Abstract
Background:
Maternal exposure to traffic-related air pollution during pregnancy has been shown to increase the risk of adverse birth outcomes and childhood disorders. High-resolution metabolomics (HRM) has previously been employed to identify metabolic responses to traffic-related air pollution in adults, including pregnant women. Thus far, no studies have examined metabolic effects of air pollution exposure in utero on neonates.
Methods:
We retrieved stored neonatal blood spots from 241 children born in California between 1998 and 2007. These healthy children were randomly selected from all California births as controls matched by birth year to children who later developed retinoblastoma. We estimated prenatal traffic-related air pollution exposure (particulate matter less than 2.5 microns (PM2.5)) during the third-trimester using the California Line Source Dispersion Model, version 4 (CALINE4) based on residential addresses recorded at birth. We employed untargeted HRM to obtain metabolic profiles, and metabolites associated with air pollution exposure were identified using partial least squares (PLS) regression and linear regressions. Biological effects were characterized using pathway enrichment analyses adjusting for potential confounders including maternal age, race/ethnicity, and education.
Results:
In total we extracted 4,038 and 4,957 metabolite features from neonatal blood spots in hydrophilic interaction (HILIC) chromatography (positive ion mode) and C18 reverse phase columns (negative ion mode), respectively. After controlling for confounding factors, partial least square regression (Variable Importance in Projection (VIP) >= 2) selected 402 HILIC positive and 182 C18 negative features as statistically significantly associated with increasing third trimester PM2.5 exposure. Using pathway enrichment analysis, we identified metabolites in oxidative stress and inflammation pathways as being altered, primarily involving lipid metabolism.
Conclusion:
The metabolite features and pathways associated with air pollution exposure in neonates suggest that maternal exposure during late pregnancy contributes to oxidative stress and inflammation in newborn children.
Keywords: High resolution metabolomics, Air pollution, Oxidative stress, Inflammation, Exposome
Introduction
Epidemiologic studies have long investigated the adverse consequences of air pollution during pregnancy, including the association with pregnancy complications, adverse birth outcomes, and serious childhood disorders including autism, childhood asthma, cancers, and obesity (Ghosh et al. 2013; Pedersen et al. 2014; Stieb et al. 2012). Biologic pathways and mechanisms underlying effects of air pollution on reproductive and child health are thought to include endocrine disruption, oxidative stress, inflammatory response, and DNA damage (Hougaard et al. 2008; Kelly 2003; Risom et al. 2005). Recently, comprehensive untargeted metabolomic studies have addressed air pollution exposure effects on human physiology as metabolites represent physiological states that are related to biological function. Metabolomic epidemiology, which aims to identify biochemical read-outs of human metabolism associated with exposures and adverse outcomes provides valuable information about biological response to exposure in human populations, and has already provided key insight into potential effects of exposure to traffic-related pollution (Jin et al. 2021). Most studies thus far have focused on short-as well as longer term air pollution exposures and fine particulate matter (PM2.5) exposures and investigated college students or adults (Breitner et al. 2016; C Chen et al. 2019; Ward-Caviness et al. 2016) (Chen 2019; Breitner, Ward-Caviness; Ladva, Golan et al. 2018, Liang, Moutinho et al. 2018, Walker, Lane et al. 2018). Fewer metabolomic study of air pollution have considered children or adolescents (C Chen et al. 2019; Z Chen et al. 2019; Wang et al. 2015), and only one investigated serum from pregnant women (Yan et al. 2019). To date, no study has examined the blood metabolome of newborn infants in response maternal air pollution exposure during pregnancy, despite pregnancy and early life being particularly vulnerable to environmental stressors. Interestingly, the metabolic pathways associated with air pollution included perturbations in lipids and lipid metabolism pathways, oxidative stress (higher levels of reactive oxygen species), inflammation, and nucleic acid damage and repair; additionally, some suggest perturbations in steroid-related pathways (e.g., glucocorticoid metabolism (Jin et al. 2021)).
We recently showed that traffic-related air pollution exposure in the first trimester affects the maternal metabolome in mid-pregnancy; however, whether these disturbances also translate to and affect the fetus has never been studied. Thus, we perform the first untargeted metabolomics study of newborn blood samples obtained from healthy infants born in California between 1998 and 2010 (Heck et al. 2013). Average air pollution exposures from traffic to PM2.5 during the third trimester of pregnancy were estimated at the residential addresses reported on birth certificates in the same manner as done in our study of mid-pregnancy maternal serum samples i.e. using an emissions based dispersion model known as the California line source dispersion model, version 4 (CALINE4) (Benson 1984). We also utilized the same high-resolution metabolomics (HRM) approach to obtain metabolic profiles in the neonates. This HRM platform has been used in four previous studies that examined ambient air pollution exposure (Ladva et al. 2018; Donghai Liang et al. 2018; Walker et al. 2018; Yan et al. 2019), and has been shown to provide a critical measure linking exposure to internal dose, biological response, and adverse health outcomes. Our approach differs from all previous air pollution metabolomics studies as it relies on the metabolome identified from dried blood spots, collected routinely during neonatal screening, providing insight into potential effects of air pollution exposure in utero.
Material and Methods
Human subject permissions were obtained from the California Committee for the Protection of Human Subjects and from the University of California, Los Angeles Institutional Review Board.
Study population
We utilized the California birth records to randomly select healthy children born between 1983 and 2011 from a previous case-control study of childhood cancer, where healthy controls were matched to children with retinoblastoma in a 1:20 ratio by birth year (see (Heck et al. 2012)). Children were eligible if they were cancer free, were born at a gestational age between 21–46 weeks and had a recorded birth weight ≥ 500g. Neonatal blood spots were collected by postpartum care service providers and mailed to the state laboratory for analyses; leftover spots were stored at −20 °C at the California Biobank. From birth records we obtained each mother-child pair’s data including information on child sex, birth year, maternal age, maternal race/ethnicity, maternal education, and parity. We were able to generate air pollution estimates only for children born between 1998 and 2007, as this was the first year that home address was reported on electronic birth certificates. Thus, out of 899 original noncancer subjects with blood spots, we were able to generate air pollution data for 248, and after excluding 7 participants with missing covariates we retained 241 subjects for analyses.
Air pollution estimation
Residential addresses as recorded on birth certificates were geocoded using OpenSource geocoding software (Goldberg et al. 2008). We estimated each woman’s average PM2.5 air pollutant exposure from traffic during the third trimester, using a modified version of CALINE4 that estimates pollution from sources within 1500 m of residential locations (for details see (Heck et al. 2013). Briefly, the prediction process used roadway geometry, traffic counts, emission factors, and meteorological parameters (wind speed, wind direction, temperature, stability class, and mixing heights) as inputs. We obtained year and season (winter, summer) and emission factors data for PM2.5 from the EMFAC2011 vehicle emissions model (California Air Resources Board 2013). Importantly, CALINE4 predictions solely represent the contribution from local traffic emissions and do not incorporate background levels of pollutants (Benson 1989; Broderick et al. 2005; Levitin et al. 2005; Marmur and Mamane 2003; Wu et al. 2016). In the following, we are only relying on PM2.5 exposure during the third trimester as the CALINE4 measures across all trimesters of pregnancy were highly correlated, as expected since they represent the same traffic sources.
High-resolution metabolomics
Neonatal blood spots were analyzed using liquid chromatography with ultra-high resolution mass spectrometry (LC-HRMS; Fusion, Thermo Scientific) using established methods (Liu et al. 2020). Samples were punched using a 5 mm hole puncher and treated with 2:1 acetonitrile in water (containing a mixture of stable isotopic internal standards). Samples were mixed on an orbital shaker at low speed for 12 hours at 0–4 °C in the dark, and then centrifuged remove particulate matter. The resulting supernatant was analyzed in triplicate using hydrophilic interaction liquid chromatography (HILIC) with positive electrospray ionization (ESI) and C18 hydrophobic reversed-phase chromatography with negative ESI to enhance the coverage of metabolic feature detection ((D. Liang et al. 2018; Liu et al. 2016). Blood spot samples were analyzed in batches of 40 that included replicate analysis of pooled plasma samples; NIST 1950 was run at the beginning and end of the study. Raw data files were extracted and aligned using apLCMS (Yu et al. 2009) with modifications by xMSanalyzer (Uppal et al. 2013). Uniquely detected ions consisted of mass-to-charge ratio (m/z), retention time (rt), and ion abundance, referred to as metabolite features. Prior to data analysis, metabolite features were batch corrected using wavelet analysis (Deng et al. 2019). For data analysis, metabolite features were limited to those detected in > 25% of blood spot samples, and median coefficients of variation (CV) among technical replicates and Pearson correlation of <30% and > 0.7, respectively. Following quality assessment, replicate intensities were summarized using the median value, log2 transformed, and auto-scaled. Missing values were imputed by one-half of the lowest signal detected for that feature across all samples.
Statistical analysis
Feature selection was conducted using a combination of univariate and multivariate analyses to discover biologically relevant metabolites while reducing the likelihood for false positive findings (Saccenti et al. 2014). Specifically, we conducted partial least squares (PLS) regression followed by linear regression to identify metabolic features associated with PM2.5 levels derived from our model. We adjusted for potential confounders selected a-priori due to their association with lifestyle factors that may be related to maternal and infant metabolite levels and also air pollution exposures including maternal age, maternal race, birth year, preterm birth, parity, and census-based neighborhood socioeconomic status (nSES) (Yost et al. 2001). California birth records did not collect maternal smoking during these years; estimating the prevalence of smoking in pregnancy according to cotinine detection in newborn blood spots will undercounted smoking if the mother did not smoke around the time of birth or did not breastfeed. Thus, we adjusted for detection of cotinine (yes/no) in blood spots as a sensitivity analysis. For PLS regression, we regressed each features’ intensity on these potential confounding variables and formed residuals as the input matrix. Ten-fold cross-validation was used to assess the performance of features selected by PLS regression. Features with Variable Importance in Projection (VIP) scores > 2 and regression p-value < 0.05 were selected.
Annotation and pathway analysis
PM2.5 -associated features were first matched to a reference database of authenticated chemical standards (identification confidence level 1) previously analyzed using the same HRM platform using an accurate mass threshold of ±5 parts-per-million; ppm and retention time error of ±15s. Details about the reference database has been published previously (Liu et al. 2020; Nagiah et al. 2015). Additional metabolomic features not matching these metabolites were annotated using xMSannotator. Accurate mass m/z for adducts formed under positive or negative ESI mode was matched to the Human Metabolome Database (HMDB) with a mass error threshold of 10 ppm. xMSannotator uses a scoring system based upon correlation modularity clustering combined with isotopic, adduct and mass defect grouping to improve annotation of high-resolution mass spectrometry data (Uppal et al. 2017). The metabolite identification confidence levels were reported for all annotation results (Schrimpe-Rutledge et al. 2016).
To facilitate biological interpretation, we conducted pathway enrichment analysis using mummichog v 2.4.2 (Li et al. 2013) and identified metabolic pathways associated with PM2.5. Mummichog uses a permutation-based framework that accounts for the complexity of untargeted mass spectral data. All metabolic features meeting the PLS VIP threshold and p<0.05 were included in pathway enrichment analysis. All metabolites annotated by mummichog were required to present in at least their primary adduct (M+H or M-H for positive and negative mode, respectively) to reduce the false positive match rate. A pathway was considered significant if gamma adjusted p-values were smaller than 0.05. Only pathways that contained at least three discriminative metabolites were interpreted.
Results
The demographic characteristics of the 241 subjects in this study are provided in Table 1. Almost half of the mothers were of Hispanic origin. Of all children, 38.2% were first born and 11.2% were born preterm, and more than half were born between 2001 and 2003. Only about 30% of mothers lived in neighborhoods ranked within the two highest levels of socio-economic status at the time of giving birth.
Table 1.
Distribution of Demographics and Exposure (PM2.5) levels
| Controls (n=241) | ||
|---|---|---|
| N | % | |
|
| ||
| Maternal Age | ||
| < 20 | 28 | 11.62 |
| 20–24 | 47 | 19.50 |
| 25–29 | 69 | 28.63 |
| 30–34 | 62 | 25.73 |
| >= 35 | 35 | 14.52 |
| Maternal Race/Ethnicity | ||
| White non-Hispanics | 70 | 29.05 |
| Hispanic of any race | 117 | 48.55 |
| Other/not specified | 54 | 22.41 |
| Birth Year | ||
| 1998–2000 | 84 | 34.85 |
| 2001–2003 | 100 | 41.49 |
| 2004–2007 | 57 | 23.65 |
| Census-based neighborhood SES level | ||
| 1 (low) | 51 | 21.16 |
| 2 | 63 | 26.14 |
| 3 | 51 | 21.16 |
| 4 | 39 | 16.18 |
| 5 (high) | 37 | 15.35 |
| Parity | ||
| 0 | 92 | 38.17 |
| 1 | 75 | 31.12 |
| >= 2 | 74 | 30.71 |
| Preterm Birth | ||
| Term birth | 214 | 88.80 |
| Preterm birth | 27 | 11.20 |
| Last trimester PM2.5(μg/m3) | ||
| Mean (SD) | 0.726 (0.732) | |
| Min | 0 | |
| Max | 5.29 | |
In total, we detected 14,555 features (6,139 in HILIC column and 8,416 in C18 column), but after filtering for missing values, 8,995 features remained (4,038 in HILIC column and 4,957 in C18 column). We selected 402 HILICpos and 182 C18neg features as significantly associated with PM2.5 (Figure 1). Among these significant features, we confirmed 6 metabolites using authentic standards (confidence level 1, Table 2). Pathway enrichment analysis showed that features associated with PM2.5 were associated with 9 enriched pathways (Table 3).
Figure 1. Identification of metabolic features associated with PM2.5 exposure among studied infants.
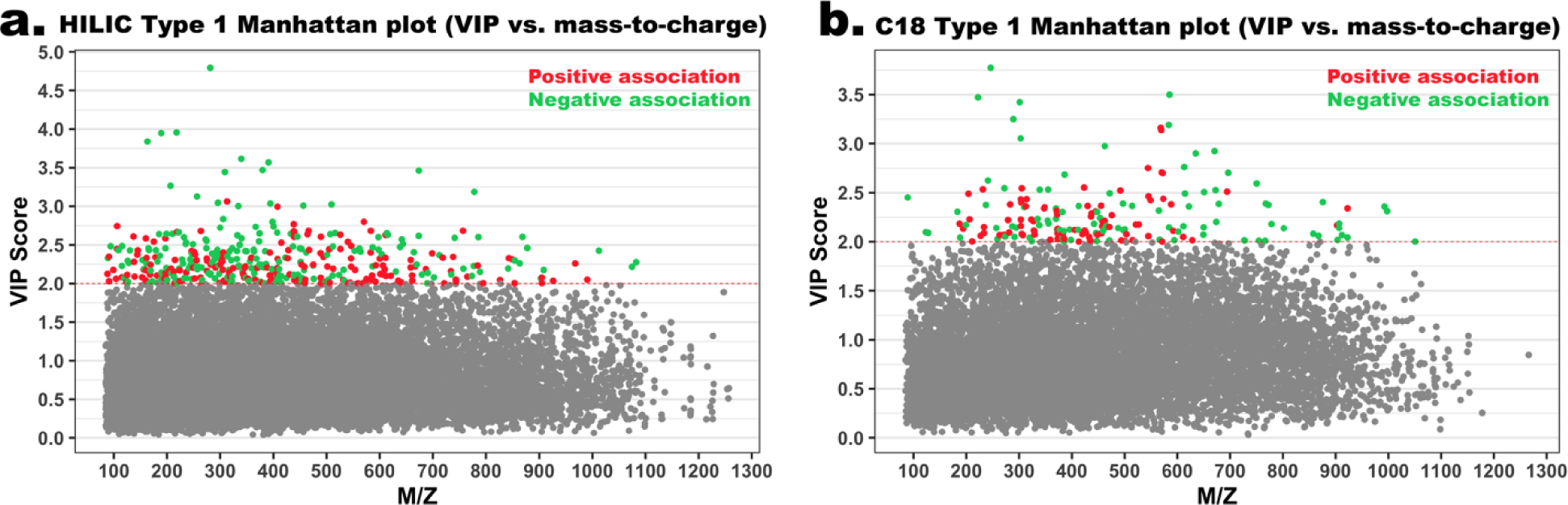
A) Type 1 Manhattan plot for features in the HILIC column (positive ion mode), VIP score vs m/z. Red dots represent features that were positively associated with PM2.5 exposure and green dots represent features that were negatively associated with PM2.5 exposure; B) Type 1 Manhattan plot for features in the C18 column (negative ion mode), VIP score vs mass-to-charge.
Table 2.
Confirmed a chemical identity of metabolic features associated with PM2.5 among studied infants
| m/z | RT (s) | Adduct Form | Metabolite | VIP | Coefficient | P-value | Mode |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 137.0458 | 40.7 | M+H | Hypoxanthine | 2.61 | 1.51E-01 | 2.47E-02 | HILICpos |
| 300.2896 | 21.8 | M+H | Sphingosine | 2.25 | 2.12E-01 | 1.93E-02 | HILICpos |
| 673.2991 | 89.8 | M+H | Methylcobalamin | 2.62 | −2.34E-01 | 2.36E-03 | HILICpos |
| 123.0450 | 18.9 | M-H | 3-Hydroxybenzyl alcohol | 2.10 | −1.90E-01 | 4.46E-03 | C18neg |
| 303.2334 | 246.5 | M-H | Arachidonic acid | 2.43 | 1.51E-01 | 6.51E-03 | C18neg |
| 327.2335 | 234.9 | M-H | Docosahexaenoic acid | 2.43 | 1.89E-01 | 2.40E-03 | C18neg |
Chemical identification was conducted by matching peaks by accurate mass and retention time to authentic reference standards in an in-house library run under identical conditions using tandem mass spectrometry.
Table 3.
Enriched metabolomic pathways associated with PM 2.5 among studied infants
| Pathways | Overlap size | Pathway size | p-value | Mode |
|---|---|---|---|---|
|
| ||||
| De novo fatty acid biosynthesis | 6 | 28 | 0.00059 | HILICpos |
| Fatty acid activation | 5 | 27 | 0.00176 | HILICpos |
| Glycerophospholipid metabolism | 6 | 51 | 0.00773 | HILICpos |
| Arachidonic acid metabolism | 4 | 25 | 0.00815 | HILICpos |
| Fatty Acid Metabolism | 3 | 17 | 0.01588 | HILICpos |
| Methionine and cysteine metabolism | 4 | 38 | 0.03353 | HILICpos |
| Carnitine shuttle | 3 | 27 | 0.04579 | HILICpos |
| De novo fatty acid biosynthesis | 3 | 30 | 0.03243 | C18neg |
| Prostaglandin formation from arachidonate | 3 | 33 | 0.04042 | C18neg |
We list annotation results of metabolites within each enriched pathways in Supplemental Table 1. Several pathways we identified as being associated with air pollution exposure are lipid-related metabolic pathways including fatty acid activation, de novo fatty acid biosynthesis, fatty acid metabolism, the carnitine shuttle, and glycerophospholipid metabolism. Changes in these pathways indicate associations between third trimester air pollution exposure and oxidative stress in newborns. Disruption of prostaglandin formation from arachidonate and the arachidonic acid metabolism reflect an inflammatory response. Finally, we also observed air pollution related differences in amino acid metabolism pathways specifically methionine and cysteine metabolism, two sulphur containing proteinogenic amino acids in the same metabolic pathway. Methionine is a key methyl-group donor and precursor for the important antioxidant glutathione and intermediate in the biosynthesis of phosphatidylcholine and other phospholipids. Sensitivity analyses in which we adjusted for the detection of cotinine in the newborns blood corroborated the identification of all lipid related pathways but not this last amino acid pathway (see supplemental table 2). All associated fatty acid metabolites associated with traffic related exposure are shown in Supplemental Table 3; and their correlations in Supplemental Figure 1.
Discussion
Our analysis of metabolites in neonatal blood spots shows that third trimester traffic related air pollution exposure experienced by pregnant California women contributes to alterations in fatty acid metabolism and biosynthesis pathways, in the carnitine shuttle, and glycerophospholipid metabolism, and disturbances in eicosanoids including prostaglandins, and in methionine and cysteine metabolism in the newborn metabolome. The pathways we identified are strongly indicative of potential alterations in inflammation and oxidative stress pathways in support of previous air pollution metabolomic studies recently summarized in (Jin et al. 2021) (see also Figure 2). Pro-inflammatory metabolites, which include arachidonic acid (precursor in the biosynthesis of prostaglandins and leukotrienes) were upregulated while some metabolites with anti-inflammation effects, such as linolenic acid, were downregulated with increased traffic related PM2.5 exposures. Thus, these results suggest that air pollutants disrupt inflammation signaling linked to the antioxidant-oxidant balance. Furthermore, identification of alterations in these pathways shows that storage conditions for infant dried blood spots were sufficient to prevent uncontrolled deterioration. This substantiates the utility of this valuable resource to support retrospective studies of environmental exposures and biologic responses.
Figure 2.
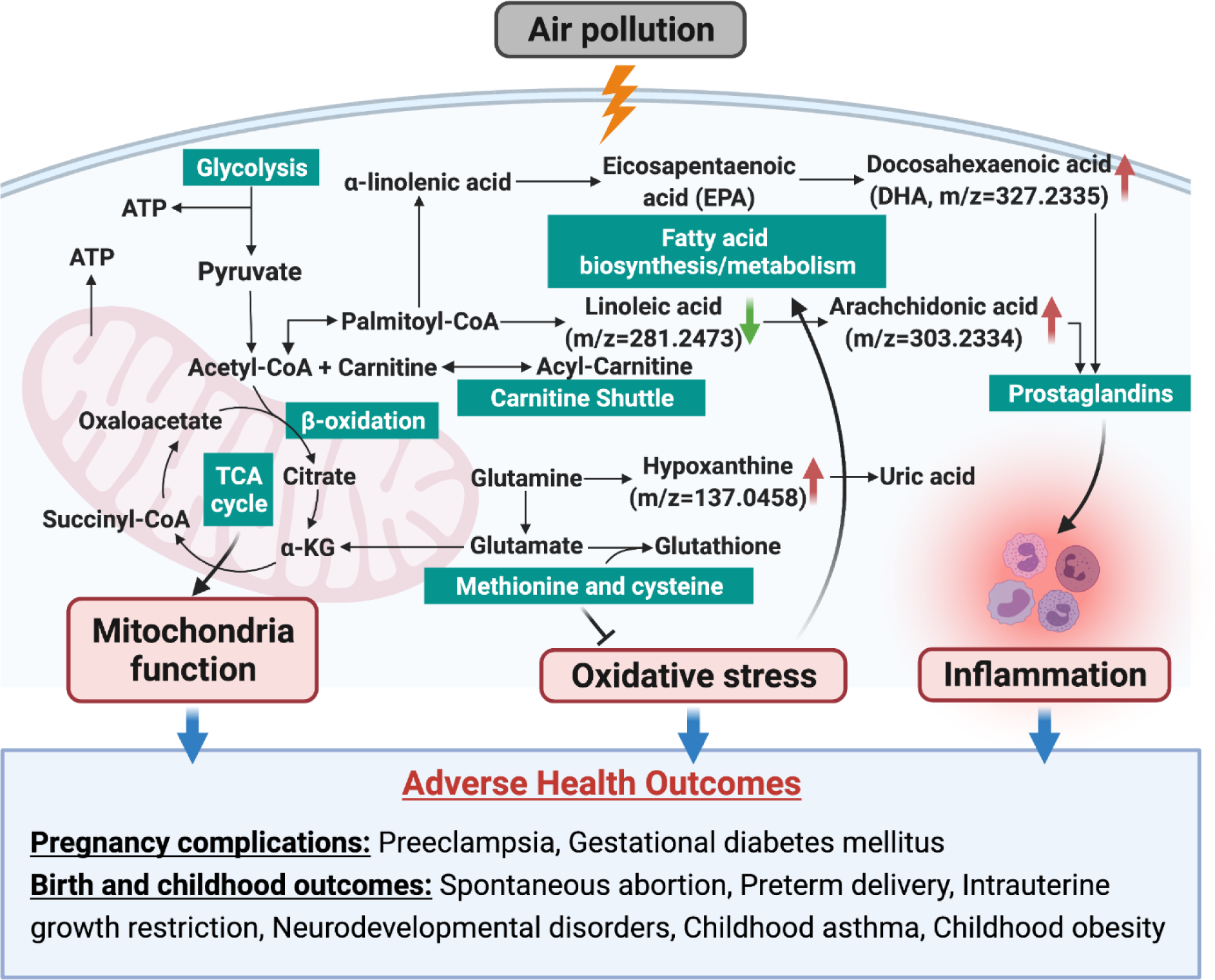
Metabolic pathways enriched in response to late pregnancy air pollution suggest increased oxidative stress and inflammation in neonates.
Constituents of coarse, fine, and ultrafine particulate matter in traffic-related air pollution are capable of producing reactive oxygen species (ROS) due to the unique physicochemistry of the inorganic and organic compounds they consist of, including metals and polycyclic aromatic hydrocarbons (PAHs) (Lai et al. 2016). Particles can also cause dysfunction of mitochondria and activate inflammatory cells that produce ROS and/or reactive nitrogen species (RNS) (Li et al. 2003; Risom et al. 2005; Tian et al. 2009). It has been repeatedly shown in in-vivo and in-vitro experiments that exposure to air pollution induces oxidative stress and inflammatory reactions (Daher et al. 2014; Dick et al. 2003; Ghio et al. 2012; Guerra et al. 2013; Happo et al. 2013). It has also been documented that air pollution exposure elicits oxidative stress responses in pregnancy (Anderson et al. 2018; Nagiah et al. 2015) that can damage the placenta and its function. Thus, oxidative stress is a mechanism that contributes to adverse birth outcomes such as spontaneous abortion, preeclampsia, intrauterine growth restriction, low birth weight, and preterm delivery (Al-Gubory et al. 2010; Duhig et al. 2016; Lavigne et al. 2018; Peter Stein et al. 2008).
Mechanistic models that use metabolomics to study biological effects of air pollution exposure show similar metabolic changes to those observed in the present study. PM2.5 samples were collected from a polluted city in China and adult male rats were exposed intratracheally with a particulate suspension once a week over three months. When extracts of the exposed lung tissue were assessed using untargeted metabolomics profiling the researchers found differences in metabolites involved in lipid and nucleotide metabolism and a disturbed pro-oxidant/antioxidant balance that suggests increased oxidative stress (Wang et al. 2017). Another group exposed female mice to inhalational concentrates of ambient PM2.5 or filtered air for 10 months and analyzed serum samples with liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) (Xu et al. 2019). The 148 metabolites that differed between exposed and unexposed mice mainly suggested pathway perturbations in amino acid and lipid metabolism, in addition to alterations in stress hormone metabolites and circadian rhythm biomarkers.
We found fatty acid and amino acid pathways in newborns to be affected by late pregnancy air pollution exposure. Almost all free polyunsaturated fatty acids including docosahexaenoic-acid (DHA) and eicosapentaenoic-acid (EPA) were positively associated with air pollution exposures (see Supplemental Table 1). DHA is not only the most abundant n-3 fatty acid in the entire nervous system but required for neuronal regeneration and formation of synapses during fetal brain development; it is also a component of prenatal supplements and infant formula (Campoy et al. 2012). These long chain polyunsaturated fatty acids are strongly related to fish intake. However, fatty acids are not only nutrients and part of complex lipids, but also act as potent and specific blood-borne signaling molecules that can act upon cells directly, as well as function as signaling molecules that accelerate or decelerate chemical reactions or specific processes in cells (Glatz and Luiken 2015). Only a minor proportion of these n-3 fatty acids are of endogenous origin (Lauritzen et al. 2016), and due to the hydrophobic nature of (long-chain) fatty acids in aqueous fluids such as blood they are overwhelmingly bound to albumin or present as fatty esters in lipoproteins leaving only nanomolar amounts of free fatty acid in serum (Richieri and Kleinfeld 1995). Thus, the free fatty acids we measured using our untargeted metabolomics approach are most likely generated by phospholipase-dependent cell signaling events, many of which are related to inflammatory responses. For example, fatty acid compounds such as linoleic acid and arachidonic acid are catalyzed by lipoxygenase enzymes expressed by circulating immune cells to generate inflammatory mediators (Mashima and Okuyama 2015). Interestingly, ours is not the first study to find higher polyunsaturated fatty acids associated with environmental exposures. One report linked higher perfluoroalkyl substance levels in elderly Swedish individuals with metabolites predominantly from lipid pathways specifically multiple glycerophosphocholines and fatty acids including DHA (Salihovic et al. 2019). Recently, researchers measured a panel of 53 eicosanoids in plasma of pregnant women and found elevated levels for a number of fatty acid parent compounds to be associated with higher levels of phthalates, PAHs, and metals in maternal urine samples that were collected together with the plasma samples (Aung et al. 2021).
The picture that emerges from our findings points to a general disturbance of mechanisms related to cell membrane function and signaling with vital importance for fetal development and growth. Lipid membranes are amongst the most vulnerable cellular components targeted by reactive oxygen species and oxidative stress (Axelsen et al. 2011) such as constituents of traffic-related air particles like PAHs or metals that have been shown to generate free radicals and oxidative stress (Kelly 2003). Oxidative stress can activate phospholipase A2 (PLA2) which then hydrolyzes cell membrane phospholipids to generate omega-6 polyunsaturated free fatty acids (PUFAs) such linoleic and arachidonic acid (Anthonymuthu et al. 2018; Sato et al. 2016). We have previously shown that exposure to traffic related air pollution in the first trimester increased maternal serum metabolites in the linoleate pathway (Yan et al. 2019), specifically we found gamma-linolenic acid to be negatively associated with air pollution exposure, which has been consistently observed in other air pollution studies that leverage an untargeted metabolomics approach (Donghai Liang et al. 2018; Walker et al. 2018). In our current study, linoleic acids measured using newborn blood spots– which is metabolized to gamma-linolenic acid - was consistently negatively associated with air pollution (see supplemental Table 1).
When the omega-6 PUFA arachidonic acid is released from cell membranes, it is either converted to eicosanoids through the lipoxygenase (LOX) pathway and then further to leukotrienes and other lipoxins or to prostaglandin via the cyclooxygenase (COX) pathway (Tam et al. 2013). Both leukotrienes and prostaglandins are major proinflammatory mediators. Previously, prostaglandins have been shown to be increased with inhalation of NO2 (Yan et al. 2016), ozone (Peden 1999; Peden 2001), PM2.5, and sulfate exposures (Li et al. 2016). In pregnancy, prostaglandins are responsible for abnormal placental and uterine blood flow and uterine contractions and possibly also preeclampsia (Kaaja et al. 1995; Ogburn et al. 1984).
Another metabolite we found to be positively associated with traffic related air pollution exposure is hypoxanthine which is oxidized to uric acid, a process during which reactive oxygen species are generated. For example hypoxanthine levels in plasma increase with cigarette smoking (Chang et al. 2005) and heavy alcohol consumption (Yamamoto et al. 2005). As previously observed in our study of mid-pregnancy serum samples from highly exposed women (Yan et al 2019), air pollution is associated with alterations in pathways of the sulfur-containing amino acids methionine and cysteine, which are readily oxidized (Berlett and Stadtman 1997; Pisoschi and Pop 2015); sulfur residues are responsible for more than 70% of ROS interactions with proteins (Johannes and Majcherczyk 2000). Interestingly, oxidation of methionine has also been described to occur in rodents’ lungs and lung fluid exposed to ambient particulate matter extracted from roadsides or high pollution haze from Chinese cities (Lai et al. 2016; Lee et al. 2014; Pardo et al. 2016). Methionine oxidation been shown to occur after carbon black, diesel exhaust, and urban dust exposures in cell-based experiments (Lai et al 2016). The methionine to cysteine pathways includes folate production, and vitamin B12 is an important co-enzyme in this pathway. In our study vitamin B 12 (methylcobalamin) was found to be decreased in association with maternal air pollution during the third trimester. Finally, we also identified sphingosine as associated with increasing exposure levels, providing additional evidence of cell membrane damage. This metabolite – together with fatty acids, phospholipids, acyl carnitines, and arachidonic acid - has also been associated with PM2.5 levels in a randomized cross over trial of acute air pollution exposure among Chinese college students (C Chen et al. 2019).
While our CALINE4 dispersion model-based estimation of traffic related air pollution has high spatial resolution, it only represents the impact from local traffic sources near the mother’s home while ignoring regional transport of pollutants. It also does not represent all personal air pollution exposures because sources other than local traffic are not included. We also do not know how much time the newborns’ mothers spent at home versus work in late pregnancy and indoors or outdoors and how much outdoor air penetrated indoors. However, for representation of localized traffic emissions sources in California, the CALINE4 model has been extensively validated (Benson 1984). Additionally, previously moderate to high correlations (R = 0.55–0.95) of CALINE4-modeled estimates with measured variability of traffic-related air pollutants [e.g., NOx and nitrogen dioxide] has been reported in urban communities (Gauderman et al. 2005; Jerrett et al. 2005). We also found high correlations (R = 0.87) of CALINE4-modeled monthly NOx concentrations with measurements at nine monitoring sites in the Long Beach study area in December 2007 and April 2008 in our own studies (Wu et al. 2009; Wu et al. 2011)
Active or passive smoking and diet may cause differences in metabolites, but we did not have information on maternal smoking behavior or dietary intake during pregnancy. In order to confound our air pollution exposure associations, smoking or diet would not only have to influence metabolite status but would also need to be related to air pollution exposures in the last trimester. We believe that we controlled at least partially for potential confounding due to lifestyle factors by adjusting for maternal age, maternal race/ethnicity, and maternal education. Additionally, we are able to identify cotinine level in the serum by matching the accurate mass m/z (177.1022) and retention time (32.8s) to an authentic standard. While previous studies have shown that cotinine is a reliable marker of active smoking (Benson 1989; Gauderman et al. 2005; Jerrett et al. 2005), cotinine levels in newborn blood spots (identified in 14 infants) are likely to not detect active smoking if women did not breastfeed, and passive smoking is unlikely to be identifiable via blood spot cotinine (Yang et al. 2013). Our sensitivity analyses in which we adjusted for cotinine detected in the DBS suggested the same pathways related to air pollution, i.e. inflammation and oxidative stress, however, residual uncontrolled confounding is possible.
Overall, this is the first study to find metabolomic changes due to third trimester prenatal air pollution exposure in newborn children’s blood spots, which included alteration in lipid and amino-acid metabolism related to oxidative stress and inflammatory pathways. Our study provides additional evidence for the long-lasting negative impacts of air pollution on developing organisms, and supports the use of newborn dried blood spots as a valuable resource for investigating biologic impacts of prenatal exposure to environmental pollutants.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health grants R03CA252788, R21ES018960, R21ES019986 and the California Tobacco-Related Disease Research Program of the University of California, grant number 24RT-0033H, T29DT0485.
The biospecimens and data used in this study were obtained from the California Biobank Program, (SIS request number 565). The California Department of Public Health is not responsible for the results or conclusions drawn by the authors of this publication.
Footnotes
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-Gubory KH, Fowler PA, Garrel C. 2010. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. The international journal of biochemistry & cell biology 42:1634–1650. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Naidoo RN, Ramkaran P, Phulukdaree A, Muttoo S, Asharam K, et al. 2018. The effect of nitric oxide pollution on oxidative stress in pregnant women living in durban, south africa. Arch Environ Contam Toxicol 74:228–239. [DOI] [PubMed] [Google Scholar]
- Anthonymuthu TS, Kenny EM, Lamade AM, Kagan VE, Bayır H. 2018. Oxidized phospholipid signaling in traumatic brain injury. Free Radic Biol Med 124:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MT, Yu Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, et al. 2021. Cross-sectional estimation of endogenous biomarker associations with prenatal phenols, phthalates, metals, and polycyclic aromatic hydrocarbons in single-pollutant and mixtures analysis approaches. Environ Health Perspect 129:37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen PH, Komatsu H, Murray IV. 2011. Oxidative stress and cell membranes in the pathogenesis of alzheimer’s disease. Physiology (Bethesda) 26:54–69. [DOI] [PubMed] [Google Scholar]
- Benson P 1989. Caline4 - a dispersion model for predicting air pollutant concentrations near roadways.California Department of Transportation, CA. [Google Scholar]
- Benson PE. 1984. Caline 4-a dispersion model for predictiong air pollutant concentrations near roadways.
- Berlett BS, Stadtman ER. 1997. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316. [DOI] [PubMed] [Google Scholar]
- Breitner S, Schneider A, Devlin RB, Ward-Caviness CK, Diaz-Sanchez D, Neas LM, et al. 2016. Associations among plasma metabolite levels and short-term exposure to pm2. 5 and ozone in a cardiac catheterization cohort. Environment international 97:76–84. [DOI] [PubMed] [Google Scholar]
- Broderick BM, Budd U, Misstear BD, Ceburnis D, Jennings SG. 2005. Validation of caline4 modelling for carbon monoxide concentrations under free-flowing and congested traffic conditions in ireland. International Journal of Environment and Pollution 24:104–113. [Google Scholar]
- California Air Resources Board. 2013. Emfac2011 - technical documentation. Sacramento, CA:California Air Resources Board. [Google Scholar]
- Campoy C, Escolano-Margarit MV, Anjos T, Szajewska H, Uauy R. 2012. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br J Nutr 107 Suppl 2:S85–106. [DOI] [PubMed] [Google Scholar]
- Chang SJ, Chen SM, Chiang SL, Chang KL, Ko YC. 2005. Association between cigarette smoking and hypoxanthine guanine phosphoribosyltransferase activity. Kaohsiung J Med Sci 21:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Li H, Niu Y, Liu C, Lin Z, Cai J, et al. 2019. Impact of short-term exposure to fine particulate matter air pollution on urinary metabolome: A randomized, double-blind, crossover trial. Environ Int 130:104878. [DOI] [PubMed] [Google Scholar]
- Chen Z, Newgard CB, Kim JS, O II, Alderete TL, Thomas DC, et al. 2019. Near-roadway air pollution exposure and altered fatty acid oxidation among adolescents and young adults - the interplay with obesity. Environ Int 130:104935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher N, Saliba NA, Shihadeh AL, Jaafar M, Baalbaki R, Shafer MM, et al. 2014. Oxidative potential and chemical speciation of size-resolved particulate matter (pm) at near-freeway and urban background sites in the greater beirut area. Science of The Total Environment 470–471:417–426. [DOI] [PubMed] [Google Scholar]
- Deng K, Zhang F, Tan Q, Huang Y, Song W, Rong Z, et al. 2019. Waveica: A novel algorithm to remove batch effects for large-scale untargeted metabolomics data based on wavelet analysis. Analytica Chimica Acta. [DOI] [PubMed] [Google Scholar]
- Dick CAJ, Singh P, Daniels M, Evansky P, Becker S, Gilmour MI. 2003. Murine pulmonary inflammatory responses following instillation of size-fractionated ambient particulate matter. Journal of Toxicology and Environmental Health, Part A 66:2193–2207. [DOI] [PubMed] [Google Scholar]
- Duhig K, Chappell LC, Shennan AH. 2016. Oxidative stress in pregnancy and reproduction. Obstet Med 9:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Lurmann F, Kuenzli N, Gilliland F, Peters J, et al. 2005. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology 16:737–743. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. 2012. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. Journal of Toxicology and Environmental Health, Part B 15:1–21. [DOI] [PubMed] [Google Scholar]
- Ghosh JK, Heck JE, Cockburn M, Su J, Jerrett M, Ritz B. 2013. Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am J Epidemiol 178:1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz JF, Luiken JJ. 2015. Fatty acids in cell signaling: Historical perspective and future outlook. Prostaglandins Leukot Essent Fatty Acids 92:57–62. [DOI] [PubMed] [Google Scholar]
- Goldberg DW, Wilson JP, Knoblock CA, Ritz B, Cockburn MG. 2008. An effective and efficient approach for manually improving geocoded data. Int J Health Geogr 7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra R, Vera-Aguilar E, Uribe-Ramirez M, Gookin G, Camacho J, Osornio-Vargas AR, et al. 2013. Exposure to inhaled particulate matter activates early markers of oxidative stress, inflammation and unfolded protein response in rat striatum. Toxicology Letters 222:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happo MS, Uski O, Jalava PI, Kelz J, Brunner T, Hakulinen P, et al. 2013. Pulmonary inflammation and tissue damage in the mouse lung after exposure to pm samples from biomass heating appliances of old and modern technologies. Science of The Total Environment 443:256–266. [DOI] [PubMed] [Google Scholar]
- Heck JE, Lombardi CA, Meyers TJ, Cockburn M, Wilhelm M, Ritz B. 2012. Perinatal characteristics and retinoblastoma. Cancer Causes & Control 23:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Wu J, Lombardi C, Qiu J, Meyers TJ, Wilhelm M, et al. 2013. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect 121:1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, Saber AT, et al. 2008. Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Part Fibre Toxicol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Arain A, Kanaroglou P, Beckerman B, Potoglou D, Sahsuvaroglu T, et al. 2005. A review and evaluation of intraurban air pollution exposure models. J Expo Anal Environ Epidemiol 15:185–204. [DOI] [PubMed] [Google Scholar]
- Jin L, Godri Pollitt KJ, Liew Z, Rosen Vollmar AK, Vasiliou V, Johnson CH, et al. 2021. Use of untargeted metabolomics to explore the air pollution-related disease continuum. Curr Environ Health Rep 8:7–22. [DOI] [PubMed] [Google Scholar]
- Johannes C, Majcherczyk A. 2000. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaja R, Tikkanen MJ, Viinikka L, Ylikorkala O. 1995. Serum-lipoproteins, insulin, and urinary prostanoid metabolites in normal and hypertensive pregnant-women. Obstet Gynecol 85:353–356. [DOI] [PubMed] [Google Scholar]
- Kelly FJ. 2003. Oxidative stress: Its role in air pollution and adverse health effects. Occup Environ Med 60:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladva CN, Golan R, Liang D, Greenwald R, Walker DI, Uppal K, et al. 2018. Particulate metal exposures induce plasma metabolome changes in a commuter panel study. PLoS One 13:e0203468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Lee CN, Bai KJ, Yang YL, Chuang KJ, Wu SM, et al. 2016. Protein oxidation and degradation caused by particulate matter. Sci Rep 6:33727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen L, Brambilla P, Mazzocchi A, Harsløf LB, Ciappolino V, Agostoni C. 2016. Dha effects in brain development and function. Nutrients 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, Burnett RT, Stieb DM, Evans GJ, Godri Pollitt KJ, Chen H, et al. 2018. Fine particulate air pollution and adverse birth outcomes: Effect modification by regional nonvolatile oxidative potential. Environ Health Perspect 126:077012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Wong CK, Chuang KJ, Bien MY, Cao JJ, Han YM, et al. 2014. Methionine oxidation in albumin by fine haze particulate matter: An in vitro and in vivo study. J Hazard Mater 274:384–391. [DOI] [PubMed] [Google Scholar]
- Levitin J, Harkonen J, Kukkonen J, Nikmo J. 2005. Evaluation of the caline4 and car-fmi models against measurements near a major road. Atmospheric Environment 39:4439–4452. [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. 2003. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, et al. 2013. Predicting network activity from high throughput metabolomics. PLoS Comput Biol 9:e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, et al. 2016. Short-term exposure to air pollution and biomarkers of oxidative stress: The framingham heart study. J Am Heart Assoc 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, et al. 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environ Int 120:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, et al. 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environment international 120:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Walker DI, Uppal K, Tran V, Rohrbeck P, Mallon TM, et al. 2016. High-resolution metabolomics assessment of military personnel: Evaluating analytical strategies for chemical detection. Journal of occupational and environmental medicine 58:S53–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Nellis M, Uppal K, Ma C, Tran V, Liang Y, et al. 2020. Reference standardization for quantification and harmonization of large-scale metabolomics. Analytical Chemistry 92:8836–8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmur A, Mamane Y. 2003. Comparison and evaluation of several mobile-source and line-source models in israel. Transportation Research Part D-Transport and Environment 8:249–265. [Google Scholar]
- Mashima R, Okuyama T. 2015. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol 6:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiah S, Phulukdaree A, Naidoo D, Ramcharan K, Naidoo RN, Moodley D, et al. 2015. Oxidative stress and air pollution exposure during pregnancy: A molecular assessment. Hum Exp Toxicol 34:838–847. [DOI] [PubMed] [Google Scholar]
- Ogburn PL Jr., Williams PP, Johnson SB, Holman RT. 1984. Serum arachidonic acid levels in normal and preeclamptic pregnancies. Am J Obstet Gynecol 148:5–9. [DOI] [PubMed] [Google Scholar]
- Pardo M, Porat Z, Rudich A, Schauer JJ, Rudich Y. 2016. Repeated exposures to roadside particulate matter extracts suppresses pulmonary defense mechanisms, resulting in lipid and protein oxidative damage. Environ Pollut 210:227–237. [DOI] [PubMed] [Google Scholar]
- Peden DB. 1999. 37 - controlled exposures of asthmatics to air pollutants. In: Air pollution and health, (Holgate ST, Samet JM, Koren HS, Maynard RL, eds). London:Academic Press, 865–880. [Google Scholar]
- Peden DB. 2001. Air pollution in asthma: Effect of pollutants on airway inflammation. Ann Allergy Asthma Immunol 87:12–17. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Stayner L, Slama R, Sørensen M, Figueras F, Nieuwenhuijsen MJ, et al. 2014. Ambient air pollution and pregnancy-induced hypertensive disorders: A systematic review and meta-analysis. Hypertension 64:494–500. [DOI] [PubMed] [Google Scholar]
- Peter Stein T, Scholl TO, Schluter MD, Leskiw MJ, Chen X, Spur BW, et al. 2008. Oxidative stress early in pregnancy and pregnancy outcome. Free Radic Res 42:841–848. [DOI] [PubMed] [Google Scholar]
- Pisoschi AM, Pop A. 2015. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 97:55–74. [DOI] [PubMed] [Google Scholar]
- Richieri GV, Kleinfeld AM. 1995. Unbound free fatty acid levels in human serum. J Lipid Res 36:229–240. [PubMed] [Google Scholar]
- Risom L, Møller P, Loft S. 2005. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res 592:119–137. [DOI] [PubMed] [Google Scholar]
- Saccenti E, Hoefsloot HCJ, Smilde AK, Westerhuis JA, Hendriks MMWB. 2014. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 10:361–374. [Google Scholar]
- Salihovic S, Fall T, Ganna A, Broeckling CD, Prenni JE, Hyötyläinen T, et al. 2019. Identification of metabolic profiles associated with human exposure to perfluoroalkyl substances. J Expo Sci Environ Epidemiol 29:196–205. [DOI] [PubMed] [Google Scholar]
- Sato H, Taketomi Y, Murakami M. 2016. Metabolic regulation by secreted phospholipase a2. Inflamm Regen 36:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA. 2016. Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom 27:1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. 2012. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res 117:100–111. [DOI] [PubMed] [Google Scholar]
- Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, et al. 2013. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 154:213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Koshland CP, Yano J, Yachandra VK, Yu IT, Lee SC, et al. 2009. Carbon-centered free radicals in particulate matter emissions from wood and coal combustion. Energy Fuels 23:2523–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, et al. 2013. Xmsanalyzer: Automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics 14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Jones DP. 2017. Xmsannotator: An r package for network-based annotation of high-resolution metabolomics data. Anal Chem 89:1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DI, Lane KJ, Liu K, Uppal K, Patton AP, Durant JL, et al. 2018. Metabolomic assessment of exposure to near-highway ultrafine particles. Journal of exposure science & environmental epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jiang S, Liu Y, Du X, Zhang W, Zhang J, et al. 2017. Comprehensive pulmonary metabolome responses to intratracheal instillation of airborne fine particulate matter in rats. Sci Total Environ 592:41–50. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zheng Y, Zhao B, Zhang Y, Liu Z, Xu J, et al. 2015. Human metabolic responses to chronic environmental polycyclic aromatic hydrocarbon exposure by a metabolomic approach. Journal of proteome research 14:2583–2593. [DOI] [PubMed] [Google Scholar]
- Ward-Caviness CK, Breitner S, Wolf K, Cyrys J, Kastenmüller G, Wang-Sattler R, et al. 2016. Short-term no2 exposure is associated with long-chain fatty acids in prospective cohorts from augsburg, germany: Results from an analysis of 138 metabolites and three exposures. Int J Epidemiol 45:1528–1538. [DOI] [PubMed] [Google Scholar]
- Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, Ritz B. 2009. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of california. Environ Health Perspect 117:1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wilhelm M, Chung J, Ritz B. 2011. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ Res 111:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Laurent O, Li L, Hu J, Kleeman M. 2016. Adverse reproductive health outcomes and exposure to gaseous and particulate-matter air pollution in pregnant women. Research report (Health Effects Institute):1–58. [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang W, Zhou J, Chen M, Huang X, Zhu Y, et al. 2019. Metabolomics analysis of a mouse model for chronic exposure to ambient pm(2.5). Environ Pollut 247:953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Moriwaki Y, Takahashi S. 2005. Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid). Clin Chim Acta 356:35–57. [DOI] [PubMed] [Google Scholar]
- Yan Q, Liew Z, Uppal K, Cui X, Ling C, Heck JE, et al. 2019. Maternal serum metabolome and traffic-related air pollution exposure in pregnancy. Environ Int 130:104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Yun Y, Ku T, Li G, Sang N. 2016. No2 inhalation promotes alzheimer’s disease-like progression: Cyclooxygenase-2-derived prostaglandin e2 modulation and monoacylglycerol lipase inhibition-targeted medication. Sci Rep-Uk 6:22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Pearl M, Jacob P 3rd, DeLorenze GN, Benowitz NL, Yu L, et al. 2013. Levels of cotinine in dried blood specimens from newborns as a biomarker of maternal smoking close to the time of delivery. American journal of epidemiology 178:1648–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost K, Perkins C, Cohen R, Morris C, Wright W. 2001. Socioeconomic status and breast cancer incidence in california for different race/ethnic groups. Cancer causes & control : CCC 12:703–711. [DOI] [PubMed] [Google Scholar]
- Yu T, Park Y, Johnson JM, Jones DP. 2009. Aplcms--adaptive processing of high-resolution lc/ms data. Bioinformatics 25:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


