model of senescence, Mech. Ageing Dev 128 (2007) 522–528, 10.1016/J.MAD.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [143].V D, J D-V, G M, A C, M P, J V, C P, Time-course of blood-brain barrier disruption in senescence-accelerated mouse prone 8 (SAMP8) mice, Int. J. Dev. Neurosci 27 (2009) 47–52, 10.1016/J.IJDEVNEU.2008.10.002. [DOI] [PubMed] [Google Scholar]
- [144].Coppé JP, Desprez PY, Krtolica A, Campisi J, The senescence-associated secretory phenotype: the dark side of tumor suppression, Annu. Rev. Pathol. Mech, Dis 5 (2010) 99–118, 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Muñoz-Espín D, Serrano M, Cellular senescence: from physiology to pathology, Nat. Rev. Mol. Cell Biol 15 (2014) 482–496, 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- [146].C T, Ja B, I H, Ie O, N VM, B V, Dp L, C G, Jj C, Hm A, Dd S, Bt H, Cc H, Radiation-induced astrocyte senescence is rescued by Δ133p53, Neuro, Oncol. 21 (2019) 474–485, 10.1093/NEUONC/NOZ001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Meldolesi J, Astrocytes: news about brain health and diseases, Biomedicines 8 (2020) 1–14, 10.3390/biomedicines8100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Schaue D, Micewicz ED, Ratikan JA, Xie MW, Cheng G, McBride WH, Radiation & inflammation, semin, Radiat. Oncol 25 (2015) 4, 10.1016/J.SEMRADONC.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Constanzo J, Midavaine É, Fouquet J, Lepage M, Descoteaux M, Kirby K, Tremblay L, Masson-Côté L, Geha S, Longpré JM, Paquette B, Sarret P, Brain irradiation leads to persistent neuroinflammation and long-term neurocognitive dysfunction in a region-specific manner, Prog. Neuro-Psychopharmacol. Biol. Psychiatry 102 (2020) 109954, 10.1016/J.PNPBP.2020.109954. [DOI] [PubMed] [Google Scholar]
- [150].Arvanitis CD, Ferraro GB, Jain RK, The blood-brain barrier and blood-tumour barrier in brain tumours and metastases, (n.d.). 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Demeule M, Régina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, Moghrabi A, Béliveau R, Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier, Vasc. Pharmacol 38 (2002) 339–348, 10.1016/S1537-1891(02)00201-X. [DOI] [PubMed] [Google Scholar]
- [152].Strugar J, Rothbart D, Harrington W, Criscuolo GR, Vascular permeability factor in brain metastases: correlation with vasogenic brain edema and tumor angiogenesis, J. Neurosurg 81 (1994) 560–566, 10.3171/jns.1994.81.4.0560. [DOI] [PubMed] [Google Scholar]
- [153].Thorsen F, Fite B, Mahakian LM, Seo JW, Qin S, Harrison V, Johnson S, Ingham E, Caskey C, Sundstrøm T, Meade TJ, Harter PN, Skaftnesmo KO, Ferrara KW, Multimodal imaging enables early detection and characterization of changes in tumor permeability of brain metastases, J. Contr. Release 172 (2013) 812–822, 10.1016/j.jconrel.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Jung BY, Lee EJ, Bae JM, Choi YJ, Lee EK, Kim DB, Differentiation between glioblastoma and solitary metastasis: morphologic assessment by conventional brain MR imaging and diffusion-weighted imaging, Investig. Magn. Reson. Imaging 25 (2021) 23, 10.13104/imri.2021.25.1.23. [DOI] [Google Scholar]
- [155].Meier R, Pahud de Mortanges A, Wiest R, Knecht U, Exploratory analysis of qualitative MR imaging features for the differentiation of glioblastoma and brain metastases, Front. Oncol 10 (2020) 1–13, 10.3389/fonc.2020.581037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Hengel K, Sidhu G, Choi J, Weedon J, Nwokedi E, Axiotis CA, Song X, Braverman AS, Attributes of brain metastases from breast and lung cancer, Int. J. Clin. Oncol 18 (2013) 396–401, 10.1007/s10147-012-0392-x. [DOI] [PubMed] [Google Scholar]
- [157].Storm AJ, Van Der Kogel AJ, Nooter K, Effect of X-irradiation on the pharmacokinetics of methotrexate in rats: alteration of the blood-brain barrier, Eur, J. Cancer Clin. Oncol 21 (1985) 759–764, 10.1016/0277-5379(85)90275-5. [DOI] [PubMed] [Google Scholar]
- [158].van der Kogel AJ, Radiation-induced damage in the central nervous system: an interpretation of target cell responses., Br, J. Cancer Suppl. 7 (1986) (2021) 207. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2149792/. (Accessed 22 August 2021). [PMC free article] [PubMed] [Google Scholar]
- [159].Remler MP, Marcussen WH, Tiller-Borsich J, The late effects of radiation on the blood brain barrier, Int, J. Radiat. Oncol. Biol. Phys 12 (1986) 1965–1969, 10.1016/0360-3016(86)90133-1. [DOI] [PubMed] [Google Scholar]
- [160].Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE, Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes, Int. J. Radiat. Oncol. Biol. Phys 66 (2006) 860–866, 10.1016/j.ijrobp.2006.06.043. [DOI] [PubMed] [Google Scholar]
- [161].Dx Q, R Z, J T, Jx L, Yh H, Influence of radiation on the blood-brain barrier and optimum time of chemotherapy, Int. J. Radiat. Oncol. Biol. Phys 19 (1990) 1507–1510, 10.1016/0360-3016(90)90364-P. [DOI] [PubMed] [Google Scholar]
- [162].Lm D, Jy D, Jb P, Radiation-induced dementia in patients cured of brain metastases, Neurology 39 (1989) 789–796, 10.1212/WNL.39.6.789. [DOI] [PubMed] [Google Scholar]
- [163].Brightman MW, Hori M, Rapoport SI, Reese TS, Westergaard E, Osmotic opening of tight junctions in cerebral endothelium, J. Comp. Neurol 152 (1973) 317–325, 10.1002/cne.901520402. [DOI] [PubMed] [Google Scholar]
- [164].Rapoport SI, Hori M, Klatzo I, Testing of a hypothesis for osmotic opening of the blood-brain barrier, Am. J. Physiol 223 (1972) 323–331, 10.1152/ajplegacy.1972.223.2.323. [DOI] [PubMed] [Google Scholar]
- [165].Zylber-Katz E, Gomori JM, Schwartz A, Lossos A, Bokstein F, Siegal T, Pharmacokinetics of methotrexate in cerebrospinal fluid and serum after osmotic blood-brain barrier disruption in patients with brain lymphoma, Clin. Pharmacol. Ther 67 (2000) 631–641, 10.1067/mcp.2000.106932. [DOI] [PubMed] [Google Scholar]
- [166].Morikawa N, Mori T, Abe T, Kawashima H, Takeyama M, Hori S, Pharmacokinetics of etoposide and carboplatin in cerebrospinal fluid and plasma during hyperosmotic disruption of the blood brain barrier and intraarterial combination chemotherapy, Biol. Pharm. Bull 22 (1999) 428–431, 10.1248/bpb.22.428. [DOI] [PubMed] [Google Scholar]
- [167].Hynynen K, Mcdannold N, Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits, Radiology (2001) 640–646. [DOI] [PubMed] [Google Scholar]
- [168].Aryal M, Arvanitis CD, Alexander PM, McDannold N, Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system, Adv. Drug Deliv. Rev 72 (2014) 94–109, 10.1016/j.addr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Meairs S, Facilitation of drug transport across the blood–brain barrier with ultrasound and microbubbles, Pharmaceutics 7 (2015) 275–293, 10.3390/pharmaceutics7030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Legon W, Adams S, Bansal P, Patel PD, Hobbs L, Ai L, Mueller JK, Meekins G, Gillick BT, A retrospective qualitative report of symptoms and safety from transcranial focused ultrasound for neuromodulation in humans,, Sci. Rep 10 (2020) 1–10, 10.1038/s41598-020-62265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Mcmahon D, Bendayan R, Hynynen K, Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome, Sci. Rep 7 (2017) 1–15, 10.1038/srep45657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kovacsa ZI, Kima S, Jikariaa N, Qureshia F, Miloa B, Lewisa BK, Breslera M, Burksa SR, Franka JA, Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation, Proc. Natl. Acad. Sci. U. S. A 114 (2017) E75–E84, 10.1073/pnas.1614777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lajoie JM, Shusta EV, Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier, Annu. Rev. Pharmacol. Toxicol 55 (2015) 613–631, 10.1146/annurev-pharmtox-010814-124852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Daniels TR, Bernabeu E, Rodríguez JA, Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera G, Penichet ML, The transferrin receptor and the targeted delivery of therapeutic agents against cancer, Biochim. Biophys. Acta - Gen, Subjectivity 1820 (2012) 291–317, 10.1016/j.bbagen.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Weaver M, Laske DW, Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) therapy of malignant gliomas, J. Neuro Oncol 65 (2003) 3–14, 10.1023/A:1026246500788. [DOI] [PubMed] [Google Scholar]
- [176].Angeli E, Nguyen TT, Janin A, Bousquet G, How to make anticancer drugs cross the blood-brain barrier to treat brain metastases, Int. J. Mol. Sci 21 (2020), 10.3390/ijms21010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].M D, A R, C C, J P, T N, R G, JP C, R B, Identification and design of peptides as a new drug delivery system for the brain, J. Pharmacol. Exp. Therapeut 324 (2008) 1064–1072, 10.1124/JPET.107.131318. [DOI] [PubMed] [Google Scholar]
- [178].Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, Mittapalli RK, Palmieri D, Steeg PS, Lockman PR, Smith QR, Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer, Pharm. Res. (N. Y.) 26 (2009) 2486–2494, 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kumthekar P, Tang SC, Brenner AJ, Kesari S, Piccioni DE, Anders C, Carrillo J, Chalasani P, Kabos P, Puhalla S, Tkaczuk K, Garcia AA, Ahluwalia MS, Wefel JS, Lakhani N, Ibrahim N, ANG1005, a brain-penetrating peptide–drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases, Clin. Cancer Res 26 (2020) 2789–2799, 10.1158/1078-0432.CCR-19-3258. [DOI] [PubMed] [Google Scholar]
- [180].Lu L, Xu H, Wang X, Guo G, Increased nitric oxide synthase activity is essential for electromagnetic-pulse-induced blood-retinal barrier breakdown in vivo, Brain Res. 1264 (2009) 104–110, 10.1016/j.brainres.2009.01.043. [DOI] [PubMed] [Google Scholar]
- [181].Ding-Zhou L, Marchand-Verrecchia C, Croci N, Plotkine M, Margaill I, L-NAME reduces infarction, neurological deficit and blood-brain barrier disruption following cerebral ischemia in mice, Eur. J. Pharmacol 457 (2002) 137–146, 10.1016/S0014-2999(02)02686-9. [DOI] [PubMed] [Google Scholar]
- [182].Boje KMK, Inhibition of nitric oxide synthase attenuates blood-brain barrier disruption during experimental meningitis, Brain Res. 720 (1996) 75–83, 10.1016/0006-8993(96)00142-4. [DOI] [PubMed] [Google Scholar]
- [183].Mohammadi MT, Shid-Moosavi SM, Dehghani GA, Contribution of nitric oxide synthase (NOS) in blood-brain barrier disruption during acute focal cerebral ischemia in normal rat, Pathophysiology 19 (2012) 13–20, 10.1016/j.pathophys.2011.07.003. [DOI] [PubMed] [Google Scholar]
- [184].Stover JF, Belli A, Boret H, Bulters D, Sahuquillo J, Schmutzhard E, Zavala E, Ungerstedt U, Schinzel R, Tegtmeier F, Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: a placebo-controlled randomized phase iia trial (NOSTRA),, J. Neurotrauma 31 (2014) 1599–1606, 10.1089/neu.2014.3344. [DOI] [PubMed] [Google Scholar]
- [185].Cardnell RJG, Mikkelsen RB, Nitric Oxide synthase inhibition enhances the antitumor effect of radiation in the treatment of squamous Carcinoma Xenografts, PLoS One 6 (2011) 1–9, 10.1371/journal.pone.0020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Goldim MP, Danielski LG, Rodrigues JF, Joaquim L, Garbossa L, de Oliveira AN Junior, Metzker KLL, Della Giustina A, Cardoso T, Barichello T, Petronilho F, Oxidative stress in the choroid plexus contributes to blood–cerebrospinal fluid barrier disruption during sepsis development, Microvasc. Res 123 (2019) 19–24, 10.1016/j.mvr.2018.12.001. [DOI] [PubMed] [Google Scholar]
- [187].Kim W, Lee S, Seo D, Kim D, Kim K, Kim EG, Kang JH, Seong KM, Youn HS, Youn BH, Cellular stress responses in radiotherapy, Cells 8 (2019) 1–18, 10.3390/cells8091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Leu D, Spasojevic I, Nguyen H, Deng B, Tovmasyan A, Weitner T, Sampaio RS, Batinic-Haberle I, Huang TT, CNS bioavailability and radiation protection of normal hippocampal neurogenesis by a lipophilic Mn porphyrin-based superoxide dismutase mimic, MnTnBuOE-2-PyP5+, Redox Biol. 12 (2017) 864–871, 10.1016/j.redox.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Spasojevic I, Miriyala S, Tovmasyan A, Salvemini D, Fan P, Vujaskovic Z, Batinic-Haberle I, Clair DKS, Lipophilicity of Mn(III) N-alkylpyridylporphyrins dominates their accumulation within mitochondria and therefore in vivo efficacy: a mouse study, Free Radic. Biol. Med 51 (2011) S98–S99, 10.1016/j.freeradbiomed.2011.10.473. [DOI] [Google Scholar]
- [190].Lehner C, Gehwolf R, Tempfer H, Krizbai I, Hennig B, Bauer HC, Bauer H, Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases, Antioxidants Redox Signal. 15 (2011) 1305–1323, 10.1089/ars.2011.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Shi YY, Cui HF, Qin BJ, Monomethyl fumarate protects cerebral hemorrhage injury in rats via activating microRNA-139/Nrf2 axis, Eur. Rev. Med, Pharmacol. Sci 23 (2019) 5012–5019, 10.26355/eurrev_201906_18093. [DOI] [PubMed] [Google Scholar]
- [192].Tovmasyan A, Sheng H, Weitner T, Arulpragasam A, Lu M, Warner DS, Vujaskovic Z, Spasojevic I, Batinic-Haberle I, Design, mechanism of action, bioavailability and therapeutic effects of Mn porphyrin-based redox modulators, Med. Princ. Pract 22 (2013) 103–130, 10.1159/000341715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Batinic-Haberle I, Rajic Z, Tovmasyan A, Reboucas JS, Ye X, Leong KW, Dewhirst MW, Vujaskovic Z, Benov L, Spasojevic I, Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics, Free Radic. Biol. Med 51 (2011) 1035–1053, 10.1016/j.freeradbiomed.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Batinic-Haberle I, Tovmasyan A, Roberts ERH, Vujaskovic Z, Leong KW, Spasojevic I, SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways, Antioxidants Redox Signal. 20 (2014) 2372–2415, 10.1089/ars.2012.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Gad SC, Sullivan DW, Spasojevic I, Mujer CV, Spainhour CB, Crapo JD, Nonclinical safety and toxicokinetics of MnTnBuOE-2-PyP5+ (BMX-001), Int. J. Toxicol 35 (2016) 438–453, 10.1177/1091581816642766. [DOI] [PubMed] [Google Scholar]
- [196].Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, Ollivier J, Petit B, Jorge PG, Syage AR, Nguyen TA, Baddour AAD, Lu C, Singh P, Moeckli R, Bochud F, Germond JF, Froidevaux P, Bailat C, Bourhis J, Vozenin MC, Limoli CL, Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species, Proc. Natl. Acad. Sci. U, S. A 166 (2019) 10943–10951, 10.1073/pnas.1901777116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Montay-Gruel P, Markarian M, Allen BD, Baddour JD, Giedzinski E, Jorge PG, Petit B, Bailat C, Vozenin MC, Limoli C, Acharya MM, Ultra-high-dose-rate FLASH irradiation limits reactive gliosis in the brain, Radiat. Res 194 (2020) 636–645, 10.1667/RADE-20-00067.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G, Patin D, Bouchaab H, Ozsahin M, Bochud F, Bailat C, Devauchelle P, Bourhis J, The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients, clin, Cancer Res. 25 (2019) 35–42, 10.1158/1078-0432.CCR-17-3375. [DOI] [PubMed] [Google Scholar]
- [199].Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, Doenlen R, Favaudon V, Bochud F, Bailat C, Bourhis J, Vozenin MC, Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s, Radiother. Oncol 124 (2017) 365–369, 10.1016/j.radonc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- [200].A V, IV K, Gm C, Mm K, G S, D G, S H, II V, K S, J C, M C, K V, L Y, L Q, Ag H, Aj M, M P, M L, Ca A, E R, J H, E D, L D, J M, C K, Ka C, A M, Tm B, FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues, While preserving sarcoma response, Cancer Res. 81 (2021), 10.1158/0008-5472.CAN-21-1500 canres.1500.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


