Abstract
Accumulating research suggests that stressful life events, especially those that threaten close intimate bonds, are associated with an increased risk of dementia. Grieving the loss of a spouse, whether in the form of caregiving or after the death, ranks among ‘life’s most significant stressors’, evoking intense psychological and physiological distress. Despite numerous studies reporting elevated dementia risk or poorer cognition among spousal caregivers and widow(er)s compared to controls, no review has summarized findings across cognitive outcomes (i.e., dementia incidence, cognitive impairment rates, cognitive performance) or proposed a theoretical model for understanding the links between partner loss and abnormal cognitive decline. The current systematic review summarizes findings across 64 empirical studies. Overall, both cross-sectional and longitudinal studies revealed an adverse association between partner loss and cognitive outcomes. In turn, we propose a biopsychosocial model of cognitive decline that explains how caregiving and bereavement may position some to develop cognitive impairment or Alzheimer’s disease and related dementias. More longitudinal studies that focus on the biopsychosocial context of caregivers and widow(er)s are needed.
Keywords: spousal caregiving, widowhood, dementia, cognition, stress, cognitive decline, interpersonal loss, close relationships, depression, cognitive impairment, Alzheimer’s disease, inflammation, glucocorticoids
1. Introduction
Normal brain aging is characterized by a decline in cognition, including learning, memory, information processing, and executive function (Alexander et al., 2012; Craik & McDowd, 1987; Harada et al., 2013; Petersen et al., 1992). The cognitive decline that exceeds age-related changes in cognition is a risk factor for dementia (Aronson et al., 1990; Ingles et al., 2003; Small et al., 19970101; Toshitake et al., 1995). There is growing evidence suggesting that stressful life events such as widowhood or spouse illness are associated with greater cognitive decline and dementia incidence (Gerritsen et al., 2017; Håkansson et al., 2009; Lee et al., 2004; Lyu et al., 2019; G. Persson & Skoog, 1996), but the consistency of these findings have not been previously evaluated.
1.1. Interpersonal losses: Spousal bereavement and spousal caregiving
Intimate relationships are particularly impactful to mental and physical well-being. A romantic partner is the primary attachment relationship for most adults. Romantic bonds serve an adaptive function, providing emotional comfort, physical security, and health benefits throughout a person’s life. Married older adults are more likely to engage in positive health behaviors (Schone & Weinick, 1998) and live longer (Kaplan & Kronick, 2006) compared to non-married older adults. However, when married individuals are faced with spousal death or spousal caregiving - two forms of interpersonal loss that typically occur later in life - the protective benefits of marriage also come at a cost. The anticipation or experience of partner death threatens years of lifelong coregulation (psychological and physiological) and interdependence between marital partners (Robles et al., 2014; Sbarra & Hazan, 2008; Wünsche et al., 2020). Indeed, spousal bereavement ranks as the most stressful life event one can experience in their lifetime on the Social Readjustment Scale (Holmes & Rahe, 1967). Spousal caregiving often serves as a classic model of chronic stress. The effects of losing an intimate partner span years: in comparison to still-married and never-married individuals, widow(er)s show significantly reduced morale and higher emotional disturbance several years after losing their spouse (Bennett, 1996).
During late-middle adulthood (55–64 years) to older adulthood (65+ years), caring for a spouse with a disability or neurodegenerative disease can impose significant mental and physical strain on the caregiver. Spousal caregivers often provide around the clock care while experiencing a form of “living bereavement,” as they watch their partners lose their personality and independence (Light & Lebowitz, 1990) and grieve the loss of personal companionship, independence, and social network (Chan et al., 2013; Roland et al., 2010). Spousal caregivers of stroke, Parkinson’s disease (PD), and Alzheimer’s disease (AD) report poorer quality of life with increasing caregiver burden (McCullagh et al., 2005; Serrano-Aguilar et al., 2006; Shin et al., 2012). Moreover, caregivers experiencing high mental and emotional strain are at increased risk of mortality than non-caregiving controls (Schulz & Beach, 1999). Caregivers also exhibit poorer cognitive function (de Vugt et al., 2006), impaired immunity (Bauer et al., 2000), and greater incidence of depressive disorders (Kiecolt-Glaser et al., 1991) compared to controls.
Spousal bereavement causes significant health disturbances (Fagundes & Wu, 2020). Bereaved spouses report more significant psychological stress, more depressive symptoms, lower self-reported health, and elevated illness burden than married individuals (Harlow et al., 1991; Jones et al., 2010; Perkins et al., 2016). Widow(er)s also exhibit poorer immune function and less adaptive autonomic nervous system functioning than nonbereaved adults (Fagundes et al., 2018; O’Connor et al., 2014; Schultze-Florey et al., 2012). Spousal bereavement is associated with poor health behavior changes that lead to increased nutritional risk, increased alcohol consumption, and poorer sleep quality (Stahl & Schulz, 2014).
While the impact of spousal bereavement and spousal caregiving on the stress response and related systems (i.e., HPA axis, autonomic nervous system, immune system) has been studied extensively (Bennett et al., 2013; Fagundes & Wu, 2020), the downstream consequences of interpersonal loss on cognition is less understood. There is growing evidence that widow(er)s and spousal caregivers exhibit more significant cognitive impairment and higher dementia incidence than nonbereaved or non-caregiving controls. For example, in a sample of 2,000 participants, Gerritsen et al. found that widowhood augmented the association between stressful life events and dementia incidence 3-fold (Gerritsen et al., 2017). Likewise, in a sample of 1,221 married couples, spouses of dementia patients were 6 times more likely to develop dementia than spouses of persons without dementia (Norton et al., 2010). To date, no review has been conducted on spousal caregiving, widowhood, and cognitive outcomes.
1.2. Stress-related changes in mood and cognition
Stressors can precipitate changes in cognition and mood. In a sample of 2,471 adults, individuals reporting more lifetime trauma (e.g., death in the family, parental divorce, assault, parent drug abuse) showed greater cognitive decline over 9 years (Lynch & Lachman, 2020). Interpersonal stressors strongly predict impending major depressive disorder (MDD) (Slavich & Irwin, 2014), a stress-related disorder characterized by changes in mood, behavior, and cognition (American Psychological Association, 2013). For example, higher rates of MDD are reported among people who experienced interpersonal losses (i.e., a recent break-up, death of a spouse, gradual loss of a spouse to chronic disease) compared to those who did not experience an interpersonal loss (Beeson, 2003; Harlow et al., 1991; Monroe et al., 1999). Notably, even though depressed mood and lack of pleasure are the most prominent symptoms of MDD, impairments in episodic memory, executive functioning, or processing speed are often present, especially in people with more severe depressive symptomology (McDermott & Ebmeier, 2009).
1.3. Stress pathways and stress-mediated changes in the brain
The stress response involves a coordinated interplay between the central nervous system (CNS) and multiple body systems (e.g., neuroendocrine, cardiovascular system). When the brain appraises a stimulus as threatening and, therefore, stressful to the individual, the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis release catecholamines and glucocorticoids, respectively, to temporarily upregulate essential functions for survival (e.g., heart rate and blood pressure) and downregulate restorative functions (e.g., digestive processes, immune system). Acute engagement of the stress response followed by successful recovery to homeostasis is adaptive, but prolonged activation of the “fight or flight” response can dysregulate physiological systems and lead to adverse health outcomes. Indeed, while glucocorticoids are typically anti-inflammatory, chronic stress reduces immune cells’ sensitivity to the inhibitory effects of glucocorticoids (termed glucocorticoid resistance), thereby, increasing inflammatory processes (Miller et al., 2002). Systemic inflammation – a phenomenon characterized by persistently elevated levels of pro-inflammatory cytokines and chronic activation of the immune system – underlies many age-related diseases, including cardiovascular disease and dementia (Franceschi & Campisi, 2014).
Stress hormones and peripheral inflammatory stimuli feed back to the brain, making them important mediators of stress-induced changes in mood and cognition (Lupien et al., 2007; McEwen & Gianaros, 2010). Glucocorticoids and cytokines can cross the blood brain barrier and directly bind to receptor sites distributed within the brain (Lupien et al., 2007; Shirazi et al., 2015; Tsyglakova et al., 2019). Cytokines, along with catecholamines, also interface with vagal afferents that relay signals from the periphery to the brainstem (Bluthe et al., 1994; Mravec, 2011). Ultimately, stress-mediated signaling promotes neural restructuring in ways that can be adaptive or maladaptive for cognition and health. For example, under normal circumstances, glucocorticoid- and immune- mediated signaling facilitate neurogenesis, learning, and memory (McGaugh, 2000; Yirmiya & Goshen, 2011). However, chronically high levels of glucocorticoids and pro-inflammatory cytokines can cause neurodegeneration and contribute to the development of cognitive and mood disorders and neurodegenerative diseases (Dantzer et al., 2008; Perry et al., 2007; Vyas et al., 2016).
1.4. A focus on two types of interpersonal losses: spousal caregiving and spousal bereavement
Although interpersonal losses are not limited to the experience of caregiving and widowhood, we focused on caregiving and widowhood because they share distinct similarities. First, caregiving and widowhood are interpersonal losses generally constrained within the same life stage: both events commonly occur in older adulthood, which contrasts from other losses such as divorce that are not typical events in older adulthood (Allred, 2019). Second, both spousal caregiving and widowhood are events that occur involuntarily and unexpectedly. Typically, life transitions entered involuntarily are more distressing than those entered voluntarily (Wade & Pevalin, 2004). A vast majority of dementia spousal caregivers report that they did not have a choice in taking up the caregiver role (Pertl et al., 2019). Even if deaths are anticipated, the financial burden of medical costs, caregiving, the context surrounding the death, gradual deterioration of spouse’s personality, and prolonged suffering can increase psychological distress among surviving spouses (Carr, 2003). Importantly, spousal caregiving and widowhood are distinct from divorce, in which people “choose to” or “grant” divorce and have forewarning to prepare for the transition emotionally. Third, previous research suggests that spousal caregiving and widowhood are related life transitions and should be examined together as a process (Keene & Prokos, 2008; Wells & Kendig, 1997). Indeed, caregiving duration (Keene & Prokos, 2008) and the distress experienced during caregiving significantly impact adjustment during bereavement (Stroebe et al., 2007). Fourth, substantial mechanistic work in both spousal caregiving and spousal bereavement literature provide a strong foundation from which we could theorize biopsychosocial pathways that link interpersonal losses to cognitive impairment.
1.4. The current review: Are spousal caregiving and bereavement associated with cognitive decline?
Guided by the literature on interpersonal losses, stress, and cognition, we examined the evidence for poorer cognitive outcomes related to spousal caregiving and spousal bereavement. Our goal was to provide a comprehensive review of the relationship between 1) spousal caregiving and cognitive outcomes and 2) widowhood and cognitive outcomes.
This review culminates with an integrative biopsychosocial model that outlines pathways through which interpersonal losses may accelerate cognitive decline. Specifically, we describe the interplay of psychological health, stress-related systems, and neurocognitive aging processes in accelerating age-related cognitive decline. We also briefly discuss how biobehavioral patterns may perpetuate or protect against abnormal cognitive aging (i.e. Alzheimer’s disease and related dementias (ADRD)). We conclude with some suggestions for future research.
2. Methods
2.1. Search Strategy
Following PRISMA guidelines for systematic reviews, a systematic literature search was conducted to assess the association between experiencing spousal bereavement or spousal caregiving and cognitive function. Sources included peer-reviewed articles from PubMed and Web of Science published online between January 1, 1964 and November 14, 2021 and articles obtained from reference lists. Grey literature, or evidence not published in commercial publications, were also examined from opengrey.eu and Proquest, two open source databases that allow access to dissertations, conference papers, and published proceedings (Paez, 2017). Searches were performed using a combination of cognitive search terms and bereavement or caregiving search terms. Separate searches were conducted for spousal bereavement and spousal caregiving and are presented in different tables. The following search terms were used to retrieve studies on spousal bereavement and cognition: “cognition” or “cognitive decline” or “cognitive activity” or “dementia” or “Alzheimer’s disease” AND “bereave*” or “widow*” or “grief.” The following search terms were used to retrieve studies on spousal caregiving and cognition: “cognition” or “cognitive decline” or “cognitive activity” or “dementia” or “Alzheimer’s disease” AND “caregiving” or “caregiver” NOT (“prenatal” or “maternal” or “birth”) AND (“spouse” or “partner”). The first author conducted the search, collated and sorted the results, checked for duplicates. All articles underwent selection twice: once by the first author and once by a coauthor or trained research assistant. Reference lists were also examined from each article. The following keywords were used in separate searches on opengrey.eu: “widow”, “widowhood”, “caregiver”, “marital status”, and “caregiver.” On ProQuest, we searched abstracts with the following keywords to find relevant bereavement literature: (“cognition” or “cognitive decline” or “cognitive activity” or “dementia” or “Alzheimer’s disease”) AND (“bereave*” or “widow*” or “grief”). Similarly, we searched abstracts with the following keywords to find relevant caregiving literature: (“cognition” or “cognitive decline” or “cognitive activity” or “dementia” or “Alzheimer’s disease”) AND (“caregiver” OR “caregiving”) AND (“spousal” or “spouse”).
Bereavement inclusion and exclusion criteria.
Inclusion criteria for studies on spousal bereavement and cognition were as follows: 1) the study compared widow(er)s to nonwidowed subjects 2) cognitive function or diagnosis of significant cognitive decline was assessed as the dependent variable. Studies were excluded for the following reasons: 1) widow(er)s were undifferentiated from divorced or single individuals, 2) the study design was qualitative, 3) the article was a review or meta-analysis, 4) the article was unavailable in English, 5) dissertation results were accounted for in a peer-review article included in the present review.
Caregiving inclusion and exclusion criteria.
Inclusion criteria for studies on spousal caregiving and cognition were as follows: 1) the sample specifically assessed spousal or partner caregivers, 2) the study compared spousal caregivers to non-caregivers, 3) cognitive function or diagnosis of significant cognitive decline was assessed as a dependent variable. Studies were excluded for the following reasons: 1) spousal caregiving was undifferentiated from other types of caregiving, 2) the study design was qualitative, 3) the article was a review or meta-analysis, 4) the article was unavailable in English, 5) dissertation results were accounted for in a peerreview article included in the present review.
2.2. Data Extraction
For both spousal bereavement and spousal caregiving searches, two researchers (primary author and one co-author) extracted the following information from each study: (a) study design, (b) reference group, (c) time since death or duration of caregiving, (d) sample characteristics (sample size, gender, mean age), (e) cognitive measure, (f) covariates, (g) the findings related to spousal loss (caregiving or bereavement) and cognitive outcomes. The primary author resolved data extraction discrepancies after careful review of the article in question.
All studies with sufficient sample size, based on the analytical method implemented, were assessed for quality. We followed Van Voorhis & Morgan (2007) sample size recommendations for detecting a medium to large effect size with 80% power. Specifically, we examined studies that met the following sample size criteria: 1) sample sizes of 30 or more per cell for pairwise comparisons and analysis of variance tests or 2) sample sizes of 50 or more for correlation and regression analyses. Three studies did not meet the sample size criteria (Mackenzie et al., 2009; O’Connor & Arizmendi, 2014; Ward et al., 2007).
The NHLBI Study Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to assess study quality (National Heart, Lung, and Blood Institute, 2021). This assessment tool consists of 14 items that evaluate factors – such as study design, sample size justification, study recruitment population, attrition, and inclusion of confounding variables – that may impact internal validity. The items are designed to guide raters toward making critical appraisals of each study’s potential for bias. Criteria are either met, not met, not applicable, not reported, or cannot be determined. Any criterion that was not met was subsequently evaluated to determine whether failure to meet it was an indication of a major flaw in study design or execution. Because the creators of the NHLBI Assessment Tool do not recommend using a tallying system, two raters, one coauthor and one senior research assistant, subjectively determined the quality of each article.
In accordance with the NHLBI Study Quality Assessment Tool rating guidelines, coders rated studies as poor, fair, or good. Studies were rated as “good” when their study designs better substantiated a causal relationship between exposure and outcome. Specifically, design characteristics that include 1) exposures occurring prior to outcomes, 2) accuracy of measurement of both exposure and outcome, 3) sufficient timeframe to see an effect, and 4) appropriate control for confounders strengthen the causal relationship between exposure and outcome. Thus, studies that met these 4 criteria were often rated as “good” (lowest risk of bias), even when not all 14 criteria were met. For example, a study was rated good because it met most criteria but did not meet criteria such as sample size justification and loss to follow-up < 20% (Håkansson et al., 2009). Studies that met some of the above criteria were often rated as “fair”; a fair rating indicates some bias but not enough to invalidate results. For example, a study was rated as fair because it met most criteria but did not meet sample size justification, exposure measured before the outcome, exposure assessed more than once over time, or timeframe sufficient to see an association between exposure and outcome (O’Connor & Arizmendi, 2014). Studies that did not meet any of these criteria were often rated as “poor”; a poor rating indicates a significant risk of bias that may compromise the conclusions of the study. For example, a study was given a poor rating because it met less than half of the 14 criteria and failed to meet criteria such as sample size justification, measuring and adjusting statistically for confounding variables, exposure measured before the outcome, exposure assessed more than once, timeframe sufficient to see an association between exposure and outcome, and exposure measures were clearly defined (Lopes et al., 2007). Intercoder reliability was 84.3%. Discrepancies across ratings were discussed and resolved among raters and the first author.
3. Results
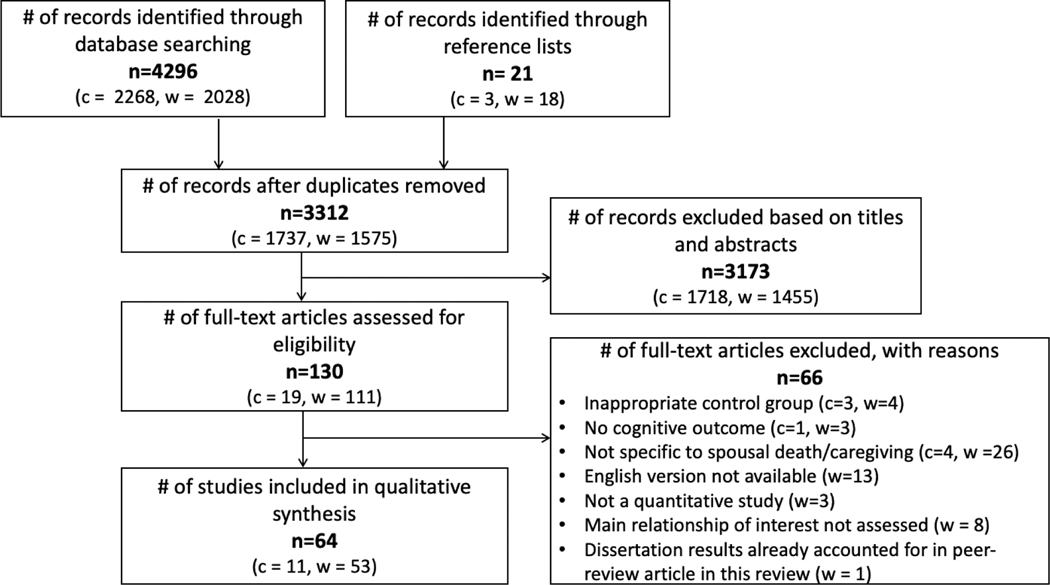
The database search strategy for spousal bereavement, spousal caregiving, and cognition generated a total of 2,595 articles from Pubmed and Web of Science and 717 from opengrey.eu and ProQuest, after excluding 1,005 duplicates. Two eligible dissertations were identified through grey literature and reference lists (Brenowitz, 2013; Hatch, 2013). One of the two dissertations (Brenowitz, 2013) was published as a peer-revied article reviewed below (Brenowitz et al., 2014) and was consequently excluded due to duplicate findings. After titles and abstracts were screened, 130 articles were eligible for full-text screening. Full-text screening resulted in 64 articles that qualified for the present review. Separate and collated screening information for spousal bereavement and spousal caregiving articles are depicted in Figure 1.
Figure 1.
Flow chart of selected articles
Note: c = articles on caregivers, w = articles on widow(er)s
Out of the 64 articles selected for the final review, 61 articles were assessed for bias, as three did not meet sample size criteria sufficient to detect a reasonable effect (Brenowitz et al., 2014). Out of the 61 evaluated, 37 articles were rated as good quality (low bias), 21 as fair (medium bias), and 3 as poor (high bias). All 64 studies are discussed in the text, and quality ratings and study and sample characteristics are detailed in Table 1 and Table 2 for caregiver and bereavement articles, respectively. Unrated studies were designated an “NA”. For a collated table of results from only good quality articles, see Table 3.
Table 1.
Study design, sample characteristics, and results for publications on spousal caregivers
| Authors (year) | Quality Rating | Study Design; Length of Observation (years) | Caregiver Type | Reference Group | Caregiving Duration in months; hours per week | Total N (% female) n Reference Group; n Caregivers | Reference Group Mean Age (SD); Caregiver Mean Age (SD) | Dependent Cognitive Measures | Cognitive Domain | Results | Controlled for age, sex, SES? |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Caswell et al. (2003) | 2 | Cross-sectional | Alzheimer’s disease | Spousal non-caregivers | nr; nr | 110 (61.8%) 66 (68.2 %); 44(52.3) 3562 (53.2%) | 70.85 (6.32); 74.27 (7.91) | DSST | Information processing speed, complex attention, concentration | Caregivers performed worse on digit symbol test than controls. | Yes |
| Chen and Botticello (2013) | 2 | Cross-sectional | Stroke | Spousal non-caregivers | nr; nr | 3562 (53.2 %) 3416 (52.5 %); 146 (68.5 %) 162 (59.3 %) | nr; nra | TICS-m | Orientation, attention, language, memory | Caregivers performed worse on episodic memory than controls but did not differ on other cognitive domains. | Yes |
| De Vugt et al. (2006) | 3 | Cross-sectional | Dementia | Non-caregivers | 24 months; 153.6 h | 108 (59.3 %); 54 (59.3 %) | 68.3 (8.4); 68.4 (8.5) | MMSE, AVLT, LDCT, SCWT, GIT-SF | Global cognitive functioning, verbal memory, information processing speed, cognitive flexibility/ inhibition, general intelligence | Caregivers had worse cognitive functioning and performed worse on verbal memory tasks than controls. Caregivers did not differ from controls on cognitive flexibility and inhibition. | Yes |
| Lee et al. (2004) | 3 | Longitudinal; 3 | Non-specific | Non-caregivers | nr | 13,740 (100%) 12,536 (100%) 1204 (100%) | 74.2 (nr); 74.6 (nr) | TICS, EBMT, EBMT, VF, DSB, composite cognitive score computed from all assessments | Global cognitive functioning, verbal memory, semantic function, executive function, attention and working memory | Caregivers had greater risk of receiving low scores on the TICS and recall/memory items on the TICS than controls. They did not differ significantly on other memory tests or overall cognitive composite than controls. | Yes |
| Mackenzie et al. (2009) | NA | Cross-sectional | Nonspecific caregiver | Married non-caregivers | 48.93 months (29.56; 12–120) | 32 (68.75 %) 16 (68.75 %); 16 (68.75 %) 2442 (50 %) 2213 (nr); | 79.37 (4.67); 78.94 (4.70) | CVLT, DSB, WAIS-arithmetic, LNST | Learning and verbal memory, working memory | Caregivers performed worse on learning and memory tasks than controls. | Yes |
| Norton et al. (2010) b | 3 | Longitudinal; 3.3 | Dementia | Spouses without dementia | 4.1 years; nr | 229 (nr) | Nrc | Dementia diagnosis (clinician) | Incident dementia | People who had a spouse with dementia had higher odds of dementia than people who didn’t have a spouse with dementia. Men whose spouses had dementia had a higher risk of dementia than women whose spouses had dementia. | Yes |
| O’Sullivan et al. (2019) | 2 | Cross-sectional | Dementia | A. Spousal non-caregivers (population sample) B. Noncaregiver (self- selecting sample) |
24+ months; 28+ hrsd | A. 179(71.0 %); 179(69.8 %) B. 155(66.5 %); 155(66.5 %) |
A. 67.06 (6.61); 67.53 (6.86) B. 66.29 (6.47); 67.02 (7.20) |
CRT, CTT, CF, LM, FCSRT, LNST | Executive functioning, processing speed, memory, working memory, verbal fluency | Caregivers had better cognitive functioning (executive functioning, faster processing speed, better memory) than controls. Caregivers did not differ from controls on working memory and verbal fluency. | Yes |
| Pertl et al. (2015) | 2 | Cross-sectional | Dementia and Nondementia | Non-caregivers | nr | 7965(50.9 %) 6247(49.7%); 179(55.3 %)e | 63.53 (nr); 66.2 (nr)f | TICS, number series test, word definition test, memory change (self-reported) | Overall cognition, reasoning, vocabulary, semantic memory, memory | Caregivers performed worse on cognitive tasks than non-caregiving controls. Dementia spousal caregivers did not differ significantly from non-caregivers on cognition. | Yes |
| Vitaliano et al. (2005) | 3 | Longitudinal; 2 | Alzheimer’s disease | Spousal noncaregivers | nr | 191 (61.2 %) 95(62 %); 96(60 %) | 71.0 (6.9); 72.2 (9.3) | SILS | Vocabulary, reasoning, | Caregivers performed worse on vocabulary but not abstract reasoning compared to controls | Nog |
| Vitaliano et al. (2007) | 2 | Longitudinal; 2 | Alzheimer’s disease | Spousal non-caregivers | 44.1 months | 1239 (63 %) 17 (64 %); 122 (62 %) | 70.2 (7.2); 71.7 (8.9) | DSST | processing speed, complex attention psychomotor speed, cognitive-motor translation, concentration processing speed, | Caregivers had poorer cognitive function than non-caregivers at each of the 3 time points. | No |
| Vitaliano et al. (2009) | 3 | Longitudinal; 2 | Alzheimer’s disease | Spousal non-caregivers | 44.1 monthsh; 7.0 h | 239 (63%) 117(64 %); 122(62 %) | 70.2 (7.2); 71.7 (8.9) | DSST | complex attention psychomotor speed, cognitive-motor translation, concentration | Caregivers had poorer baseline cognitive performance and faster declines over time than non-caregivers. | Yesi |
Notes: nr = not reported; NA = not applicable; AVLT = Auditory Verbal Learning Test; CF = Category Fluency; CTT = Color Trails Test; CRT = Choice Reaction Time; CVLT = California Verbal Learning Test; DS = Digit Span; Digit Span Backwards = DSB; DSST = Digit Symbol Substitution Test; EBMT = East Boston Memory Test; FCSRT = Free and Cued Selective Reminding Test; GIL-SF = Groninger Intelligence Test Short Form; LDCT = letter Digit Coding Test; LM = Logical Memory Test from WAIS; LNST = Letter-Number Sequencing Test; MMSE = Mini Mental State Exam; SCWT = Stroop Colour-Word Test; SILS = Shipley Institute of Living Scale; TICS = Telephone Interview for Cognitive Status; TICS-m = TICS-modified; VF = Verbal fluency test; WAIS = Wechsler Adult Intelligence Scale.
This study only reported age in categories. 80.4 % of reference group was 55+ years old and 85.6 % of caregiver group was 55+ years old.
Spouses of persons with dementia were not explicitly identified as “caregivers”.
Did not report age for total sample but they did stratify age by gender: Male: 75.7 (5.9), Female = 73.1 (5.3).
~88 % of sample has been caregiving for 2+ years. ~82 % provides care for 4+ hours a day or 28+ hours a week.
The sample was stratified by spouse’s dementia status and spouse’s caregiver status. The 2 remaining groups not mentioned in the table have the following Total N (% female): dementia non-caregivers = 120(66.7 %), Non-dementia caregivers = 1419(54.3 %).
Values were calculated by Wu et al. using information provided by the study, as sample statistics were stratified by caregiver status x spouse’s dementia status.
Did not control for these covariates in analyses but in univariate analyses, the two groups did not differ on age, sex, or SES.
44.1 months is median.
For baseline/cross-sectional test, analyses controlled for age, education and sex. for change analyses, education was not accounted for.
Table 3.
Summary of findings across caregiver and bereavement studies with minimal bias
| Authors (year) | Results | Evidence of poorer cognitive function in widow(er)s and caregivers?* |
|---|---|---|
|
| ||
| Aartsen et al. (2005) | Widow(er)s had greater memory decline than control | Yes |
| Amieva et al. (2010) | Being widowed was not associated with greater odds of dementia or AD than being married | No |
| Widow(er)s had poorer cognitive function than married adults, with longer widowhood duration associated with | Yes | |
| Barragán-García et al. (2021) | lower overall cognition. | |
| Bickel & Cooper (1994) | Being widowed was not associated with increased odds of dementia | No |
| Biddle et al. (2020) | Widow(er)s declined in cognitive performance compared to married subjects | Yes |
| Brenowitz et al. (2014). | Widowers had lower risk of MCI than controls. | No |
| Widowed men had higher rates of cognitive impairment than married/re-partnered men. Widowed women did not | Yes | |
| Brown et al. (2020) | show differences in cognitive function compared to married women. | |
| Caregivers had worse cognitive functioning and performed worse on verbal memory tasks than controls. Caregivers | Yes | |
| De Vugt et al. (2006) | did not differ from controls on cognitive flexibility and inhibition. | |
| Losing a spouse is associated with receiving a dementia diagnosis within 3 and 6 months of the loss but not | Yes | |
| Forbes et al. (2019) | associated with long-term risk of dementia | |
| Those who were widowed at midlife were more likely to show cognitive impairment later in life than those cohabiting with a partner in mid-life. Still widowed individuals had 3× risk of cognitive impairment than married/cohabiting | Yes | |
| Håkansson et al. (2009) | people. | |
| Hatch (2013) | There was no association between widowhood and risk of dementia. | No |
| Helmer et al. (1999) | Widowed adults did not differ from married adults in dementia risk. | No |
| Jia et al. (2020) | Being widowed was associated with greater risk of dementia or mild cognitive impairment. | Yes |
| Widow(er)s had faster declines on total cognition and recall than married adults. Baseline marital status was not | Yes | |
| Karlamangla et al. (2009) | associated with baseline cognitive function. | |
| Lee et al. (2019). | There were no significant association between widowhood and cognitive function across a 4-year period. | No |
| Caregivers had greater risk of receiving low scores on the TICS and recall/memory items on the TICS than controls. | Yes | |
| Lee et al. 2004 | They did not differ significantly on other memory tests or overall cognitive composite than controls. | |
| Widow(er)s had greater cognitive impairment in memory, executive function and orientation than married adults. | Yes | |
| Liu et al. (2019) | They also had higher prevalence rates of cognitive impairment (not dementia) and dementia. | |
| Widowed respondents (and all unmarried groups) had higher odds of dementia than married respondents. The | Yes | |
| Liu et al. (2020) | transition into widowhood, but not to divorce or remarriage, is associated with higher odds of dementia. | |
| Widow(er)s had greater cognitive impairment in memory, executive function and orientation than married adults. | Yes | |
| Liu et al. (2019) | They also had higher prevalence rates of cognitive impairment (not dementia) and dementia. | |
| Lyu et al. (2019) | Widow(er)s bereaved for 4–6 years had steeper declines in global cognitive functioning than nonwidowed individuals. | Yes |
| Widowed women had lower initial levels of cognitive function and slower rates of cognitive decline than married | Yes | |
| Monserud et al. (2019) | men. | |
| Widow(er)s had better overall episodic memory than singles but showed faster rates of declines in episodic memory (recognition) than married or divorced adults. Older widow(er)s had poorer semantic memory performance (vocabulary) than older married people. Widow(er)s showed no differences in recall or fluency from married, | Yes | |
| Mousavi-Nasab et al. (2012) | divorced, single adults. | |
| People who had a spouse with dementia had higher odds of dementia than people who didnť have a spouse with dementia. Men whose spouses had dementia had a higher risk of dementia than women whose spouses had | Yes | |
| Norton et al. (2010) b | dementia. | |
| Being widowed was not associated with cognitive outcomes. Bereaved men did worse than nonbereaved men on immediate story recall task. Bereaved younger adults did worse on immediate and delayed story recall compared to | Yes | |
| Rosnick et al (2010). | nonbereaved younger adults. | |
| Widow(er)s had lower global cognition than married subjects. Cognition scores also declined significantly as time | Yes | |
| Shin et al. (2018) | since spousal loss increased. | |
| Sundström et al. (2016) | Being widowed was associated with increased odds of dementia for young-old (50–64 yrs) and middle-old (65–74 yrs) | Yes |
| Sundström et al. (2014) | Widow(er)s have an increased risk of dementia compared to married adults. | Yes |
| Widow(er)s did not differ on cognitive performance than married individuals and did not have greater risk of dementia or mild cognitive impairment. Widowed women had lower executive functioning performance than | Yes | |
| Vidarsdottir et al. (2014) | married women but no differences were seen across widowed and married men. | |
| Vitaliano et al. (2005) | Caregivers performed worse on vocabulary but not abstract reasoning compared to controls | Yes |
| Vitaliano et al. (2009) | Caregivers had poorer baseline cognitive performance and faster declines over time than noncaregivers. | Yes |
| Widowed men had lower risk of dementia than married men. No differences in dementia risk between widowed and | No | |
| Wändell et al. (2020) | married women. | |
| Bereaved women performed showed decrease in reasoning performance compared to married women; no differences in memory, global cognitive functioning, and processing speed were found. No cognitive differences | Yes | |
| Wörn et al. (2020) | between bereaved men and nonbereaved men. Widow(er)s were more likely to experience cognitive decline than married subjects. When stratified by gender, wk owhood negatively impacted cognitive function in men but did not impact women. When widowhood was divided. to 5 ategories of widowhood duration, men bereaved for 5 years or less, 16–20 years, or 21+ years were more likely to have lower cognitive scores than married men. Women bereaved for 21+ years had worse cognitive function | |
| Xiang et al. (2021) | than currently married women. | |
| Yin et al. (2019) | Married individuals have lower risk of developing cognitive impairment than widowed individuals | Yes |
| Continually widowed had greater decline in episodic memory over 2 years than continually married adults. Newly | Yes | |
| Zhang, Z et al. (2019) | widowed adults did not differ from continually married adults in episodic memory changes | |
| Zhang et al. (2021) | Being widowed is associated with greater odds of dementia in both blacks and whites. | Yes |
| Being widowed was negatively associated with global cognition, objective memory, crystallized cognition and fluid | Yes | |
| Zhao et al. (2021) | cognition. Being widowed was not associated with backward counting, subjective memory, or date naming. | |
Note.
Articles are marked as yes if a minimum of one finding provides evidence for a relationship between widowhood and caregiving and poorer cognitive outcomes.
Below, we organized the results section into two parts based on the type of interpersonal loss: caregiving and widowhood. These two sections are subdivided by study design (cross-sectional, longitudinal/prospective). At the end of each section, we also summarize the role of additional factors (i.e., sex and psychological distress) that influenced the relationship between interpersonal losses and cognitive function. A majority of the studies derived their samples from longitudinal, population- or community-based studies. Sample sizes ranged from 32 to 30,578 subjects. Unless otherwise mentioned, the associations reported below were found after accounting for the effects of age, sex, and socioeconomic status (SES), at minimum. This was done either by having a control group matched on these characteristics or adjusting for these variables in analyses.
3.1. Spousal caregivers
Eleven studies on spousal caregiving were identified and all but three studies (Chen & Botticello, 2013; S. Lee et al., 2004; Mackenzie et al., 2009) focused on dementia spousal caregivers. Spousal non-caregivers were used as reference groups for all studies except for two, for which non-caregivers were used as the comparator (de Vugt et al., 2006; Lee et al., 2004). A majority of the studies conducted in the United States (U.S.) derived their study sample from the same longitudinal dataset but differed in the number of waves used in analyses. Specifically, four studies utilized a longitudinal dataset collected in Washington, U.S. (Caswell et al., 2003; Vitaliano et al., 2005, 2007, 2009), and two studies used data from the Health and Retirement Study, a nationally represented sample of the U.S. collected biannually since 1992 (Chen & Botticello, 2013; Pertl et al., 2015). The remaining study samples came from separate datasets in the U.S. (Lee et al., 2004; Norton et al., 2010), Netherlands (de Vugt et al., 2006), Ireland (O’Sullivan et al., 2019), and Canada (Mackenzie et al., 2009).
3.1.1. Cross-sectional associations between caregiving status and cognition
Six studies on spousal caregiving utilized a cross-sectional research design. One was rated good quality (de Vugt et al., 2006), one was unrated (Mackenzie et al., 2009), and the remaining four were rated as fair. Studies used a variety of cognitive measures to assess overall cognition (i.e., Mini-Mental State Exam (MMSE), Telephone Interview for Cognitive Status (TICS), Montreal Cognitive Assessment (MoCA)) and specific domains of cognition, including learning and memory (i.e., California Verbal Learning Test (CVLT), Logical Memory Test, Free and Cued Selective Reminding Test, self-reported memory), information processing and attention (i.e., Digit Symbol Substitution Test (DSST), Color Trails Test, Choice Reaction Time, Letter Digit Coding), cognitive flexibility (i.e., Stroop test), working memory (i.e., working memory index from Wechsler Adult Intelligence Scale (WAIS)), verbal ability and fluency (i.e., Category Fluency Test, word definition test from WAIS-Revised), and reasoning (i.e., number series test from Woodcock Johnson III test battery). One study did not account for sex or gender in analyses (Caswell et al., 2003).
All but one study reported poorer cognitive function in spousal caregivers than non-caregivers, but the affected cognitive domain varied across studies. Specifically, spousal caregivers performed worse on tasks measuring information processing and attention (Caswell et al., 2003; de Vugt et al., 2006), learning and memory (Chen & Botticello, 2013; de Vugt et al., 2006; Mackenzie et al., 2009, 2009), and global cognition (de Vugt et al., 2006; Pertl et al., 2015). Contrary to the rest of the studies, one study reported reverse associations between caregiving status and cognitive function: dementia caregivers had faster cognitive reaction times and better memory (i.e., free recall) than matched, spousal non-caregivers, even after controlling for demographics and I.Q. (O’Sullivan et al., 2019). Spousal caregivers showed no significant difference in cognitive flexibility (de Vugt et al., 2006), working memory (Chen & Botticello, 2013; Mackenzie et al., 2009; O’Sullivan et al., 2019), and semantic memory (Chen & Botticello, 2013) compared to non-caregivers.
3.1.2. Longitudinal associations between caregiving status and cognition
Five studies, four of which were also rated good quality, adopted prospective or longitudinal research designs that involved follow-ups 1 to 9 years later (S. Lee et al., 2004; Norton et al., 2010; Vitaliano et al., 2005, 2009). Two studies did not control for SES (Lee et al., 2004; Vitaliano et al., 2005), but caregiving and non-caregiving groups did not differ on educational level. These studies assessed dementia incidence, global cognition (i.e., TICS, z-scored average of multiple cognitive assessments), verbal knowledge and reasoning (i.e., Shipley Institute of Living Scale), and information processing and attention (i.e., DSST).
In all five studies, spousal caregivers either exhibited a higher incidence of dementia, higher risk for cognitive impairment, poorer cognitive function at each time point, or a more rapid decrease in cognitive function over time than non-caregivers. Norton et al. (2010) reported that spouses of people with dementia had 6 times greater risk of incident dementia than spouses of people without dementia. In a sample of female nurses, those providing care to a spouse had higher cognitive impairment risk (measured by a low TICS score) 4 years later (Lee et al., 2004). Moreover, women who consistently provided spousal care across the 4 years had a higher risk of overall cognitive impairment than non-caregiving women, while those who provided caregiving at only one time point exhibited no differences in cognitive risk compared to non-caregiving women (Lee et al., 2004). However, when composite cognitive scores derived from multiple cognitive measures (i.e., East Boston Memory Test (EBMT), Verbal Fluency Test, Digit Span Backwards (DSB)) were assessed in relation to caregiver status, no significant associations were found (Lee et al., 2004). Caregivers also showed greater declines in information processing and attention (Vitaliano et al., 2009), even after having lower cognitive function than non-caregivers at baseline and every time point thereafter (Vitaliano et al., 2007, 2009). Similarly, Vitaliano and colleagues found that caregivers exhibited greater decreases in vocabulary skill, but not in abstract reasoning, compared to non-caregivers (Vitaliano et al., 2005).
3.1.3. Additional factors that influence the relationship between caregiving status and cognition
Sex.
Some studies also considered the contribution of sex to the relationship between caregiving status and cognition. While having a spouse who requires care was associated with poorer cognitive performance than non-caregivers, male caregivers exhibited steeper declines in cognitive functioning than female caregivers (Pertl et al., 2015). Male dementia spousal caregivers had higher incident dementia than female dementia spousal caregivers (Norton et al., 2010).
Depression and distress.
Due to the association between depression and cognitive impairment, studies controlled for depression in several ways yet still found significant impacts of depression on the associations between caregiver status and cognitive function (Chen & Botticello, 2013; de Vugt et al., 2006; Lee et al., 2004; Pertl et al., 2015; Vitaliano et al., 2009). However, a few studies found that depression or distress explained the relationship between caregiving status and cognitive function. For example, one study found that depression fully mediated the association between caregiving status and some cognitive domains, including processing speed (Vitaliano et al., 2009), but not vocabulary skill (Vitaliano et al., 2005). Caswell et al. (2003) reported that when caregiver distress was included in the model instead of depression, the relationship between caregiving status and cognition was no longer significant. In another study, psychological distress partially mediated the association between caregiving status and episodic memory and working memory performance (Mackenzie et al., 2009).
3.2. Bereaved spouses
A majority of the studies used similar reference groups and were conducted outside of the U.S. using separate datasets. For all studies except five (Hatch, 2013; Y. B. Kim & Lee, 2019; Rosnick et al., 2010; Ward et al., 2007; Yin et al., 2020), married subjects served as the reference group. The remaining studies used nonbereaved subjects as the reference group (Hatch, 2013; Rosnick et al., 2010; Ward et al., 2007) or used widow(er)s as the reference group (Kim & Lee, 2019; Yin et al., 2020). Seventeen studies were conducted in the U.S., while 36 studies were conducted outside of the U.S. Study samples were derived from the following countries: Amsterdam (Aartsen et al., 2005; Wörn et al., 2020), United Kingdom (Forbes et al., 2019), France (Amieva et al., 2010; Helmer et al., 1999), Germany (Bickel & Cooper, 1994), Italy (Guaita et al., 2015), Sweden (MousaviNasab et al., 2012; Sundström et al., 2016; Sundström, Westerlund, et al., 2014; Wändell et al., 2020), Iceland (Vidarsdottir et al., 2014), Finland (Håkansson et al., 2009), Australia (Byrne & Raphael, 1997; Ward et al., 2007), Mexico (Barragán-García et al., 2021), Ecuador (Espinosa del Pozo et al., 2020), Brazil (Lopes et al., 2007; Ribeiro et al., 2013), Portugal (Paúl et al., 2010), Singapore (Feng et al., 2014), Korea (Kim & Lee, 2019; Lyu et al., 2019), India (Farron et al., 2020; Perkins et al., 2016; Saha et al., 2010; Subramanian et al., 2021), Taiwan (Fan et al., 2015), and China (Jia et al., 2020; Sun et al., 2021; Xiang et al., 2021; Xu et al., 2020; Yin et al., 2020; Y. Zhang et al., 2019; Z. Zhang et al., 2019; Z.-X. Zhang et al., 2006). The two studies in Korea, France, and Amsterdam were derived from the Korean Longitudinal Study of Aging (Kim & Lee, 2019; Lyu et al., 2019), Personnes Agees QUID cohort (Amieva et al., 2010; Helmer et al., 1999), Longitudinal Aging Study in Amsterdam (Aartsen et al., 2005; Wörn et al., 2020), respectively. Two Swedish samples were derived from the Betula Project (Mousavi Nasab et al., 2012; Sundström, Westerlund, et al., 2014) and the remaining two Swedish studies came from the same national registry (Sundström et al., 2016; Wändell et al., 2020). From the 17 studies conducted in the United States (U.S.), 12 studies had samples consisting of primarily non-Hispanic Caucasian subjects (>70%) and 4 studies did not report race categories (Hatch, 2013; Li et al., 2018; Shahar et al., 2001; Zhao et al., 2021). One study sample consisted of only Mexican Americans (Monserud, 2019). Six U.S. studies were taken from the Health and Retirement Study (Brown et al., 2020; Y. Lee et al., 2019; Liu, Zhang, Choi, et al., 2019; S. H. Shin et al., 2018; Z. Zhang et al., 2021; Zhao et al., 2021).
3.2.1. Cross-sectional associations between widowhood status and cognition
Twenty-three examined cross-sectional relationships between bereavement status and cognitive function. Out of the 23, two studies were unrated (O’Connor & Arizmendi, 2014; Ward et al., 2007), three studies were rated as good quality (Barragán-García et al., 2021; Jia et al., 2020; Rosnick et al., 2010), three studies were rated as poor (Byrne & Raphael, 1997; Lopes et al., 2007; Saha et al., 2010), and the remaining were rated as fair. Six of the 23 studies did not account for age, sex, and/or socioeconomic status in their analyses (Kramer et al., 1985; O’Connor & Arizmendi, 2014; Paúl et al., 2010; Saha et al., 2010; Shahar et al., 2001; Sun et al., 2021). Studies assessing overall cognition uniformly utilized the MMSE, except three studies that derived a composite score from multiple domain-specific cognitive tests (Farron et al., 2020; Rosnick et al., 2010) or administered a cross-cultural cognitive exam (Barragán-García et al., 2021). A variety of assessments were used to test memory (i.e., 10-word recall, delayed story recall, disc spatial memory test, Rey Auditory Verbal Learning Test, Benton Visual Retention Test), cognitive flexibility (i.e., Wisconsin Card Sorting Task), working memory (i.e., DSB), information processing and attention (i.e., emotional counting Stroop, Trail Making Test, elevator counting with distraction, Symbol Digit Modalities Test), verbal ability (i.e., EBMT, Controlled Oral Word Association Test), visuospatial ability (i.e., copying four objects, Rey-Osterrieth Complex Figure test) and reasoning (i.e., Raven’s Colored Progressive Matrices (CPM)). For studies that examined risk of dementia or cognitive impairment, categorization was determined by a clinician (criteria not specified) (Sun et al., 2021) or in accordance with clinical diagnostic standards (e.g., DSM, National Institute of Neurological and Communicative Disorders and Stroke, Alzheimer’s Disease and Related Disorders Association, National Institute on Aging and the Alzheimer’s Association) (Fan et al., 2015; Guaita et al., 2015; Jia et al., 2020; Ribeiro et al., 2013; Y. Zhang et al., 2019; Z.-X. Zhang et al., 2006). Other studies assigned categorization based off a respondent-based questionnaire (Espinosa del Pozo et al., 2020) or cut-off points on the MMSE (Feng et al., 2014; Kramer et al., 1985; Paúl et al., 2010; Saha et al., 2010; Subramanian et al., 2021) or on multiple global cognitive assessments
Findings across the 23 studies were mixed. Fourteen studies reported significant associations between marital status and cognitive function/dementia risk; two out of the 14 studies only examined the relationship between widowhood on cognition by gender (Perkins et al., 2016; Zhang et al., 2019) (see Section 3.2.3). Nine studies provided tenuous to no evidence for the main effect of marital status on cognitive function/dementia risk. In studies that reported significant associations between marital status and cognitive function, the relationship was in the hypothesized direction: widow(er)s exhibited poorer cognitive function or were more likely to be diagnosed with dementia or mild cognitive impairment (MCI) than non-widowed subjects. Specifically, widowed individuals exhibited poorer overall cognition (Barragán-García et al., 2021; Farron et al., 2020; O’Connor & Arizmendi, 2014), greater cognitive interference (O’Connor & Arizmendi, 2014), poorer attention and processing speed (Ward et al., 2007), and higher prevalence rates of cognitive impairment (Jia et al., 2020; Kramer et al., 1985; Saha et al., 2010; Subramanian et al., 2021; Sun et al., 2021) and dementia (Fan et al., 2015; Jia et al., 2020; Zhang et al., 2006). However, several studies found no differences between bereaved and nonbereaved individuals on dementia incidence (Guaita et al., 2015; Ribeiro et al., 2013), overall cognition (Byrne & Raphael, 1997; Espinosa del Pozo et al., 2020; Feng et al., 2014; Lopes et al., 2007; Paúl et al., 2010; Rosnick et al., 2010; Shahar et al., 2001; Ward et al., 2007; Xu et al., 2020), episodic memory (Rosnick et al., 2010; Ward et al., 2007), visuospatial memory, reasoning, verbal ability, visuospatial ability (Ward et al., 2007), working memory, and executive function (O’Connor & Arizmendi, 2014). Notably, some studies that reported null findings did find that gender moderated the association between widowhood and cognitive function (see Section 3.2.3).
3.2.2. Longitudinal and prospective associations between widowhood status and cognition
Thirty studies, all rated good quality except one (Li et al., 2018), used a prospective or longitudinal research design to evaluate the association between bereavement and cognitive function/dementia risk. Average observation period from baseline to the last-follow-up visit was 11.5 years (range: 2–32 years). All studies except four accounted for age, sex, and SES in their analyses (Bickel & Cooper, 1994; Li et al., 2018; Mousavi Nasab et al., 2012; Sundström, Westerlund, et al., 2014). Studies that assessed overall cognition used standardized overall cognitive measures (i.e., TICS, MMSE) or developed a cognitive composite with a host of cognitive measures (Biddle et al., 2020; Y. Lee et al., 2019; Shin et al., 2018). There was heterogeneity in the cognitive tests used to assess episodic memory (i.e., 15-words test, 10-word immediate and delayed recall, cued recall tasks, recognition tasks, California Verbal Learning Test), semantic memory (i.e., synonym test, fluency tests), orientation (i.e., knowledge of time, day and current national leaders), cognitive flexibility (i.e., Stroop test), working memory (i.e., DSB, Spatial Working Memory Test), information processing (i.e., DSST, figure comparison test, coding task), broad executive functioning skills (i.e., clock drawing test), and reasoning (i.e., CPM). For studies that examined risk of dementia or cognitive impairment, the diagnosis was ascertained through patient medical records (Forbes et al., 2019; Sundström et al., 2016; Wändell et al., 2020) or by a clinical team adhering to predetermined clinical criteria (e.g., DSM III or IV, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association, National Institute on Aging and the Alzheimer’s Association, CAMDEX) (Amieva et al., 2010; Bickel & Cooper, 1994; Brenowitz et al., 2014; Håkansson et al., 2009; Hatch, 2013; Helmer et al., 1999; Li et al., 2018; Sundström, Rönnlund, et al., 2014). Other studies assigned categorization based off cut-off points on the MMSE (Yin et al., 2020), TICS (Brown et al., 2020; Liu, Zhang, Choi, et al., 2019; Zhang et al., 2021), or a composite risk score using multiple cognitive assessments (Liu, Zhang, Burgard, et al., 2019)
In twenty-one studies, widow(er)s exhibited more significant cognitive impairment or higher dementia or cognitive impairment rates over time than nonbereaved controls. Widow(er)s showed worse overall cognition over time than nonbereaved controls (Biddle et al., 2020; Y. B. Kim & Lee, 2019; S. H. Shin et al., 2018; Xiang et al., 2021; Zhao et al., 2021). Compared to married controls, widow(er)s also showed faster declines in overall cognition (Aartsen et al., 2005; Biddle et al., 2020; Karlamangla et al., 2009; Lyu et al., 2019), memory (Aartsen et al., 2005), and semantic memory (Mousavi Nasab et al., 2012). Widowed subjects, relative to controls, had significantly higher odds of being diagnosed with all-cause dementia (Forbes et al., 2019; Li et al., 2018; Liu, Zhang, Choi, et al., 2019; Sundström et al., 2016; Sundström, Westerlund, et al., 2014; Z. Zhang et al., 2021) or with cognitive impairment (Håkansson et al., 2009; Liu, Zhang, Burgard, et al., 2019; Yin et al., 2020), especially subjects who were widowed from mid-life onwards (Håkansson et al., 2009). Specifically, widowed individuals had a higher prevalence of impairment in executive functioning, orientation, and memory (Liu, Zhang, Burgard, et al., 2019). The remaining two of the 21 studies only examined the relationship between marital status and cognition by gender (Brown et al., 2020; Wörn et al., 2020). They are discussed in detail in Section 3.2.3.
Three studies reported significant relationships between widowhood and cognitive outcomes that were opposite from the hypothesized directions. Brenowitz et al. (2014) found that widow(er)s had a lower risk of MCI than married individuals, while no differences in risk were observed between separated/divorced or unmarried individuals compared to married subjects. Two studies only examined associations between widowhood and dementia risk by sex (Monserud, 2019; Wändell et al., 2020) (See Section 3.2.3).
Six studies found no differences in dementia risk (Hatch, 2013) or overall cognition over time across widowed and married groups (Amieva et al., 2010; Bickel & Cooper, 1994; Helmer et al., 1999; Y. Lee et al., 2019; Vidarsdottir et al., 2014).
3.2.3. Additional factors that influence the associations between widowhood status and cognition
Sex.
Four longitudinal studies and two cross-sectional studies sex-stratified the sample before examining the main effect of widowhood on cognitive outcomes by sex. Five out of the six studies provided some support for the association between widowhood and poorer cognitive outcomes. Wörn et al. (2020) reported that widowed women two years after the loss exhibited greater decreases in the reasoning domain from pre-loss baseline than controls, but no differences were found among men. Wörn et al. (2020) also assessed global cognition, memory, and processing speed, but widowed men and women did not perform differently from married men and women. In a prospective study conducted in the U.S., Brown et al. (2020) reported that the association between widowhood and rates of cognitive impairment existed in men, not women: widowed men had higher rates of cognitive impairment than married/re-partnered men. Similar results were found in a cross-sectional, population-based study in rural China: widowed men had greater odds of cognitive impairment than married men but no differences in widowed and married women were found (Zhang et al., 2019). Monserud (2019) included married men as the reference group and found that widowed men and women had lower cognitive functioning than married men at baseline, while married women had better cognitive functioning than married men. However, when cognitive trajectories were assessed, widowed and married women had slower rates of decline across waves than married men. Perkins et al. (2016) found that widowed men and women exhibited poorer episodic memory performance than married men and women, respectively. Moreover, duration of widowhood altered the relationship between widowhood and episodic memory performance among women, such that only women who were bereaved for the shortest (0–4 years) and longest duration (10+ years) had worse memory performance than married women. In contrast to the aforementioned five studies, Wändell et al. (2020) found that widowed men had lower risk of dementia than married men widowed; married and widowed women exhibited no differences in dementia risk.
Of the studies that examined the associations between widowhood and cognitive outcomes in the entire sample (i.e., results described in Section 3.2.1 and 3.2.2), twelve studies also examined whether the relationship between widowhood and cognitive outcomes depended on sex. In 6 of the 12 studies, the relationship between widowhood and adverse cognitive outcomes depended on gender/sex. Specifically, Liu and colleagues (2019) reported that both widowed men and women had greater odds of dementia than married men and women, respectively. In main effect analyses, both Rosnick et al. (2010) and Vidarsdottir et al. (2014) found no cognitive function differences cross-sectionally or longitudinally in widowed and married subjects. However, when sex was assessed as a moderator, bereaved men had worse episodic memory than nonbereaved men (Rosnick et al., 2010) and widowed women had poorer executive functioning than married women during the first two years after their husbands’ death (Vidarsdottir et al., 2014). Similarly, Xiang and colleagues demonstrated that men who were bereaved for 5 or less or 16–20 years were more likely to have lower cognitive scores than married men; women who were bereaved for more than 21 years were more likely to have worse cognitive function than married women (Xiang et al., 2021). Feng et al. also found cross-sectional evidence that widowed men had 5× higher risk of cognitive impairment (MMSE < 24) compared to married men (Feng et al., 2014). Kramer and colleagues found that rates of severe cognitive impairment were higher for widowed females than married females. Still, no such association was found among males (Kramer et al., 1985). The remaining 6 of the 12 studies provided no evidence for a moderating effect of sex on the relationship between widowhood and cognitive outcomes, despite reporting a significant main effect of widowhood on cognitive outcomes (Aartsen et al., 2005; Helmer et al., 1999; Mousavi Nasab et al., 2012; Sundström et al., 2016; Z. Zhang et al., 2019, 2021).
Depression and stress.
When studies controlled for psychological symptoms, the association between widowhood and cognitive outcomes became insignificant or remained unchanged. For example, after accounting for anxiety, stress, and depression, the effect of group no longer was associated with attention, processing speed, and verbal fluency (Ward et al., 2007). Similarly, when accounting for several covariates, including psychological distress, being a widower was no longer associated with odds of cognitive impairment. Instead, psychological distress continued to predict cognitive impairment (Paúl et al., 2010). However, a majority of studies did find a significant effect of widowhood on cognitive function, even after controlling for depression (Biddle et al., 2020; Brown et al., 2020; Feng et al., 2014; Forbes et al., 2019; Håkansson et al., 2009; Y. B. Kim & Lee, 2019; Lyu et al., 2019; Rosnick et al., 2010; S. H. Shin et al., 2018; Sundström, Westerlund, et al., 2014; Z. Zhang et al., 2019).
4. Discussion
This systematic review examined the relationship between interpersonal losses in older adulthood (e.g., spousal caregiving and spousal bereavement) and cognitive outcomes. Our results provide preliminary evidence for an adverse association between spousal caregiving and spousal bereavement and cognitive functioning. Evidence from both cross-sectional and longitudinal studies suggests that spousal caregivers and widow(er)s exhibit poorer overall cognitive function, greater cognitive decline, and higher rates of dementia than control subjects. Regarding affected cognitive domains, caregivers and widow(er)s performed worse than controls on tasks testing information processing, attention, and memory. However, results on specific cognitive domains were less robust (supported by fewer studies) compared to results on overall cognition and rates of dementia, because only some studies examined specific cognitive domains and those that did, differed in the types of domain-specific tests administered. To our knowledge, this is the first review to focus on the impact of caregiving and widowhood on cognitive function.
Ninety percent (10 out of 11 articles) of cross-sectional and longitudinal studies provided strong support for the association between spousal caregiving and poor cognitive function. These findings align with the broader understanding that caregiving and the stress associated with the experience adversely impact health (Bennett et al., 2013; Schulz & Beach, 1999; Shaw et al., 1997). Spousal caregivers may be at a disadvantage because they are more likely to be caring for a spouse for long hours (21+ hours weekly, often 40+ hours) without additional aid and are older in age (National Alliance for Caregiving and AARP, 2015). In a sample of 1,517 cancer caregivers, spousal caregivers were more likely than family non-spousal caregivers to develop chronic back pain and arthritis 5–8 years after the initial caregiving experience (Kim et al., 2015). As evident from the limited studies identified for the present review, there is sparse but growing work examining cognitive outcomes among spousal caregivers. The scarcity of studies may be attributed to the challenges of recruitment; for instance, spousal caregivers may be difficult to recruit because they make up 10% of all caregivers (National Alliance for Caregiving and AARP, 2015), have less flexible schedules, or be less likely to return to follow-up visits.
There was some evidence that caregivers and widow(er)s had poorer episodic memory and executive functioning (i.e., attention) performance than controls. This finding is notable, given that poorer episodic memory performance (i.e., CVLT) predicts progression from to mild cognitive impairment in normal adults (Blacker et al., 2007). Baseline executive function (i.e., Trail Making Test) and episodic memory also predict progression to AD among adults with mild cognitive impairment (Blacker et al., 2007). Poorer memory function following partner loss may signify elevated risk for ADRD. Early intervention efforts may be particularly beneficial for caregivers and widow(er)s who exhibit abnormal deficits in these cognitive domains.
Compared to the caregiving literature, the bereavement literature produced more varied conclusions regarding the relationship between widowhood and cognitive outcomes. Seventy-three percent (22 out of 30) of longitudinal studies reported significant cognitive decline or poorer overall cognition in widow(er)s compared to married adults; 65% (15 out of 23) of the cross-sectional studies reviewed reported associations in the hypothesized direction. Inconsistencies across studies may be attributed to variability surrounding the widowhood context. Because most samples came from large epidemiological studies, widowhood status was most often ascertained solely by indication of one’s marital status without regard for widowhood duration, which was often not reported or, if reported, spanned a wide range. Though more work is needed to clarify if and when there is a time window for greater cognitive vulnerability, some studies reported that the relationship between widowhood and cognitive functioning depended on widowhood duration (Y. B. Kim & Lee, 2019; Lyu et al., 2019; Perkins et al., 2016; Xiang et al., 2021).
Inconsistent findings across studies may partially be confounded by differences in sociocultural and the economic environment. Studies on widowhood derived samples from both industrialized and developing Western/Eastern countries, whereas studies on caregiving were only obtained from Western countries. In fact, all but two caregiving studies came from samples in the U.S. and Canada (de Vugt et al., 2006; O’Sullivan et al., 2019). Cognitive aging is universal but culture has been found to modulate neurocognitive aging, especially cognitive tasks that depend on acquired knowledge (also called cognitive pragmatics) (Park & Gutchess, 2006). Even among Western societies, baseline cognition and rates of cognitive decline significantly vary across European regions, with people in Scandinavia showing higher baseline cognition but faster rates of cognitive decline than people in Western Europe, Central/Eastern Europe, and Mediterranean countries (Formanek et al., 2019). Nevertheless, the current review’s findings suggest that the adverse association between widowhood and cognitive outcomes is evident across multiple countries.
4.1. A biopsychosocial model of stress, cognition, and dementia
The notion that stressors impact cognition is not new, but the mechanisms linking major life stressors (e.g., spousal caregiving, widowhood) to abnormal cognitive decline are not clearly delineated. Stress, especially when prolonged, imposes widespread effects on multiple biological systems. Spousal caregiving and widowhood exert significant wear and tear on the body. Indeed, a growing body of literature has identified behavioral, immune, cardiovascular, and/or neural pathways through which caregiving and widowhood confer risk for depression and other comorbid disorders. Importantly, stress-related mechanisms are the common denominator across depression, AD (the most common form of dementia and characterized by severe memory loss), and related dementias.
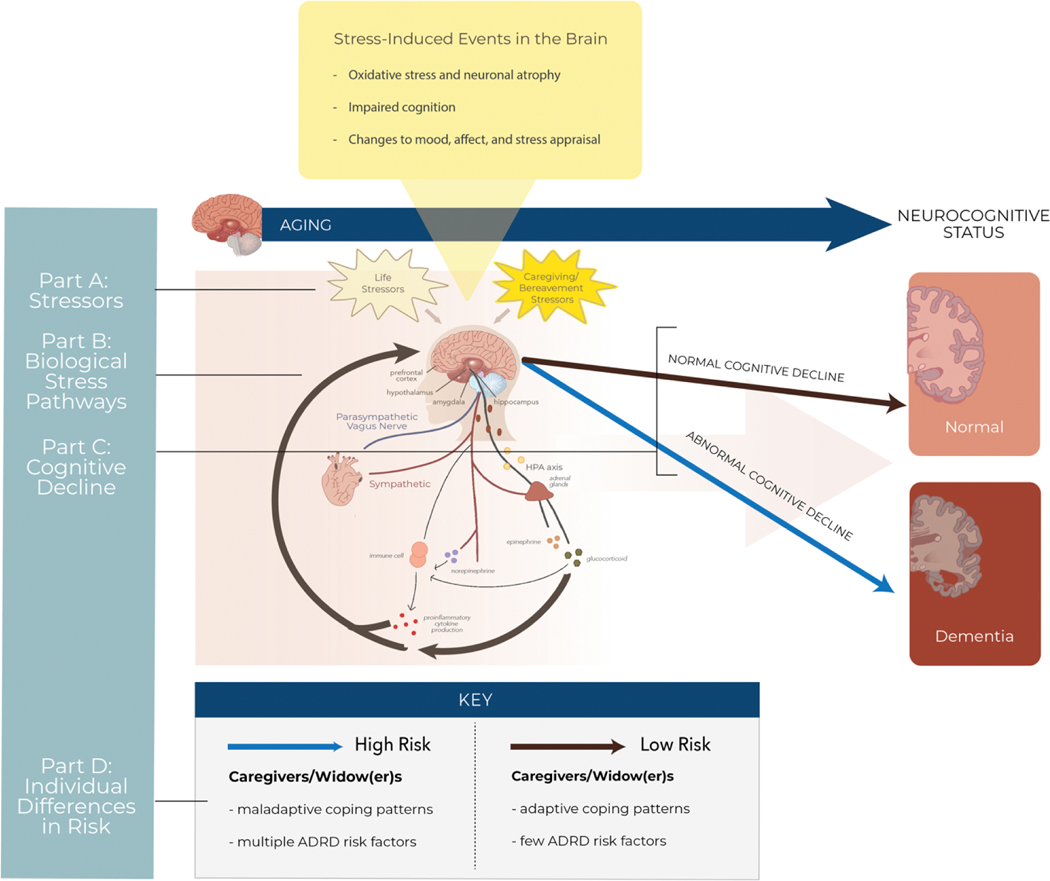
The development of neurodegenerative diseases is likely attributed to multiple converging factors. We propose that the biopsychosocial consequences of caregiving and partner loss exacerbate age-related processes and ongoing neuropathological changes at multiple levels of organization, from cells to body systems. Biological vulnerability coupled with maladaptive psychological patterns and changes in the socio-environment may position spousal caregivers and widow(er)s to experience more stressors or to be less resilient psychologically and physiologically against the consequences of stress. Over time, individuals who adjusted maladaptively to caregiving or widowhood and who possess multiple risk factors for ADRD are at high risk for abnormal cognitive decline. Integrating current work to date among spousal caregivers and widow(er)s with mechanistic work in ADRD, we expound upon this model (depicted in Figure 2) in the sections below.
Figure 2.
Biobehavioral mechanisms linking interpersonal losses to poorer cognitive outcomes.
A. Spousal caregivers and widow(er)s face caregiving/bereavement-related stressors on top of typical life stressors. B. Chronic stress dysregulates biological stress pathways, whose mediators (i.e., glucocorticoids, cytokines, epinephrine, norepinephrine) feedback to the brain to impact neurobiology, neurocognitive function, mood, affect, and stress appraisal. C. Prolonged dysregulation of biological stress pathways and stress-induced changes in the brain can contribute to abnormal cognitive decline. D. Individual differences in risk exist: caregivers and widow(er)s with multiple Alzheimer’s disease and related dementias (ADRD) risk factors and who adopt maladaptive coping mechanisms may be more likely to develop dementia than individuals with fewer ADRD risk factors and who adopt adaptive coping mechanisms.
4.1.1. The psychological health of spousal caregivers and widow(er)s (Part A in the figure)
Daily stressors.
Caregivers experience significant daily stress associated with caring for the ill spouse. Caregiving stress can last years, depending on the chronicity and severity of the illness. The transition to a caregiver is marked by a gradual loss of reciprocity within the relationship, which increases the caregiving burden (Reid et al., 2005). Over a 24-hour period, dementia caregivers are more likely to experience multiple stressors than non-caregiving controls (Gouin et al., 2012). Caregivers are forced to adapt to the patient’s constantly changing physical and cognitive limitations, which places a toll on the caregivers’ health. Indeed, in AD spousal caregivers, providing more activities of daily living assistance is associated with greater risk for hospitalization, low medical rating at check-ups, or extended disability or physical illness in AD spousal caregivers (Shaw et al., 1997). Caregiving burden is associated with social and behavioral problems of the dementia patient and perceiving the caregiving role as a threat (Wijngaart et al., 2007). As the disease progresses, caregivers also report experiencing “little deaths” as they gradually lose social networks and their independence as a couple (Roland et al., 2010).
Following the death of a spouse, widow(er)s experience two main types of stressors: loss-oriented and restoration-oriented stressors. Daily loss-oriented stressors relate to the presence or absence of the deceased person. For example, these stressors could arise from thoughts and feelings toward objects, places, events, or conversations related to when the deceased individual was alive; the absence of the spouse could be felt more significantly when the bereaved individual experiences primary needs alone (i.e., sleeping alone, eating alone, or yearning to talk with the deceased person). Restoration-oriented stressors derive from consequences of the loss, such as the financial, social, and lifestyle changes that come with being a widow(er). Restoration-oriented stressors may include performing or learning a task previously performed by the deceased (ex: cooking or repairing appliances), taking on a new identity as a widow(er), making decisions without the advice of the deceased, or losing social circles. Notably, while widow(er)s may experience fewer interpersonal stressors than married adults, they exhibit greater physical reactivity to home-related stressors (Hahn et al., 2014).
According to the dual-process model, healthy coping requires a dynamic oscillation between confrontation of the loss (loss-oriented stressors) and avoidance of the loss by concentrating on other things (restoration-oriented stressors); over time, bereaved individuals report fewer loss-oriented stressors and more restoration-oriented stressors (Caserta & Lund, 2007; Lundorff et al., 2019; Stroebe & Schut, 1999). However, elderly bereaved participants experience twice as many loss-oriented stressors as restoration-oriented stressors even after a year post-spousal death (Ryckebosch-Dayez et al., 2016).
Emotional states.
There are strong associations between daily stress and negative affect in older adults (Mroczek & Almeida, 2004). Both married and bereaved spouses experience higher negative affect on days when they report an interpersonal stressor (Hahn et al., 2014). However, bereaved spouses report higher negative affect even on days when no interpersonal stressors are reported (Hahn et al., 2014). In caregivers who report less interdependence (sense of mutual need between relationship partners) with the ill spouse, actively helping or monitoring the spouse’s safety is associated with higher negative affect (Poulin et al., 2010).
Emotional states are linked to mental and physical health outcomes. Negative affective states are associated with anxiety and depression; negative affect, especially distress experienced only internally and not expressed externally, in the elderly is also related to increased mortality risk (Wilson et al., 2003). A higher positive affect to negative affect ratio is associated with better mental health (Diehl et al., 2011).
Loneliness.
Spousal caregivers and widow(er)s experience more severe loneliness than controls (Beeson, 2003; Golden et al., 2009; McRae et al., 2009). Loneliness also explained a significant portion of the excess risk of depression observed in both population (Beeson, 2003; Golden et al., 2009). Among PD caregivers, lower education, longer duration of caregiving, and lower perceived self-efficacy are associated with more loneliness among caregivers (McRae et al., 2009). Loneliness in older adults is associated with more rapid cognitive decline and an increased risk for AD (Wilson et al., 2007; Zhong et al., 2017); however, given that caregiving and bereavement commonly occur in older adulthood but was not accounted for in the study, the relationship between loneliness and AD may be primarily driven by widowhood or caregiving status.
Loneliness may partially explain the sex effect we found in the current review, in which men generally fared worse cognitively than women. In the bereavement literature, widowers suffer relatively more significant health consequences than widows (Streeter, 2020; Stroebe et al., 2001). Although the reasons are far from conclusive, differences in health outcomes have been attributed, in part, to differences in social support resources and grief coping strategies (Stroebe et al., 2001). Even though women experience higher rates of loneliness due to widowhood being more common among women than men (Aartsen & Jylhä, 2011), loneliness is more potent to older men’s cognitive health than older women’s cognitive health (Zhou et al., 2018).
Mental health.
Depression often follows stressful life events. Depressive symptoms are commonly observed in both caregivers and widow(er)s. In spousal caregivers of dementia, 60% developed a depressive/anxiety disorder within two years of caregiving (Joling et al., 2015). Despite reporting reduced stress and negative affect over 3 years, former caregivers’ level of depressive symptoms and loneliness were not comparable to levels of non-caregivers and, instead, remained similar to levels of current caregivers (Robinson-Whelen et al., 2001). Among widow(er)s, depressive symptoms remain significantly higher than nonbereaved adults for the first five years after the loss (Harlow et al., 1991; Kristiansen et al., 2019).
In our review of the literature, depressive symptoms sometimes altered the relationship between the experience of interpersonal stressors and cognitive function. In some studies, caregiving status and bereavement status were no longer associated with cognitive function after accounting for depressive symptomology; in other studies, the relationship between interpersonal stressors and cognitive function remained even after accounting for depressive symptoms. These findings suggest that the psychological consequences of major life stressors partially contribute to cognitive changes in older adults. Some work suggests that past or current depression distinguishes widow(er)s who are more at risk for dementia and poorer cognitive health than nondepressed widow(er)s. For example, Hatch et al. (2015) reported that widow(er)s with a depression/antidepressant use history had a greater risk of incident A.D. than widow(er)s without a depression/antidepressant use history. Aartsen et al. (2005), one of the studies reviewed here, reported that among widow(er)s, higher levels of depressive symptoms were related to a decrease in memory performance over 6 years.
In the general population, depressive symptomology is a risk factor for cognitive decline and dementia (Dotson et al., 2010; Heun et al., 2002; Jorm, 2000; Ownby et al., 2006; Saczynski et al., 2010; Wilson, Barnes, et al., 2002). Depression across the lifespan (early-, mid-, and late-life) increases risk of dementia (Barnes et al., 2012; Byers & Yaffe, 2011). However, whether late-life depression is a risk factor for dementia or a prodromal condition of dementia remains unclear (Brommelhoff et al., 2009; Schweitzer et al., 2002); some evidence suggests that recurrent depression is a risk factor of vascular dementia while depression that begins in late-life may indicate prodromal AD (Barnes et al., 2012). Comorbid depressive symptomology and MCI hasten dementia onset (Gabryelewicz et al., 2007), and AD patients with comorbid depression showed more severe AD neuropathology (Rapp et al., 2008). Their common co-occurrence has led to the theory that depression, MCI, and dementia represent a cognitive and mood continuum that is driven by stress and stress-related mechanisms (Sotiropoulos et al., 2008).
4.1.2. Biological mechanisms linking partner loss to poor cognitive health (Part B in Figure 2)
The autonomic nervous system, HPA axis, and immune system have garnered growing attention for their potential roles in aging and disease. Because their mediators (e.g., epinephrine, norepinephrine, glucocorticoids, cytokines) subserve both peripheral systems and the brain, dysregulation to these pathways can have large implications to health. Importantly, stress-related pathways are significantly dysregulated in spousal caregivers and bereaved spouses compared to age-matched controls, making these pathways important avenues through which interpersonal stressors confer risk for mental and physical illness.
Autonomic and neuroendocrine dysregulation.
Autonomic dysfunction may be involved in the pathogenesis of dementia. The autonomic nervous system’s sympathetic and parasympathetic branches regulate several important functions, including blood pressure and heart rate. Hence, autonomic imbalance is central to the etiology of abnormal blood pressure levels (Guzzetti et al., 1988). While high systolic blood pressure in mid-life is a risk factor for cognitive decline, low systolic blood pressure in late-life (65+ years) is associated with depression (Barrett-Connor & Palinkas, 1994; Paterniti et al., 2000), cognitive decline, and ADRD incidence (Licht et al., 2009; Molander et al., 2010; Walker, Sharrett, et al., 2019). Though the exact mechanisms are unclear, abnormal blood pressure levels likely contribute to dementia-related pathology by impairing arterial walls and disrupting cerebral perfusion (Roman, 2004); these events ultimately obstruct blood flow to cortical and subcortical regions and lead to hypoxic environments that facilitate increased protein deposition and neurodegeneration (Kennelly et al., 2009).
Evidence from demented and non-demented samples suggests that HPA axis dysfunction contributes to neurodegeneration. According to the glucocorticoid hypothesis of brain aging, aging enhances glucocorticoid efficacy in some cell types (i.e., neurons) but decreases glucocorticoid efficacy in other cell types (i.e., astrocytes); together, these changes weaken glucocorticoid-driven anti-inflammatory activity and catalyze downstream events that impair neurocognitive function (Landfield et al., 2007). Indeed, cortisol, the primary glucocorticoid in humans, can exert effects on cortical and subcortical structures implicated in various cognitive functions. In general, most studies have found that higher cortisol levels are associated with poorer overall cognitive performance (Geerlings et al., 2015; Lupien et al., 2007; Sang et al., 2018) and poorer episodic memory in non-demented older adults (Beluche et al., 2010; Sang et al., 2018; Segerstrom et al., 2016). These findings infer that even relatively small differences in cortisol levels can impart noticeable cognitive changes in relatively healthy older adults. Concurrent with neurocognitive changes, higher cortisol levels are also linked to hippocampal atrophy (Lupien et al., 1998) and reduced brain volume (Geerlings et al., 2015). Higher cortisol levels are also associated with increased ADRD risk (Ennis et al., 2017) and a greater degree of cognitive impairment in ADRD patients (Zvěřová et al., 2013). Cortisol may augment ADRD pathology by promoting oxidative stress and Aβ neurotoxicity (Goodman et al., 1996).
Maladaptive patterns of sympathetic, parasympathetic, and neuroendocrine activity are observed in caregivers and widow(er)s. Relative to non-caregivers, caregivers exhibit higher basal sympathetic activity and neuroendocrine activity (Bauer et al., 2000; Cacioppo et al., 2000; de Vugt et al., 2005; Grant et al., 2002). Widow(er)s exhibit poorer parasympathetic function (Fagundes et al., 2018), higher sympathetic activity (Buckley et al., 2011), and dysregulated neuroendocrine patterns (e.g., diurnal cortisol levels and slope) than nonbereaved adults (Holland et al., 2014; O’Connor et al., 2012; Richardson et al., 2015).
Stress neurobiology.
The stress neurobiology literature corroborates links between stress and cognitive decline. The hippocampus, prefrontal cortex, and amygdala play integral roles in memory, cognitive control, and emotional arousal, respectively. In addition, these three brain regions comprise a neural circuitry responsible for appraising threat and regulating behavioral and physiological responses to situations deemed stressful to the individual (McEwen & Gianaros, 2010). Since glucocorticoid and -adrenergic receptors can be found in the prefrontal cortex, hippocampus, and amygdala, stress hormones can bind to these receptors and modulate biobehavioral, cognitive, and emotional processes (Lupien et al., 2007).
Stress mediators alter brain morphology (Lupien et al., 2007), memory (McGaugh, 2000), and mood (Pariante & Miller, 2001; Slavich & Irwin, 2014). Chronic exposure to glucocorticoids is associated with hippocampal volume loss (Sheline et al., 1996; Starkman et al., 1992). Beyond the effects of aging on hippocampal volume (Scahill et al., 2003), hippocampal volume reduction is evident in depression (Elgh et al., 2006; Sheline et al., 1996) and AD (Convit et al., 1997; Shi et al., 2009; West et al., 1994). Exogenously (i.e., are significantly elevated in depressed individuals (Slavich & Irwin, 2014; Zunszain et al., 2011) and AD patients (Elgh et al., 2006; Hartmann et al., 1997; Walker et al., 2017; Walker, Gottesman, et al., 2019).
Impaired neurogenesis and altered neuronal morphology and synaptic functioning underlie stress-mediated structural and functional changes in the brain (Arnsten, 2009; Tata & Anderson, 2010). Elevated glucocorticoid levels inhibit hippocampal neurogenesis and decrease the size and complexity of dendritic arborization in neurons (Anacker et al., 2013). Further, because glucocorticoids also modulate the immune system, brain-resident macrophages called microglia are also impacted by stress (Sierra et al., 2008). In fact, chronic stress alters microglial density and morphology, which are implicated in prefrontal-dependent cognitive impairments (Hinwood et al., 2012). Damage to microglia has widespread implications to brain integrity because microglia coordinate the neuroinflammatory response and, under homeostatic conditions, also facilitate synaptogenesis and neurogenesis (Kim et al., 2016).
Inflammation.
Growing research suggests that inflammatory processes drive cognitive decline and ADRD pathology (Akiyama et al., 2000; Darweesh et al., 2018). Inflammation is the immune system’s response to foreign invasion, infection, and injury (Medzhitov, 2007, 2008). Although acute inflammation is adaptive and maintains homeostasis, chronic inflammation can damage cells, tissues, and organs. Notably, inflammation increases with age, making it a likely contributor to many age-related diseases (Franceschi & Campisi, 2014).
Systemic inflammation also contributes to neurodegenerative processes. Systemic inflammatory markers (e.g., cytokines, C-reactive protein, fibrinogen, white blood cell count) are significantly associated with declines in cognitive functioning (Marioni et al., 2009; Walker, Gottesman, et al., 2019), volume reductions in signature AD brain regions (e.g., hippocampus, entorhinal cortex, etc.) (Walker et al., 2017), and dementia incidence (Kempuraj et al., 2016; Koyama et al., 2013). Peripheral cytokines communicate with the brain through multiple pathways, including signal transduction at the blood-brain barrier, vagal stimulation, or direct infiltration around circumventricular organs of the blood-brain barrier (Lyman et al., 2014). Interestingly, since the discovery of brain-immune pathways, substantial work supports an inflammatory role in the development/progression of depression (Dantzer et al., 2008; Slavich & Irwin, 2014). The overlapping role of inflammation in both depression and dementia has important implications for understanding the intersection between stress and neurocognitive disorders.
Neuroinflammation, which is local inflammation in the central nervous system, can damage the structural and functional integrity of the brain. In the brain, microglia repair synapses, promote neurogenesis, and eliminate tissue debris (Heneka et al., 2014). Infection, injury or misfolded proteins activate microglia to release pro-inflammatory mediators (e.g., cytokines, chemokines, free radicals, and complement proteins), thereby exciting a neuroinflammatory response. Because pro-inflammatory mediators initiate further production of inflammatory signaling molecules, peripheral inflammation likely propagates and sustains neuroinflammation (Lyman et al., 2014). Evidence from animal and human subjects substantiate the causative and accelerative role that neuroinflammation has in the development of neurodegenerative diseases (Kempuraj et al., 2016; Lyman et al., 2014).
The chronic stress of caregiving accelerates age-related declines of the immune system (Bennett et al., 2013). Compared to age-matched controls, dementia spousal caregivers show poorer antibody response to vaccines; these findings suggest that caregiving stress increases vulnerability to infection beyond age-related changes in immune function (Kiecolt-Glaser et al., 1996). Elderly caregivers show impaired immune function, as evidenced by immune cells’ sensitivity to the anti-inflammatory effects of glucocorticoids (Bauer et al., 2000). Inflammatory levels are also more elevated and remain elevated after caregiving: Average rate of increases in the pro-inflammatory cytokine, interleukin-6 (IL-6), is 4x greater in spousal caregivers of dementia than in non-caregiving older adults and yearly average levels of inflammation are similar across current and former caregivers (Kiecolt-Glaser et al., 2003).
Widow(er)s also exhibit maladaptive patterns of immune activity. Compared to nonbereaved adults, widow(er)s have higher levels of systemic inflammation, indexed by the IL-6 and tumor necrosis factor-alpha (TNF-α) (Fagundes et al., 2018). Elevated levels of inflammation are cross-sectionally and prospectively associated with higher levels of depressive symptoms in widow(er)s (Fagundes et al., 2019; Wu et al., 2021), which align with the broader literature supporting positive cross-sectional and longitudinal relationships between inflammatory levels and depressive symptomology (Slavich & Irwin, 2014).
4.1.3. Normal and pathological cognitive aging (Part C in Figure 2)
Cognitive aging.
Age is the greatest risk factor for cognitive decline and dementia incidence. A vague distinction between normal and pathological aging confounds the current understanding of dementia pathogenesis. For example, it is not known whether age-related changes in cognition are mainly attributed to premorbid pathological changes; one group of scientists found that age-related cognitive decline may be minimal, as those deficits were mostly accounted for by person-level factors and by those who died within years of the baseline assessment (Wilson et al., 2003; Wilson, Beckett, et al., 2002). To understand the intricacies of the disease, some argue that researchers first must understand why ADRD affects older brains (Fjell et al., 2014). Nevertheless, normal cognitive aging is typically characterized by declines in some functions and preservation of others: Older adults show declines in mental speed, executive function, and episodic memory, but their verbal abilities and world knowledge remain relatively intact (Fjell et al., 2014). In particular, episodic memory starts declining between 50–60 years of age (Rönnlund et al., 2005). Age-related changes in cognition are explained by volume reductions in frontal and temporal regions (e.g., hippocampus) (Fjell et al., 2014; Goukasian et al., 2019). Functional magnetic resonance imaging (fMRI) studies reveal that aging adults exhibit increased activity in the hippocampus. However, hippocampal hyperactivity is followed by decreases in hippocampal activity when memory impairment becomes severe (Furst & Mormino, 2010; J. Persson et al., 2012). At the cellular level, altered synaptic activity between neurons reduces cognitive functioning with age (Mostany et al., 2013; Torres & Cardenas, 2020). Because declines in cognition and neuronal atrophy characterize both normal and pathological aging, the rate of atrophy and distinct patterns of brain changes can serve as indicators of pathological cognitive aging (Apostolova & Thompson, 2008; Driscoll et al., 2009; McEvoy et al., 2009, 2011).
Alzheimer’s disease and related dementias pathology and risk.
Abnormal accumulation of harmful proteins is the most established cause of ADRD (Mucke, 2009). These proteins include amyloid-beta(A||) peptides,microtuble-associated tau protein, apolipoprotein, and presynaptic protein ||-synuclein (Mucke, 2009). Although scientists debate the exact mechanisms, protein accumulation likely impairs synaptic connectivity and promotes cell death, thereby, disrupting neural networks supporting learning, memory, executive functioning, and other cognitive functions (Mucke, 2009). Over time, neurobiological changes manifest as cognitive deficits in one (e.g., learning and memory) and eventually multiple domains. Clinical diagnosis of ADRD currently requires clinically significant cognitive impairment that impairs daily functioning and that cannot be better explained by another comorbid disorder (McKhann et al., 2011). Biomarker evidence of neuropathology is not necessary for diagnosis, but can enhance certainty (McKhann et al., 2011).
ADRD neuropathology occurs years before clinical diagnosis, even before protein deposition (Reiman et al., 2012). Stress-induced immune, autonomic, and neuroendocrine dysregulation may compound existing health conditions or initiate new health conditions; together, these events accelerate neuropathological events and promote abnormal cognitive aging. Although the biological mechanisms preceding dementia onset are theoretically driven, they require further scientific validation. However, it is well known that cardiovascular conditions, which have strong links to stress mechanisms, increase cognitive decline and dementia (Whitmer et al., 2005; Yaffe et al., 2020). Several additional factors – including education, health behaviors, neuropsychiatric conditions, genetic predisposition – increase the risk for dementia. The presence of multiple risk factors for the cognitive decline may increase dementia risk in caregivers and widow(er)s, especially since the relationship between risk of cognitive decline/dementia incidence and the number of intraindividual risk factors increases in a dose-dependent manner (Peters et al., 2019).
4.1.4. Individual differences in risk: Biobehavioral coping patterns perpetuate or mitigate the stress dysregulation cycle (Part D in Figure 2)
Priming, threat sensitivity, and stress regulation are important constructs to a biopsychosocial framework of stress and cognition. Caretaking stressors, bereavement-related stressors, and daily hassles are inevitable. Still, an individual’s pattern of responses to a stressor can shape or prime immediate and future psychological and physiological reactions to stressors. Our model proposes that maladaptive biobehavioral patterns perpetuate an ongoing, chronic cycle of stress dysregulation. In contrast, adaptive biobehavioral patterns may buffer against the negative effects of stress by reducing the intensity and frequency of stressors, ultimately mitigating the stress dysregulation cycle. We only highlight a few biobehavioral coping patterns that have been studied within the context of interpersonal loss and health. Still, there are many important ones that we do not have the space to discuss in detail, such as health behaviors (e.g., physical activity, smoking, alcohol consumption), social integration, and spirituality to name a few. Importantly, modifiable risk factors such as physical activity, diet, and management of cardiovascular risk factors can reduce the risk of cognitive decline and dementia risk (Baumgart et al., 2015). These effects occur in a dose-response manner (Peters et al., 2019).
Adult attachment patterns.
The attachment system functions to ensure protection and reduce fear by promoting proximity-seeking behaviors to key attachment figures (Bowlby, 1973). Based upon prior relationships beginning in early childhood, people develop relatively stable patterns of cognitions, emotions, and behaviors, denoted as attachment orientations (Mikulincer & Shaver, 2003). In the adult attachment literature, attachment insecurity is represented across two orthogonal dimensions of anxiety and avoidance. Individuals low on attachment anxiety and attachment avoidance have secure attachment orientation (Mikulincer & Shaver, 2003). Those high on attachment anxiety intensify proximity seeking efforts and are hypervigilant to threat cues; those high on attachment avoidance disengage from proximity seeking behaviors in favor of self-reliance. In contrast, people with secure attachment can obtain “felt-security” and effectively manage responses to threat cues (Mikulincer & Shaver, 2003). Notably, insecure attachment is a risk factor for mental and physical health problems. In contrast, secure attachment orientation is associated with better health outcomes (McWilliams & Bailey, 2010).
Attachment orientations are particularly informative for understanding how individuals cope with interpersonal stressors (Mikulincer & Shaver, 2003). Securely attached caregivers perceive fewer caregiving difficulties and less psychological strain, while anxiously attached caregivers report more significant psychological strain (Crispi et al., 1997; Daire, 2002). Spousal caregivers with secure attachment have better emotional well-being than spousal caregivers with insecure attachment styles (Perren et al., 2007). Among widow(er)s, securely attached individuals adaptively adjust to loss. In contrast, anxious and avoidant individuals are at greater risk for pathological grief reactions (Mikulincer & Shaver, 2008). Insecure attachment patterns also have translational effects on a widow(er)’s physical health: widow(er)s high on attachment anxiety exhibit higher levels of inflammation and grief symptoms and poorer self-reported mental and physical health (LeRoy et al., 2020).
Emotion regulation.
An individual’s ability to independently regulate their emotional response to caregiving and post-bereavement stress is critical to health. Losing a lifetime partner severs years of interpersonal regulation between partners to maintain emotional and physiological stability (Butler & Randall, 2013; Zaki & Williams, 20131007). Caregivers who “sought distraction” or “fostered reassuring thoughts” experienced less negative reactivity to daily stressful events (Knippenberg et al., 2018). These qualitative findings align with quantitative results related to the construct of emotion regulation, which involves managing and responding to emotional experiences, via one or more regulation strategies (Gross, 1998). Frequent use of maladaptive emotion regulation strategies such as suppressing one’s emotions (termed emotional suppression) during bereavement is associated with immune dysregulation (Lopez et al., 2020). In contrast, cognitive change-based emotion regulation strategies such as mindfulness, which is the mindful awareness of one’s current mental and physical state in the current moment, have been found to improve mental well-being in widow(er)s (O’Connor et al., 2014) and are negatively correlated with caregiver burden (Klietz et al., 2020). Mindfulness was also found to improve emotion regulation and executive control function in bereaved individuals (Huang et al., 2019). Altogether, these findings suggest that adaptive emotion regulation strategies are critical to preserving mental and physical well-being in caregivers and bereaved spouses.
4.1.5. Summarizing the biopsychosocial model of interpersonal loss and cognition
Our biopsychosocial model provides an integrative framework for understanding how significant interpersonal losses, specifically spousal caregiving, and spousal bereavement, jeopardize cognitive health and increase the risk for ADRD. We argue that the context of caregiving and widowhood, mainly the biopsychosocial aspects of caring for, losing, or adjusting to life without a lifelong intimate partner, exacerbates ongoing age-related changes occurring in the brain. The autonomic nervous system, HPA axis, and the immune system transduce psychological experiences to physiological repercussions; chronic activation of these stress pathways can promote neuroinflammation and other neurodegenerative processes that manifest as cognitive dysfunction. When these biological events are accompanied by additional ADRD risk factors and maladaptive biobehavioral patterns of coping, abnormal cognitive decline and ADRD neuropathology becomes evident over time. Indeed, two studies (also reviewed here) corroborated our conceptual framework: Biddle et al. (2020) found that windowers with high ||–amyloid levels declined 3 times faster congnitively than married subjects with high ||-amyloid|| levels. Håkansson et al. (2009) demonstrated that the highest risk for AD was in those who lost their partner at midlife, were still widowed 20 years later, and were carriers of the apolipoprotein E e4 allele. These findings suggest that the combination of genetic, environmental, and biological risk factors substantially increases the risk for pathological cognitive decline.
4.2. Limitations, implications, and future directions
Limitations.
Some limitations should be noted. While most of the studies (58%) were rated as having minimal bias, 42% of studies were rated as having medium or high bias or were not rated due to low sample sizes. In addition, even though a significant association between interpersonal loss and poor cognitive outcomes was identified across most low-bias articles, the present review does not quantify the strength of this relationship. Because the primary intentions of this review were to 1) draw attention to a possible relationship between interpersonal losses and cognitive outcomes and 2) to propose a cross-disciplinary, theoretical framework to explain this pattern, a quantitative analysis was not prioritized. Considerable study heterogeneity complicates the ability to perform quantitative analyses. Studies varied widely in the way cognitive outcomes were assessed (dementia/cognitive impairment diagnosis vs. relative cognitive performance), the types of cognitive domains examined, the types of cognitive tests used, how marital status/caregiving status was evaluated, and whether widowhood/caregiving duration was considered, among others. Evaluating the size of the relationship between interpersonal losses and cognitive outcomes and understanding how heterogeneity in study design and sample characteristics alters this relationship warrants complex quantitative procedures (i.e., meta-analysis), an important direction for future research.
Some articles used the same datasets, which may overestimate the confidence of observing these relationships in the general population. However, these articles also used substantial sample sizes (n=1000+), which improves the reliability of the results reported in those studies. As the current study was a systematic review of the literature, the findings we report here were obtained qualitatively. Thus, the strength of the association between caregiving and widowhood status is not known. However, given that our findings were largely supported in longitudinal studies that tended to have low bias, we believe these results remain valid and pose significant implications to the broader scientific community. Further, only 10 caregiving articles were reviewed, which is substantially smaller than the number of bereavement articles identified. While this low number is attributed primarily to the scarcity of studies conducted on this topic, we believe these findings are robust, given that 89% of the studies reported relationships in the hypothesized direction. Lastly, some parts of the biopsychosocial model are more speculative than others and require further investigation; for example, it is unclear whether coping patterns (i.e., attachment orientations, emotion regulation) have noticeable effects on long-term cognitive outcomes after accounting for established ADRD risk factors. More studies that examine the biopsychosocial health of caregivers and widow(er)s over time are necessary to test and validate the model.
Implications.
Our findings and proposed theoretical model contribute to early prevention research in ADRD. ADRD is the only disease in the top ten causes of death without a means to prevent, cure, or slow its progression (Alzheimer’s Association, 2020). Because no drugs to date effectively alter the course of ADRD (Schneider et al., 2014), interventionists have redirected their efforts toward targeting high-risk healthy adults as a way to reduce ADRD prevalence and incidence (Crous-Bou et al., 2017). Our model provides an integrative framework for testing and identifying a combination of factors that impair or protect cognitive health. Diagnostic tools that evaluate clinical, biological, and psychological health may help researchers and doctors identify at-risk individuals early before the disease’s hallmark features appear and become irreversible. Importantly, promising work suggests that multi-faceted approaches can reduce the risk for cognitive decline. Multi-faceted approaches address multiple risk factors simultaneously and have proven to improve or maintain cognitive functioning in elderly samples (Ngandu et al., 2015).
Future directions.
Future work should test various components of the proposed theoretical model. While research on the bereaved and caregiving population’s physiological health is growing (especially in terms of immune health), little work has examined how stress-related mediators vary with cognitive functioning post-bereavement. Even less work has followed bereaved spouses or spousal caregivers beyond two-time points; more longitudinal studies are warranted. As technology advances, it would be valuable to examine cognitive function, neural activity, and stress physiology simultaneously over time to better understand how body systems interact to promote normal or abnormal cognitive aging.
More research is needed to determine whether certain cognitive domains are more susceptible to interpersonal loss than others. Global cognitive functioning, episodic memory, attention, and information processing were most commonly assessed. Still, heterogeneity across studies precluded the ability to make conclusive assumptions about which cognitive domains were most affected by interpersonal losses. Among the studies summarized in this review, different assessments were used to measure overall cognitive function and specific cognitive domains. Many studies utilized the Mini-Mental State Exam or the Telephone Interview for Cognitive Status to measure overall cognitive function; some even decomposed these assessments to examine cognitive domains separately. However, the MMSE is not sensitive to subtle changes in cognition within a healthy population, often displaying ceiling effects (Trzepacz et al., 2015). Future research examining changes in cognition may consider using more sensitive cognitive tasks that have the potential to detect early signs of ADRD. For example, pattern separation tasks – tasks that test one’s ability to remember experiences with overlapping content as distinct from one another – depend on hippocampal function and are more sensitive to memory loss than traditional neuropsychological testing (Leutgeb et al., 2007; Stark et al., 2010). Mounting evidence suggests that pattern separation deficits distinguish mild cognitive impaired adults from age-matched controls and healthy adults with worse delayed recall performance from healthy adults with better recall performance (Stark et al., 2013); some AD clinical trials and early detection trials have started implementing these tasks as promising indicators of decline in the preclinical stages of AD (Sperling et al., 2014). For studies that examine nondemented older adults (e.g., spousal caregivers or widow(er)s), pattern separation tasks, in conjunction with neurobiological measures, may capture subtle but significant changes in memory that would otherwise go unnoticed until disease diagnosis. Future studies may also consider deriving composite scores from multiple domain-specific cognitive tests. Researchers should implement multiple validated neuropsychological assessments per cognitive domain to accurately represent a subject’s cognitive function when examining specific cognitive domains.
As evident from our search results on spousal caregiving and cognition, more work on spousal caregivers and their cognitive health is needed. It would be interesting to examine whether widow(er)s who were previously spousal caregivers experience different cognitive outcomes than widow(er)s who were not previously spousal caregivers. For some caregivers, bereavement marks a turning point in health and social networks, such that health declines abated and social networks regrew after spousal loss. Differences in post-bereavement cognitive health may depend on marital quality, caregiving duration, helping behavior, caregiving burden, and caregiving stress. These factors differentially impact mental health in widowhood (Beach et al., 2000; Prokos & Keene, 2005; Schaan, 2013). In addition, certain types of spousal caregiving may be more detrimental to health and cognition than others such that dementia caregiving elicits greater cognitive impairment than non-dementia caregiving. For example, in a sample of 1,255 caregivers, dementia spousal caregivers showed more significant cognitive impairment than non-dementia spousal caregivers across periods preceding spousal loss and following spousal loss (Dassel et al., 2017). However, in a separate study, caring for a spouse with dementia was not significantly associated with cognitive performance (Pertl et al., 2015)
It is important to note that most widow(er)s are resilient. Only a small percentage continue to show maladjustment beyond the first year. Further, mortality risk and distress are highest during the first few months after the loss (Moon et al., 2014; Zisook & Shuchter, 1991) and typically return to pre-loss levels after 1.5 years (Bonanno et al., 2002). However, given that some studies still found a relationship between interpersonal loss and poor cognitive health even 4+ years into widowhood (Lyu et al., 2019; Perkins et al., 2016), more longitudinal research is needed to identify what factors perpetuate these risks beyond the first 2 years of bereavement. One potential factor is social connectedness. In a longitudinal study spanning 2 years before and 4 years after spousal death, Wilson and colleagues found that socially isolated dementia spousal caregivers had poorer self-reported health and immune function than socially connected caregivers both before and after bereavement. Importantly, even though socially connected caregivers reported decreases in self-reported health years leading up to the death, they showed a pattern of recovery after the loss that exceeded prior levels. (Wilson et al., 2020).
It is unclear whether ADRD pathology can be reversed with early cognitive interventions or if the cognitive decline is inevitable in those with certain risk factors. Were the widow(er)s who developed dementia already on a trajectory toward pathological cognitive decline? To what extent can post-bereavement interventions delay or reverse cognitive health trajectories? Is there a sensitive period during which interventions are most effective and least effective at offsetting dementia risk? Theoretically and empirically-driven intervention work may help address some of these questions. Frequently, cognitive interventions are implemented in people with noticeable cognitive impairment (i.e., MCI or mild AD). Future work could examine whether incorporating cognitive interventions before clinically significant cognitive impairment (i.e., in recently bereaved spouses) may be more impactful to cognition. Importantly, interventions that target multiple factors, such as comprehensive cognitive rehabilitation therapy, may be more promising because the etiology of ADRD is multi-factorial. Comprehensive cognitive rehabilitation therapy integrates cognitive training with psychotherapeutic or lifestyle interventions that help manage stress, neuropsychiatric symptoms, and improve health behaviors. Even though comprehensive cognitive rehabilitation therapy is typically used in clinical samples (Cicerone et al., 2011; Tsolaki et al., 2011), the fundamental concepts - mainly the combination of cognitive training and lifestyle changes/stress management skills – may prove to be highly effective for individuals who are cognitively intact but who have multiple risk factors for dementia.
One novel, underexplored field is the impact of the musical arts on cognition and stress. Music has beneficial effects on stress reduction (Pelletier, 2004), and growing evidence suggests that music-support therapy induces brain plasticity and enhances functional recovery in stroke patients (Ripollés et al., 2016). Among healthy older adults, the combination of physical exercise and music produced more improvements in cognition than exercise alone (Satoh et al., 2014). Because of music’s ability to reduce stress and promote cognitive flexibility, interventions with a music creativity component may help preserve cognitive ability in older adulthood.
5. Conclusion
Qualitative, systematic analysis of 64 empirical studies supported the association between interpersonal stressors and poorer cognitive health in older adulthood. Using a biopsychosocial framework within the context of cognitive aging, we proposed an integrative model for understanding how the psychological, biological, and social environment during spousal caregiving and widowhood accelerates the risk for cognitive decline and ADRD. The model integrates theories across multiple disciplines and serves as a valuable framework for examining mechanisms and implementing multi-faceted approaches to enhance risk identification and stifle abnormal cognitive aging.
Table 2.
Study design, sample characteristics, and study results for articles on widow(er)s
| Authors (year) | Quality | Study Design; Length of Observation (years) | Reference Group | Time Since Loss | Total N (% female) n Reference Group; n Widow(er)s | Mean Age (SD) or Age Range or % 65+ | Cognitive Assessment(s) | Cognitive Domain | Results | Controlled for age, sex, SES? |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Aartsen et al. (2005) | 3 | Longitudinal; 6 | Married subjects | 37 months | 1164 (40.7%) 940 (36 %); 204 (66 %) 2089 (59.9 %) | 60–85 | AVLT | Memory | Widow(er)s had greater memory decline than control | Yes |
| Amieva et al. (2010) | 3 | Longitudinal; 15 | Married subjects | nr | 1267 (nr); 679 (nr) | 73.7 (6.0) | Dementia diagnosis (clinician) | Incident dementia Verbal memory, | Being widowed was not associated with greater odds of dementia or AD than being married Widow(er)s had poorer cognitive function than married | Yes |
| Barragán- García et al. (2021) | 3 | Cross-sectional | Married subjects | 0–11+ years | 6898 (59.3 %) 4349 (50 %); 1624 (76.8 %) | 70.86 (7.43) | CCCE | Verbal memory, visuospatial ability/ memory | Widow(er)s had poorer cognitive function than married adults, with longer widowhood duration associated with lower overall cognition. | Yes |
| Bickel and Cooper (1994) | 3 | Longitudinal; 7.5 | Married subjects | nr | 314 (65.0 %) 133 (nr); 149 (nr) | 73.8 (6.0) | Dementia diagnosis (clinician, patient records, proxy report) | Incident dementia | Being widowed was not associated with increased odds of dementia | No |
| Biddle et al. (2020) | 3 | Longitudinal; 3.1 | Married subjects | nr | 257 (59.5 %) 145 (45.5 %); 35 (88.6 %) | 73.5 (6.1) | Preclinical Alzheimer Cognitive Composite included: MMSE, LMDR, DSST, FCSRT | Overall cognitive function, memory, executive function | Widow(er)s declined in cognitive performance compared to married subjects | Yes |
| Brenowitz et al. (2014). | 3 | Prospective; 3.2 | Married subjects | nr | 5335 (67.1 %) 3106 (nr); 1263 (nr) 10,940 (48.4 %) | 83.7 % | MCI diagnosis (clinician) | nr | Widowers had lower risk of MCI than controls. | Yes |
| Brown et al. (2020) | 3 | Longitudinal; 14 | Married subjects | 1–14 years | 9951 (53.2 %); 419 (79.5 %) | 50–65 | TICS-m | Global cognition; cognitive impairment | Widowed men had higher rates of cognitive impairment than married/re-partnered men. Widowed women did not show differences in cognitive function compared to married women. | Yes |
| Byrne and Raphael (1997) | 1 | Cross-sectional | Married subjects | 6 weeks | 114 (0%) 57 (0%); 57 (0%) | 74.94 (nr)* | MMSE | Global cognition | Widowed men did not differ from married men on overall cognitive performance. | Yes |
| Espinosa del Pozo et al. (2020) | 2 | Cross-sectional | Married subjects | nr | 80 (67 %) 48(nr); 16(nr) 7964 (50.5 %) | 100% | MMSE, AD8 | global cognition, self-report dementia symptoms | Widow(er)s did not differ on global cognition or risk for dementia than married adults. | Yes |
| Fan et al. (2015) | 2 | Cross-sectional | Married subjects | nr | 5111 (nr); 2471(nr) 2874 (nr) | 75.69 (nr)* | CDR, MMSE | Global cognition; cognitive impairment | Being widowed was associated with increased odds of dementia. | Yes |
| Farr on et al. (2020) | 2 | Cross-sectional | Married subjects | nr | nr; nr 2498 (56.6 %) 1857 (56.6 %); | 68.6 (nr) | Summary cognition score of 18 cognitive tests: WR, animal naming, MoCA CDT, HMSE, DSF, symbol cancellation, LM, constructional praxis, hand sequence, token test, judgment, problem solving, CSI-D, CPM, GNG | Overall cognition: memory, retrieval fluency, language, visuospatial skills, executive function, orientation, attention/speed, judgment | Widow(er)s had worse overall cognition than married adults. | Yes |
| Feng et al. (2014) | 2 | Cross-sectional | Married subjects | nr | 414 (90.8 %) | 66.0 | MMSE (cognitive impairment = <24) | Global cognition | Widowed men had greater odds of cognitive impairment than married men. No differences were found between widowed and married women. No differences found when widow (er)s were compared with married adults. | Yes |
| Forbes et al. (2019) | 3 | Longitudinal; 1.9–8 | Married subjects | 0–5 years | 247,586 (65.7 %) 123,793 (65.7 %) 123,793(65.7%) | 74.2c | Dementia diagnosis (patient records) | Incident dementia | Losing a spouse is associated with receiving a dementia diagnosis within 3 and 6 months of the loss but not associated with long-term risk of dementia | Yes |
| Guaita et al. (2015) | 2 | Cross-sectional | Married subjects | nr | 1321 (54.1 %) 872 (nr); 325 (nr) 1999 (72.4 %) 1157 (56.4%); | 71.69 (1.45) | Diagnosis of dementia or cognitive impairment no dementia (clinician) | incident dementia or mild cognitive impairment | Being widowed was not associated with increased odds of dementia or cognitive impairment Those who were widowed at midlife were more likely to show cognitive impairment later in | Yes |
| Hakansson et al. (2009) | 3 | Longitudinal; 21 | Married/cohabiting subjects | nr | 116 (95.7%) 3633 (nr) | 50.4 (6.0)d | Incident dementia | Incident dementia | life than those cohabiting with a partner in mid-life. Still widowed individuals had 3× risk of cognitive impairment thaan married/cohabiting people. | Yes |
| Hatch (2013) | 3 | Longitudinal; 13 | Nonbereaved subjects | nr | 2395 (47.2 %); 1088 (42.0 %) | 74.5 (nr)* | Dementia diagnosis (clinician) | Incident dementia | There was no association between widowhood and risk of dementia. | Yes |
| Helmer et al. (1999) | 3 | Longitudinal; 5 | Married subjects | nr | 3675 (58 %) 2106 (nr); 1287 (nr) | 100 % | Dementia diagnosis (clinician) | Incident dementia | Widowed adults did not differ from married adults in dementia risk. | Yes |
| Jia et al. (2020) | 3 | Cross-sectional | Married subjects | nr | 39,938 (nr); 3676 (nr) 6476 (61.3 %) | 70.26 (7.51) | Dementia diagnosis (clinician) | Incident dementia | Being widowed was associated with greater risk of dementia or mild cognitive impairment. | Yes |
| Karlamangla et al. (2009) | 3 | Longitudinal; 9 | Married subjects | nr | 3354 (nr); 2583 (nr) | 77.1 (nr) | TICS | Global cognition | Widow(er)s had faster declines on total cognition and recall than married adults. Baseline marital status was not associated with baseline cognitive function. Widow(er)s had greater | Yes |
| Kim et al. (2019) | 3 | Longitudinal; 2 | Married subjects with diverse social networka | nr | 1960 (48.2 %) 781 (36.5 %); 321 (80.7 %) 923 (62.0 %) | 72.05 (5.35) | MMSE | Global cognition | cognitive decline than married adults who had close contact with social groups. Widow(er)s have higher rates of cognitive impairment than married adults. Widowed | Yes |
| Kramer et al. (1985). | 2 | Cross-sectional | Married subjects | nr | 395 (41.0 %); 393 (83.7 %) 2618 (58.6 %) | 65–74 | MMSE (severe impairment = 0—17) | Global cognition | females have higher rates of cognitive impairment than married females. No differences across males by marital status. | No |
| Lee et al. (2019). | 3 | Longitudinal; 4 | Married subjects | nr | 2072 (51.5 %); 546 (85.5 %) 2462 (50.4 %) | 51–93 | Total cognition: WR, SS7, Backward counting | memory, working memory, attention and processing speed | There were no significant association between widowhood and cognitive function across a 4-year period. | Yes |
| Li et al. (2018) | 2 | Longitudinal; 30 | Married subjects | 2131 (nr); 61 (nr) | 40–65 | Dementia diagnosis (clinician) | Incident dementia | Widowhood was associated with increased risk of dementia for those aged 50 +. | No | |
| Liu et al. (2019) | 3 | Longitudinal; 7 | Married subjects | nr | 7508(58.2%) 3609 (41.1%); 2542 (81.2%) 15,379 (54.97 %) | 100% | Overall cognition and independent domains assessed using the following assessments: WR, CDT, time orientation. If unable to complete the tests, cognitive status measured by AD diagnosis or AD8. Overall cognitive score was categorized into 3 groups: dementia, cognitive impairment, normal cognition. | Memory, orientation, executive function | Widow(er)s had greater cognitive impairment in memory, executive function and orientation than married adults. They also had higher prevalence rates of cognitive impairment (not dementia) and dementia. | Yes |
| Liu et al. (2020) | 3 | Longitudinal; 14 | Married subjects | nr | 10,105(46.2%); 3007 (81.9 %) 1145 (63.4%) | 65.91 (9.88) | TICS, proxy assessment of memory and IADLs | Global cognition/ dementia category | Widowed respondents (and all unmarried groups) had higher odds of dementia than married respondents. The transition into widowhood, but not to divorce or remarriage, is associated with higher odds of dementia. | Yes |
| Lopes et al. (2007) | 1 | Cross-sectional | Married subjects | nr | 660 (nr); 315 (nr) 3660 (42.5 %) | 70.9 (60–100) | MMSE (< 26), FOME (< 35), IQCODE (> 3.40), B-ADL (> 3.19) | global cognition: language, attention, memory, verbal fluency, verbal and tactile recognition; activities of daily living | Widowhood was not associated with cognitive and functional impairment | Yes |
| Lyu et al. (2019) | 3 | Longitudinal; 8 | Married subjects | 0–6+ years | 3266(nr); 394 (nr) 3329 (nr) | 68.5 (6.16) | MMSE | Global cognition | Widow(er)s bereaved for 4—6 years had steeper declines in global cognitive functioning than nonwidowed individuals. Widowed women had lower initial levels of cognitive | Yes |
| Monserud (2019). | 3 | Longitudinal; 17 | Married men | nr | nr; nr 1882 (54.5 %) 1422 (47.3 %); | 65–109 | MMSE | Global cognition | Widow(er)s bereaved for 4—6 years had steeper declines in global cognitive functioning than nonwidowed individuals. Widowed women had lower initial levels of cognitive | Yes |
| Mousavi-Nasab et al. (2012) | 3 | Longitudinal; 5 | Married or single subjects | nr | 242 (86.8 %) 77 (72 %) 32 (68.8 %); | 35–85b | Free/cued recall and recognition tests, vocabulary and fluency tests | Episodic memory, semantic memory | divorced adults. Older widow (er)s had poorer semantic memory performance (vocabulary) than older married people. Widow(er)s showed no differences in recall or fluency from married, divorced, single adults. Widow(er)s with complicated | No |
| O’Connor and Arizmendi (2014) | NA | Cross-sectional | Married subjects | nr | 45(73.3%) | 71.85 (nr)* | e-Stroop, MMSE, DSB, WCST | Global cognition, working memory, executive function, emotional inhibition | grief had poorer overall cognitive performance and exhibited greater interference (slower reaction times) to emotional words, than nonbereaved group. No | No |
| Paul et al. (2010). | 2 | Cross-sectional | Married or single subjects | nr | 1268(70.4%) 704(nr); 442(nr) 9171 (52.8 %) 5586 (33.8 %); | 70.3 (8.7) | MMSE | Global cognition | differences in working memory or executive functioning. Widow(er)s did not have higher rates of cognitive impairment than nonbereaved group (single or married). Widows and widowers had worse memory performance than married counterparts. When widowhood duration was | No |
| Perkins et al. (2016) | 2 | Cross-sectional | Married subjects | 0–10+ years | 3585 (82.5 %) | 64.9 % | WR | Memory | accounted for, men bereaved for 5—9 years had lower memory ability than married men. Women bereaved for 0—4 years or 10+ years had lower memory ability than married women. Being widowed was not | Yes |
| Ribeiro et al. (2013) | 2 | Cross-sectional | Married subjects | nr | 683 (70.9 %) 284 (nr); 279 (nr) 211 (85.3 %) 84(nr); | 78.24 (6.95) | Dementia diagnosis (clinician) | Incident dementia | associated with greater odds of dementia than being married. Being widowed was not associated with cognitive | Yes |
| Rosnick et al (2010). | 3 | Cross-sectional | Nonbereaved subjects | 6 months | 127(nr) | 69.97 (6.33) | SR, disc positioning on a board, BNT, WAIS-R Similarities subtest, copying objects, overall cognitive score (summary statistic of all assessments) | Episodic and spatial memory, visuospatial skills, verbal ability, reasoning | outcomes. Bereaved men did worse than nonbereaved men on immediate story recall task. Bereaved younger adults did worse on immediate and delayed story recall compared to nonbereaved younger adults. | Yes |
| Saha et al. (2010) | 1 | Cross-sectional | Married subjects | nr | 179 (100 %) nr; nr | 64 (7.6) | MMSE (cognitive impairment<24) | Global cognition | Being widowed was associated with cognitive impairment | No |
| Shahar et al. (2001) | 2 | Cross-sectional | Married subjects | 2.9 months | 126 (82 %) 58 (82 %); 58 (82 %) 6766 (51.01%) | 77.6 (nr) | Combined score: MMSE, digit symbol substitution | Global cognition | No significant difference in cognitive functioning between widowed and married subjects. Widow(er)s had lower global | No |
| Shin et al. (2018) | 3 | Longitudinal; 17 | 0-16 years | Married subjects | 4040 (nr); 2726 (65.1 %) 240 (62.5 %) | 72.79(5.46) | Total cognition score (WR, mental status summary score) | Global cognition | cognition than married subjects. Cognition scores also declined significantly as time since spousal loss increased. Being widowed was associated | Yes |
| Subramanian et al. (2021) | 2 | Cross-sectional | Married subjects | nr | 160 (nr); 80 (nr) 1177 (57.4 %) | 63.9 (7.1) | HMSE (cognitive impairment <26) | Global cognition | with greater prevalence of cognitive impairment than being married. Being widowed was associated | Yes |
| Sun et al. (2021) | 2 | Cross-sectional | Married subjects | nr | 402(nr); 775(nr) 2,288,489 (51 %) | 81.25 (nr)* | MCI (clinician) | Cognitive impairment | with greater risk of severe cognitive impairment Being widowed was associated | No |
| Sundstrom et al. (2016). | 3 | Longitudinal; 10 | Married subjects | nr | 1,484,754 (48.6 %); 191,401 (79.8 %) | 60.5 (7.3) | Dementia diagnosis (patient records) | Incident dementia | with increased odds of dementia for young-old (50—64 yrs) and middle-old (65—74 yrs) | Yes |
| 3 | Married subjects | nr | 74.74 (nr)* | Dementia diagnosis (clinician) | Incident dementia | No | ||||
| Sundstrom et al.(2014b) 524 (nr) | Longitudinal; 8.6 | 1609 (56.12 %) 926 (nr); 4370 (45.6 %) 3007 (45.1 %); | Widow(er)s have an increased risk of dementia compared to married adults. | |||||||
| Vidarsdottir et al. (2014) | 3 | Longitudinal; 25 | Married subjects | 0–10+ years | 1363 (74.9 %) 537,513 (46.4 %) | 76.68 (nr) | composite cognition score (CVLT, DSST, Figure comparison test, Stroop, DSB, SWM of CANTAB); dementia diagnosis (clinician), MCI diagnosis (clinician) | Memory, processing speed, executive function | Widow(er)s did not differ on cognitive performance than married individuals and did not have greater risk of dementia or mild cognitive impairment. Widowed women had lower executive functioning performance than married women but no differences were seen across widowed and married men. | Yes |
| Wandell et al. (2020) | 3 | Longitudinal; 5.4 | Married subjects | nr | 292,736(36.0 %); 133,627 (74.3 %) 50 (72 %) 25(72 %); | 77.1 % | Dementia diagnosis (patient records) | Incident dementia | Widowed men had lower risk of dementia than married men. No differences in dementia risk between widowed and married women. | Yes |
| Ward et al. (2007) | NA | Cross-sectional | Non-bereaved subjects | < 18 months | 25(72 %) 1269 (42%) 850 (nr); | 65–80 | WTAR, MMSE, AVLT, Benton VRT, TEA subtests: elevator counting and visual elevator, TMT, RCFT, COWAT, SDMT MMSE, Coding task, CPM, AVLT MMSE | Intelligence quotient, global cognitive ability, memory, attention, information processing, visuospatial ability, verbal fluency | Widow(er)s performed worse on tests of attention, processing speed, and verbal fluency than nonbereaved group but after depression, stress and anxiety were accounted for, these differences were no longer significant. | Yes |
| Worn et al. (2020) | 3 | Longitudinal; 20 | Married subjects | 0–4+ years | 419 (64.4 %) 5872 (55.81 %) 3267 (nr); | 75.98 (6.60) | MMSE, Coding task, CPM, AVLT | MMSE, Coding task, CPM, AVLT | Global cognition, processing speed, reasoning, memory Bereaved women performed showed decrease in reasoning performance compared to married women; no differences in memory, global cognitive functioning, and processing speed were found. No cognitive differences between bereaved men and nonbereaved men. Widow(er)s were more likely to experience cognitive decline than married subjects. When stratified by gender, widowhood negatively impacted cognitive function in men but did not impact women. | Yes |
| Xiang et al. (2021) | 3 | Longitudinal; 9 | Married subjects | nr | 2605 (nr) | 80.64 (10.32) | MMSE | Global cognition | When widowhood was divided into 5 categories of widowhood duration, men bereaved for 5 years or less, 16—20 years, or 21+ years were more likely to have lower cognitive scores than married men. Women bereaved | Yes |
| Xu et al. (2020) | 2 | Cross-sectional | Married subjects | nr | 1018 (50.20 %); 285 (70.53 %) 5897(51%) | 71 (60–94) | MMSE | Global cognition | for 21+ years had worse cognitive function than currently married women. Window(er)s did not differ from married adults on global cognitive functioning. No effect of gender. | Yes |
| Yin et al. (2020) | 3 | Longitudinal; 5.32 | Widowed individuals | nr | 2448(nr); 3253(nr) 5550 (55.5 %) | 81.7 (9.7) | MMSE | Global cognition, cognitive impairment | Married individuals have lower risk of developing cognitive impairment than widowed individuals Widowed men have greater odds | Yes |
| Zhang et al. (2019a) | 2 | Cross-sectional | Married subjects | nr | 940 (61.2 %); 352 (64.8 %) | 73.30 (7.56) | MMSE, CDR, cognitive impairment (clinician) | Incident cognitive impairment no | of cognitive impairment than married men. No differences | Yes |
| 352 (64.8 %) | dementia | between widowed and married women | ||||||||
| Zhang et al. (2006) | 2 | Cross-sectional | Married subjects | nr | 34,807 (53.8 %) 25,910 (nr); | 56.3 % | Dementia diagnosis (clinician) | Incident AD or incident vascular dementia | Widowed adults had higher prevalence of AD cases and lower prevalence of vascular | Yes |
| Zhang et al. (2019b) | 3 | Longitudinal; 2 | Married subjects | nr | 1293 (72.2 %o) | 40.9 % | WR, TICS (orientation and subtraction) | Memory, orientation, calculation | continually married adults. Newly widowed adults did not differ from continually married adults in episodic memory changes | Yes |
| Zhang et al. (2021) | 3 | Longitudinal; 16 | Married subjects | nr | 14,788 (55.2 %) 9700 (nr); 2975 (nr) 30,578 (nr) 27,732 (49 %); | 65.89 (nr) | TICS, proxy assessment of memory and IADLs | Global cognition/ dementia category | Being widowed was associated with greater odds of dementia in both blacks and whites. Being widowed was negatively associated with global cognition, objective memory, crystallized | Yes |
| Zhao et al., 2021 | 3 | Longitudinal; 18 | Married subjects | 0–4+ years | 2846 (69 %); | 67.49 (nr)* | Cognitive constructs formed from: WR, backward counting, SS, TICS | Global cognition, memory, mental status | cognition and fluid cognition. Being widowed was not associated with backward counting, subjective memory, or date naming. | Yes |
Notes: nr = not reported; NA = not applicable; CE 8 = Eight-item Informant Interview to Differentiate Aging and Dementia; AVLT = Auditory Verbal Learning Test; B-ADL = Bayer-Activities of daily living scale; BNT = Boston Naming Test; CCCE = Cross Cultural Cognitive Examination; CDR = Clinical Dementia Rating Scale; CDT = clock drawing test; COWAT = Controlled Oral Word Association Test; CVLT = California Verbal Learning Test; DSB = Digit Span Backward; DSF = Digit Span Forward; DSST = Digit Symbol Substitution Test; e-Stroop = emotional counting-Stroop; FOME = Fuld Object Memory Evaluation; FCSRT = Free and Cued Selective Reminding Test; GNG = Go-No-Go Test; HMSE = Hindi Mental State Exam; IADLs = Instrumental Activities of Daily Living; IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly; LMDR = Logical Memory Delayed Recall; MCI = mild cognitive impairment; MMSE = Mini Mental State Exam; RCFT = Rey-Osterrieth Complex Figure Test ; CPM = Raven’s Coloured Progressive Matrices; TEA = Test of Everyday Attention; TICS = Telephone Interview for Cognitive Status; TMT = Trail Making Test; SDMT = Symbol Digit Modalities Test; SS = Serial 7 Test; SR = Story Recall (immediate and delayed); TICS-m = modified TICS; WAIS- R = Wechsler Adult Intelligence Scale Revised; WR = Word recall (immediate and/or delayed); VRT = Visual Retention Test ; WCST = Wisconsin Card Sorting Task; WTAR = Wechsler Test of Adult Reading.
Reference group is widowed network (mostly widowed individuals) but reported results are based on comparison between widowed network and married couples with diverse networks.
Age was divided into two cohorts, 35–60 and 65–85.
Value is median age.
Age at mid-life/baseline.
Indicates estimates calculated by Wu et al. based on information provided by authors.
Highlights.
Spousal caregivers and widow(er)s have worse cognitive outcomes than controls
Spousal caregivers and widow(er)s have increased long-term risk for dementia
Stress-related pathways may contribute to abnormal cognitive decline
We propose a biopsychosocial model linking partner loss to increased ADRD risk
Longitudinal research on stress, health, and cognition in this population is needed
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute (Grant No. 1R01HL127260–01) and National Institute on Aging (R01AG062690 and R21AG061597). We would like to acknowledge Ms. Yoully Kang for creating the figure and for our senior research assistants, Ms. Shelly Zhou and Mr. Russell Ku who helped with data extraction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aartsen M, & Jylhä M. (2011). Onset of loneliness in older adults: Results of a 28 year prospective study. European Journal of Ageing, 8(1), 31–38. 10.1007/s10433-011-0175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsen M, Tilburg TV, Smits C, Comijs H, & Knipscheer K. (2005). Does widowhood affect memory performance of older persons? Psychological Medicine, 35(2), 217–226. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, … Wyss– Coray T. (2000). Inflammation and Alzheimer’s disease. Neurobiology of Aging, 21(3), 383–421. 10.1016/S0197-4580(00)00124-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE., Ryan L., Bowers D., Foste TC., Bizon JL., Geldmacher DS., & Glisky EL. (2012). Characterizing cognitive aging in humans with links to animal models. Frontiers in Aging Neuroscience, 4. 10.3389/fnagi.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred C. (2019). Age variation in the divorce rate, 1990 & 2017 (No. 13). National Center for Family & marriage Research. 10.25035/ncfmr/fp-19-13. [DOI] [Google Scholar]
- Alzheimer’s Association. (2020). Facts and Figures. Alzheimer’s Disease and Dementia. https://www.alz.org/alzheimers-dementia/facts-figures
- American Psychological Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Association Publishing. [Google Scholar]
- Amieva H, Stoykova R, Matharan F, Helmer C, Antonucci TC, & Dartigues J-F (2010). What Aspects of Social Network Are Protective for Dementia? Not the Quantity But the Quality of Social Interactions Is Protective Up to 15 Years Later. Psychosomatic Medicine, 72(9), 905–911. 10.1097/PSY.0b013e3181f5e121 [DOI] [PubMed] [Google Scholar]
- Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, & Pariante CM (2013). Glucocorticoid-Related Molecular Signaling Pathways Regulating Hippocampal Neurogenesis. Neuropsychopharmacology, 38(5), 872–883. 10.1038/npp.2012.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG., & Thompson PM. (2008). Mapping progressive brain structural changes in early Alzheimer’s disease and mild cognitive impairment. Neuropsychologia, 46(6), 1597–1612. 10.1016/j.neuropsychologia.2007.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410–422. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson MK, Ooi WL, Morgenstern H, Hafner A, Masur D, Crystal H, Frishman WH, Fisher D, & Katzman R. (1990). Women, myocardial infarction, and dementia in the very old. Neurology, 40(7), 1102–1106. 10.1212/WNL.40.7.1102 [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, & Whitmer RA (2012). Midlife vs Late-Life Depressive Symptoms and Risk of Dementia: Differential Effects for Alzheimer Disease and Vascular Dementia. Archives of General Psychiatry, 69(5), 493–498. 10.1001/archgenpsychiatry.2011.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán-García M, Ramírez-Aldana R, López-Ortega M, Sánchez-García S, & García-Peña C. (2021). Widowhood Status and Cognitive Function in Community-Dwelling Older Adults from the Mexican Health and Aging Study (MHAS). Journal of Population Ageing. 10.1007/s12062-020-09322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, & Palinkas LA (1994). Low blood pressure and depression in older men: A population based study. BMJ, 308(6926), 446–449. 10.1136/bmj.308.6926.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer ME, Vedhara K, Perks P, Wilcock GK, Lightman SL, & Shanks N. (2000). Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. Journal of Neuroimmunology, 103(1), 84–92. 10.1016/S0165-5728(99)00228-3 [DOI] [PubMed] [Google Scholar]
- Baumgart M., Snyder HM., Carrillo MC., Fazio S., Kim H., & Johns H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s & Dementia, 11(6), 718–726. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Beach SR, Schulz R, Yee JL, & Jackson S. (2000). Negative and positive health effects of caring for a disabled spouse: Longitudinal findings from the Caregiver Health Effects Study. Psychology and Aging, 15(2), 259. 10.1037/0882-7974.15.2.259 [DOI] [PubMed] [Google Scholar]
- Beeson RA (2003). Loneliness and depression in spousal caregivers of those with Alzheimer’s disease versus non-caregiving spouses. Archives of Psychiatric Nursing, 17(3), 135–143. 10.1016/S0883-9417(03)00057-8 [DOI] [PubMed] [Google Scholar]
- Beluche I, Carrière I, Ritchie K, & Ancelin M-L (2010). A prospective study of diurnal cortisol and cognitive function in community-dwelling elderly people. Psychological Medicine, 40(6), 1039–1049. 10.1017/S0033291709991103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JM, Fagundes CP, & Kiecolt-Glaser JK (2013). The chronic stress of caregiving accelerates the natural aging of the immune system. In Bosch JA, Phillips AC, & Lord JM (Eds.), Immunosenescence: Psychosocial and Behavioral Determinants (pp. 35–46). Springer. 10.1007/978-1-4614-4776-4_3 [DOI] [Google Scholar]
- Bennett KM (1996). A Longitudinal Study of Wellbeing in Widowed Women. International Journal of Geriatric Psychiatry, 11(11), 1005–1010. [DOI] [PubMed] [Google Scholar]
- Bickel H, & Cooper B. (1994). Incidence and relative risk of dementia in an urban elderly population: Findings of a prospective field study. Psychological Medicine, 24(1), 179–192. 10.1017/S0033291700026945 [DOI] [PubMed] [Google Scholar]
- Biddle KD, Jacobs HIL, Uquillas F.d’Oleire, Zide BS, Kirn DR, Properzi MR, Rentz DM, Johnson KA, Sperling RA, & Donovan NJ (2020). Associations of Widowhood and β-Amyloid With Cognitive Decline in Cognitively Unimpaired Older Adults. JAMA Network Open, 3(2), e200121–e200121. 10.1001/jamanetworkopen.2020.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Moss M, & Albert M. (2007). Neuropsychological Measures in Normal Individuals That Predict Subsequent Cognitive Decline. Archives of Neurology, 64(6), 862–871. 10.1001/archneur.64.6.862 [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, & Dantzer R. (1994). Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. Comptes Rendus de l’Academie Des Sciences - Serie III, 317(6), 499–503. [PubMed] [Google Scholar]
- Bonanno GA., Wortman CB, Lehman DR, Tweed RG, Haring M, Sonnega J, Carr D, & Nesse RM (2002). Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss. Journal of Personality and Social Psychology, 83(5), 1150. 10.1037/0022-3514.83.5.1150 [DOI] [PubMed] [Google Scholar]
- Bowlby J. (1973). Attachment and loss: Vol. 2. Separation: Anxiety and anger (Vol. 2). Basic Books. [Google Scholar]
- Brenowitz WD (2013). The associations of social relationships with risk of incident mild cognitive impairment in older adults. [Thesis]. https://digital.lib.washington.edu:443/researchworks/handle/1773/21808 [Google Scholar]
- Brenowitz WD, Kukull WA, Beresford SAA, Monsell SE, & Williams EC (2014). Social Relationships and Risk of Incident Mild Cognitive Impairment in U.S. Alzheimer’s Disease Centers. Alzheimer Disease and Associated Disorders, 28(3), 253–260. 10.1097/WAD.0000000000000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommelhoff JA, Gatz M, Johansson B, McArdle JJ, Fratiglioni L, & Pedersen NI (2009). Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychology and Aging, 24(2), 373–384. 10.1037/a0015713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Lin I-F, Vielee A, & Mellencamp KA (2020). Midlife Marital Dissolution and the Onset of Cognitive Impairment. The Gerontologist, gnaa193. 10.1093/geront/gnaa193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T., Mihailidou AS, Bartrop R, McKinley S, Ward C, Morel-Kopp M-C, Spinaze M, & Tofler GH (2011). Haemodynamic changes during early bereavement: Potential contribution to increased cardiovascular risk. Heart, Lung and Circulation, 20(2), 91–98. 10.1016/j.hlc.2010.10.073 [DOI] [PubMed] [Google Scholar]
- Butler EA, & Randall AK (2013). Emotional coregulation in close relationships. Emotion Review, 5(2), 202–210. 10.1177/1754073912451630 [DOI] [Google Scholar]
- Byers AL, & Yaffe K. (2011). Depression and Risk of Developing Dementia. Nature Reviews. Neurology, 7(6), 323–331. 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne GJA, & Raphael B. (1997). The Psychological Symptoms of Conjugal Bereavement in Elderly Men Over the First 13 Months. International Journal of Geriatric Psychiatry, 12(2), 241–251. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Burleson MH, Poehlmann KM, Malarkey WB, Kiecolt-Glaser JK, Berntson GG, Uchino BN, & Glaser R. (2000). Autonomic and neuroendocrine responses to mild psychological stressors: Effects of chronic stress on older women. Annals of Behavioral Medicine, 22(2), 140–148. 10.1007/BF02895778 [DOI] [PubMed] [Google Scholar]
- Carr D. (2003). A “good death” for whom? Quality of spouse’s death and psychological distress among older widowed persons. Journal of Health and Social Behavior, 44(2), 215–232. 10.2307/1519809 [DOI] [PubMed] [Google Scholar]
- Caserta MS, & Lund DA (2007). Toward the Development of an Inventory of Daily Widowed Life (IDWL): Guided by the Dual Process Model of Coping with Bereavement. Death Studies, 31(6), 505–535. 10.1080/07481180701356761 [DOI] [PubMed] [Google Scholar]
- Caswell LW, Vitaliano PP, Croyle KL, Scanlan JM, Zhang J, & Daruwala A. (2003). Negative Associations of Chronic Stress and Cognitive Performance in Older Adult Spouse Caregivers. Experimental Aging Research, 29(3), 303–318. 10.1080/03610730303721 [DOI] [PubMed] [Google Scholar]
- Chan D., Livingston G, Jones L, & Sampson EL (2013). Grief reactions in dementia carers: A systematic review. International Journal of Geriatric Psychiatry, 28(1), 1–17. 10.1002/gps.3795 [DOI] [PubMed] [Google Scholar]
- Chen P, & Botticello AL (2013). Spouses of Stroke Survivors May Be at Risk for Poor Cognitive Functioning: A Cross-sectional Population-Based Study. Topics in Stroke Rehabilitation, 20(4), 369–378. 10.1310/tsr2004-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, Felicetti T, Laatsch L, Harley JP, Bergquist T, Azulay J, Cantor J, & Ashman T. (2011). Evidence-Based Cognitive Rehabilitation: Updated Review of the Literature From 2003 Through 2008. Archives of Physical Medicine and Rehabilitation, 92(4), 519–530. 10.1016/j.apmr.2010.11.015 [DOI] [PubMed] [Google Scholar]
- Convit A, De Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, & George A. (1997). Specific Hippocampal Volume Reductions in Individuals at Risk for Alzheimer’s Disease. Neurobiology of Aging, 18(2), 131–138. 10.1016/S0197-4580(97)00001–8 [DOI] [PubMed] [Google Scholar]
- Craik FIM, & McDowd JM (1987). Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition, 13(3), 474. 10.1037/0278-7393.13.3.474 [DOI] [Google Scholar]
- Crispi EL, Schiaffino K, & Berman WH (1997). The Contribution of Attachment to Burden in Adult Children of Institutionalized Parents With Dementia1. The Gerontologist, 37(1), 52–60. 10.1093/geront/37.1.52 [DOI] [PubMed] [Google Scholar]
- Crous-Bou M, Minguillón C, Gramunt N, & Molinuevo JL (2017). Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer’s Research & Therapy, 9(1), 71. 10.1186/s13195-017-0297-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daire AP (2002). The Influence of Parental Bonding on Emotional Distress in Caregiving Sons for a Parent With Dementia. The Gerontologist, 42(6), 766–771. 10.1093/geront/42.6.766 [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews. Neuroscience, 9(1), 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, & Hofman A. (2018). Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimer’s & Dementia, 14(11), 1450–1459. 10.1016/j.jalz.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Dassel KB, Carr DC, & Vitaliano P. (2017). Does Caring for a Spouse With Dementia Accelerate Cognitive Decline? Findings From the Health and Retirement Study. The Gerontologist, 57(2), 319–328. 10.1093/geront/gnv148 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ-F, Roozendaal B, Nitsch RM, McGaugh JL, & Hock C. (2000). Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience, 3(4), 313–314. 10.1038/73873 [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Jolles J, van Osch L, Stevens F, Aalten P, Lousberg R, & Verhey FRJ (2006). Cognitive functioning in spousal caregivers of dementia patients: Findings from the prospective MAASBED study. Age and Ageing, 35(2), 160–166. 10.1093/ageing/afj044 [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Nicolson NA, Aalten P, Lousberg R, Jolle J, & Verhey FRJ (2005). Behavioral Problems in Dementia Patients and Salivary Cortisol Patterns in Caregivers. The Journal of Neuropsychiatry and Clinical Neurosciences, 17(2), 201–207. 10.1176/jnp.17.2.201 [DOI] [PubMed] [Google Scholar]
- Diehl M., Hay EL, & Berg KM (2011). The ratio between positive and negative affect and flourishing mental health across adulthood. Aging & Mental Health, 15(7), 882–893. 10.1080/13607863.2011.569488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Beydoun MA, & Zonderman AB (2010). Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology, 75(1), 27–34. 10.1212/WNL.0b013e3181e62124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, & Resnick SM (2009). Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology, 72(22), 1906–1913. 10.1212/WNL.0b013e3181a82634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgh E, Lindqvist Åstot A, Fagerlund M, Eriksson S, Olsson T, & Näsman B. (2006). Cognitive Dysfunction, Hippocampal Atrophy and Glucocorticoid Feedback in Alzheimer’s Disease. Biological Psychiatry, 59(2), 155–161. 10.1016/j.biopsych.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Ennis GE, An Y, Resnick SM, Ferrucci L, O’Brien RJ, & Moffat SD (2017). Longterm cortisol measures predict Alzheimer disease risk. Neurology, 88(4), 371–378. 10.1212/WNL.0000000000003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa del Pozo PH, Espinosa PS, Donadi E, Rogel L, Naranjo R, Haro GE, Riera DC, Mendoza GS, Garzón NA, & Andrade NF (2020). Detecting Cognitive Decline and Dementia in Santa Cruz, Galápagos Islands, Ecuador. Cureus, 12(10). 10.7759/cureus.10826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Brown RL, Chen MA, Murdock KW, Saucedo L, LeRoy A, Wu EL, Garcini LM, Shahane AD, Baameur F, & Heijnen C. (2019). Grief, depressive symptoms, and inflammation in the spousally bereaved. Psychoneuroendocrinology, 100, 190–197. 10.1016/j.psyneuen.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP., Murdock KW, LeRoy A, Baameur F, Thayer JF, & Heijnen C. (2018). Spousal bereavement is associated with more pronounced ex vivo cytokine production and lower heart rate variability: Mechanisms underlying cardiovascular risk? Psychoneuroendocrinology, 93, 65–71. 10.1016/j.psyneuen.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Fagundes CP, & Wu EL (2020). Matters of the Heart: Grief, Morbidity, and Mortality. Current Directions in Psychological Science, 0963721420917698. 10.1177/0963721420917698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L-Y, Sun Y, Lee H-J, Yang S-C, Chen T-F, Lin K-N, Lin C-C, Wang P-N, Tang L-Y, & Chiu M-J (2015). Marital Status, Lifestyle and Dementia: A Nationwide Survey in Taiwan. PLoS ONE, 10(9). 10.1371/journal.pone.0139154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farron MR, Kabeto MU, Dey AB, Banerjee J, Levine DA, & Langa KM (2020). Hypertension and Cognitive Health Among Older Adults in India. Journal of the American Geriatrics Society, 68(S3), S29–S35. 10.1111/jgs.16741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Ng X-T, Yap P, Li J, Lee T-S, Håkansson K, Kua E-H, & Ng T-P (2014). Marital Status and Cognitive Impairment among Community-Dwelling Chinese Older Adults: The Role of Gender and Social Engagement. Dementia and Geriatric Cognitive Disorders Extra, 4(3), 375–384. 10.1159/000358584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, & Walhovd KB (2014). What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Progress in Neurobiology, 117, 20–40. 10.1016/j.pneurobio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes HJ, Wong AYS, Morton C, Bhaskaran K, Smeeth L, Richards M, Schmidt SAJ, Langan SM, & Warren-Gash C. (2019). Partner Bereavement and Detection of Dementia: A UK-Based Cohort Study Using Routine Health Data. Journal of Alzheimer’s Disease, 72(2), 653–662. 10.3233/JAD-190571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formanek T., Kagstrom A, Winkler P, & Cermakova P. (2019). Differences in cognitive performance and cognitive decline across European regions: A population-based prospective cohort study. European Psychiatry, 58, 80–86. 10.1016/j.eurpsy.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Franceschi C, & Campisi J. (2014). Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. The Journals of Gerontology: Series A, 69(Suppl_1), S4–S9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- Furst AJ, & Mormino EC (2010). A BOLD move: Clinical application of fMRI in aging. Neurology, 74(24), 1940–1941. 10.1212/WNL.0b013e3181e533f8 [DOI] [PubMed] [Google Scholar]
- Gabryelewicz T, Styczynska M, Luczywek E, Barczak A, Pfeffer A, Androsiuk W, Chodakowska Zebrowska M, Wasiak B, Peplonska B, & Barcikowska M. (2007). The rate of conversion of mild cognitive impairment to dementia: Predictive role of depression. International Journal of Geriatric Psychiatry, 22(6), 563–567. 10.1002/gps.1716 [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Sigurdsson S, Eiriksdottir G, Garcia ME, Harris TB, Gudnason V, & Launer LJ (2015). Salivary cortisol, brain volumes, and cognition in community-dwelling elderly without dementia. Neurology, 85(11), 976–983. 10.1212/WNL.0000000000001931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen L, Wang H-X, Reynolds CA, Fratiglioni L, Gatz M, & Pedersen NL (2017). Influence of Negative Life Events and Widowhood on Risk for Dementia. The American Journal of Geriatric Psychiatry, 25(7), 766–778. 10.1016/j.jagp.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J, Conroy RM, Bruce I, Denihan A, Greene E, Kirby M, & Lawlor BA (2009). Loneliness, social support networks, mood and wellbeing in community-dwelling elderly. International Journal of Geriatric Psychiatry, 24(7), 694–700. 10.1002/gps.2181 [DOI] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, & Mattson MP (1996). Estrogens Attenuate and Corticosterone Exacerbates Excitotoxicity, Oxidative Injury, and Amyloid β-Peptide Toxicity in Hippocampal Neurons. Journal of Neurochemistry, 66(5), 1836–1844. 10.1046/j.1471-4159.1996.66051836.x [DOI] [PubMed] [Google Scholar]
- Gouin J-P., Glaser R, Malarkey WB, Beversdorf D, & Kiecolt-Glaser J. (2012). Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology, 31(2), 264–268. 10.1037/a0025536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goukasian N, Porat S, Blanken A, Avila D, Zlatev D, Hurtz S, Hwang KS, Pierce J, Joshi SH, Woo E, & Apostolova LG (2019). Cognitive Correlates of Hippocampal Atrophy and Ventricular Enlargement in Adults with or without Mild Cognitive Impairment. Dementia and Geriatric Cognitive Disorders Extra, 9(2), 281–293. 10.1159/000490044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Adler KA, Patterson TL, Dimsdale JE, Ziegler MG, & Irwin MR (2002). Health Consequences of Alzheimer’s Caregiving Transitions: Effects of Placement and Bereavement. Psychosomatic Medicine, 64(3), 477–486. [DOI] [PubMed] [Google Scholar]
- Gross JJ (1998). The emerging field of emotion regulation: An integrative review. Review of General Psychology, 2(3), 271–299. [Google Scholar]
- Guaita A, Vaccaro R, Davin A, Colombo M, Vitali SF, Polito L, Abbondanza S, Valle E, Forloni G, Ferretti VV, & Villani S. (2015). Influence of socio-demographic features and apolipoprotein E epsilon 4 expression on the prevalence of dementia and cognitive impairment in a population of 70–74-year olds: The InveCe.Ab study. Archives of Gerontology and Geriatrics, 60(2), 334–343. 10.1016/j.archger.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Piccaluga E, Casati R, Cerutti S, Lombardi F, Pagani M, & Malliani A. (1988). Sympathetic predominance in essential hypertension: A study employing spectral analysis of heart rate variability. Journal of Hypertension, 6(9), 711–717. 10.1097/00004872-198809000-00004 [DOI] [PubMed] [Google Scholar]
- Hahn EA, Cichy KE, Small BJ, & Almeida DM (2014). Daily Emotional and Physical Reactivity to Stressors Among Widowed and Married Older Adults. The Journals of Gerontology: Series B, 69B(1), 19–28. 10.1093/geronb/gbt035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson K., Rovio S, Helkala E-L, Vilska A-R, Winblad B, Soininen H, Nissinen A, Mohammed AH, & Kivipelto M. (2009). Association between mid-life marital status and cognitive function in later life: Population based cohort study. BMJ, 339. 10.1136/bmj.b2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada CN, Natelson Love MC, & Triebel K. (2013). Normal Cognitive Aging. Clinics in Geriatric Medicine, 29(4), 737–752. 10.1016/j.cger.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Goldberg EL, & Comstock GW (1991). A Longitudinal Study of the Prevalence of Depressive Symptomatology in Elderly Widowed and Married Women. Archives of General Psychiatry, 48(12), 1065–1068. 10.1001/archpsyc.1991.01810360029004 [DOI] [PubMed] [Google Scholar]
- Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, & Heuser I. (1997). Twenty-Four Hour Cortisol Release Profiles in Patients With Alzheimer’s and Parkinson’s Disease Compared to Normal Controls: Ultradian Secretory Pulsatility and Diurnal Variation. Neurobiology of Aging, 18(3), 285–289. 10.1016/S0197-4580(97)80309-0 [DOI] [PubMed] [Google Scholar]
- Hatch DJ (2013). The influence of widowhood and sociodemographic moderators on dementia and Alzheimer’s disease risk [Ph.D., Utah State University]. https://www.proquest.com/docview/1330768813/abstract/650338E420C74F23PQ/1
- Hatch DJ, Schwartz S, & Norton MC (2015). Depression and antidepressant use moderate association between widowhood and Alzheimer’s disease. International Journal of Geriatric Psychiatry, 30(3), 292–299. 10.1002/gps.4140 [DOI] [PubMed] [Google Scholar]
- Helmer C., Damon D, Letenneur L, Fabrigoule C, Barberger-Gateau P, Lafont S, Fuhrer R, Antonucci T, Commenges D, Orgogozo JM, & Dartigues JF (1999). Marital status and risk of Alzheimer’s disease: A French population-based cohort study. Neurology, 53(9), 1953–1953. 10.1212/WNL.53.9.1953 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, & Latz E. (2014). Innate immune activation in neurodegenerative disease. Nature Reviews Immunology, 14(7), 463–477. 10.1038/nri3705 [DOI] [PubMed] [Google Scholar]
- Heun R, Kockler M, & Ptok U. (2002). Depression in Alzheimer’s disease: Is there a temporal relationship between the onset of depression and the onset of dementia? European Psychiatry, 17(5), 254–258. 10.1016/S0924-9338(02)00678-8 [DOI] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, & Walker FR (2012). Evidence that Microglia Mediate the Neurobiological Effects of Chronic Psychological Stress on the Medial Prefrontal Cortex. Cerebral Cortex, 22(6), 1442–1454. 10.1093/cercor/bhr229 [DOI] [PubMed] [Google Scholar]
- Holland JM, Rozalski V, Thompson KL, Tiongson RJ, Schatzberg AF, O’Hara R, & Gallagher-Thompson D. (2014). The Unique Impact of Late-Life Bereavement and Prolonged Grief on Diurnal Cortisol. The Journals of Gerontology: Series B, 69B(1), 4–11. 10.1093/geronb/gbt051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes T, & Rahe R. (1967). The social readjustment rating scale. Journal of Psychosomatic Research, 11, 213–218. [DOI] [PubMed] [Google Scholar]
- Huang F-Y, Hsu A-L, Hsu L-M, Tsai J-S, Huang C-M, Chao Y-P, Hwang T-J, & Wu CW (2019). Mindfulness Improves Emotion Regulation and Executive Control on Bereaved Individuals: An fMRI Study. Frontiers in Human Neuroscience, 12. 10.3389/fnhum.2018.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles JL, Fisk JD, Merry HR, & Rockwood K. (2003). Five-Year Outcomes for Dementia Defined Solely by Neuropsychological Test Performance. Neuroepidemiology, 22(3), 172–178. 10.1159/000069891 [DOI] [PubMed] [Google Scholar]
- Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, Li Y, Li Y, Zhu M, Jiao H, Song Y, Shi Y., Zhang H., Gong M., Wei C., Tang Y., Fang B., Guo D., Wang F., … Qiu Q. (2020). Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. The Lancet Public Health, 5(12), e661–e671. 10.1016/S2468-2667(20)30185-7 [DOI] [PubMed] [Google Scholar]
- Joling KJ, van Marwijk HWJ, Veldhuijzen AE, van der Horst HE, Scheltens P, Smit F, & van Hout HPJ (2015). The Two-Year Incidence of Depression and Anxiety Disorders in Spousal Caregivers of Persons with Dementia: Who is at the Greatest Risk? The American Journal of Geriatric Psychiatry, 23(3), 293–303. 10.1016/j.jagp.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Jones MP, Bartrop RW, Forcier L, & Penny R. (2010). The long-teråm impact of bereavement upon spouse health: A 10-year follow-up. Acta Neuropsychiatrica, 22(5), 212–217. 10.1111/j.1601-5215.2010.00482.x [DOI] [PubMed] [Google Scholar]
- Jorm AF (2000). Is Depression a Risk Factor for Dementia or Cognitive Decline? Gerontology, 46(4), 219–227. 10.1159/000022163 [DOI] [PubMed] [Google Scholar]
- Kaplan RM, & Kronick RG (2006). Marital status and longevity in the United States population. Journal of Epidemiology & Community Health, 60(9), 760–765. 10.1136/jech.2005.037606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, & Chodosh J. (2009). Trajectories of Cognitive Function in Late Life in the United States: Demographic and Socioeconomic Predictors. American Journal of Epidemiology, 170(3), 331–342. 10.1093/aje/kwp154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene JR, & Prokos AH (2008). Widowhood and the end of spousal care-giving: Relief or wear and tear? Ageing and Society, 28(4), 551–570. 10.1017/S0144686X07006654 [DOI] [Google Scholar]
- Kempuraj D, Thangavel R, Natteru P, Selvakumar G, Saeed D, Zahoor H, Zaheer S, Iyer S, & Zaheer A. (2016). Neuroinflammation Induces Neurodegeneration. Journal of Neurology, Neurosurgery and Spine, 1(1). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5260818/ [PMC free article] [PubMed] [Google Scholar]
- Kennelly SP., Lawlor BA, & Kenny RA (2009). Review: Blood pressure and dementia — a comprehensive review. Therapeutic Advances in Neurological Disorders, 2(4), 241–260. 10.1177/1756285609103483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, & Glaser R. (1991). Spousal caregivers of dementia victims: Longitudinal changes in immunity and health.: Psychosomatic Medicine, 53(4), 345–362. 10.1097/00006842-199107000-00001 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, & Sheridan J. (1996). Chronic stress alters the immune response to influenza virus vaccine in older adults. Proceedings of the National Academy of Sciences, 93(7), 3043–3047. 10.1073/pnas.93.7.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, & Glaser R. (2003). Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences, 100(15), 9090–9095. 10.1073/pnas.1531903100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YB, & Lee SH (2019). Social network types and cognitive decline among older Korean adults: A longitudinal population-based study. International Journal of Geriatric Psychiatry, 34(12), 1845–1854. 10.1002/gps.5200 [DOI] [PubMed] [Google Scholar]
- Kim Y., Carver CS, Shaffer KM, Gansler T, & Cannady RS (2015). Cancer caregiving predicts physical impairments: Roles of earlier caregiving stress and being a spousal caregiver. Cancer, 121(2), 302–310. 10.1002/cncr.29040 [DOI] [PubMed] [Google Scholar]
- Kim Y-K, Na K-S, Myint A-M, & Leonard BE (2016). The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 277–284. 10.1016/j.pnpbp.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, & Hellhammer DH (1996). Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sciences, 58(17), 1475–1483. 10.1016/0024-3205(96)00118-X [DOI] [PubMed] [Google Scholar]
- Klietz M, Drexel SC, Schnur T, Lange F, Groh A, Paracka L, Greten S, Dressler D, Höglinger GU, & Wegner F. (2020). Mindfulness and Psychological Flexibility are Inversely Associated with Caregiver Burden in Parkinson’s Disease. Brain Sciences, 10(2), 111. 10.3390/brainsci10020111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Knippenberg RJM, de Vugt ME, Ponds RW, Verhey FRJ, & Myin-Germeys I. (2018). Emotional reactivity to daily life stress in spousal caregivers of people with dementia: An experience sampling study. PLOS ONE, 13(4), e0194118. 10.1371/journal.pone.0194118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A, O’Brien J, Weuve J, Blacker D, Metti AL, & Yaffe K. (2013). The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: A meta-analysis. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 68(4), 433–440. 10.1093/gerona/gls187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M, German PS, Anthony JC, Von Korff M, & Skinner EA (1985). Patterns of mental disorders among the elderly residents of eastern Baltimore. Journal of the American Geriatrics Society, 33(4), 236–245. 10.1111/j.1532-5415.1985.tb07110.x [DOI] [PubMed] [Google Scholar]
- Kristiansen CB., Kjær JN, Hjorth P, Andersen K, & Prina AM (2019). The association of time since spousal loss and depression in widowhood: A systematic review and meta-analysis. Social Psychiatry and Psychiatric Epidemiology, 54(7), 781–792. 10.1007/s00127-019-01680-3 [DOI] [PubMed] [Google Scholar]
- Kuhlmann S. (2005). Impaired Memory Retrieval after Psychosocial Stress in Healthy Young Men. Journal of Neuroscience, 25(11), 2977–2982. 10.1523/JNEUROSCI.5139-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Blalock EM, Chen K-C, & Porter NM (2007). A New Glucocorticoid Hypothesis of Brain Aging: Implications for Alzheimer’s Disease. Current Alzheimer Research, 4(2), 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kawachi I, & Grodstein F. (2004). Does Caregiving Stress Affect Cognitive Function in Older Women? The Journal of Nervous and Mental Disease, 192(1), 51–57. 10.1097/01.nmd.0000106000.02232.30 [DOI] [PubMed] [Google Scholar]
- Lee Y, Chi I, & Palinkas AL (2019). Widowhood, leisure activity engagement, and cognitive function among older adults. Aging & Mental Health, 23(6), 771–780. 10.1080/13607863.2018.1450837 [DOI] [PubMed] [Google Scholar]
- LeRoy AS, Gabert T, Garcini L, Murdock KW, Heijnen C, & Fagundes CP (2020). Attachment orientations and loss adjustment among bereaved spouses. Psychoneuroendocrinology, 112, 104401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M-B, & Moser EI (2007). Pattern Separation in the Dentate Gyrus and CA3 of the Hippocampus. Science, 315(5814), 961–966. 10.1126/science.1135801 [DOI] [PubMed] [Google Scholar]
- Li J., Ogrodnik M, Kolachalama VB, Lin H, & Au R. (2018). Assessment of the Mid-Life Demographic and Lifestyle Risk Factors of Dementia Using Data from the Framingham Heart Study Offspring Cohort. Journal of Alzheimer’s Disease, 63(3), 1119–1127. 10.3233/JAD-170917 [DOI] [PubMed] [Google Scholar]
- Licht CMM, de Geus EJC, Seldenrijk A, van Hout HPJ, Zitman FG, van Dyck R, & Penninx BWJH (2009). Depression Is Associated With Decreased Blood Pressure, but Antidepressant Use Increases the Risk for Hypertension. Hypertension, 53(4), 631–638. 10.1161/HYPERTENSIONAHA.108.126698 [DOI] [PubMed] [Google Scholar]
- Light E, & Lebowitz B. (1990). Alzheimer’s Disease Treatment and Family Stress: Directions for Research. Taylor & Francis. [Google Scholar]
- Liu H, Zhang Y, Burgard SA, & Needham BL (2019). Marital status and cognitive impairment in the United States: Evidence from the National Health and Aging Trends Study. Annals of Epidemiology, 38, 28–34.e2. 10.1016/j.annepidem.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang Z, Choi S, & Langa KM (2019). Marital Status and Dementia: Evidence from the Health and Retirement Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 75(8), 1783–1795. 10.1093/geronb/gbz087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MA, Hototian SR, Bustamante SEZ, Azevedo D, Tatsch M, Bazzarella MC, Litvoc J, & Bottino CMC (2007). Prevalence of cognitive and functional impairment in a community sample in Ribeirão Preto, Brazil. International Journal of Geriatric Psychiatry, 22(8), 770–776. 10.1002/gps.1737 [DOI] [PubMed] [Google Scholar]
- Lopez RB, Brown RL, Wu E-LL, Murdock KW, Denny BT, Heijnen C, & Fagundes C. (2020). Emotion Regulation and Immune Functioning During Grief: Testing the Role of Expressive Suppression and Cognitive Reappraisal in Inflammation Among Recently Bereaved Spouses. Psychosomatic Medicine, 82(1), 2–9. 10.1097/PSY.0000000000000755 [DOI] [PubMed] [Google Scholar]
- Lundorff M, Thomsen DK, Damkier A, & O’Connor M. (2019). How do loss- and restoration-oriented coping change across time? A prospective study on adjustment following spousal bereavement. Anxiety, Stress, & Coping, 32(3), 270–285. 10.1080/10615806.2019.1587751 [DOI] [PubMed] [Google Scholar]
- Lupien SJ.,de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, & Meaney MJ (1998). Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience, 1(1), 69–73. 10.1038/271 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, & Schramek TE (2007). The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition, 65(3), 209–237. 10.1016/j.bandc.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, & Ma D. (2014). Neuroinflammation: The role and consequences. Neuroscience Research, 79, 1–12. 10.1016/j.neures.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Lynch KS, & Lachman ME (2020). The Effects of Lifetime Trauma Exposure on Cognitive Functioning in Midlife. Journal of Traumatic Stress, 33(5), 773–782. 10.1002/jts.22522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J, Min J, & Kim G. (2019). Trajectories of cognitive decline by widowhood status among Korean older adults. International Journal of Geriatric Psychiatry, 34(11), 1582–1589. 10.1002/gps.5168 [DOI] [PubMed] [Google Scholar]
- Mackenzie CS, Wiprzycka UJ, Hasher L, & Goldstein D. (2009). Associations Between Psychological Distress, Learning, and Memory in Spouse Caregivers of Older Adults. The Journals of Gerontology: Series B, 64B(6), 742–746. 10.1093/geronb/gbp076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE., Stewart MC, Murray GD, Deary IJ, Fowkes FGR, Lowe GDO, Rumley A, & Price JF (2009). Peripheral levels of fibrinogen, C-reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosomatic Medicine, 71(8), 901–906. 10.1097/PSY.0b013e3181b1e538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh E, Brigstocke G, Donaldson N, & Kalra L. (2005). Determinants of caregiving burden and quality of life in caregivers of stroke patients. Stroke, 36(10), 2181–2186. 10.1161/01.STR.0000181755.23914.53 [DOI] [PubMed] [Google Scholar]
- McDermott LM, & Ebmeier KP (2009). A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders, 119(1), 1–8. 10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ, Holland D, Karow DS, Pung CJ, Brewer JB, & Dale AM (2009). Alzheimer Disease: Quantitative Structural Neuroimaging for Detection and Prediction of Clinical and Structural Changes in Mild Cognitive Impairment. Radiology, 251(1), 195–205. 10.1148/radiol.2511080924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Holland D, Hagler DJ, Fennema-Notestine C, Brewer JB, & Dale AM (2011). Mild Cognitive Impairment: Baseline and Longitudinal Structural MR Imaging Measures Improve Predictive Prognosis. Radiology, 259(3), 834–843. 10.1148/radiol.11101975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL (2000). Memory—A Century of Consolidation. 287, 5. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, & Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae C, Fazio E, Hartsock G, Kelley L, Urbanski S, & Russell D. (2009). Predictors of loneliness in caregivers of persons with Parkinson’s disease. Parkinsonism & Related Disorders, 15(8), 554–557. 10.1016/j.parkreldis.2009.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams LA, & Bailey SJ (2010). Associations between adult attachment ratings and health conditions: Evidence from the National Comorbidity Survey Replication. Health Psychology, 29(4), 446. 10.1037/a0020061 [DOI] [PubMed] [Google Scholar]
- Medzhitov R. (2007). Recognition of microorganisms and activation of the immune response. Nature, 449(7164), 819–826. 10.1038/nature06246 [DOI] [PubMed] [Google Scholar]
- Medzhitov R. (2008). Origin and physiological roles of inflammation. Nature, 454(7203), 428–435. 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- Mikulincer M, & Shaver PR (2003). The Attachment Behavioral System in Adulthood: Activation, Psychodynamics, and Interpersonal Processes. In Advances in experimental social psychology, Vol. 35 (pp. 53–152). Elsevier Academic Press. 10.1016/S0065-2601(03)01002-5 [DOI] [Google Scholar]
- Mikulincer M, & Shaver PR (2008). An attachment perspective on bereavement. In Handbook of bereavement research and practice: Advances in theory and intervention (pp. 87–112). American Psychological Association. 10.1037/14498-005 [DOI] [Google Scholar]
- Miller GE, Cohen S, & Ritchey AK (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology, 21(6), 531. 10.1037/0278-6133.21.6.531 [DOI] [PubMed] [Google Scholar]
- Molander L, Gustafson Y, & Lövheim H. (2010). Longitudinal Associations between Blood Pressure and Dementia in the Very Old. Dementia and Geriatric Cognitive Disorders, 30(3), 269–276. 10.1159/000320252 [DOI] [PubMed] [Google Scholar]
- Monroe SM., Rohde P, Seeley JR, & Lewinsohn PM (1999). Life events and depression in adolescence: Relationship loss as a prospective risk factor for first onset of major depressive disorder. Journal of Abnormal Psychology, 108(4), 606. 10.1037/0021-843X.108.4.606 [DOI] [PubMed] [Google Scholar]
- Monserud MA (2019). Later-Life Trajectories of Cognitive Functioning among Married and Widowed Older Men and Women of Mexican Origin. Journal of Cross-Cultural Gerontology, 34(3), 307–324. 10.1007/s10823-019-09380-w [DOI] [PubMed] [Google Scholar]
- Moon JR, Glymour MM, Vable AM, Liu SY, & Subramanian SV (2014). Short- and long-term associations between widowhood and mortality in the United States: Longitudinal analyses. Journal of Public Health, 36(3), 382–389. 10.1093/pubmed/fdt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostany R, Anstey JE, Crump KL, Maco B, Knott G, & Portera-Cailliau C. (2013). Altered Synaptic Dynamics during Normal Brain Aging. Journal of Neuroscience, 33(9), 4094–4104. 10.1523/JNEUROSCI.4825-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi Nasab, Hossein S-M, KormiNouri R, Sundström A, & Nilsson L. (2012). The effects of marital status on episodic and semantic memory in healthy middleaged and old individuals. Scandinavian Journal of Psychology, 53(1), 1–8. 10.1111/j.1467-9450.2011.00926.x [DOI] [PubMed] [Google Scholar]
- Mravec B. (2011). Role of catecholamine-induced activation of vagal afferent pathways in regulation of sympathoadrenal system activity: Negative feedback loop of stress response. Endocrine Regulations, 45(1), 37–41. [PubMed] [Google Scholar]
- Mroczek DK, & Almeida DM (2004). The Effect of Daily Stress, Personality, and Age on Daily Negative Affect. Journal of Personality, 72(2), 355–378. 10.1111/j.0022-3506.2004.00265.x [DOI] [PubMed] [Google Scholar]
- Mucke L. (2009). Alzheimer’s disease. Nature, 461(7266), 895–897. 10.1038/461895a [DOI] [PubMed] [Google Scholar]
- National Alliance for Caregiving and AARP. (2015). Caregiving in the U.S. [Google Scholar]
- National Heart, Lung, and Blood Institute. (2021). Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomileht J., … Kivipelt M. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. The Lancet, 385(9984), 2255–2263. 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- Norton MC, Smith KR, Østbye T, Tschanz JT, Corcoran C, Schwartz S, Piercy KW, Rabins PV, Steffens DC, Skoog I, Breitner JCS, & WelshBohmer KA (2010). Greater Risk of Dementia When Spouse Has Dementia? The Cache County Study. Journal of the American Geriatrics Society, 58(5), 895–900. 10.1111/j.1532-5415.2010.02806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M, Piet J, & Hougaard E. (2014). The Effects of Mindfulness-Based Cognitive Therapy on Depressive Symptoms in Elderly Bereaved People with Loss-Related Distress: A Controlled Pilot Study. Mindfulness, 5(4), 400–409. 10.1007/s12671-013-0194-x [DOI] [Google Scholar]
- O’Connor M-F, & Arizmendi BJ (2014). Neuropsychological Correlates of Complicated Grief in Older Spousally Bereaved Adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 69B(1), 12–18. 10.1093/geronb/gbt025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Schultze-Florey CR, Irwin MR, Arevalo JMG, & Cole SW (2014). Divergent gene expression responses to Complicated Grief and Non-complicated Grief. Brain, Behavior, and Immunity, 37, 78–83. 10.1016/j.bbi.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Wellisch DK, Stanton AL, Olmstead R, & Irwin MR (2012). Diurnal cortisol in Complicated and Non-Complicated Grief: Slope differences across the day. Psychoneuroendocrinology, 37(5), 725–728. 10.1016/j.psyneuen.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M., Brennan S, Lawlor BA, Hannigan C, Robertson IH, & Pertl MM (2019). Cognitive functioning among cognitively intact dementia caregivers compared to matched self-selected and population controls. Aging & Mental Health, 23(5), 566–573. 10.1080/13607863.2018.1428937 [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, & Loewenstein D. (2006). Depression and Risk for Alzheimer Disease: Systematic Review, Meta-analysis, and Metaregression Analysis. Archives of General Psychiatry, 63(5), 530–538. 10.1001/archpsyc.63.5.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez A. (2017). Gray literature: An important resource in systematic reviews. Journal of Evidence-Based Medicine, 10(3), 233–240. 10.1111/jebm.12266 [DOI] [PubMed] [Google Scholar]
- Pariante CM, & Miller AH (2001). Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biological Psychiatry, 49(5), 391–404. 10.1016/S0006-3223(00)01088-X [DOI] [PubMed] [Google Scholar]
- Park D, & Gutchess A. (2006). The Cognitive Neuroscience of Aging and Culture. Current Directions in Psychological Science, 15(3), 105–108. 10.1111/j.0963-7214.2006.00416.x [DOI] [Google Scholar]
- Paterniti S., Verdier-Taillefer M-H, Geneste C, Bisserbe J-C, & Alpérovitch A. (2000). Low blood pressure and risk of depression in the elderly: A prospective community-based study. The British Journal of Psychiatry, 176(5), 464–467. 10.1192/bjp.176.5.464 [DOI] [PubMed] [Google Scholar]
- Paúl C, Ribeiro O, & Santos P. (2010). Cognitive impairment in old people living in the community. Archives of Gerontology and Geriatrics, 51(2), 121–124. 10.1016/j.archger.2009.09.037 [DOI] [PubMed] [Google Scholar]
- Pelletier CL (2004). The Effect of Music on Decreasing Arousal Due to Stress: A Meta-Analysis. Journal of Music Therapy, 41(3), 192–214. [DOI] [PubMed] [Google Scholar]
- Perkins JM, Lee H, James KS, Oh J, Krishna A, Heo J, Lee J, & Subramanian SV (2016). Marital status, widowhood duration, gender and health outcomes: A cross-sectional study among older adults in India. BMC Public Health, 16(1). 10.1186/s12889-016-3682-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren S, Schmid R, Herrmann S, & Wettstein A. (2007). The impact of attachment on dementia-related problem behavior and spousal caregivers’ well-being. Attachment & Human Development, 9(2), 163–178. 10.1080/14616730701349630 [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, & Holmes C. (2007). Systemic infections and inflammation affect chronic neurodegeneration. Nature Reviews Immunology, 7(2), 161–167. 10.1038/nri2015 [DOI] [PubMed] [Google Scholar]
- Persson G, & Skoog I. (1996). A Prospective Population Study of Psychosocial Risk Factors for Late Onset Dementia. International Journal of Geriatric Psychiatry, 11(1), 15–22. [DOI] [Google Scholar]
- Persson J, Pudas S, Lind J, Kauppi K, Nilsson L-G, & Nyberg L. (2012). Longitudinal Structure–Function Correlates in Elderly Reveal MTL Dysfunction with Cognitive Decline. Cerebral Cortex, 22(10), 2297–2304. 10.1093/cercor/bhr306 [DOI] [PubMed] [Google Scholar]
- Pertl MM, Lawlor BA, Robertson IH, Walsh C, & Brennan S. (2015). Risk of Cognitive and Functional Impairment in Spouses of People With Dementia: Evidence From the Health and Retirement Study. Journal of Geriatric Psychiatry and Neurology, 28(4), 260–271. 10.1177/0891988715588834 [DOI] [PubMed] [Google Scholar]
- Pertl MM, Sooknarine-Rajpatty A, Brennan S, Robertson IH, & Lawlor BA (2019). Caregiver Choice and Caregiver Outcomes: A Longitudinal Study of Irish Spousal Dementia Caregivers. Frontiers in Psychology, 10. 10.3389/fpsyg.2019.01801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Booth A, Rockwood K, Peters J, D’Este C, & Anstey KJ (2019). Combining modifiable risk factors and risk of dementia: A systematic review and meta-analysis. BMJ Open, 9(1), e022846. 10.1136/bmjopen-2018-022846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC., Smith G, Kokmen E, Ivnik RJ, & Tangalos EG (1992). Memory function in normal aging. Neurology, 42(2), 396–401. 10.1212/wnl.42.2.396 [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Brown SL, Ubel PA, Smith DM, Jankovic A, & Langa KM (2010). Does a helping hand mean a heavy heart? Helping behavior and well-being among spouse caregivers. Psychology and Aging, 25(1), 108. 10.1037/a0018064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokos AH, & Keene JR (2005). The Long-Term Effects of Spousal Care Giving on Survivors’ Well-Being in Widowhood*. Social Science Quarterly, 86(3), 664–682. 10.1111/j.0038-4941.2005.00323.x [DOI] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, & Sano M. (2008). Increased Neurofibrillary Tangles in Patients With Alzheimer Disease With Comorbid Depression. The American Journal of Geriatric Psychiatry, 16(2), 168–174. 10.1097/JGP.0b013e31816029ec [DOI] [PubMed] [Google Scholar]
- Reid CE, Moss S, & Hyman G. (2005). Caregiver Reciprocity: The effect of reciprocity, carer self-esteem and motivation on the experience of caregiver burden. Australian Journal of Psychology, 57(3), 186–196. 10.1080/00049530500141022 [DOI] [Google Scholar]
- Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Sperling RA, Dickerson B, Stern CE, Tirado V, Munoz C, Reiman RA, Huentelman MJ., … Loper F. (2012). Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: A case-control study. The Lancet Neurology, 11(12), 1048–1056. 10.1016/S1474-4422(12)70228-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro PCC, Lopes C.de S., & Lourenço RA (2013). Prevalence of Dementia in Elderly Clients of a Private Health Care Plan: A Study of the FIBRA-RJ, Brazil. Dementia and Geriatric Cognitive Disorders, 35(1–2), 77–86. 10.1159/000345984 [DOI] [PubMed] [Google Scholar]
- Richardson VE, Bennett KM, Carr D, Gallagher S, Kim J, & Fields N. (2015). How Does Bereavement Get Under the Skin? The Effects of Late-Life Spousal Loss on Cortisol Levels. The Journals of Gerontology: Series B, 70(3), 341–347. 10.1093/geronb/gbt116 [DOI] [PubMed] [Google Scholar]
- Ripollés P, Rojo N, Grau-Sánchez J, Amengual JL, Càmara E, Marco-Pallarés J, Juncadella M, Vaquero L, Rubio F, Duarte E, Garrido C, Altenmüller E, Münte TF, & Rodríguez-Fornells A. (2016). Music supported therapy promotes motor plasticity in individuals with chronic stroke. Brain Imaging and Behavior, 10(4), 1289–1307. 10.1007/s11682-015-9498-x [DOI] [PubMed] [Google Scholar]
- Robinson-Whelen S, Tada Y, MacCallum RC, McGuire L, & Kiecolt-Glaser JK (2001). Long-term caregiving: What happens when it ends? Journal of Abnormal Psychology, 110(4), 573. 10.1037/0021-843X.110.4.573 [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, & McGinn MM (2014). Marital quality and health: A meta-analytic review. Psychological Bulletin, 140(1). 10.1037/a0031859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland KP., Jenkins ME, & Johnson AM (2010). An exploration of the burden experienced by spousal caregivers of individuals with Parkinson’s disease. Movement Disorders, 25(2), 189–193. 10.1002/mds.22939 [DOI] [PubMed] [Google Scholar]
- Roman GC (2004). Brain hypoperfusion: A critical factor in vascular dementia. Neurological Research, 26(5), 454–458. 10.1179/016164104225017686 [DOI] [PubMed] [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, & Nilsson L-G (2005). Stability, Growth, and Decline in Adult Life Span Development of Declarative Memory: Cross-Sectional and Longitudinal Data From a Population-Based Study. Psychology and Aging, 20(1), 3–18. 10.1037/0882-7974.20.1.3 [DOI] [PubMed] [Google Scholar]
- Rosnick CB, Small BJ, & Burton AM (2010). The Effect of Spousal Bereavement on Cognitive Functioning in a Sample of Older Adults. Aging, Neuropsychology, and Cognition, 17(3), 257–269. 10.1080/13825580903042692 [DOI] [PubMed] [Google Scholar]
- Ryckebosch-Dayez A-S, Zech E, Cord JM, & Taverne C. (2016). Daily life stressors and coping strategies during widowhood: A diary study after one year of bereavement. Death Studies. http://www.tandfonline.com/doi/abs/10.1080/07481187.2016.1177750 [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, & Au R. (2010). Depressive symptoms and risk of dementia. Neurology, 75(1), 35–41. 10.1212/WNL.0b013e3181e62138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Sanyal D, Bhattacharyya A, Bhattacharyya R, Barman N, & Mukherjee A. (2010). A study on cognitive status of 50 years and above aged non-demented women in a rural area of West Bengal. Journal of the Indian Medical Association, 108(11), 726–729. [PubMed] [Google Scholar]
- Sang YM, Wang LJ, Mao HX, Lou XY, & Zhu YJ (2018). The association of shortterm memory and cognitive impairment with ghrelin, leptin, and cortisol levels in nondiabetic and diabetic elderly individuals. Acta Diabetologica, 55(6), 531–539. 10.1007/s00592-018-1111-5 [DOI] [PubMed] [Google Scholar]
- Satoh M, Ogawa J, Tokita T, Nakaguchi N, Nakao K, Kida H, & Tomimoto H. (2014). The Effects of Physical Exercise with Music on Cognitive Function of Elderly People: Mihama-Kiho Project. PLOS ONE, 9(4), e95230. 10.1371/journal.pone.0095230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra DA., &Hazan C. (2008). Coregulation, Dysregulation, Self-Regulation: An Integrative Analysis and Empirical Agenda for Understanding Adult Attachment, Separation, Loss, and Recovery. Personality and Social Psychology Review, 12(2), 141–167. 10.1177/1088868308315702 [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, & Fox NC (2003). A Longitudinal Study of Brain Volume Changes in Normal Aging Using Serial Registered Magnetic Resonance Imaging. Archives of Neurology, 60(7), 989–994. 10.1001/archneur.60.7.989 [DOI] [PubMed] [Google Scholar]
- Schaan B. (2013). Widowhood and Depression Among Older Europeans—The Role of Gender, Caregiving, Marital Quality, and Regional Context. The Journals of Gerontology: Series B, 68(3), 431–442. 10.1093/geronb/gbt015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, & Kivipelto M. (2014). Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. Journal of Internal Medicine, 275(3), 251–283. 10.1111/joim.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schone BS, & Weinick RM (1998). Health-Related Behaviors and the Benefits of Marriage for Elderly Persons. The Gerontologist, 38(5), 618–627. 10.1093/geront/38.5.618 [DOI] [PubMed] [Google Scholar]
- Schultze-Florey CR., Martinez-Maza O, Magpantay L, Breen EC, Irwin MR, Gündel H, & O’Connor M-F (2012). When grief makes you sick: Bereavement induced systemic inflammation is a question of genotype. Brain, Behavior, and Immunity, 26(7), 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, & Beach SR (1999). Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA, 282(23), 2215–2219. 10.1001/jama.282.23.2215 [DOI] [PubMed] [Google Scholar]
- Schweitzer I, Tuckwell V, O’Brien J, & Ames D. (2002). Is late onset depression a prodrome to dementia? International Journal of Geriatric Psychiatry, 17(11), 997–1005. 10.1002/gps.525 [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Geiger PJ, Boggero IA, Schmitt FA, & Sephton SE (2016). Endogenous cortisol exposure and declarative verbal memory: A longitudinal study of healthy older adults. Psychosomatic Medicine, 78(2), 182–191. 10.1097/PSY.0000000000000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Aguilar PG, Lopez-Bastida J, & Yanes-Lopez V. (2006). Impact on Health-Related Quality of Life and Perceived Burden of Informal Caregivers of Individuals with Alzheimer’s Disease. Neuroepidemiology, 27(3), 136–142. 10.1159/000095760 [DOI] [PubMed] [Google Scholar]
- Shahar DR, Schultz R, Shahar A, & Wing RR (2001). The effect of widowhood on weight change, dietary intake, and eating behavior in the elderly population. Journal of Aging and Health, 13(2), 186–199. 10.1177/089826430101300202 [DOI] [PubMed] [Google Scholar]
- Shaw WS, Patterson TL, Semple SJ, Ho S, Irwin MR, Hauger RL, & Grant I. (1997). Longitudinal analysis of multiple indicators of health decline among spousal caregivers. Annals of Behavioral Medicine, 19(2), 101–109. 10.1007/BF02883326 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, & Vannier MW (1996). Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences, 93(9), 3908–3913. 10.1073/pnas.93.9.3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, & Jiang T. (2009). Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus, 19(11), 1055–1064. 10.1002/hipo.20573 [DOI] [PubMed] [Google Scholar]
- Shin H, Lee J-Y, Youn J, Kim JS, & Cho JW (2012). Factors Contributing to Spousal and Offspring Caregiver Burden in Parkinson’s Disease. European Neurology, 67(5), 292–296. 10.1159/000335577 [DOI] [PubMed] [Google Scholar]
- Shin SH, Kim G, & Park S. (2018). Widowhood Status as a Risk Factor for Cognitive Decline among Older Adults. The American Journal of Geriatric Psychiatry, 26(7), 778–787. 10.1016/j.jagp.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Shirazi SN., Friedman AR., Kaufer D., & Sakhai SA. (2015). Glucocorticoids and the Brain: Neural Mechanisms Regulating the Stress Response. In Wang J-C & Harris C (Eds.), Glucocorticoid Signaling: From Molecules to Mice to Man (pp. 235–252). Springer. 10.1007/978-1-4939-2895-8_10 [DOI] [PubMed] [Google Scholar]
- Sierra A, GottfriedBlackmore A, Milner TA, McEwen BS, & Bulloch K. (2008). Steroid hormone receptor expression and function in microglia. Glia, 56(6), 659–674. 10.1002/glia.20644 [DOI] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Herlitz A, Fratiglioni L, Almkvist O, & Bäckman L. (1997. 0101). Cognitive predictors of incident Alzheimer’s disease: A prospective longitudinal study. Neuropsychology, 11(3), 413. 10.1037/0894-4105.11.3.413 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos I, Cerqueira JJ, Catania C, Takashima A, Sousa N, & Almeida OFX (2008). Stress and glucocorticoid footprints in the brain—The path from depression to Alzheimer’s disease. Neuroscience & Biobehavioral Reviews, 32(6), 1161–1173. 10.1016/j.neubiorev.2008.05.007 [DOI] [PubMed] [Google Scholar]
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, & Aisen P. (2014). The A4 Study: Stopping AD before Symptoms Begin? Science Translational Medicine, 6(228), 228fs13. 10.1126/scitranslmed.3007941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl ST, & Schulz R. (2014). Changes in routine health behaviors following late-life bereavement: A systematic review. Journal of Behavioral Medicine, 37(4), 736–755. 10.1007/s10865-013-9524-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM.,Yassa MA, Lacy JW, & Stark CEL (2013). A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia, 51(12), 2442–2449. 10.1016/j.neuropsychologia.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, & Stark CEL (2010). Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learning & Memory (Cold Spring Harbor, N.Y.), 17(6), 284–288. 10.1101/lm.1768110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, & Schteingart DE (1992). Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biological Psychiatry, 32(9), 756–765. 10.1016/0006-3223(92)90079-f [DOI] [PubMed] [Google Scholar]
- Streeter JL (2020). Gender differences in widowhood in the short-run and long-run: Financial, emotional, and mental wellbeing. The Journal of the Economics of Ageing, 17, 100258. 10.1016/j.jeoa.2020.100258 [DOI] [Google Scholar]
- Stroebe M, & Schut H. (1999). The Dual Process Model of Coping with Bereavement: Rationale and Description. Death Studies, 23(3), 197–224. 10.1080/074811899201046 [DOI] [PubMed] [Google Scholar]
- Stroebe M, Schut H, & Stroebe W. (2007). Health outcomes of bereavement. The Lancet, 370(9603), 1960–1973. 10.1016/S0140-6736(07)61816-9 [DOI] [PubMed] [Google Scholar]
- Stroebe M, Stroebe W, & Schut H. (2001). Gender Differences in Adjustment to Bereavement: An Empirical and Theoretical Review. Review of General Psychology, 5(1), 62–83. 10.1037/1089-2680.5.1.62 [DOI] [Google Scholar]
- Subramanian M, Vasudevan K, & Rajagopal A. (2021). Cognitive Impairment Among Older Adults With Diabetes Mellitus in Puducherry: A Community-Based Cross-Sectional Study. Cureus, 13(1). 10.7759/cureus.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Jia R, Guo C, Sun T, Dong X, Li L, & Yang P. (2021). Synergistic influence of education and marriage on the risk for cognition loss among the older people in China. Nursing Open, 00, 1–6. 10.1002/nop2.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström A., Rönnlund M, Adolfsson R, & Nilsson L-G (2014). Stressful life events are not associated with the development of dementia. International Psychogeriatrics, 26(1), 147–154. 10.1017/S1041610213001804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström A, Westerlund O, & Kotyrlo E. (2016). Marital status and risk of dementia: A nationwide population-based prospective study from Sweden. BMJ Open, 6(1), e008565. 10.1136/bmjopen-2015-008565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström A, Westerlund O, Mousavi-Nasab H, Adolfsson R, & Nilsson L-G (2014). The relationship between marital and parental status and the risk of dementia. International Psychogeriatrics, 26(5), 749–757. 10.1017/S1041610213002652 [DOI] [PubMed] [Google Scholar]
- Tata DA, & Anderson BJ (2010). The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: Implications for hippocampal volume reductions in depression. Physiology & Behavior, 99(2), 186–193. 10.1016/j.physbeh.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres DMC, & Cardenas FP (2020). Synaptic plasticity in Alzheimer’s disease and healthy aging. Reviews in the Neurosciences, 31(3), 245–268. 10.1515/revneuro-2019-0058 [DOI] [PubMed] [Google Scholar]
- Toshitake T, Kiyohara Y, Kato I, Ohmura T, & et al. (1995). Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: The Hisayama study. Neurology, 45(6), 1161–1168. 10.1212/WNL.45.6.1161 [DOI] [PubMed] [Google Scholar]
- Trzepacz PT., Hochstetler H, Wang S, Walker B, & Saykin AJ (2015). Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatrics, 15. 10.1186/s12877-015-0103-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolaki M, Kounti F, Agogiatou C, Poptsi E, Bakoglidou E, Zafeiropoulou M, Soumbourou A, Nikolaidou E, Batsila G, Siambani A, Nakou S, Mouzakidis C, Tsiakiri A, Zafeiropoulos S, Karagiozi K, Messini C, Diamantidou A, & Vasiloglou M. (2011). Effectiveness of Nonpharmacological Approaches in Patients with Mild Cognitive Impairment. Neuro - Degenerative Diseases; Basel, 8(3), 138–145. http://dx.doi.org.ezproxy.rice.edu/10.1159/000320575 [DOI] [PubMed] [Google Scholar]
- Tsyglakova M, McDaniel D, & Hodes GE (2019). Immune mechanisms of stress susceptibility and resilience: Lessons from animal models. Frontiers in Neuroendocrinology, 54, 100771. 10.1016/j.yfrne.2019.100771 [DOI] [PubMed] [Google Scholar]
- Van Voorhis CRW, & Morgan BL (2007). Understanding Power and Rules of Thumb for Determining Sample Sizes. Tutorials in Quantitative Methods for Psychology, 3(2), 43–50. 10.20982/tqmp.03.2.p043 [DOI] [Google Scholar]
- Vidarsdottir H, Fang F, Chang M, Aspelund T, Fall K, Jonsdottir MK, Jonsson PV, Cotch MF, Harris TB, Launer LJ, Gudnason V, & Valdimarsdottir U. (2014). Spousal loss and cognitive function in later life: A 25-year follow-up in the AGES-Reykjavik study. American Journal of Epidemiology, 179(6), 674–683. 10.1093/aje/kwt321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaliano PP., Echeverria D, Shelkey M, Jianping Zhang, & Scanlan J. (2007). A Cognitive Psychophysiological Model to Predict Functional Decline in Chronically Stressed Older Adults. Journal of Clinical Psychology in Medical Settings, 14(3), 177–190. 10.1007/s10880-007-9071-x [DOI] [Google Scholar]
- Vitaliano PP, Echeverria D, Yi J, Phillips PEM, Young H, & Siegler IC (2005). Psychophysiological Mediators of Caregiver Stress and Differential Cognitive Decline. Psychology and Aging, 20(3), 402–411. 10.1037/0882-7974.20.3.402 [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Zhang J, Young HM, Caswell LW, Scanlan JM, & Echeverria D. (2009). Depressed Mood Mediates Decline in Cognitive Processing Speed in Caregivers. The Gerontologist, 49(1), 12–22. 10.1093/geront/gnp004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas S, Rodrigues AJ, Silva JM, Tronche F, Almeida OFX, Sousa N, & Sotiropoulos I. (2016). Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural Plasticity, 2016, e6391686. 10.1155/2016/6391686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TJ, & Pevalin DJ (2004). Marital Transitions and Mental Health. Journal of Health and Social Behavior, 45(2), 155–170. 10.1177/002214650404500203 [DOI] [PubMed] [Google Scholar]
- Walker KA, Gottesman RF, Wu A, Knopman DS, Gross AL, Mosley TH, Selvin E, & Windham BG (2019). Systemic inflammation during midlife and cognitive change over 20 years. Neurology, 92(11), e1256–e1267. 10.1212/WNL.0000000000007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA., Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Jack CR, & Gottesman RF (2017). Midlife systemic inflammatory markers are associated with late-life brain volume: The ARIC study. Neurology, 89(22), 2262–2270. 10.1212/WNL.0000000000004688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen-Roche K, Coresh J, Gross AL, Windham BG, Knopman DS, Power MC, Rawlings AM, Mosley TH, & Gottesman RF (2019). Association of Midlife to Late-Life Blood Pressure Patterns With Incident Dementia. JAMA, 322(6), 535–545. 10.1001/jama.2019.10575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wändell P, Carlsson AC, Li X, Gasevic D, Sundquist J, & Sundquist K. (2020). The association between sociodemographic characteristics and dementia in patients with atrial fibrillation. Aging Clinical and Experimental Research, 32(11), 2319–2327. 10.1007/s40520-019-01449-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L, Mathias JL, & Hitchings SE (2007). Relationships between bereavement and cognitive functioning in older adults. Gerontology, 53(6), 362–372. 10.1159/000104787 [DOI] [PubMed] [Google Scholar]
- Wells YD, & Kendig HL (1997). Health and Well-Being of Spouse Caregivers and the Widowed1. The Gerontologist, 37(5), 666–674. 10.1093/geront/37.5.666 [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, & Troncoso JC (1994). Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. The Lancet, 344(8925), 769–772. 10.1016/S0140-6736(94)92338-8 [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, & Yaffe K. (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology, 64(2), 277–281. 10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- Wijngaart MAGVD., Vernooij-Dassen MJFJ, & Felling AJA (2007). The influence of stressors, appraisal and personal conditions on the burden of spousal caregivers of persons with dementia. Aging & Mental Health, 11(6), 626–636. 10.1080/13607860701368463 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, & Bennett DA (2002). Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology, 59(3), 364–370. 10.1212/wnl.59.3.364 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, & Bennett DA (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17(2), 179–193. 10.1037/0882-7974.17.2.179 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, & Bennett DA (2003). Terminal decline in cognitive function. Neurology, 60(11), 1782–1787. 10.1212/01.WNL.0000068019.60901.C1 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bienias JL, Mendes de Leon CF, Evans DA, & Bennett DA (2003). Negative Affect and Mortality in Older Persons. American Journal of Epidemiology, 158(9), 827–835. 10.1093/aje/kwg224 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, & Bennett DA (2007). Loneliness and Risk of Alzheimer Disease. Archives of General Psychiatry, 64(2), 234–240. 10.1001/archpsyc.64.2.234 [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Padin AC, Bailey BE, Laskowski B, Andridge R, Malarkey WB, & Kiecolt-Glaser JK (2020). Spousal bereavement after dementia caregiving: A turning point for immune health. Psychoneuroendocrinology, 118, 104717. 10.1016/j.psyneuen.2020.104717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wörn J, Comijs H, & Aartsen M. (2020). Spousal Loss and Change in Cognitive Functioning: An Examination of Temporal Patterns and Gender Differences. The Journals of Gerontology: Series B, 75(1), 195–206. 10.1093/geronb/gby104 [DOI] [PubMed] [Google Scholar]
- Wu EL, LeRoy AS, Heijnen CJ, & Fagundes CP (2021). Inflammation and future depressive symptoms among recently bereaved spouses. Psychoneuroendocrinology, 128, 105206. 10.1016/j.psyneuen.2021.105206 [DOI] [PubMed] [Google Scholar]
- Wünsche J, Weidmann R, & Grob A. (2020). Until death do us part: The codevelopment of life satisfaction in couples preceding the death of one partner. Journal of Personality and Social Psychology, 119(4), 881. 10.1037/pspi0000228 [DOI] [PubMed] [Google Scholar]
- Xiang N, Liu E, Li H, Qin X, Liang H, & Yue Z. (2021). The Association between Widowhood and Cognitive Function among Chinese Elderly People: Do Gender and Widowhood Duration Make a Difference? Healthcare, 9(8), 991. 10.3390/healthcare9080991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Wei R, Cheng B, Wang A, Li X, Li H, Sun L, Du J, Sheng J, Liu K, Tao F, & Yang L. (2020). The association of marital status with cognitive function and the role of gender in Chinese community-dwelling older adults: A cross-sectional study. Aging Clinical and Experimental Research. 10.1007/s40520-020-01743-5 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Bahorik AL, Hoang TD, Forrester S, Jacobs DR, Lewis CE, Lloyd-Jones DM, Sidney S, & Reis JP (2020). Cardiovascular risk factors and accelerated cognitive decline in midlife: The CARDIA Study. Neurology, 95(7), e839–e846. 10.1212/WNL.0000000000010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Yang Q, Xiong J, Li T, & Zhu X. (2020). Social Support and the Incidence of Cognitive Impairment Among Older Adults in China: Findings From the Chinese Longitudinal Healthy Longevity Survey Study. Frontiers in Psychiatry, 11. 10.3389/fpsyt.2020.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R., & Goshen I. (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity, 25(2), 181–213. 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Zaki J, & Williams WC (2013. 1007). Interpersonal emotion regulation. Emotion, 13(5), 803. 10.1037/a0033839 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guan Y, Shi Z, Yue W, Liu S, Liu S, Lu H, Zhao L, Zhang Y, Su W, & Ji Y. (2019). Sex Differences in the Prevalence of and Risk Factors for Cognitive Impairment No Dementia among the Elderly in a Rural Area of Northern China: A Population-Based Cross-Sectional Study. Neuroepidemiology, 52(1–2), 25–31. 10.1159/000493141 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li LW, Xu H, & Liu J. (2019). Does widowhood affect cognitive function among Chinese older adults? SSM - Population Health, 7, 100329. 10.1016/j.ssmph.2018.100329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu H, & Choi SE (2021). Marital loss and risk of dementia: Do race and gender matter? Social Science & Medicine, 275, 113808. 10.1016/j.socscimed.2021.113808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-X, Zahner GEP, Román GC, Liu X-H, Wu C-B, Hong Z, Hong X, Tang M-N, Zhou B, Qu Q-M, Zhang X-J, & Li H. (2006). Socio-Demographic Variation of Dementia Subtypes in China: Methodology and Results of a Prevalence Study in Beijing, Chengdu, Shanghai, and Xian. Neuroepidemiology, 27(4), 177–187. 10.1159/000096131 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Inder B, & Kim JS (2021). Spousal bereavement and the cognitive health of older adults in the US: New insights on channels, single items, and subjective evidence. Economics & Human Biology, 43, 101055. 10.1016/j.ehb.2021.101055 [DOI] [PubMed] [Google Scholar]
- Zhong B-L, Chen S-L, Tu X, & Conwell Y. (2017). Loneliness and Cognitive Function in Older Adults: Findings From the Chinese Longitudinal Healthy Longevity Survey. The Journals of Gerontology: Series B, 72(1), 120–128. 10.1093/geronb/gbw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang P, & Fang Y. (2018). Loneliness and the risk of dementia among older Chinese adults: Gender differences. Aging & Mental Health, 22(4), 519–525. 10.1080/13607863.2016.1277976 [DOI] [PubMed] [Google Scholar]
- Zisook S, & Shuchter SR (1991). Depression through the first year after the death of a spouse. American Journal of Psychiatry, 148(10), 1346–1352. Scopus. 10.1176/ajp.148.10.1346 [DOI] [PubMed] [Google Scholar]
- Zunszain PA., Anacker C., Cattane A., Carvalho LA., & Pariante CM. (2011). Glucocorticoids, cytokines and brain abnormalities in depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 35(3), 722–729. 10.1016/j.pnpbp.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvěřová M, Fišar Z, Jirák R, Kitzlerová E, Hroudová J, & Raboch J. (2013). Plasma cortisol in Alzheimer’s disease with or without depressive symptoms. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research, 19, 681–689. 10.12659/MSM.889110 [DOI] [PMC free article] [PubMed] [Google Scholar]