Abstract
Background
Daptomycin pulmonary eosinophilia (DPE) has been well described in case reports and reporting from the Food and Drug Administration. We report 3 eosinophilic syndromes associated with daptomycin use.
Methods
This is a retrospective review of all patients who received daptomycin (inpatient or outpatient) from 2010 to 2020 at the Veterans Affairs Portland Healthcare System. Patients who developed DPE while receiving daptomycin were evaluated to determine risk factors. Data collected included daptomycin dose and duration, body mass index, creatinine clearance, and peripheral eosinophilia.
Results
Of 330 patients who received daptomycin, 81.5% developed a peripheral eosinophilia, with 109 (33%) developing peripheral eosinophilia ≥5%. Fifty-one (16%) met criteria for DPE. Primary DPE occurred in 38 of the 51 patients with a median 26 days of treatment, and 49% had peripheral eosinophilia ≥5%. Re-exposure DPE occurred in the other 13 patients and occurred a median of 3 days after initiation of daptomycin. The presence of an elevated peripheral eosinophilia of ≥5% during daptomycin usage was significantly associated with primary (odds ratio [OR], 2.23; 95% CI, 1.2–4.09; P = .008) and re-exposure DPE (OR, 12; 95% CI, 1.6–103; P = .003). All patients recovered after withdrawal of daptomycin without complications.
Conclusions
There are 3 daptomycin eosinophilic syndromes: peripheral eosinophilia, primary DPE occurring about 4 weeks into therapy, and re-exposure DPE. Elevated peripheral eosinophilia ≥5% was a risk factor for both primary and re-exposure DPE, but still identified about half the cases. Peripheral eosinophilia should be carefully monitored during daptomycin treatment, and clinicians should be aware that prior eosinophilia may predict an acute pulmonary reaction upon daptomycin re-exposure.
Keywords: daptomycin, daptomycin pulmonary eosinophilia, eosinophilia, hypersensitivity pneumonitis
Daptomycin is a lipoglycopeptide antibiotic with potent bactericidal activity against most gram-positive infections, with current indications for treatment of skin and soft tissue infections and Staphylococcus aureus bloodstream infections, but it also has been used off-label for many infections including osteomyelitis and prosthetic joint infections [1, 2]. Daptomycin has been shown to be safe, with the once-daily dosing limiting rhabdomyolysis events and allowing the dosing to be increased for more difficult-to-treat infections such as vancomycin-resistant enterococci (VRE), as recent changes in Clinical and Laboratory Standards Institute breakpoints recommend [3, 4]. With its increased usage, reports of pulmonary eosinophilia (DPE) have been observed [5]. The description of DPE is the onset of fever, dyspnea, infiltrates on chest imaging, and >25% eosinophils on bronchoalveolar lavage (BAL) about 4 weeks after starting therapy with a rapid improvement after drug withdrawal [5]. The lack of peripheral eosinophilia and need for BAL for diagnosis contribute to the likely under-reporting of the condition. Also, factors associated with the causes of eosinophilia (obesity and daptomycin dosing) have been inferred from case reports, but not clearly demonstrated [6, 7]. With increased dosing of daptomycin to 8 mg/kg recommended for S. aureus infections, there has been no guidance on whether this should be on actual body weight (as recommended by the Food and Drug Administration [FDA]) or adjusted body weight, making it complicated for dosing in morbidly obese patients [8, 9].
Our site began observing an acute hypersensitivity pneumonitis several days after initiation of daptomycin in several patients who did not meet the FDA criteria. Therefore, this paper presents 10 years of complete inpatient/outpatient monitoring data on daptomycin usage that have identified 3 eosinophilic syndromes associated with use of daptomycin.
METHODS
Design and Location
This is a retrospective chart review of all patients at the Veterans Affairs (VA) Portland Healthcare System who received >1 dose of daptomycin either as an inpatient or through the outpatient parenteral antibiotic program (OPAT) from 2010 to 2020. All patients were closely monitored with weekly creatine phosphokinases (CPKs), serum chemistry, liver function tests, and complete blood counts (CBCs) with differential as per our OPAT protocol either through our local laboratory or faxed to us from our home health care infusion services. All patients on OPAT regardless of antibiotic class had a CBC with differential, as this was part of our protocol for the early detection of allergic drug reactions by elevations in eosinophils. All adverse events, hospitalizations, or deaths were available and extracted from the VA electronic health record (CPRS, version 37). Outside hospital admission reports were available as they were routinely collected and uploaded into CPRS for review. Mortality during pneumonia episodes was collected.
Patient Consent
This study received exempt approval by the medical ethics committee of the VA Portland Healthcare System. Informed consent was waived as no interventions were performed and patient information was protected. Patient care was conducted in compliance with the Declaration of Helsinki.
Patient Monitoring
The VA Antimicrobial Stewardship Team was established in August 2008, and the same Infectious Diseases clinical pharmacist (IDCP) and Infectious Diseases physician were responsible for approval for initiating daptomycin for both inpatient and outpatient use. The same IDCP monitored daptomycin usage for the whole study period, recording and uploading weekly laboratories and adverse events into CPRS. Patients in the outpatient setting could be anywhere in Oregon or Washington state and had to have received drug from home health care infusion groups that were contracted with the VA and kept in close contact with the IDCP during their treatment.
Definitions
All daptomycin cases suspected of DPE were initially extracted from the hospital pharmacy allergy and adverse event reporting system. All other daptomycin use was manually extracted, and suspected cases of abnormal reactions were reviewed for causality. We also reviewed all use to determine total number of elevated creatine phosphokinase (CPK) levels within the same period. We used FDA definitions for rhabdomyolysis, which was an elevation of CPK ≥5 times the upper limit of normal [8]. We also used the FDA package insert definition of a creatine clearance (CrCl) <30 mL/min being considered impaired kidney function for adjusting daptomycin dosing; this level was used for assessing impact of kidney function.
We defined a second treatment course of daptomycin for a patient as requiring treatment of a new infection >8 weeks after completion of an initial course. Interrupted courses and renally dosed daptomycin were considered part of the primary treatment of the same infection.
Peripheral Eosinophilia
We collected all instances of an elevation of the eosinophil count that were >1% of the total white cell count during the duration of daptomycin therapy. Any elevation over this was recorded as an abnormal laboratory value for this review. However, peripheral eosinophilia was considered related to daptomycin when it was ≥5% of the total white cell count on 2 consecutive blood draws while on treatment to meet the criteria for a persistent peripheral eosinophilia.
The criteria used to determine proven and probable DPE from other causes are available in Supplementary Table 1 . Patients with proven DPE should have at least 2 clinical factors, including hypoxia (which was either a baseline oxygen saturation on presentation of 89% on room air or the need for supplemental oxygen by any route), abnormal imaging, and sputum or bronchoalveolar lavage demonstrating ≥25% eosinophils, while probable DPE was similar but with ≤25% eosinophils. Re-exposure DPE was any patient who was on a second treatment course and presented with a clinical syndrome consistent with DPE. Any patient who had the diagnosis of pneumonia on hospital admission while on daptomycin was further evaluated for closer review: Patients with a proven bacterial infection, congestive heart failure, or other cause unlikely to be due to daptomycin were deemed not to have DPE. Patients with dyspnea, cough, fevers, and infiltrates on chest imaging, with or without eosinophilia either from BAL or peripherally, were counted as suspected cases based on FDA criteria. Also, those deemed by an Infectious Diseases practitioner as proven DPE were reviewed as proven cases [5]. These suspected cases were then evaluated separately by an independent reviewer (A.S.) for concordance with a final diagnosis of daptomycin pulmonary eosinophilia, as set out in the criteria.
Data Collection
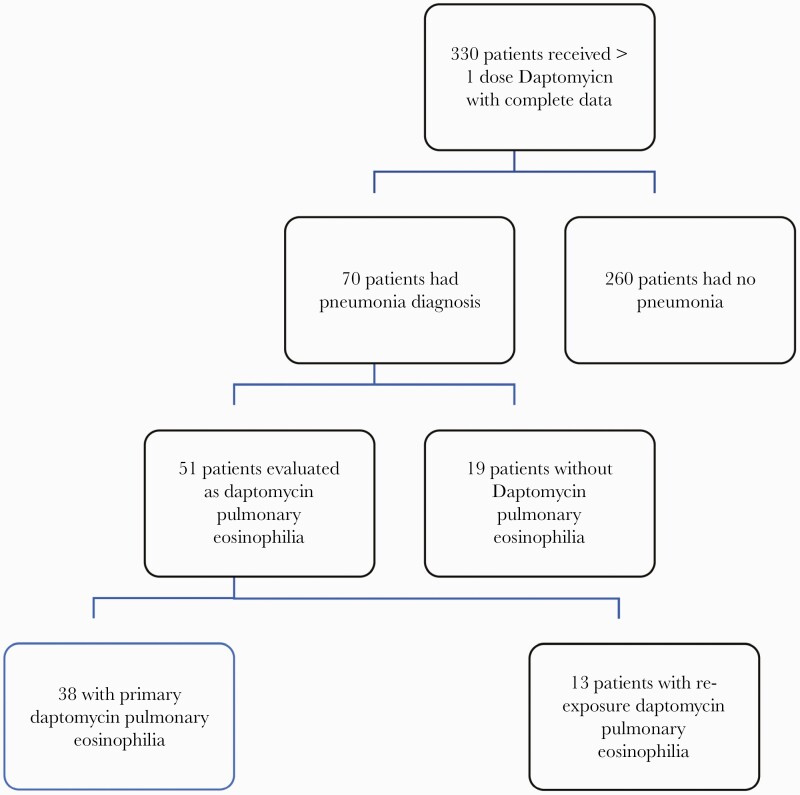
All patients who received >1 dose of daptomycin from 2010 to 2020 and for whom reported data were available were evaluated. Figure 1 describes the final numbers for evaluation.
Figure 1.
Daptomycin Pulmonary Eosinophilla (DPE) Criteria Flow sheet.
Patient demographics, weight, body mass index (BMI), duration of daptomycin, dosing (mg/kg), CrCl at baseline and minimum while on daptomycin, baseline chemistry, eosinophilia while on daptomycin, and number of courses of daptomycin were documented. Mortality, pneumonia, and treatment of DPE were also collected where possible. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were fully applied to ensure the reporting of the study (Supplementary Table 2).
Statistical Analysis
Continuous variables with non–normally distributed continuous variables were expressed as median (range) and compared with Mann-Whitney U test. Categorical variables were reported as number and proportion, which were compared between groups using the χ2 test or Fisher exact test, as appropriate. Categorical variables across ordered groups were accessed using a nonparametric test for trend. Cox proportional hazards were used to identify independent prognostic factors using variables with a P value of <.10 in univariate analysis. A P value of <.05 was considered statistically significant, along with an associated odds ratio (OR) with 95% CIs not crossing 1. All statistics were performed with SPSS, version 26, software (IBM, Minneapolis, MN, USA).
RESULTS
Demographics and Characteristics
There were 330 patients who received daptomycin during the period who met our criteria for evaluation (Figure 1). The main demographics of the total population exposed are summarized in Table 1. Nearly all patients were men (96.4%), White (75.2%), with an elevated BMI (28.7 m/kg2), and 26% had a CrCl <30 mL/min. Eighty-three patients (25%) received a second course of daptomycin treatment. Two hundred sixty-nine of the 330 patients (81.5%) developed measurable peripheral eosinophilia during their daptomycin treatment, with 109 (33%) having >5% on their first daptomycin course. Of the patients who received a second daptomycin treatment course, 46 of the 83 (53%) developed a peripheral eosinophilia >5%. Seventy patients who were evaluated for pneumonia, of whom 51 (15.5%) met criteria for proven and probable DPE, 38 of 330 (13.6%) on their primary treatment (14 proven, 24 probable) course and 13 of 83 (16%) with a re-exposure DPE (all proven). There were 279 patients in the comparator group (including 19 patients with pneumonia deemed not to have DPE). Only 3 of 1120 CPK tests sent in this cohort had an elevated CPK ≥5ULN, and all 3 were concomitantly receiving a statin drug.
Table 1.
Major Characteristics of 330 Patients Exposed to Daptomycin
| Baseline Characteristics | No. (%) | Range |
|---|---|---|
| Median age, y | 64 | 26–94 |
| Male gender | 322 (97.5) | |
| Race | ||
| -White | 253 (76.6) | |
| -Black | 62 (18.8) | |
| -Hispanic/other | 7 (2.1) | |
| Median weight, kg | 91.8 | 39–225 |
| Median BMI, kg/m2 | 28.7 | 13–60 |
| BMI >30, kg/m2 | 134 (40.6) | |
| CrCl <30 mL/min | 87 (26) | |
| Median initial daptomycin treatment duration, d | 14 | 2–154 |
| No. of patients receiving second daptomycin treatment | 83 (25) | |
| Median second treatment duration, d | 5 | 2–78 |
| Daptomycin dosing ≥8 mg/kg | 48 (15) | |
| No. of patients with any detectable eosinophilia after starting daptomycin | 269 (81.5) | |
| Peripheral eosinophilia ≥5% on first treatment course (n = 330) | 109 (33) | |
| Peripheral eosinophilia ≥5% on second treatment course (n = 83) | 46 (55) | |
| Eosinophilic pneumoniaa | 51 (16) | |
| -Initial treatment (n = 330) | 38 (11.5) | |
| -Second treatment (n = 83) | 13 (15) | |
| Median duration to pneumonia onset | ||
| -Initial daptomycin treatment, d | 26 | 2–60 |
| -Re-exposure daptomycin treatment, d | 3 | 2–58 |
| CPK tests | 780 | |
| -No. 5X upper limit of normal | 3 (0.04) |
Abbreviations: BMI, body mass index; CrCl, creatinine clearance; CPK, creatine phosphokinase; ULN, upper limit of normal.
All patients met proven/probable criteria.
Daptomycin Pulmonary Eosinophilia
Table 2 compares patients with DPE with those who did not have DPE in the cohort. There were no differences in age, BMI, initial duration of therapy, or decreased CrCl between the DPE group and those without. Patients with a BMI >30 kg/m2 had a lower odds ratio of developing DPE, but they were more likely to have received a higher dose of daptomycin. On closer review, daptomycin dosing varied when weight was >100 kg to adjusted body weight from actual body weight, impacting our analysis.
Table 2.
Comparing Patients With Daptomycin Pneumonia and Those Without who Received Daptomycin
| Characteristic | DPE (n = 51) | No DPE (n = 279) | P Value (OR; 95% CI) |
|---|---|---|---|
| Median age, y | 66 (41–85) | 63 (26–92) | .09 |
| Median BMI, kg/m2 | 26.4 (15–54) | 28.7 (16–60) | .344 |
| BMI >30, kg/m2 | 9 (17.6) | 125 (44.8) | <.001 (0.36; 0.26–0.75) |
| CrCl <30 mL/min | 14 (27.5) | 73 (26.2) | .85 |
| Daptomycin dosing ≥8 mg/kg | 12 (24) | 22 (8) | .001 (3.6; 1.6–7.8) |
| Primary DPE | 38/279 (13.6) | ||
| Median initial daptomycin treatment duration, d | 14 (2–60) | 14 (2–154) | .89 |
| No. of patients with eosinophils present after starting daptomycin | 48 (94.1) | 221 (79) | .03 |
| Peripheral eosinophilia ≥5% on first treatment course | 25 (49) | 84 (30.1) | .008 (2.23; 1.2–4.09) |
| ICU stay | 12 (32) | ||
| Received antibiotics for pneumonia | 37 (97) | ||
| Re-exposure DPE | 13/83 (16) | ||
| No. of patients receiving second daptomycin treatment | 13 (100) | 70 (22) | |
| Median second treatment duration, d | 13 (2–58) | 17 (2–78) | .55 |
| No. of patients with eosinophils present after starting daptomycin | 12 (92.3) | 55 (79) | .23 |
| Peripheral eosinophilia ≥5% on second treatment course | 12/13 (92.3) | 34/70 (49) | .003 (12; 1.6–103) |
| ICU stay | 9 (70) | ||
| Received antibiotics for pneumonia | 10 (77) |
Abbreviations: BMI, body mass index; CrCl, creatinine clearance; CPK, creatine phosphokinase; DPE, daptomycin pulmonary eosinophilia; ICU, intensive care unit; OR, odd ratio; ULN, upper limit of normal.
The major observations appear to be in the development of DPE in the initial daptomycin treatment group and the re-exposure group.
In the primary DPE group, there was no difference in median duration of therapy and development of DPE between those who developed pneumonia and those who did not; however, the presence of any peripheral eosinophilia and an eosinophilia ≥5% was greater in the DPE group (49% vs 30%; 0.008; OR, 2.23; 95% CI, 1.2–4.09).
The re-exposure DPE group, all except 1 patient, had elevated peripheral eosinophilia ≥5% at time of diagnosis, and this was significant compared with the re-exposed patients who did not develop pneumonia (0.003; OR, 12; 95% CI, 1.6–103). These 13 re-exposure patients developed a re-exposure pulmonary eosinophilia within 7 days (median, 3 days), which was a faster onset than those patients who developed DPE on a primary exposure (median, 28 days). All 13 patients had a peripheral eosinophilia ≥5% on their primary exposure to daptomycin (Table 3).
Table 3.
Clinical Presentation of Hyperacute Pneumonitis After Daptomycin Re-exposure
| Clinical Factors | No. (%) |
|---|---|
| Cough | 13 (100) |
| Shortness of breath | 13 (100) |
| Fevers >38°C | 11 (78) |
| Rash | 1 (7) |
| Abnormal chest x-ray—diffuse infiltrates | 13 (100) |
| Peripheral eosinophilia >5% | 12 (92.5) |
| Corticosteroid therapy | 7 (54) |
| ICU admission | 7 (54) |
| Renal insufficiency | 1 (7) |
| Prior eosinophilia >5% of previous treatment | 13 (100) |
| Median time to onset of symptoms, d | 3 |
Abbreviation: ICU, intensive care unit.
There were no deaths in either the primary group or re-exposure group, with all patients recovering upon withdrawal of daptomycin. Daptomycin was discontinued immediately on all occasions due to the presence of pneumonia. Corticosteroid therapy was administered to 25 (51%) patients with DPE.
When comparing our DPE patients with the all the other patients in the outpatient antibiotic programs who were also monitored, there was a stark difference. There were 51/330 (15%) who developed pneumonia while on daptomycin compared with only 1 case out of 1534 patients (0.06%) on other licensed antibiotic therapy reported to our program in that same period.
DISCUSSION
This is the largest cohort of patients on daptomycin with continuous monitoring of their clinical laboratories and clinical status. From these data we can identify 3 types of daptomycin eosinophilic syndromes: (1) a low grade peripheral eosinophilia that develops 10–14 days after starting therapy; (2) DPE on primary exposure to daptomycin that occurs about 28 days after starting therapy; and (3) a re-exposure DPE to daptomycin on a second treatment course within 7 days of exposure due to having an elevated peripheral eosinophilia on a prior treatment course.
A low-grade peripheral eosinophilia may develop at any time and is often ignored. In effect it is benign and may not be identified in patients on shorter durations of daptomycin therapy or those who have had the drug discontinued before monitoring occurs. Also, patients who are on corticosteroids, have hematologic malignancy, or are on another chemotherapy may never develop eosinophilia. The primary DPE we saw reflects the FDA initial case definitions and occurred around 28 days after starting therapy; in this review, all cases were all recognized by ID consultants. The re-exposure DPE group rapidly developed their pneumonitis within days after initiation of the drug. These patients had prior elevated peripheral eosinophilia on their previous daptomycin treatment course. The positive predictive value of an elevated peripheral eosinophilia ≥5% on prior daptomycin therapy for re-exposure pneumonitis is 100%, but has only a 51% negative predictive value, so its presence if helpful when there is a pneumonia. Our hypothesis appears to be that there may be priming of the surfactant in the alveoli with the first exposure, reflected by the elevated peripheral eosinophilia, and then on re-exposure to daptomycin a more aggressive pulmonary response with systemic symptoms occurs. This type of re-exposure event to daptomycin has only been reported once before, where the patient never had evidence of pneumonitis on first exposure [10].
Despite the clinical syndromes we have described, there was no mortality related to these DPE events with either on primary or on re-exposure as the process is self-limiting and responds rapidly to discontinuation of the drug. However, as an entity, it is frequently missed as only 21 of the 51 cases were initially recognized as DPE by clinicians on admission; all cases were eventually correctly identified by an Infectious Diseases physician or by the IDCP. The missed cases all received broad-spectrum antibiotics for presumed hospital-acquired pneumonia, which meant that the daptomycin was discontinued anyway, helping to treat the underlying problem [11]. Unfortunately, several patients required intensive care unit support, so prompt recognition of these syndromes could avoid unnecessary antibiotics and procedures. Even though we downgraded 19 patients admitted with a diagnosis of pneumonia based on microbiological or other causes, pneumonia was rarely seen using other OPAT antibiotics, which suggests that anyone on daptomycin with pneumonia has a greater chance of the pneumonia being related to the drug.
On initial analysis of the data, there was concern that patients with lower BMI and lower doses of daptomycin had a significant association with DPE. In reviewing these patients, we acknowledge that in patients with a body weight >120 kg, our IDCP used an adjusted body weight to calculate the dose of daptomycin. Dosing of daptomycin in patients with weight >100 kg has not been clearly established and can vary by site of infection, indication, and clinical pharmacist. In comparing adjusted with actual body weight dosing of daptomycin, there was no difference in clinical outcomes or CPK elevations in a retrospective review [9]. Our site changed from actual to adjusted body weight in our period of review, which impacted our ability to determine the effect of elevated BMI on eosinophilia.
Therefore, we cannot conclude that body weight, BMI, or dose is associated with the development of DPE. Lastly, patients who are on corticosteroids (organ transplant, stem cell transplant, etc.) are unlikely to develop DPE as eosinophils are suppressed, so the new increased dosing of daptomycin for VRE bacteremia as recommended by the Clinical and Laboratory Standards Institute in these patients should not cause them to develop DPE [3].
In reviewing 10 years of our data, the role of weekly CPK monitoring should probably be re-assessed. We found it of little value given that cases were exceedingly uncommon and only seen when combined with statin use [12, 13]. This was confirmed by Samura et al., who showed that the association of CPK elevation was greatest with daptomycin use with statins and diphenhydramine [14]. Most patients with daptomycin rhabdomyolysis have symptoms, so CPK could be checked at that time. CPK could be checked on a clinical basis, perhaps only testing those who are nonverbal or who cannot stop statins.
The limitations of this study are that this is a mostly homogeneous group of patients: White males with an elevated BMI and poor kidney function but not taking any immunosuppressive therapy. This limits any generalizability to women, healthier patients with lower BMIs, and immunosuppressed patients. Even with this large review, peripheral eosinophilia was still only 50% of DPE cases, and the diagnosis relied on the clinical presentation and the provider having a high index of suspicion. However, we have a complete set of 10-year data that have been routinely collected to determine clinical effects, with all patients having CPKs and eosinophils collected and responses of daptomycin, and that give a clearer insight into exposure risks. We cannot address if the use of prophylactic steroids would prevent re-exposure fulminant DPE.
In conclusion, daptomycin remains a safe and effective treatment for gram-positive infections. However, there appear to be 3 eosinophilic syndromes that can develop with its use but are non-life-threatening: These are a benign peripheral eosinophilia, a primary DPE occurring ~4 weeks into initial therapy, and a re-exposure DPE after having a prior elevated peripheral eosinophilia on a past treatment. Clinical monitoring of peripheral eosinophilia while on daptomycin therapy could be a future predictor for a re-exposure fulminant DPE; however, despite this, we would still miss half the patients at risk. These syndromes respond rapidly to removal of the drug, and early recognition of DPE will result in earlier discontinuation of daptomycin and prevent unnecessary antibiotics and procedures. Weekly monitoring of CPK does not appear to be beneficial unless the patient is on a statin or is unable to verbalize.
Supplementary Material
Acknowledgments
The authors thank the VA, Infectious Diseases team, and clinical pharmacists for their contribution and participation in this study.
Financial support. This work was unsupported.
Disclaimer. The VA Office of Research and Development had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript. The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs or the United States Government.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. G.F. and K.W. designed this study. K.M. provided all daptomycin data and allergy documentation. K.W. and A.S. collected the data from medical records. A.S. independently reviewed all cases based on definitions. K.W., G.F., and A.S. analyzed and interpreted the data. K.W., A.S., K.M., and G.F. were equal contributors in writing the manuscript. All authors read and approved the final manuscript.
References
- 1. OlesonBerman FBCL, Kirkpatrick JB, Regan KS, et al. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob Agents Chemother 2000; 44:2948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. FowlerBoucher VGHW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–65. [DOI] [PubMed] [Google Scholar]
- 3. Satlin MJ, Nicolau DP, Humphries RM, et al. Development of daptomycin susceptibility breakpoints for Enterococcus faecium and revision of the breakpoints for other enterococcal species by the clinical and laboratory standards institute. Clin Infect Dis 2020; 70:1240–6. [DOI] [PubMed] [Google Scholar]
- 4. Parra-Ruiz J, Dueñas-Gutiérrez C, Tomás-Jiménez C, et al. Safety analysis of high dose (>6 mg/kg/day) daptomycin in patients with concomitant statin therapy. Eur J Clin Microbiol Infect Dis 2012; 31:1771–4. [DOI] [PubMed] [Google Scholar]
- 5. Kim PW, Sorbello AF, Wassel RT, Pham TM, Tonning JM, Nambiar S.. Eosinophilic pneumonia in patients treated with daptomycin: review of the literature and US FDA adverse event reporting system reports. Drug Saf 2012; 35:447–57. [DOI] [PubMed] [Google Scholar]
- 6. Kumar S, Acosta-Sanchez I, Rajagopalan N.. Daptomycin-induced acute eosinophilic pneumonia. Cureus 2018; 10:e2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phillips J, Cardile AP, Patterson TF, Lewis JS 2nd. Daptomycin-induced acute eosinophilic pneumonia: analysis of the current data and illustrative case reports. Scand J Infect Dis 2013; 45:804–8. [DOI] [PubMed] [Google Scholar]
- 8. Dvorchik BH, Damphousse D.. The pharmacokinetics of daptomycin in moderately obese, morbidly obese, and matched nonobese subjects. J Clin Pharmacol 2005; 45:48–56. [DOI] [PubMed] [Google Scholar]
- 9. Fox AN, Smith WJ, Kupiec KE, et al. Daptomycin dosing in obese patients: analysis of the use of adjusted body weight versus actual body weight. Ther Adv Infect Dis 2019; 6:2049936118820230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higashi Y, Nakamura S, Tsuji Y, et al. Daptomycin-induced eosinophilic pneumonia and a review of the published literature. Intern Med 2018; 57:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basnet S, Tachamo N, Dhital R, Tharu B.. Daptomycin associated eosinophilic pneumonia: case report and differential diagnoses. J Community Hosp Intern Med Perspect 2018; 8:152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dare RK, Tewell C, Harris B, et al. Effect of statin coadministration on the risk of daptomycin-associated myopathy. Clin Infect Dis 2018; 67:1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golightly LK, Barber GR, Barron MA, Page RL 2nd. Statins and daptomycin: safety assessment of concurrent use and evaluation of drug interaction liability. Drug Metabol Drug Interact 2013; 28:49–58. [DOI] [PubMed] [Google Scholar]
- 14. Samura M, Hirose N, Kurata T, et al. Identification of risk factors for daptomycin-associated creatine phosphokinase elevation and development of a risk prediction model for incidence probability. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.