Abstract
Purpose
Obesity is prevalent in Saudi Arabia and is associated with adverse clinical features and poor breast cancer (BC) outcomes. We determined the distribution of body mass index (BMI) and evaluated its association with disease characteristics and outcomes in women with non-metastatic BC.
Patients and Methods
We conducted a retrospective analysis of a prospectively collected database of consecutive patients treated for non-metastatic BC between 2002 and 2014. Patients were categorized into the following groups: underweight/normal weight (BMI <25 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥30 kg/m2). Regression analysis was used to evaluate clinicopathological factors associated with BMI and clinical stage.
Results
A total of 2212 patients were enrolled. The median age was 45 years (interquartile range [IQR], 39–52 years), and the median BMI was 30 kg/m2 (IQR, 26–34 kg/m2). Most patients were premenopausal (63.6%), nearly half of the patients had stage III disease, and 11.2% were screen-detected. The prevalence of obesity was 53.4%, with a significant difference between the peri/premenopausal (49.4%) and postmenopausal (61.7%) groups (p < 0.001). Obese patients were more likely to be aged >40 years, be postmenopausal, have a history of oral contraceptive pills, have advanced-stage disease, and have undergone radiation therapy, and were less likely to have human epithelial growth factor 2 (HER2)+ disease than non-obese patients. Premenopausal obese women had fewer hormone receptor-positive and more triple-negative cancers than postmenopausal obese women did. Obesity, non-screening-detected BC, and HER+ status were independent prognostic factors for advanced-stage presentation.
Conclusion
The prevalence of obesity and its significant association with advanced BC justify the upscaling of screening services and instituting weight-reduction strategies.
Keywords: obesity, body mass index, non-metastatic breast cancer, clinical stage, Saudi Arabia
Introduction
Breast cancer (BC) in women has the highest global age-standardized incidence rate (ASR) per 100,000 persons per year and is the most common cause of death due to cancer worldwide,1 including in Saudi Arabia (SA).2 Although the ASR in the Saudi Kingdom is far less than the global figure (27.7 vs 89.9 per 100,000 persons per year, respectively),3 over the past three decades, a sharp rise in local cancer incidence and mortality has been reported,4,5 which is in concordance with the global transition cancer theory and changes in risk factors.6 Obesity (body mass index [BMI] ≥30 kg/m2) is a major global health problem, especially in wealthy or transitional economies. Between 1980 and 2015, its prevalence doubled in >70 countries, and the numbers continue to increase in most other nations,7 thereby affecting 20–41% of the populations.8 Among Saudi women, the prevalence of obesity has significantly increased over the past several decades (14.3% in 1975, 30% in 2001, and 41.2% in 2016),9 and in conjunction with being overweight (BMI ≥25 kg/m2), the prevalence is approximately 70%.9 Excess body weight is associated with increased cancer incidence, and poor overall and BC-specific survival.10,11 The disease characteristics of obesity include larger tumors, more positive nodes, higher tumor grades, more triple-negative disease in premenopausal women, and more hormone-positive tumors in postmenopausal women.12–15 Ethnicity is associated with variations in age and tumor stage at diagnosis. Moreover, race, age, menopausal status, and BMI at diagnosis are associated with variations in the molecular subtype distribution.16–21
We observed a high rate of obesity and a high rate of adverse clinical features of non-metastatic BC at diagnosis in Saudi women. However, there are no data on BMI prevalence or the impact of BMI on BC. This study aimed to evaluate the BMI impact on non-metastatic BC among Saudi women and evaluate the associated factors with advanced clinical stage.
Patients and Methods
This retrospective study used data from consecutive patients with non-metastatic BC treated at the Oncology Center of the King Faisal Specialist Hospital and Research Center, Riyadh, between January 2002 and December 2014. The study protocol was approved by the Research Advisory Council of King Faisal Specialist Hospital and Research Centre. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki (2000).
The data were obtained from a prospective BC database. Data on the following demographic and clinicopathological parameters were retrieved: age at the time of diagnosis, presentation (screen-detected or non-screen-detected), history of oral contraceptive pill (OCP) use, menopausal status, height, weight, tumor histology and grade, clinical tumor and nodal stages, estrogen receptor (ER) and progesterone receptor (PR) status, and human epithelial growth factor 2 (HER2) overexpression. Data on management, chemotherapy (adjuvant and neoadjuvant), surgery type, radiation therapy, hormonal therapy, and anti-HER2 therapy were also retrieved.
A specialized BC pathologist confirmed all pathology slides. ER- or PR-positive tumors were defined as tumor cells with ≥10% immunohistochemistry (IHC) staining for ER or PR. Tumors were defined as hormone receptor (HR)-positive if ER, PR, or both were positive. HER2 status was determined by IHC scoring as follows: 0 or +1, negative; +2, positive (confirmed by fluorescence in situ hybridization); and +3, positive. Tumor grade was determined based on the Nottingham histological score. Weight and height were measured and documented at the time of diagnosis. BMI was computed as weight divided by height2 and expressed as kg/m2. The patients were divided according to BMI classification as described by the World Health Organization: underweight, <18.5 kg/m2; normal weight, 18.5–24.9 kg/m2; overweight, 25–29.9 kg/m2; and obese, ≥30 kg/m2. BC was clinically staged according to the TNM staging system of the UICC/AJCC.
Statistical Analysis
Patient characteristics are expressed as frequencies for continuous variables and as medians with interquartile ranges (IQRs) for categorical variables. Continuous comparisons were performed using the Mann–Whitney U-test or Kruskal–Wallis test, and categorical variables were compared using the chi-squared test. Regression analysis was used to determine the factors associated with BMI and clinical stage. Covariates with a proven or potential association in previous studies were selected. Multicollinearity was avoided by including only variables with an intervariable correlation coefficient ≤0.7, tolerance ≥0.1, and variance inflation factor (VIF) <10. The covariates included were age, menopausal status, mode of presentation (screen-detected vs symptomatic), history of OCP use, histology, grade, clinical stage, chemotherapy, surgery, and radiation therapy. Hormonal and anti-HER2 therapy were not included because of high collinearity with ER/PR and HER2 status. In the final model, the VIF and tolerance of all included variables were <2.5 and >0.4, respectively. Disease-free survival and overall survival probabilities were plotted using the Kaplan–Meier estimator. Statistical computations were performed using IBM SPSS Statistics for Mac, version 27.0.
Results
The study enrolled 2212 women with non-metastatic BC, with the majority being premenopausal (63.6%). The median BMI was 30 kg/m2 (IQR, 26–34 kg/m2). At the time of diagnosis, 53.4% of patients were obese, 30.9% were overweight, and 15.6% were normal weight/underweight. Table 1 shows patient demographics, clinicopathological characteristics, and management.
Table 1.
Patients’ Demographic and Clinicopathological Characteristics and Management of the Study Group Stratified by BMI
| Parameter | All | BMI (kg/m2) Groups | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal Weight | Overweight | Obese | |||||||
| No. | % | No. | % | No. | % | No. | % | ||
| No. of patients | 2212 | 100 | 346 | 15.6 | 684 | 30.9 | 1182 | 53.4 | <0.001 |
| Age group, years | |||||||||
| ≤40 | 717 | 32.4 | 166 | 48 | 253 | 37 | 298 | 25.2 | <0.001 |
| >40 | 1495 | 67.6 | 180 | 52 | 431 | 63 | 884 | 74.8 | |
| Menopausal status | |||||||||
| Pre | 1407 | 65.2 | 249 | 73.5 | 473 | 70.7 | 685 | 59.5 | <0.001 |
| Peri | 62 | 2.9 | 9 | 2.7 | 13 | 1.9 | 40 | 3.5 | |
| Post | 690 | 32 | 81 | 23.9 | 183 | 27.4 | 426 | 37 | |
| NA | 53 | 2.0 | |||||||
| History of taking OCP | 1164 | 70.9 | 148 | 61.7 | 369 | 70.3 | 647 | 73.8 | 0.001 |
| Screen detected | 247 | 11.2 | 40 | 12.4 | 72 | 10.5 | 135 | 11.4 | 0.67 |
| Histology | |||||||||
| Invasive ductal carcinoma | 1985 | 89.7 | 303 | 88.2 | 615 | 90.3 | 1067 | 90.2 | 0.94 |
| Invasive lobular carcinoma | 148 | 6.7 | 26 | 7.5 | 38 | 5.6 | 84 | 7.1 | |
| Others | 79 | 3.5 | |||||||
| Tumor grade | |||||||||
| G1 | 102 | 4.7 | 17 | 5.1 | 27 | 4.1 | 58 | 5 | 0.21 |
| G2 | 970 | 45.1 | 162 | 48.4 | 286 | 43.3 | 522 | 45.2 | |
| G3 | 877 | 40.8 | 117 | 34.9 | 285 | 43.2 | 475 | 41.1 | |
| Gx | 263 | 11.8 | 39 | 11.6 | 62 | 9.4 | 100 | 8.7 | |
| ER | |||||||||
| Positive | 1380 | 63 | 224 | 65.3 | 422 | 62.2 | 734 | 62.7 | 0.61 |
| Negative | 811 | 37 | 119 | 34.7 | 256 | 37.8 | 436 | 37.3 | |
| Missing | 21 | <1 | |||||||
| PR | |||||||||
| Positive | 1159 | 52.9 | 173 | 50.4 | 352 | 51.9 | 634 | 54.2 | 0.39 |
| Negative | 1032 | 47.1 | 170 | 49.6 | 326 | 48.1 | 536 | 45.8 | |
| Missing | 21 | <1 | |||||||
| HER2 | 0.03 | ||||||||
| Positive | 704 | 32.6 | 109 | 32.1 | 243 | 36.3 | 352 | 30.5 | |
| Negative | 1458 | 67.4 | 231 | 67.9 | 426 | 63.7 | 801 | 69.5 | |
| Missing | 50 | ||||||||
| Molecular profiles | 0.24 | ||||||||
| ER/PR+/HER2– | 1012 | 46.9 | 159 | 46.9 | 295 | 44.2 | 558 | 48.4 | |
| ER/PR+/HER2+ | 380 | 17.6 | 64 | 18.9 | 131 | 19.6 | 185 | 16 | |
| ER/PR-/HER2+ | 322 | 14.9 | 44 | 13 | 111 | 16.6 | 167 | 14.5 | |
| ER/PR-HER2– | 446 | 20.6 | 72 | 21.2 | 131 | 19.6 | 243 | 21.1 | |
| Missing | 52 | 2.3 | |||||||
| Clinical T stage | |||||||||
| T1 | 258 | 12.3 | 24 | 12.7 | 85 | 13.1 | 131 | 11.7 | 0.04 |
| T2 | 887 | 42.3 | 155 | 46.8 | 291 | 44.9 | 441 | 39.4 | |
| T3 | 511 | 24.3 | 75 | 22.7 | 149 | 23 | 287 | 25.6 | |
| T4 | 443 | 21.1 | 59 | 17.8 | 123 | 19 | 261 | 23.3 | |
| Tx | 113 | 5 | |||||||
| Nodal stage | |||||||||
| N0 | 618 | 29 | 96 | 29 | 203 | 30.9 | 319 | 27.9 | 0.07 |
| N1 | 968 | 45 | 165 | 49.8 | 295 | 44.9 | 508 | 44.4 | |
| N2 | 376 | 17.6 | 55 | 16.6 | 111 | 16.9 | 210 | 18.4 | |
| N3 | 169 | 7.9 | 15 | 4.5 | 48 | 7.3 | 106 | 9.3 | |
| Nx | 81 | 3.6 | |||||||
| Management | |||||||||
| Neoadjuvant chemotherapy | 879 | 40.5 | 139 | 41.4 | 260 | 38.8 | 480 | 41.3 | 0.54 |
| Type of breast surgery | |||||||||
| BCS | 789 | 36.6 | 124 | 37.1 | 250 | 37.4 | 415 | 35.9 | 0.02 |
| MRM | 1188 | 55.1 | 183 | 54.7 | 343 | 51.4 | 662 | 57.4 | |
| SSM | 70 | 3.2 | 13 | 3.8 | 29 | 4.3 | 28 | 2.4 | |
| SM | 97 | 4.5 | 14 | 4.1 | 40 | 5.9 | 43 | 3.7 | |
| Missing | 10 | ||||||||
| Axillary dissection | 1860 | 85 | 283 | 84 | 568 | 83.8 | 1009 | 86.1 | 0.11 |
| Adjuvant chemotherapy | 1335 | 61.5 | 210 | 62.3 | 422 | 62.9 | 703 | 60.4 | 0.53 |
| Radiation therapy | 1795 | 82.8 | 258 | 77.2 | 545 | 81.2 | 992 | 85.2 | 0.001 |
| Hormonal therapy | 1355 | 63.4 | 212 | 63.3 | 417 | 63.3 | 726 | 63.6 | 0.92 |
| Anti-HER2 therapy | 548 | 25.3 | 87 | 25.7 | 181 | 27.1 | 280 | 24.1 | 0.37 |
Abbreviations: BCS, breast-conserving surgery; BMI, body mass index; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IQR, interquartile range; MRM, modified radical mastectomy; NA, not available; OCP, oral contraceptive pill; PR, progesterone receptor; SM, simple mastectomy; SSM, skin-sparing mastectomy.
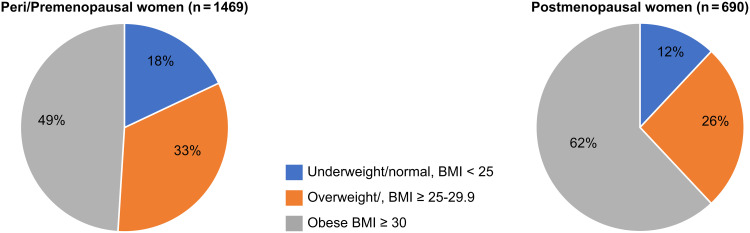
The median age at diagnosis was 45.7 ± 10.4 years (IQR, 39–52 years), with a significant difference between the BMI groups as follows: underweight/normal weight, 41 years (IQR, 34–50 years); overweight, 44 years (IQR, 37–50 years); and obese, 47 years (IQR, 40–53 years) (p < 0.001). The distribution of BMI of pre- and postmenopausal patients is shown in Figure 1.
Figure 1.
Pie charts demonstrating the distribution of body mass index (BMI) groups in peri/pre- and postmenopausal women (p < 0.001).
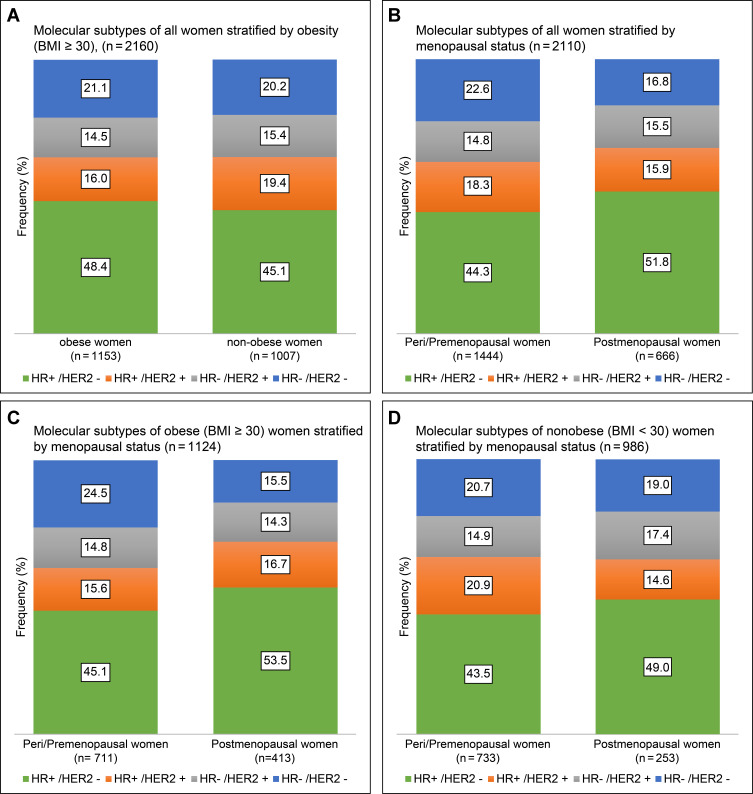
Menopausal Status and Molecular Subtypes in Obese Patients
The molecular subtype distribution for all women stratified by obesity (BMI ≥30 kg/m2) and menopausal status is illustrated in Figure 2A and B. Obese premenopausal patients demonstrated a lower rate of HR+/HER− (45.1% vs 53.5%) and a higher number of triple-negative tumors (24.5% vs 15.5%) than postmenopausal obese patients and almost similar rates of HER2-enriched subtypes (HR+, 15.6% vs 16.7%; HR−, 14.8% vs 14.3%; overall, 30.4% vs 31%; p = 0.003, Figure 2C). There was no significant difference in molecular subtype distribution in the non-obese group (p = 0.10, Figure 2D).
Figure 2.
Bar graphs demonstrating molecular subtype distribution stratified by (A) obesity and (B) menopausal status in all patients and molecular subtype distribution in (C) obese and (D) non-obese women stratified by menopausal status in newly diagnosed non-metastatic breast cancer.
Factors Associated with Obesity
Table 2 presents the logistic regression of variables associated with obesity (BMI ≥30 kg/m2). The factors independently associated with obesity were ≥40 years of age (odds ratio [OR], 1.90; 95% CI, 1.49–2.43; p < 0.001), history of OCP use (OR, 1.33; 95% CI, 1.05–1.67; p = 0.01), postmenopausal status (OR, 1.37; 95% CI, 1.06–1.76; p = 0.01), stage III vs stage I/II disease (OR, 1.25; 95% CI, 1.01–1.56; p = 0.04), radiation therapy (OR, 1.63; 95% CI, 1.22–2.209; p = 0.001), and HER2 overexpression (OR, 0.73; 95% CI, 0.59–0.92; p = 0.008). A subgroup analysis revealed that premenopausal obese women were more likely to present with a triple-negative molecular subtype (OR, 1.30; 95% CI 1.01–1.69; p = 0.04) and more advanced disease (stage III vs stage I/II) (OR, 1.27; 95% CI, 1.03–1.5; p = 0.02) than non-obese premenopausal women. In contrast, postmenopausal obese patients were only significantly associated with more stage III (OR, 1.45; 95% CI 1.05–2.01; p = 0.02) than non-obese postmenopausal women.
Table 2.
Regression Analysis of Variables Associated with Obesity (BMI ≥30 Kg/M2)
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age, years | ||||||
| ≤40 | 1 | 1 | ||||
| >40 | 2.03 | 1.69–2.43 | <0.001 | 1.90 | 1.49–2.43 | <0.001 |
| Menopausal status | ||||||
| Pre/perimenopause | 1 | 1 | ||||
| Postmenopause | 1.65 | 1.37–1.99 | <0.001 | 1.37 | 1.06–1.76 | 0.01 |
| History of OCP | 1.34 | 1.09–1.67 | 0.006 | 1.33 | 1.05–1.67 | 0.01 |
| Screen detected | 1.05 | 0.81–1.37 | 0.68 | |||
| ER+ vs ER- | 0.97 | 0.82–1.12 | 0.97 | |||
| PR+ vs PR- | 1.11 | 0.94–1.32 | 0.19 | |||
| HER2+ vs HER2- | 0.82 | 0.68–0.98 | 0.03 | 0.73 | 0.59–0.92 | <0.01 |
| TN vs others | 1.05 | 0.85–1.30 | 0.60 | |||
| Grade ≤II vs III | 0.94 | 0.80–1.12 | 0.54 | |||
| Clinical stage I/II vs III | 1.35 | 1.14–1.61 | 0.001 | 1.25 | 1.01–1.56 | 0.04 |
| NAC vs no NAC | 1.07 | 0.90–1.27 | 0.43 | |||
| MRM vs BCS | 0.97 | 0.77–1.23 | 0.83 | |||
| Radiation vs no radiation | 1.45 | 1.16–1.81 | 0.001 | 1.63 | 1.22–2.20 | 0.001 |
| Axillary dissection | 1.19 | 0.94–1.50 | 0.14 | |||
| ACT vs no ACT | 0.90 | 0.76–1.07 | 0.27 | |||
Abbreviations: ACT, adjuvant chemotherapy; BCS, breast conservative surgery; BMI, body mass index; CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; MRM, modified radical mastectomy; NAC, neoadjuvant chemotherapy; OR, odds ratio; PR, progesterone receptor; TN, triple-negative.
Factors Associated with Clinical Stage
Almost 50% of this cohort presented with stage III disease. The following factors were associated with advanced clinical stage (stage III vs stage I/II): obesity (p = 0.001), screen-detected tumors (p = 0.01), and molecular subtype (p < 0.002). The following factors were not associated with advanced stage at presentation: age ≤40 vs >40 years (p = 0.14), pre- vs postmenopausal status (p = 0.33), history of OCP use (p = 0.11), or tumor grade (G1 and G2 vs G3; p < 0.11). However, G3 was more strongly associated with advanced stage than G1 (p = 0.04). There was no significant association between overweight and clinical stage (p = 0.33).
Multivariate logistic regression revealed that screen-detected tumors were associated with less stage III vs stage I and II disease (OR, 0.69; 95% CI, 0.51–0.93; p < 0.01), and obese patients were at a greater risk of presenting with advanced disease (stage III vs I and II; OR, 1.52; 95% CI, 1.15–1.9; p < 0.001). In addition, in comparison to HR+/HER2− disease, the HR+/HER2+ subtype was associated more with advanced stage at diagnosis (OR, 1.46; 95% CI, 1.14–1.88; p < 0.002), as was the HR−/HER2+ subtype (OR, 1.54; 95% CI, 1.18–2.00; p = 0.001), whereas the triple-negative subtype did not reach statistical significance (p = 0.09).
The screen-detected tumors were associated with more stage I/II vs III in premenopausal women only, whereas HER2+ status and obesity were associated with advanced stage at diagnosis (stage III vs I/II) in pre- and postmenopausal women. However, the association of HER2+ with advanced stage at presentation was higher in premenopausal women (OR, 1.68; 95% CI, 1.33–2.11; p < 0.001) than in postmenopausal women (OR, 1.52; 95% CI, 1.08–2.13; p < 0.01), whereas the association of obesity with advanced stage was higher in postmenopausal women (OR, 1.46; 95% CI, 1.04–1.60; p = 0.02) than in premenopausal women (OR, 1.29; 95% CI, 1.04–1.60; p = 0.01).
Survival Analysis
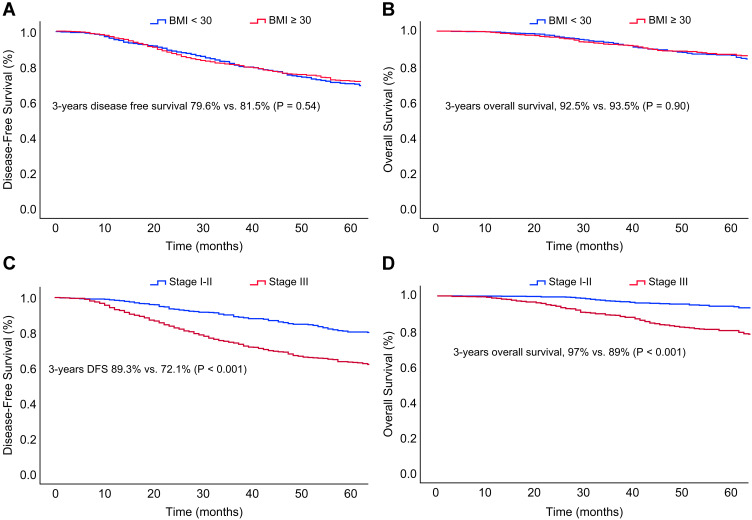
The median follow-up period was 39 months (IQR, 22–66 months), with 44 patients (2%) lost to follow-up. During this brief period, disease-free survival and overall survival did not differ significantly among the various BMI groups. However, there were significant differences among the women grouped by clinical stage (Figure 3A–D).
Figure 3.
Kaplan–Meier curves of (A) disease-free survival and (B) overall survival stratified by obesity and (C) disease-free survival and (D) overall survival stratified by clinical stage (I and II vs III) in patients with non-metastatic breast cancer.
Discussion
We present the first study to examine the BMI distribution in Saudi women with newly diagnosed non-metastatic BC and clarify the association between BMI and clinicopathological characteristics and outcomes from the largest prospective database in SA. The results revealed that the prevalence of excess weight and obesity in newly diagnosed non-metastatic BC patients (84.4%) was higher than the prevalence in the general population of Saudi women (70.2%).9 Obesity was less prevalent in premenopausal women (49.4%) than in postmenopausal women (61.7%).
Most newly diagnosed patients with non-metastatic BC were premenopausal (62%) and/or obese (53%), and nearly half presented with stage III disease. Only 11.2% of cases were screen-detected BC. Obesity in our patients was positively associated with an advanced clinical stage, regardless of menopausal status, and was associated with more triple-negative disease in premenopausal women. The following factors were independently associated with obesity: age ≥40 years, history of OCP use, postmenopausal status, HER2 overexpression, stage III vs stage I/II, and radiation therapy. The association between excess weight and adverse BC characteristics demonstrated in this study is comparable to that demonstrated in previous studies.13–15,18,22–24 However, the higher prevalence of excess weight in Saudi women is associated with higher adverse pathological features. Independently, obesity, HER2-positive disease, and non-screen-detected tumors were associated with an advanced clinical stage. The higher prevalence of obesity and lower screening detection rate in our patients necessitates further research and encourages prompt intervention.
The vast majority of newly diagnosed BC patients have one risk factor at diagnosis.25 The risk factors for BC in SA include a decrease in the age at menarche, increase in the age at marriage and first-time pregnancy, decrease in parity and breastfeeding duration, and obesity and physical inactivity.26,27 Obesity is more prevalent in women than in men in SA,28 and physical inactivity is especially widespread in women.29
Obesity is linked to BC pathogenesis through multiple mechanisms. It is associated with decreased blood flow and oxygenation of adipose tissue, upregulation of leptin levels and vascular endothelial growth factor via hypoxia-inducible factor-1, and inhibition of adiponectin expression. Circulating insulin and insulin-like growth factor-1 act as potent growth factors. Various processes increase aromatase-mediated estrogen production and stimulate the nuclear factor-kappa B pathway. These processes promote inflammation and trigger anti-apoptotic genes that facilitate BC proliferation, invasion, angiogenesis, and metastasis.30–32
The median age among Saudi non-metastatic BC patients at diagnosis was 45.7 years, which is in concordance with previous data from Arab countries (44.5–48.5 years),33 Asia (47.3 years),24 and the Caribbean region (49 years),34 but lower than that in the United States (60 vs 63 years in black and white women, respectively).35 Determining the reason for this apparent difference is beyond the scope of this study, but it could be attributed to the younger age structure of the population in these regions compared to that in the United States, which might be due to environmental and genetic factors.36 Furthermore, most of the Saudi women (62%) included in the study were premenopausal at diagnosis, which was higher than what was reported in a previous study from SA (49.7%)37 and from Jourdan (44%).14 However, both studies included metastatic disease, which we are not including here. In general, a BC diagnosis before the age of 50 years is higher in developing countries (47–56.9%) than in developed countries (16–21.5%).38 The median age at diagnosis increased proportionally with an increase in BMI, which is in agreement with previous studies.14,22
We found that the rate of obesity was higher in older patients (age ≥40 years) than in younger patients (59.1% vs 41.6%; p < 0.001). Furthermore, age ≥40 years was an independent risk factor for obesity. However, age was not associated with an advanced clinical stage. Indeed, advanced tumor stage III vs stage I/II was more strongly associated with obesity, regardless of age and menopausal status.
This study also found an association between oral contraceptive use and obesity (BMI ≥30 kg/m2) at BC diagnosis. A review of 49 clinical trials did not show a significant association between combination contraceptives and weight gain.39 However, OCPs have been found to be a risk factor associated with BC in Arabian women.40 In addition, the association between obesity and radiation therapy can be explained by the more advanced stages of BC among obese women in this population.
Three factors were independently associated with advanced clinical stage: obesity, HER2-positive disease, and non-screen-detected tumors. Interestingly, obesity was inversely associated with HER2+ disease (30.5% vs 34.9%), and both were independently associated with more advanced stage. However, we cannot exclude the aggressive influence of obesity on molecular subtypes, as we observed that in premenopausal women, obesity was associated with more triple-negative diseases (24.5% vs 20.7%). Screen-detected BC was not associated with obesity. However, both obesity and screen-detected BC were independently associated with the clinical stage. Therefore, the higher stage III disease in our patients cannot be explained by a low screening rate or factors related to screening only, such as lack of mammograms or mammogram inaccuracy in obese women, but is probably related more to the biological impact of obesity on BC development, as explained earlier. However, some researchers have suggested that obesity is a potential barrier for screening compliance and effectiveness.41–44 In contrast, other studies have shown no effect of obesity on screening compliance45 and have reported that mammography use and accuracy are not the reasons for the higher clinical stage in obese patients.46 Moreover, another study reported that obese women had higher sensitivity for screening mammography with similar specificity, regardless of their BMI group.47 Delayed diagnosis of obese women has been reported as a reason for the advanced stage owing to the inability to feel the lumps because of breast size and embarrassment to seek medical help because of their body appearance.48
The rate of screen-detected tumors in our patients was far lower than has been reported internationally, 11.2% vs 22–36% and up to 48% in women aged >50 years.49,50 The benefits of mammography screening have been evident since the mid-1980s based on results from randomized controlled trials; every 2–3 years, mammograms for women aged 40–74 years result in a one-third reduction in mortality from BC and a one-fourth reduction in advanced stage at diagnosis.51 Given that BC incidence in SA has increased significantly in the last three decades and that the pattern is similar to that in the United States during the pre-screening mammography era,26 intervention is warranted. National screening programs for BC in SA have not yet been established, and there is no widespread explicit action to overcome obesity. However, the Ministry of Health has promoted awareness for early BC detection52 and provided obesity prevention and management guidelines.53 The public acceptance of screening programs is encouraging;54 however, 92% of women aged 50 years or older who participated in the Saudi Health Interview Survey 2013 reported never having had a mammogram, although mammography screening in SA is free.55
Obesity is a modifiable risk factor that merits further investigation and intervention to decrease its effects on the adverse features of BC. The inflammatory process in adipose tissue is reversible; research on interrupting the link between obesity and BC requires further work, and its impact on patients with BC is yet to be studied.32
In the present study, disease-free survival and overall survival were not affected by obesity, possibly because of the limited follow-up period. In a previous study, the significance of obesity on BC progression-free survival was observed after 5 years, and the risk of death increased after 10 years of follow-up.56 Extended follow-up periods may provide greater insight into the effects of BMI on cancer recurrence and survival rates.
The strength of our study is that it targeted an unselected aggregate of women with non-metastatic BC from the largest prospectively collected database in SA, which makes the findings likely to be generalized in our region. Second, it provides a comprehensive and in-depth view of patient characteristics and healthcare system challenges in managing non-metastatic BC that necessitates prompt intervention in SA. We acknowledge that the duration of follow-up was relatively short for localized BC. Our facility is a broad-based referral center, and many patients continue their follow-up at local hospitals after completing their therapy.
Conclusion
The high prevalence of obesity and low screen-detection rate in SA significantly contributes to the high rate of adverse clinical features of non-metastatic BC in Saudi women. Given the significant increase in BC ASR in our population, these findings necessitate prompt intervention, including implementation of weight reduction strategies, a widespread mandatory screening program, and screening at earlier ages, particularly for obese women.
Acknowledgments
The authors thank Ms. Romellia, a breast cancer database coordinator, for her assistance with the data retrieval from the database. Early results were presented as an abstract at the American Society of Clinical Oncology Conference (ASCO) 2020. The abstract was published in the Journal of Clinical Oncology as an e-supplement (e12602). Available at: https://ascopubs.org/doi/10.1200/JCO.2020.38.15_suppl.e12602.
Funding Statement
There is no funding to report.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Research Advisory Council (RAC) of King Faisal Specialist Hospital and Research Centre, Riyadh (RAC number 2051-029). The RAC committee at King Faisal Specialist Hospital and Research Centre, Riyadh, waived the requirement for informed consent. All methods were performed in accordance with relevant guidelines and regulations.
Disclosure
Dr Taher Al-Tweigeri reports personal fees from Roche, Novartis, Lilly, during the conduct of the study. The authors declare that the research was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 2.Saudi Health Council National Health Information Center Saudi Cancer Registry. Cancer incidence report; 2016. Available from: https://nhic.gov.sa/en/eServices/Pages/TumorRegistration.aspx. Accessed May 4, 2021.
- 3.Global Cancer Observatory: Cancer Today. Estimated age-standardized incidence rates (World) in 2020. Available from: https://gco.iarc.fr/today/online-analysis-map. Accessed March 2, 2021.
- 4.Chaudhri E, Fathi W, Hussain F, Hashmi SK. The increasing trends in cases of the most common cancers in Saudi Arabia. J Epidemiol Glob Health. 2020;10(4):258–262. doi: 10.2991/jegh.k.200515.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Althubiti MA, Nour Eldein MM. Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med J. 2018;39(12):1259–1262. doi: 10.15537/smj.2018.12.23348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5 [DOI] [PubMed] [Google Scholar]
- 7.Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Prevalence of obesity among adults. Available from: https://www.who.int/data/gho/data/indicators/indicator-details. Accessed January 2, 2022.
- 10.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627–635. doi: 10.1007/s10549-010-0990-0 [DOI] [PubMed] [Google Scholar]
- 11.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose DP, Vona-Davis L. Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther. 2009;9(8):1091–1101. doi: 10.1586/ERA.09.71 [DOI] [PubMed] [Google Scholar]
- 13.Copson ER, Cutress RI, Maishman T, et al. Obesity and the outcome of young breast cancer patients in the UK: the POSH study. Ann Oncol. 2015;26(1):101–112. doi: 10.1093/annonc/mdu509 [DOI] [PubMed] [Google Scholar]
- 14.Ayoub NM, Yaghan RJ, Abdo NM, Matalka II, Akhu-Zaheya LM, Al-Mohtaseb AH. Impact of obesity on clinicopathologic characteristics and disease prognosis in pre- and postmenopausal breast cancer patients: a retrospective institutional study. J Obes. 2019;2019:1–11. doi: 10.1155/2019/3820759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-de La Roche LA, Steljes I, Janni W, Friedl TWP, De Wilde RL. The association between obesity and premenopausal breast cancer according to intrinsic subtypes - a systematic review. Geburtshilfe Frauenheilkd. 2020;80(6):601–610. doi: 10.1055/a-1170-5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86(9):705–712. doi: 10.1093/jnci/86.9.705 [DOI] [PubMed] [Google Scholar]
- 17.Hahn KME, Bondy ML, Selvan M, et al. Factors associated with advanced disease stage at diagnosis in a population-based study of patients with newly diagnosed breast cancer. Am J Epidemiol. 2007;166(9):1035–1044. doi: 10.1093/aje/kwm177 [DOI] [PubMed] [Google Scholar]
- 18.Stark A, Kapke A, Schultz D, Brown R, Linden M, Raju U. Advanced stages and poorly differentiated grade are associated with an increased risk of HER2/neu positive breast carcinoma only in White women: findings from a prospective cohort study of African-American and White-American women. Breast Cancer Res Treat. 2008;107(3):405–414. doi: 10.1007/s10549-007-9560-5 [DOI] [PubMed] [Google Scholar]
- 19.Verma R, Bowen RL, Slater SE, Mihaimeed F, Jones JL. Pathological and epidemiological factors associated with advanced stage at diagnosis of breast cancer. Br Med Bull. 2012;103(1):129–145. doi: 10.1093/bmb/lds018 [DOI] [PubMed] [Google Scholar]
- 20.Gershuni V, Li YR, Williams AD, et al. Breast cancer subtype distribution is different in normal weight, overweight, and obese women. Breast Cancer Res Treat. 2017;163(2):375–381. doi: 10.1007/s10549-017-4192-x [DOI] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492 [DOI] [PubMed] [Google Scholar]
- 22.Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111(2):329–342. doi: 10.1007/s10549-007-9785-3 [DOI] [PubMed] [Google Scholar]
- 23.Stark A, Stahl MS, Kirchner HL, Krum S, Prichard J, Evans J. Body mass index at the time of diagnosis and the risk of advanced stages and poorly differentiated cancers of the breast: findings from a case-series study. Int J Obes. 2010;34(9):1381–1386. doi: 10.1038/ijo.2010.69 [DOI] [PubMed] [Google Scholar]
- 24.Chen FY, Ou HY, Wang SM, Wu YH, Yan GJ, Tang LL. Associations between body mass index and molecular subtypes as well as other clinical characteristics of breast cancer in Chinese women. Ther Clin Risk Manag. 2013;9(1):131–137. doi: 10.2147/TCRM.S41203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engmann NJ, Golmakani MK, Miglioretti DL, et al. Population-attributable risk proportion of clinical risk factors for breast cancer. Mitochondrial DNA. Part B, Resources. 2018;3(9):1228–1236. doi: 10.1001/jamaoncol.2016.6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim EM, Zeeneldin AA, Bin SB, Ezzat AA. The present and the future of breast cancer burden in the Kingdom of Saudi Arabia. Med Oncol. 2008;25(4):387–393. doi: 10.1007/s12032-008-9051-5 [DOI] [PubMed] [Google Scholar]
- 27.Almutlaq BA, Almuazzi RF, Almuhayfir AA, et al. Breast cancer in Saudi Arabia and its possible risk factors. J Cancer Policy. 2017;12:83–89. doi: 10.1016/j.jcpo.2017.03.004 [DOI] [Google Scholar]
- 28.Memish ZA, El bcheraoui CE, Tuffaha M, et al. Obesity and associated factors—Kingdom of Saudi Arabia, 2013. Prev Chronic Dis. 2014;11(10):1–10. doi: 10.5888/pcd11.140236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Hazzaa HM. Prevalence of physical inactivity in Saudi Arabia: a brief review. East Mediterr Health J. 2004;10:663–670. doi: 10.26719/2004.10.4-5.663 [DOI] [PubMed] [Google Scholar]
- 30.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- 31.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484–498. doi: 10.1038/nrc3967 [DOI] [PubMed] [Google Scholar]
- 32.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270–4276. doi: 10.1200/JCO.2016.67.4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Najjar H, Easson A. Age at diagnosis of breast cancer in Arab nations. Int J Surg. 2010;8(6):448–452. doi: 10.1016/j.ijsu.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 34.Fadelu T, Damuse R, Lormil J, et al. Patient characteristics and outcomes of nonmetastatic breast cancer in Haiti: results from a retrospective cohort. Oncologist. 2020;25(9):1372–1381. doi: 10.1634/theoncologist.2019-0951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 36.United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Prospects 2019. Available from: https://population.un.org/wpp/DataQuery/. Accessed January 20, 2021.
- 37.Elkum N, Al-Tweigeri T, Ajarim D, Al-Zahrani A, Amer SMB, Aboussekhra A. Obesity is a significant risk factor for breast cancer in Arab women. BMC Cancer. 2014;14(1):1–10. doi: 10.1186/1471-2407-14-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghiasvand R, Adami HO, Harirchi I, Akrami R, Zendehdel K. Higher incidence of premenopausal breast cancer in less developed countries; myth or truth? BMC Cancer. 2014;14:343. doi: 10.1186/1471-2407-14-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallo MF, Lopez LM, Grimes DA, Carayon F, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014;2014(1):CD003987. doi: 10.1002/14651858.CD003987.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karim SM, Baeshen W, Neamatullah SN, Bin B. Oral contraceptives, abortion and breast cancer risk: a case control study in Saudi Arabia. Asian Pac J Cancer Prev. 2015;16(9):3957–3960. doi: 10.7314/APJCP.2015.16.9.3957 [DOI] [PubMed] [Google Scholar]
- 41.Wee CC, McCarthy EP, Davis RB, Phillips RS. Obesity and breast cancer screening. J Gen Intern Med. 2004;19:324–331. doi: 10.19080/JOJPH.2018.03.555616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maruthur NM, Bolen S, Brancati FL, Clark JM. Obesity and mammography: a systematic review and meta-analysis. J Gen Intern Med. 2009;24(5):665–677. doi: 10.1007/s11606-009-0939-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldstein AC, Perrin N, Rosales AG, Schneider J, Rix MM, Glasgow RE. Patient barriers to mammography identified during a reminder program. J Womens Health (Larchmt). 2011;20(3):421–428. doi: 10.1089/jwh.2010.2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elmore JG, Carney PA, Abraham LA, et al. The association between obesity and screening mammography accuracy. Arch Intern Med. 2004;164(10):1140–1147. doi: 10.1001/archinte.164.10.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miles RC, Lehman CD, Mercaldo SF, Tamimi RM, Dontchos BN, Narayan AK. Obesity and breast cancer screening: cross-sectional survey results from the behavioral risk factor surveillance system. Cancer. 2019;125(23):4158–4163. doi: 10.1002/cncr.32430 [DOI] [PubMed] [Google Scholar]
- 46.Kerlikowske K, Walker R, Miglioretti DL, Desai A, Ballard-Barbash R, Buist DSM. Obesity, mammography use and accuracy, and advanced breast cancer risk. J Natl Cancer Inst. 2008;100(23):1724–1733. doi: 10.1093/jnci/djn388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Njor SH, Von Euler-chelpin M, Tjønneland A, Vejborg I, Lynge E. Body weight and sensitivity of screening mammography. Eur J Cancer. 2016;60:93–100. doi: 10.1016/j.ejca.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 48.Deglise C, Bouchardy C, Burri M, et al. Impact of obesity on diagnosis and treatment of breast cancer. Breast Cancer Res Treat. 2010;120(1):185–193. doi: 10.1007/S10549-009-0459-1 [DOI] [PubMed] [Google Scholar]
- 49.McPherson CP, Swenson KK, Jolitz G, Murray CL. Survival of women ages 40–49 years with breast carcinoma according to method of detection. Cancer. 1997;79(10):1923–1932. doi: [DOI] [PubMed] [Google Scholar]
- 50.Jiang L, Gilbert J, Langley H, Moineddin R, Groome PA. Breast cancer detection method, diagnostic interval and use of specialized diagnostic assessment units across Ontario, Canada. Heal Promot Chronic Dis Prev Can. 2018;38(10):358–367. doi: 10.24095/hpcdp.38.10.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabár L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;325(8433):829–832. doi: 10.1016/s0140-6736(85)92204-4 [DOI] [PubMed] [Google Scholar]
- 52.Saudi Ministry of Health. National program for early detection for breast cancer. Available from: https://www.moh.gov.sa/en/Ministry/Projects/breast-cancer/Pages/default.aspx. Accessed January 26, 2021.
- 53.Alfadda AA, Al-Dhwayan MM, Alharbi AA, et al. The Saudi clinical practice guideline for the management of overweight and obesity in adults. Saudi Med J. 2016;37(10):1151–1162. doi: 10.15537/smj.2016.10.14353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abulkhair O, Al Tahan F, Young S, Musaad S, Jazieh AR. The first national public breast cancer screening program in Saudi Arabia. Ann Saudi Med. 2010;30(5):350–357. doi: 10.4103/0256-4947.67078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bcheraoui CE, Basulaiman M, Wilson S, et al. Breast cancer screening in Saudi Arabia: free but almost no takers. PLoS One. 2015;10(3):e0119051. doi: 10.1371/journal.pone.0119051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ewertz M, Jensen MB, Gunnarsdóttir KÁ, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614 [DOI] [PubMed] [Google Scholar]