Abstract
Background:
Repetitive head impacts (RHI) from contact sports have been associated with cognitive and neuropsychiatric disorders. However, not all individuals exposed to RHI develop such disorders. This may be explained by the reserve hypothesis. It remains unclear if the reserve hypothesis accounts for the heterogenous symptom presentation in RHI-exposed individuals. Moreover, optimal measurement of reserve in this population is unclear and likely unique from non-athlete populations.
Objective:
We examined the association between metrics of reserve and cognitive and neuropsychiatric functioning in 89 symptomatic former National Football League players.
Methods:
Individual-level proxies (e.g., education) defined reserve. We additionally quantified reserve as remaining residual variance in 1) episodic memory and 2) executive functioning performance, after accounting for demographics and brain pathology. Associations between reserve metrics and cognitive and neuropsychiatric functioning were examined.
Results:
Higher reading ability was associated with better attention/information processing (β=0.25; 95%CI, 0.05–0.46), episodic memory (β=0.27; 95%CI, 0.06–0.48), semantic and phonemic fluency (β=0.24; 95%CI, 0.02–0.46; β=0.38; 95%CI, 0.17–0.59), and behavioral regulation (β=–0.26; 95%CI, –0.48, –0.03) performance. There were no effects for other individual-level proxies. Residual episodic memory variance was associated with better attention/information processing (β=0.45; 95%CI, 0.25, 0.65), executive functioning (β=0.36; 95%CI, 0.15, 0.57), and semantic fluency (β=0.38; 95%CI, 0.17, 0.59) performance. Residual executive functioning variance was associated with better attention/information processing (β=0.44; 95%CI, 0.24, 0.64) and episodic memory (β=0.37; 95%CI, 0.16, 0.58) performance.
Conclusion:
Traditional reserve proxies (e.g., years of education, occupational attainment) have limitations and may be unsuitable for use in elite athlete samples. Alternative approaches of reserve quantification may prove more suitable for this population.
Keywords: Chronic traumatic encephalopathy, cognition, cognitive reserve, football, neurodegenerative diseases, repetitive head impacts, resilience
INTRODUCTION
Exposure to repetitive head impacts (RHI), particularly from contact sports, has been associated with later-life cognitive and neuropsychiatric disturbances, as well as the development of neurodegenerative diseases (e.g., chronic traumatic encephalopathy [CTE]) and other pathologies (e.g., white matter degeneration) [1–15]. However, not all individuals exposed to RHI develop cognitive or neuropsychiatric disorders [2, 16–19]. Among those who do, there is heterogeneity in the presence and severity of the presentation and underlying neuropathology [2, 5, 9, 20]. Identification of risk and resilience factors of the late effects of RHI is critical to facilitate disease detection, diagnosis, and treatment and prevention strategies.
The heterogeneous expression of cognitive and neuropsychiatric deficits following RHI exposure may be explained by the reserve hypothesis. Reserve is a heuristic to describe individual variations in cognition and/or function and can be conceptualized as two main components: brain reserve (BR) and cognitive reserve (CR) [21–23]. BR refers to an individual’s neurobiological capital, i.e., resistance to pathology [24–26]. It is often measured by dimensions like gray matter (GM) volume, white matter (WM) volume, or estimated intracranial volume (eTIV) [24–27]. Recent literature has identified eTIV as an appropriate operationalization of BR as it encompasses GM, WM, and cerebrospinal fluid (CSF) measures [27, 28]. CR represents the varying susceptibility of an individual’s cognitive abilities to neuropathology, i.e., resilience to the clinical manifestation of pathology. CR is typically estimated by measures referred to as proxies (i.e., representative measures of reserve). Common CR proxies include estimated premorbid intelligence/reading ability [29, 30], years or level of education [22, 31], and occupational attainment [32, 33]. Higher scores on proxy measures are typically associated with better cognitive performance [34]. The definitions and quantifying methods of reserve are continuously evolving and while recent attempts to clarify these concepts have been made [22, 24], there is currently no consensus. Moreover, evidence suggests that BR and CR independently and interactively contribute to the heterogeneity in one’s resistance and resilience to brain insult [35–37]. As a result, the current investigation refers to CR and BR collectively as reserve, i.e., an entity that accounts for the discrepancy between the degree of brain pathology and the clinical manifestation of pathology [35–37].
Reserve has been extensively studied in Alzheimer’s disease (AD) and AD-related dementias. Higher educational and occupational attainment have been shown to mitigate the clinical and neuropathological expression of AD [38–40], even in APOE s4 carriers [33]. Moreover, higher estimated premorbid intelligence/reading ability has also been associated with improved cognitive recovery following traumatic brain injury (TBI) [29, 30]. Extant studies have also alluded to the possible role of reserve in the heterogeneous presentation of RHI-related deficits [4, 9, 41]; however, formal investigations in this area are limited. Alosco et al. [42] examined the association between occupational attainment and years of education and age of symptom onset among 25 deceased American football players with severe CTE. Here, higher occupational attainment was found to be associated with a later age of symptom onset, but no effect was found for years of education. In addition to highlighting the need for further RHI-focused reserve research, this investigation raised the question of the suitability of traditional proxies for reserve quantification, particularly in elite former contact sport athletes.
Despite the convenience of single proxies for reserve estimation, reserve is a dynamic concept not sufficiently summarized by one component [22, 43]. Representing reserve in this way fails to distinguish between reserve itself and its contributing factors, likely resulting in significant measurement error [43, 44]. Individual proxy use for reserve quantification may also be problematic when considering RHI exposure. Firstly, years of education may not reflect quality of education in former elite athletes [42]. Most former elite athletes obtain 16 years of education with restricted variability due to the close relationship between sporting expertise and the American educational system. As a result, for athletic samples, education may not appropriately reflect complete reserve capacity. It is further considered that occupational attainment may be a poor measure of reserve in samples with RHI exposure. While Alosco et al. [42] found an effect for occupational attainment in their sample, they argue that low occupational attainment may be a consequence of RHI-related pathology (i.e., the pathology leads to functional impairment) rather than reflecting an individual’s complete reserve capacity. Therefore, a more refined measure of reserve is required to further our knowledge of its potential role in RHI-exposed samples and to gain an understanding of how it may contribute to clinical heterogeneity.
One alternative method of reserve quantification is the residual memory variance calculation [45]. Here, variance refers to the variability of a value(s) from the average. To calculate reserve using this method, the residual variance of cognitive performance (e.g., on an episodic memory task) is derived, after accounting for demographic factors (e.g., age, sex) and structural brain characteristics (e.g., total brain volume). This residual component encompasses all individual differences in cognition that cannot be explained by brain structure and demographic variables, thus representing a measure of reserve. This residual method of reserve quantification defines reserve as the discrepancy between one’s predicted and one’s observed level of cognitive performance [45, 46]. Higher positive residuals represent better than expected performance. The residual measure was shown to be correlated with traditional proxy measures of CR, highlighting the plausibility of this method. These findings have since been replicated and extended to different cognitive domains (e.g., executive functioning, language) across multiple samples [46–50]. Moreover, a recent review and meta-analysis of residual methods of reserve quantification strongly supports this technique as a measure of resilience and resistance in aging samples [51]. An important limitation of this approach, however, is that while a proportion of the residual likely represents reserve, there is also likely to be a significant but unknown proportion that is random error [49]. Nonetheless, applying such methodology to an RHI-exposed cohort would be a novel approach towards exploring the evident heterogeneity in symptom expression, or lack of thereof, following RHI exposure.
The objective of this study was to examine the association between reserve and cognitive and neuropsychiatric function in symptomatic former National Football League (NFL) players. The primary objective was to identify optimal measurement of reserve in this population. To do so, we used traditional individual proxy measures of reserve, in addition to the residual variance method of reserve quantification. Episodic memory and executive dysfunction are prominent symptoms following RHI exposure [2, 8, 9, 52–54]. Therefore, we targeted both the episodic memory and executive functioning domains to create residual episodic memory and executive functioning variance variables. The quantification of reserve by means of single proxies is problematic and the residual variance method may prove a suitable alternative to overcome these aforementioned issues in athletic samples.
MATERIALS AND METHODS
Participants and study design
The current sample included former NFL players from the National Institute of Neurological Disorders and Stroke–funded study entitled ‘Diagnosing and Evaluating Traumatic Encephalopathy Using Clinical Tests’ (DETECT; R01NS078337). Recruitment and data collection occurred between November 2011 and October 2015. Inclusion criteria included: male, aged 40–69 years, self-reported complaints of cognitive, behavior and/or mood symptoms at telephone screening, and a minimum of 12 years of organized American football, with at least two seasons in the NFL. Former NFL players were recruited to ensure the selected sample represented a population with high risk for CTE. Exclusion criteria included MRI and/or lumbar puncture contraindications, concussion history within 1 year of study entry, visual or hearing impairment that would compromise neuropsychological testing, lack of adequate decisional capacity to provide consent to participate, and/or a primary language other than English.
Upon enrollment to the DETECT study, participants completed a 2-to-3-day visit to undergo neuropsychological testing, neuroimaging, and demographic, medical, neurological, psychiatric, and physical evaluations. Only data relevant to the current investigation was examined. All participants provided written informed consent prior to participation. All study protocols were approved by the Boston University Medical Center and Brigham and Women’s Hospital Institutional Review Boards.
Measures
Traditional reserve proxies
A number of reserve proxies were derived. Participants’ level of education was defined by the number of formal years attained [22, 31]. An individual’s level of occupational attainment following their NFL career was obtained by categorizing participants’ occupation as high or low according to the U.S. Department of Labors’ Dictionary of Occupational Titles (DOT) [32, 33, 55]. High attainment was classified as any professional, technical, or managerial profession (DOT codes 0–1). Low attainment was defined as work such as clerical or sales positions, processing occupations, and structural occupations (DOT codes 2–8). Miscellaneous occupations were classified with a DOT code of 9. For participants with multiple occupations, the highest level of attainment was used. One participant fell into the category of miscellaneous occupations (DOT code 9); however, due to similarities to occupations covered under DOT codes 2–8, this participant was classified as having low occupational attainment. The Wide Range Achievement Test-4 (WRAT-4) [56] reading subtest was used as a representative of an individual’s reading ability [29, 30]. As with most other reading ability estimates, the WRAT-4 measures the accumulation of learning at the age prior to insult, in addition to the altered trajectory of learning and development following the insult. Lastly, eTIV served as an additional proxy of reserve [27].
Neuropsychological and neuropsychiatric measurements
A comprehensive neuropsychological battery was administered to all participants to assess cognitive functioning. To limit the number of analyses, a subset of tests were selected a priori based on their ability to assess cognitive domains impaired following RHI exposure [8, 9, 53] and included: the Trail Making Test (TMT) Parts A and B [57], Neuropsychological Assessment Battery (NAB) List Learning Long Delay Recall [58], Controlled Oral Word Association Test (COWAT) [59], and Animal Fluency Test [59]. In order to describe the clinical sample, neuropsychological test raw scores were converted into standardized scores accounting for age, sex, and/or educational attainment. However, as educational attainment is a well-recognized traditional proxy for reserve [22, 31], all statistical analyses used neuropsychological test raw scores. TMT Parts A and B were reverse coded to correct directionality (i.e., lower scores represent worse performance).
Symptoms of depression and neurobehavioral dysregulation were assessed using the Beck Depression Inventory-II (BDI-II) [60] and the Behavior Rating Inventory of Executive Functioning – Adult Version (BRIEF-A) Behavioral Regulation Index (BRI) [61], respectively. Raw BRIEF-A BRI scores were converted into standardized age-adjusted T-scores.
Cumulative Head Impact Index (CHII)
The CHII is a retrospective estimate of total cumulative exposure to RHI from participation in American football [4]. This value is derived using two sources of information; 1) self-reported athletic exposure (i.e., level of play, number of seasons played, position[s] played), and 2) objective estimates of head impact frequency based on position played, as determined by published helmet accelerometer studies. The development of the CHII has been previously described and validated [4]. The CHII was developed in former youth, high school, and college American football players (for which helmet accelerometer studies are available) to estimate the frequency of head impacts. However, published helmet accelerometer data are non-existent for professional level American football. Therefore, college-level estimates of head impact frequencies were applied to the DETECT sample to estimate their professional-level exposure. Higher CHII values indicate greater exposure to RHI.
MRI acquisition and processing
All participants underwent structural MRI on a 3-Tesla Siemens Verio MRI scanner with a 32-channel head coil and the Syngo MR-B17 software suite. Three-dimensional T1-weighted scans (MPRAGE [1 X 1 X 1 mm3; TR = 1800 ms; TE = 3.36 ms; acquisition matrix = 256 x 256; flip angle = 7°]) were acquired. We examined total brain volume, and hippocampal and white matter hypointensity (WM-hypo) volumes. Hippocampal and WM-hypo volumes served as specific regions of interest (ROIs) because of their known effects on cognitive and neuropsychiatric function, as well as their association with RHI exposure [62–65]. WM-hypo also captures cerebrovascular contributions to cognitive impairment and dementia. All imaging variables were only used to derive metrics of reserve. FreeSurfer 5.3 was used for automated segmentation of brain tissue and has been described in detail elsewhere [66, 67]. This program subdivides brain tissue into areas of GM, WM, and CSF. Total eTIV is also calculated. Visual quality assessment was conducted to ensure correct detection and automated segmentation occurred. Manual adjustments of the hippocampus were performed using Slicer 4.1 [68], as previously described [62, 69]. A trained neuroanatomist was involved in defining the criteria for these manual adjustments and intra- and inter-reader reliability was tested. Coronal slices were used to correct ROIs, from anterior to posterior, referring to sagittal slices for verification and volumes were subsequently extracted from the label maps of the left and right hippocampi. All images were processed by the Psychiatry Neuroimaging Laboratory at Brigham and Women’s Hospital.
Statistical analyses
Statistical analyses were performed in R version 3.6.0 [70]. To normalize their distribution, WM-hypo volumes were log-transformed. Before analysis, all MRI variables were coded to ensure that higher scores indicated greater pathology (i.e., left hippocampal, right hippocampal, total GM, total WM, and eTIV were reverse coded). Listwise deletion was applied, omitting any observations with missing data on variables of interest. Statistical significance was defined by p < 0.05, with false discovery rate (FDR)-adjusted corrections for multiple comparisons. The above-described neuropsychological and neuropsychiatric test scores acted as primary outcome measures.
Traditional reserve proxies
Five separate linear regression analyses were conducted to assess the association between traditional reserve proxies (i.e., level of education, occupational attainment, reading ability, and eTIV) and participants’ neuropsychological test performance and reported neuropsychiatric symptoms. To examine each proxy’s representation of reserve and to avoid multicollinearity, separate regression analyses were performed. The following covariates were included due to their suggested contribution to reserve and their previous association with cognitive and behavioral/mood symptom expression: age [71–73], racial identity [74–76], CHII [4, 20], and APOE genotype [33, 78–80]. CHII was chosen as a representative measure of RHI exposure as it encompasses several estimates of RHI into a single metric. Racial identity was coded as 0 and 1 for White and Black or African American race, respectively. APOE genotype was coded as 0 and 1 for non-carriers (absence of an s4 allele) and carriers (presence of at least one s4 allele), respectively.
Residual episodic memory and executive functioning variance
Residual episodic memory variance was computed by regressing the residuals of NAB List Learning Long Delay Recall raw scores on age, racial identity, APOE s4 status, CHII, logWM-hypo, total brain matter volume, and total hippocampal volume. Episodic memory was chosen as it is one of the most prominent symptoms associated with RHI exposure [2, 9]. All variance of the NAB List Learning Long Delay Recall variable that could be explained by demographics or brain pathology was removed. That is, the residuals represented the discrepancy between a participant’s observed NAB List Learning Long Delay Recall performance and the performance predicted by the aforementioned predictors. Executive dysfunction has also been identified as a prominent RHI-related symptom [8, 52, 53]. Residual executive functioning variance was computed using this same method, substituting NAB List Learning Long Delay Recall raw scores for TMT Part B raw scores. The resulting unstandardized residual terms were then used individually as representative metrics of reserve for this sample. All variables were coded to ensure higher residual scores indicated higher reserve, i.e., better performance than predicted. All subject-specific residuals were calculated using the leave-one-out cross validation technique, i.e., with the subject deleted when estimating the predicted value of that subject. Next, the linear regressions were repeated separately for each of the residual variables, the newly created reserve term acting as the predictor and the neuropsychological and neuropsychiatric test raw scores (not including NAB List Learning Long Delay Recall or TMT Part B, where applicable) as the outcomes. To determine how well these predictive models performed, leave-one-out cross validation was completed. Bivariate Pearson correlations were conducted to investigate the relationship between the individual newly created residual variables and the four traditional reserve proxies.
RESULTS
Sample derivation
The final sample size for all analyses was 89. This was derived following exclusion of one participant due to poor effort, as reflected by failure on numerous symptom validity tests, multiple neuropsychological scores at floor (i.e., lower limit), and the presence of external evidence supporting this conclusion. Two more participants were removed due to missing occupational attainment data. Neuroimaging data was available for 81 participants, following the exclusion of those who did not complete MRI and those with inadequate data quality due to motion artifact. Table 1 summarizes the sample characteristics. Tables 2 and 3 display the neuropsychological, neuropsychiatric, and MRI characteristics for the current sample.
Table 1.
Demographic, athletic, medical, and APOE carrier status characteristics in former NFL players
| Characteristic | Total Sample (n = 89) |
|---|---|
| Age, mean (SD) y | 55.63 (7.77) |
| Education, mean (SD) y | 16.44 (0.99) |
| Occupational attainment, n (%) | |
| High | 59 (66.3) |
| Low | 30 (33.7) |
| Racial Identity, n (%) | |
| White | 52 (58.4) |
| Black or African American | 37 (41.6) |
| AFE, mean (SD) y | 11.96 (2.52) |
| Duration of football play, mean (SD) y | 18.19 (3.54) |
| Duration of play in the NFL, mean (SD) y | 8.04 (2.79) |
| CHII, mean (SD) | 20,089.58 (6,943.69) |
| Primary position group, n (%) | |
| Offensive Line | 27 (30.3) |
| Running Back | 8 (9.0) |
| Tight End | 5 (5.6) |
| Offensive Skill | 1 (1.1) |
| Defensive Line | 12 (13.5) |
| Linebacker | 19 (21.3) |
| Defensive Back | 17 (19.1) |
| APOE s4 status, n (%) | |
| 23 | 9 (10.3) |
| 24 | 2 (2.3) |
| 33 | 52 (59.8) |
| 34 | 21 (24.1) |
| 44 | 3 (3.4) |
SD, standard deviation; y, years; AFE, age of first exposure; CHII, cumulative head impact index; APOE, Apolipoprotein E.
Table 2.
Neuropsychiatric and neuropsychological raw scores and standardized scores in the former NFL player sample (N = 89)
| Neuropsychiatric & Neuropsychological Tests1 | Raw Scores2 |
T-Scores3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI |
Median | Mean | SD | 95% CI |
Median | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||||
| BDI-II: Total score | 16.61 | 12.23 | 14.07 | 19.15 | 15.00 | – | – | – | – | – |
| BRIEF-A BRI | 54.39 | 11.81 | 51.94 | 56.85 | 55.00 | 63.47 | 12.85 | 60.78 | 66.15 | 64.00 |
| TMT Part A Time4 | 32.69 | 10.94 | 30.41 | 34.96 | 31.00 | 49.27 | 11.46 | 46.88 | 51.67 | 51.00 |
| TMT Part B Time4 | 83.53 | 46.45 | 73.88 | 93.18 | 70.00 | 44.14 | 16.01 | 40.79 | 47.48 | 49.00 |
| NAB List Learning Long Delay Recall | 6.11 | 2.88 | 5.51 | 6.71 | 6.00 | 41.92 | 13.86 | 39.03 | 44.82 | 40.00 |
| COWAT | 43.09 | 13.10 | 40.37 | 45.81 | 41.00 | 49.57 | 11.20 | 47.23 | 51.91 | 49.00 |
| Animal Fluency | 20.16 | 5.66 | 18.98 | 21.33 | 20.00 | 49.14 | 11.69 | 46.69 | 51.58 | 49.00 |
TMT, Trail Making Test; NAB, Neuropsychological Assessment Battery; COWAT, Controlled Oral Word Association Test; BRIEF-A BRI, Behavior Rating Inventory of Executive Functioning – Adult Version Behavioral Regulation Index; BDI-II, Beck Depression Inventory-II.
To limit the number of analyses, a subset of tests were selected a priori based on their ability to assess cognitive domains impaired following RHI exposure [8, 9, 53]
All statistical analyses used neuropsychological test raw scores
In order to describe the clinical sample, neuropsychological test raw scores were converted into standardized scores accounting for age, sex, and/or educational attainment. Raw BRIEF-A BRI scores were converted into standardized age-adjusted T-scores
Before analysis, Trails A and B were reverse coded to correct directionality (i.e., lower scores represented worse performance).
Table 3.
Summary statistics of MRI indices measured in former NFL players (N = 81)1
| MRI Indices2 (mm3) | Mean | SD | 95% CI |
Median | Interquartile Range | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| White Matter Hypointensities | 2116.72 | 2009.24 | 1679.16 | 2554.28 | 1521.70 | 989.90 |
| Log-White Matter Hypointensities3 | 7.43 | 0.60 | 7.30 | 7.56 | 7.33 | 0.63 |
| Left Hippocampal Volume | 3381.67 | 386.33 | 3465.80 | 3297.53 | 3374.00 | 474.00 |
| Right Hippocampal Volume | 3381.56 | 379.79 | 3464.26 | 3298.85 | 3387.00 | 363.00 |
| Total Gray Matter Volume | 617993.74 | 47254.12 | 628284.45 | 607703.03 | 617091.48 | 71148.78 |
| Total White Matter Volume | 480610.40 | 47633.12 | 490983.64 | 470237.16 | 473970.02 | 72295.49 |
| Estimated Intracranial Volume | 1498424.53 | 237880.08 | 1550228.57 | 1446620.48 | 1546022.42 | 266048.64 |
Neuroimaging data was available for 81 participants, following the exclusion of those who did not complete MRI and those with inadequate data quality due to motion artifact
Before analysis, all variables were coded to ensure that higher scores indicated greater pathology (i.e., left hippocampal volume, right hippocampal volume, total gray matter volume, total white matter volume, and estimated intracranial volume were reverse coded)
To normalize their distribution, white matter hypointensity volumes were log-transformed.
Traditional reserve proxies
Linear regression analyses
Regression analyses revealed a statistically significant association between age and racial identity, and several of the neuropsychological and neuropsychiatric outcomes (Supplementary Table 1). Older age correlated with worse performance on TMT Part B (p < 0.01) and NAB List Learning Long Delay Recall (p = 0.01). Black or African American race was associated with worse performance on TMT Parts A (p = 0.01) and B (p = 0.01), NAB List Learning Long Delay Recall (p = 0.03), Animal Fluency (p = 0.03), and the COWAT (p = 0.05). No significant effects were found for the CHII or APOE s4 status on any of the neuropsychological or neuropsychiatric test scores (Supplementary Table 1).
The WRAT-4 had a significant effect on TMT Part A (p = 0.04), NAB List Learning Long Delay Recall (p = 0.04), Animal Fluency (p = 0.04), and the COWAT (p < 0.01). Higher WRAT-4 scores were associated with better test performance. Higher WRAT-4 scores were also associated with lower BRI (p = 0.04) scores, indicating less impairment. The largest effect was evident for the COWAT (β=0.38), followed by NAB List Learning Long Delay Recall (β=0.27), BRI (β=–0.26), TMT Part A (β=0.25), and Animal Fluency (β=0.24) (Fig. 1A; Supplementary Table 2). No significant effects were found for years of education, occupational attainment, or eTIV on any of the neuropsychological or neuropsychiatric test scores (Supplementary Table 2).
Fig. 1.
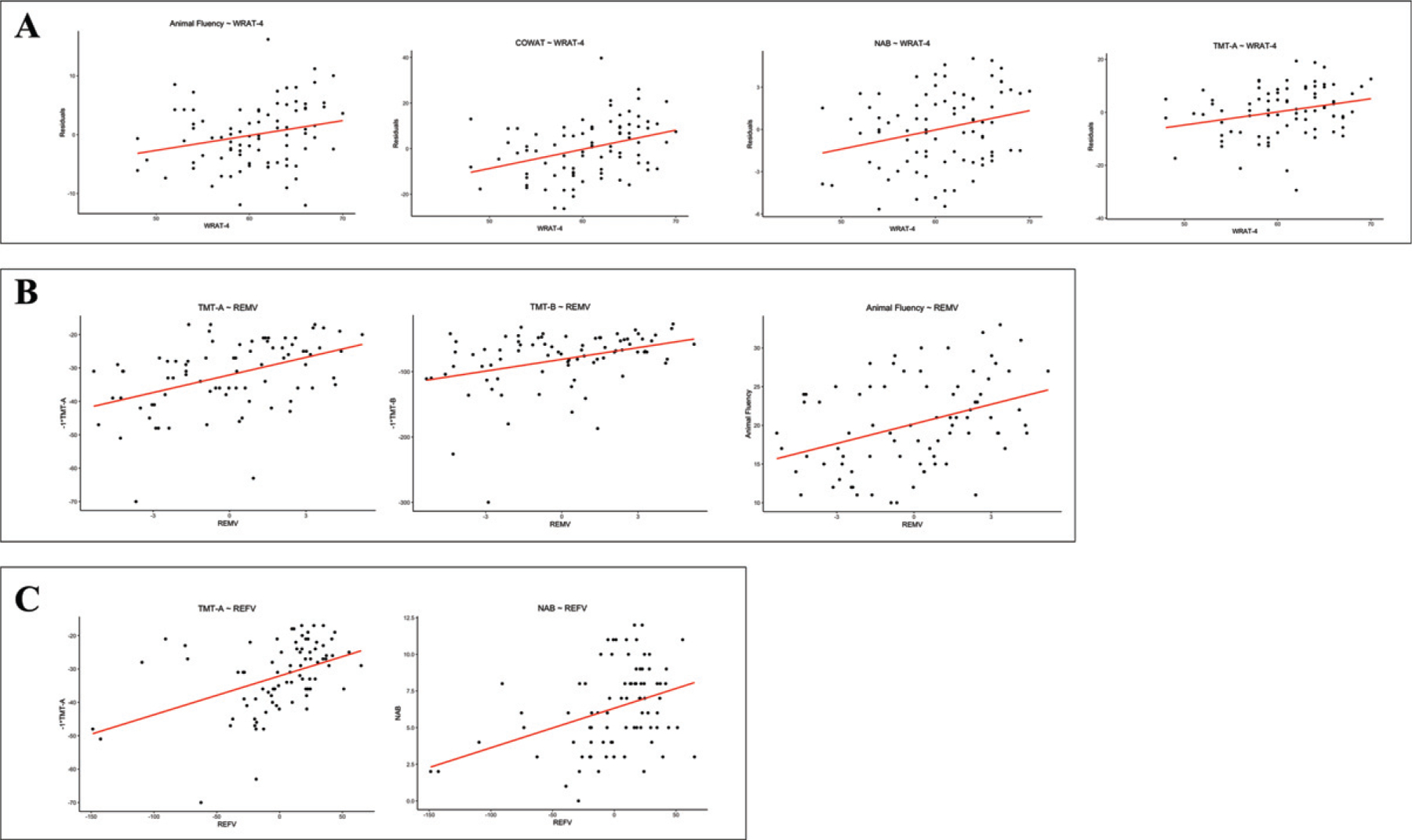
Scatterplots of significant relationships between reserve proxies and neuropsychological test scores. A) WRAT-4 and animal fluency (p = 0.04), the COWAT (p < 0.01), NAB (p = 0.04), and TMT A (p = 0.04). B) Residual episodic memory variance and TMT A (p < 0.01), TMT B (p < 0.01), and animal fluency (p < 0.01). C) Residual executive functioning variance and TMT A (p < 0.01) and NAB (p < 0.01). Statistical significance was defined by a false discovery rate adjusted p-value<0.05. Age, racial identity, CHII, and APOE genotype were included as covariates. Non-significant associations presented in Table 4 and Supplementary Table 1 not shown. CHII, cumulative head impact index; WRAT-4, Wide Range Achievement Test-4; COWAT, Controlled Oral Word Association Test; NAB, Neuropsychological Assessment Battery List Learning Long Delay Recall; TMT A, Trail Making Test Part A; TMT B, Trail Making Test Part B; REMV, residual episodic memory variance; REFV, residual executive functioning variance.
Residual Variance
Residual Episodic Memory Variance
Residual episodic memory variance scores were associated with better TMT Part A (p < 0.01), TMT Part B (p < 0.01), and Animal Fluency (p < 0.01) performance (Fig. 1B). The standardized regression coefficients for these analyses are reported in Table 4. Larger effects for residual episodic memory variance were seen for TMT Parts A and B and Animal fluency, as compared to the WRAT-4. The leave-one-out cross validation technique validated our results (Table 5, Supplementary Figure 1).
Table 4.
Standardized regression coefficients of residual episodic memory and executive functioning variance and neuropsychological and neuropsychiatric tests
| Neuropsychiatric & Neuropsychological Tests | Residual Episodic Memory Variance |
Residual Executive Functioning Variance |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | T | p2 | 95% CI |
β | T | p2 | 95% CI |
|||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||||
| TMT Part A1 | 0.45 | 4.43 | <0.01 | 0.25 | 0.65 | 0.44 | 4.34 | <0.01 | 0.24 | 0.64 |
| TMT Part B1 | 0.36 | 3.40 | <0.01 | 0.15 | 0.57 | – | – | – | – | – |
| NAB List Learning Long Delay Recall | – | – | – | – | – | 0.37 | 3.55 | <0.01 | 0.16 | 0.58 |
| COWAT | 0.22 | 1.97 | 0.08 | 0.00 | 0.43 | 0.18 | 1.64 | 0.21 | −0.04 | 0.40 |
| Animal Fluency | 0.38 | 3.67 | <0.01 | 0.17 | 0.59 | 0.08 | 0.69 | 0.49 | −0.15 | 0.30 |
| BRIEF-A BRI | −0.16 | −1.40 | 0.20 | −0.38 | 0.07 | −0.09 | −0.82 | 0.49 | −0.31 | 0.13 |
| BDI-II | −0.12 | −1.05 | 0.30 | −0.34 | 0.11 | −0.14 | −1.29 | 0.30 | −0.37 | 0.08 |
TMT, Trail Making Test; NAB, Neuropsychological Assessment Battery; COWAT, Controlled Oral Word Association Test; BRIEF-A BRI, Behavior Rating Inventory of Executive Functioning – Adult Version Behavioral Regulation Index; BDI-II, Beck Depression Inventory-II.
TMT Parts A and B were reverse coded to correct directionality (i.e., lower scores represented worse performance)
Statistical significance was defined by a false discovery rate adjusted p-value<0.05. Age, racial identity, CHII, and APOE genotype were included as covariates.
Table 5.
Standardized regression coefficients with leave-one-out residuals as metric of reserve
| Neuropsychiatric & Neuropsychological Tests | Leave-one-out residuals for episodic memory |
Leave-one-out residuals for executive functioning |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | T | p2 | 95% CI |
β | T | p2 | 95% CI |
|||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||||
| TMT Part A1 | 0.45 | 4.51 | <0.01 | 0.25 | 0.65 | 0.44 | 4.37 | <0.01 | 0.24 | 0.64 |
| TMT Part B1 | 0.37 | 3.59 | <0.01 | 0.17 | 0.58 | – | – | – | – | – |
| NAB List Learning Long Delay Recall | – | – | – | – | – | 0.37 | 3.54 | <0.01 | 0.16 | 0.58 |
| COWAT | 0.22 | 2.01 | 0.07 | 0.00 | 0.44 | 0.18 | 1.62 | 0.22 | −0.04 | 0.40 |
| Animal Fluency | 0.39 | 3.75 | <0.01 | 0.18 | 0.60 | 0.09 | 0.81 | 0.50 | −0.13 | 0.31 |
| BRIEF-A BRI | −0.15 | −1.33 | 0.23 | −0.37 | 0.07 | −0.07 | −0.64 | 0.53 | −0.29 | 0.15 |
| BDI-II | −0.12 | −1.04 | 0.30 | −0.34 | 0.11 | −0.14 | −1.30 | 0.30 | −0.37 | 0.08 |
TMT, Trail Making Test; NAB, Neuropsychological Assessment Battery; COWAT, Controlled Oral Word Association Test; BRIEF-A BRI, Behavior Rating Inventory of Executive Functioning – Adult Version Behavioral Regulation Index; BDI-II, Beck Depression Inventory-II.
TMT Parts A and B were reverse coded to correct directionality (i.e., lower scores represented worse performance)
Statistical significance was defined by a false discovery rate adjusted p-value <0.05. Age, racial identity, CHII, and APOE genotype were included as covariates.
Residual Executive Functioning Variance
Residual executive functioning variance scores were associated with better TMT Part A (p < 0.01) and NAB List Learning Long Delay Recall (p < 0.01) performance (Fig. 1C). The standardized regression coefficients for these analyses are reported in Table 4. Larger effects for residual executive functioning variance were seen for TMT Part A and NAB List Learning Long Delay Recall, as compared to the WRAT-4. The leave-one-out cross validation technique validated our results (Table 5, Supplementary Figure 1).
Post-Hoc analyses
Post-hoc analyses were performed to investigate the potential moderating effect of 1) the WRAT-4 (as the only significant traditional reserve proxy), 2) residual executive functioning variance, and 3) residual episodic memory variance on the relationship between the CHII and neuropsychological test performance and neuropsychiatric symptoms. Separate linear regression analyses were performed as above, with the addition of a 1) CHII x WRAT-4, 2) CHII x residual executive functioning variance, and 3) CHII x residual episodic memory variance interaction term. None of the interaction terms had a statistically significant effect on any of the neuropsychological or neuropsychiatric tests.
Bivariate Pearson correlations
The WRAT-4 was found to be statistically significantly correlated with residual episodic memory variance (r = 0.24, p = 0.03). No significant correlations were identified between residual episodic memory variance and the remaining three traditional reserve proxies, years of education, occupational attainment, and eTIV (Table 6). Additionally, no significant correlations were identified between residual executive functioning variance and the four traditional reserve proxies (Table 6).
Table 6.
Bivariate Pearson correlations between residual variance variables and four traditional reserve proxies
| Neuropsychiatric & Neuropsychological Tests | Residual Episodic Memory Variance |
Residual Executive Functioning Variance |
||
|---|---|---|---|---|
| r | p | r | p | |
| WRAT-4 | 0.24 | 0.03 | 0.07 | 0.55 |
| Years of Education | −0.05 | 0.74 | −0.01 | 0.97 |
| Occupational Attainment | −0.10 | 0.30 | −0.06 | 0.52 |
| Estimated Intracranial Volume | −0.02 | 0.94 | 0.02 | 0.82 |
WRAT-4, The Wide Range Achievement Test-4.
DISCUSSION
This study investigated the role of reserve in 89 male, symptomatic, former NFL players and examined multiple methods for the measurement of reserve. Similar to other populations, higher reserve may contribute to the symptom heterogeneity observed within and across samples of former elite American football players. Residual episodic memory variance scores were shown to be associated with better performance on tests of attention and information processing, executive functioning, and semantic fluency, while residual executive functioning variance scores were associated with better performance on tests of attention and information processing and episodic memory. We additionally found that a measure traditionally believed to estimate reading ability was associated with better neuropsychological test performance and decreased neurobehavioral dysregulation, after accounting for covariates. Years of education, occupational attainment, and eTIV were not associated with cognitive test performance or neuropsychiatric symptoms.
Higher reading ability has been shown to mitigate cognitive dysfunction in neurological disorders such as AD and TBI [30, 81–83]. The current investigation indeed observed significant effects for the WRAT-4. Although word-reading tests such as the WRAT-4 have been a commonly used proxy of reserve, they may underestimate premorbid intelligence in individuals who have been exposed to head impacts at an early age [30, 77]. Moreover, the WRAT-4 provides an estimation of one’s reading ability rather than being a direct measure of intelligence itself. While this estimation may capture a proportion of reserve, it likely also encompasses a significant proportion of measurement error. A more comprehensive estimate of premorbid intelligence is needed for future studies in this population. We additionally hypothesized that reserve metrics (e.g., WRAT-4) may moderate the relationship between exposure to RHI and cognitive test performance. No such effects were found. RHI exposure in our sample of former professional athletes was retrospectively estimated using the CHII by applying accelerometer data of American football players at collegiate level. Helmet accelerometer studies are not available for the NFL and thus, as this study focused on professional athletes, the CHII may have underestimated RHI exposure in our sample. Nevertheless, the CHII remains one of the most refined metrics for quantifying RHI exposure. Accelerometer data of professional American football players is needed to overcome this limitation in future studies of this population.
No associations were identified between years of education and cognitive test performance or neuropsychiatric symptom expression. This was also the case for occupational attainment. In former professional American football players, years of education may not capture complete reserve capacity [42]. Most NFL players attain 16 years of education, which may explain the restricted variability in education duration in the current sample. In this all-male sample, variability is also lacking in eTIV [84, 85]. Lastly, in contrast to Alosco et al.’s [42] findings, the current investigation found no significant effect for occupational attainment. Again, >65% of participants in the current study reported high occupational attainment. There are also substantial differences between this sample and Alosco et al., the latter of which included a small sample of brain donors with autopsy-confirmed stage III/IV CTE. Alosco et al. also examined informant-reported age of symptom onset, whereas the present study used objective measures of cognitive and neuropsychiatric function.
In response to the aforementioned limitations of the individual reserve proxies, this study examined more refined approaches of reserve quantification. Residual episodic memory and executive functioning variance variables were created and tested for their association with cognitive and neuropsychiatric function. Residual episodic memory variance showed a significant association with better performance on tests of attention and information processing speed, executive functioning, and semantic fluency. Residual executive functioning variance was similarly associated with better performance on tests of episodic memory and attention and information processing speed performance. Larger effect sizes were evident for the residual variance approach as compared to a test of reading ability in regard to associations with neuropsychological test performance. Unlike reading ability score, the residual cognitive variance scores were not associated with any of the neuropsychiatric measures. This may be because risk factors for neuropsychiatric symptoms in this population are distinct from those used to create the residual cognitive variance scores. Behavioral disturbances in former elite contact sport athletes may also have distinct causes and/or etiologies compared with cognitive impairments. Correlation analyses revealed that a significant correlation existed between a measure of reading ability and residual episodic memory variance. This remains consistent with our findings that, out of the four traditional proxies measured in this study, reading ability was superior. On the other hand, CR is a multidimensional construct and previous research has shown that traditional reserve proxies represent distinct components of reserve, thus each providing a unique contribution to individual performance on cognitive tests [86–88]. Our findings could alternatively suggest that the newly created residual variance variables capture different aspects of reserve than the traditional reserve proxies and reading ability most closely resembles that which is measured by these variables.
The cognitive and neuropsychiatric symptomatology associated with RHI is heterogenous. Even among those with comparable neuropathological load, there can be differences in symptom presence and severity [9, 65]. Not all those subject to RHI experience subsequent cognitive dysfunction or neurobehavioral dysregulation [18, 19, 89]. High reserve in individuals exposed to RHI may be protective against cognitive decline and may explain some of the inconsistent findings in the literature [18, 90, 91]. Individual differences in the susceptibility to age-related brain changes have been examined extensively with regard to reserve [23, 38, 40]. Previous research has shown that high reserve, as measured by traditional reserve proxies, is protective of cognitive decline [92, 93] and the presence of neuropsychiatric symptoms [94, 95]. Furthermore, despite higher severity of AD-related pathology, those with high reserve can still appear clinically similar to those with low reserve and less AD pathology [39]. The exact neural mechanism underlying reserve is currently unknown [39]. Colangeli et al. [96] showed that reserve proxies (i.e., education, occupation, and leisure activities) were associated with activation in the anterior cingulate cortex. Also, Wilson et al. [97] demonstrated that reduced neuronal density (i.e., reserve at the neuronal level) in the locus coeruleus and brain stem neurofibrillary tangles and Lewy bodies were predictive of the rate of cognitive decline in AD. Research has also indicated that the relationship between amyloid plaques and cognitive functioning is moderated by formal years of education [98]. Reserve factors may similarly influence the expression of ptau and other neuropathologies, as well as resulting clinical syndromes associated with RHI.
There are limitations to our findings. While a number of significant effects were found, the meaningfulness of these effects remains unclear (as shown in Fig. 1). Moreover, the sample included male former NFL players, and it is unclear if our findings generalize to the broader American football population, other contact sports, or female athletes. Future research should focus on exploring alternative methods of reserve/resilience quantification in these other samples. The current research was also cross-sectional. Prospective studies are needed to determine how reserve factors might mitigate cognitive and neuropsychiatric decline. Defining and quantifying reserve, particularly in groups with RHI exposure, is problematic and no consensus has yet been reached. The comparison of results between reserve-based research investigations remains difficult and there is a great need for a more exhaustive and standardized method of reserve estimation. The residual variance method shows promise towards finding a solution to these issues by encompassing multiple aspects of reserve into a single component. Although, the proportion of the residual scores that represent reserve versus random error is unknown, significant measurement error also exists when estimating reserve using traditional proxies. Future research should focus on investigating the applicability of the residual variance approach to reserve quantification in a sample with neuropathologically confirmed CTE to provide further insight into the role of reserve in the clinical expression of this pathology. Furthermore, more refined measures of reserve are required to further understand its predictors. This investigation made use of total brain, hippocampal, and WM-hypo volumes to derive residual variance scores in order to capture the effects of atrophy and cerebrovascular disease. Other brain regions and types of pathology might be more suitable in people exposed to RHI, such as microstructural injury from diffusion tensor imaging. Therefore, future research should focus on exploring alternative methods of capturing the effects of atrophy and cerebrovascular disease.
Conclusions
Reading ability, residual episodic memory variance, and residual executive functioning variance as proxies of reserve were associated with better cognitive test performance in former NFL players. Larger effects were evident for the residual cognitive variance approach. The quantification of reserve by means of single proxies (e.g., years of education, occupational attainment) remains problematic and may be unsuitable for use in this population. The residual variance method may be a suitable alternative to overcome these issues. Continued research is needed to better define optimal measurement of reserve and resilience in former elite American football players and other contact sport populations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant funding from the NIH (R01NS078337; R56 9500304025; U01NS093334; K23AG046377; K23NS102399; P30AG013846).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21–0379r3).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-210379.
REFERENCES
- [1].Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, DeSaro P, Boylan KB, Graff-Radford NR, Wszolek ZK, Rademakers R, Boeve BF, McKee AC, Dickson DW (2015) Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 130, 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, Alosco ML, Solomon TM, Nowinski CJ, McHale L, Cormier KA, Kubilus CA, Martin BM, Murphy L, Baugh CM, Montenigro PH, Chaisson CE, Tripodis Y, Kowall NW, Weuve J, McClean MD, Cantu RC, Goldstein LE, Katz DI, Stern RA, Stein TD, Mckee AC (2017) Clinicopathological evaluation of chronic traumatic encephalopathy in players of American Football. JAMA 318, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wright MJ, Woo E, Birath JB, Siders CA, Kelly DF, Wang C, Swerdloff R, Romero E, Kernan C, Cantu RC, Guskiewicz K (2016) An index predictive of cognitive outcome in retired professional American Football players with a history of sports concussion. J Clin Exp Neuropsychol 38, 561–571. [DOI] [PubMed] [Google Scholar]
- [4].Montenigro PH, Alosco ML, Martin BM, Daneshvar DH, Mez J, Chaisson CE, Nowinski CJ, Au R, Mckee AC, Tripodis Y, Mcclean MD, Stern RA, Cantu RC, Mcclean MD, Stern RA, Tripodis Y (2017) Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma 34, 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Roberts AL, Pascual-Leone A, Speizer FE, Zafonte RD, Baggish AL, Taylor HJ, Nadler LM, Courtney TK, Connor A, Grashow R, Stillman AM, Marengi DA, Weisskopf MG (2019) Exposure to American Football and neuropsychiatric health in former National Football League players: findings from the football players health study. Am J Sports Med 47, 2871–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alosco ML, Tripodis Y, Baucom ZH, Mez J, Stein TD, Martin B, Haller O, Conneely S, McClean M, Nosheny R, Mackin S, McKee AC, Weiner MW, Stern RA (2020) Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology 95, e793–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alosco ML, Stein TD, Tripodis Y, Chua AS, Kowall NW, Huber BR, Goldstein LE, Cantu RC, Katz DI, Palmisano JN, Martin B, Cherry JD, Mahar I, Killiany RJ, McClean MD, Au R, Alvarez V, Stern RA, Mez J, McKee AC (2019) Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol 76, 1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee H-S, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, Fritts NG, Stamm JM, Robbins CA, McHale L, Simkin I, Stein TD, Alvarez VE, Goldstein LE, Budson AE, Kowall NW, Nowinski CJ, Cantu RC, McKee AC (2013) Clinical presentation of chronic traumatic encephalopathy. Neurology 81, 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ling H, Morris HR, Neal JW, Lees AJ, Hardy J, Holton JL, Revesz T, Williams DDR (2017) Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 133, 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte T, Gavett BE, Budson AE, Santini VE, Lee H-S, Kubilus CA, Stern RA (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy following repetitive head injury. J Neuropathol Exp Neurol 68, 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mez J, Daneshvar DH, Abdolmohammadi B, Chua AS, Alosco ML, Kiernan PT, Evers L, Marshall L, Martin BM, Palmisano JN, Nowinski CJ, Mahar I, Cherry JD, Alvarez VE, Dwyer B, Huber BR, Stein TD, Goldstein LE, Katz DI, Cantu RC, Au R, Kowall NW, Stern RA, McClean MD, Weuve J, Tripodis Y, McKee AC (2020) Duration of American football play and chronic traumatic encephalopathy. Ann Neurol 87, 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, Perl DP, Stein TD, Vonsattel J-P, Stewart W, Tripodis Y, Crary JF, Bieniek KF, Dams-O’Connor K, Alvarez VE, Gordon WA, the TBI/CTE group (2016) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M, Jordan BD, Randolph C, Dvořák J, Alix Hayden K, Tator CH, McCrory P, Iverson GL (2017) A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med 51, 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Levitch CF, Zimmerman ME, Lubin N, Kim N, Lipton RB, Stewart WF, Kim M, Lipton ML (2018) Recent and long-term soccer heading exposure is differentially associated with neuropsychological function in amateur players. J Int Neuropsychol Soc 24, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kuhn AW, Zuckerman SL, Solomon GS, Casson IR, Viano DC (2017) Interrelationships among neuroimaging biomarkers, neuropsychological test data, and symptom reporting in a cohort of retired National Football League players. Sports Health 9, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Solomon GS, Kuhn AW, Zuckerman SL, Casson IR, Viano DC, Lovell MR, Sills AK (2016) Participation in pre-high school football and neurological, neuroradiological, and neuropsychological findings in later life: a study of 45 retired National Football League players. Am J Sports Med 44, 1106–1115. [DOI] [PubMed] [Google Scholar]
- [18].Iverson GL, Terry DP, Caccese JB, Büttner F, Merz ZC (2021) Age of first exposure to football is not associated with midlife brain health problems. J Neurotrauma 38, 538–545. [DOI] [PubMed] [Google Scholar]
- [19].Iverson GL, Gardner AJ, Shultz SR, Solomon GS, McCrory P, Zafonte R, Perry G, Hazrati L-N, Keene CD, Castellani R (2019) Chronic traumatic encephalopathy neuropathology might not be inexorably progressive or unique to repetitive neurotrauma. Brain 142, 3672–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McKee AC, Alosco ML, Huber BR (2016) Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg Clin N Am 27, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stern Y, Barnes CA, Grady C, Jones RN, Raz N (2019) Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol Aging 83, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, Vuoksimaa E, the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup (2020) Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement 16, 1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stern Y, Barulli D (2019) Cognitive reserve. Handb Clin Neurol 167, 181–190. [DOI] [PubMed] [Google Scholar]
- [24].Montine TJ, Cholerton BA, Corrada MM, Edland SD, Flanagan ME, Hemmy LS, Kawas CH, White LR (2019) Concepts for brain aging: resistance, resilience, reserve, and compensation. Alzheimers Res Ther 11, 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fratiglioni L, Wang H (2007) Brain reserve hypothesis in dementia. J Alzheimers Dis 12, 11–22. [DOI] [PubMed] [Google Scholar]
- [26].Valenzuela MJ (2008) Brain reserve and the prevention of dementia. Curr Opin Psychiatry 21, 296–302. [DOI] [PubMed] [Google Scholar]
- [27].Groot C, Loenhoud AC Van, Barkhof F, van Berckel BMN, Koene T, Tenuissen CC, Scheltens P, van der Flier WM, Ossenkoppele R (2018) Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology 90, 149–156. [DOI] [PubMed] [Google Scholar]
- [28].van Loenhoud AC, Groot C, Vogel JW, van der Flier WM, Ossenkoppele R (2018) Is intracranial volume a suitable proxy for brain reserve? Alzheimers Res Ther 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Donders J, Stout J (2019) The influence of cognitive reserve on recovery from traumatic brain injury. Arch Clin Neuropsychol 34, 91. [DOI] [PubMed] [Google Scholar]
- [30].Steward KA, Kennedy R, Novack TA, Crowe M, Marson DC, Triebel KL (2018) The role of cognitive reserve in recovery from traumatic brain injury. J Head Trauma Rehabil 33, E18–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Habeck C, Gazes Y, Razlighi Q, Stern Y (2020) Cortical thickness and its associations with age, total cognition and education across the adult lifespan. PLoS One 15, e0230298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, Sorbi S, Cappa SF, Padovani A, Fazio F, Perani D (2008) Education and occupation as proxies for reserve in aMCI converters and AD. Neurology 71, 1342–1349. [DOI] [PubMed] [Google Scholar]
- [33].Garibotto V, Borroni B, Sorbi S, Cappa SF, Padovani A, Perani D (2012) Education and occupation provide reserve in both ApoE ε4 carrier and noncarrier patients with probable alzheimer’s disease. Neurol Sci 33, 1037–1042. [DOI] [PubMed] [Google Scholar]
- [34].Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R (1994) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271, 1004–1010. [PubMed] [Google Scholar]
- [35].Tucker AM, Stern Y (2011) Cognitive reserve in aging. Curr Alzheimer Res 8, 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McDaniel MA (2005) Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence 33, 337–346. [Google Scholar]
- [37].Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT (2002) Exercise, experience and the aging brain. Neurobiol Aging 23, 941–955. [DOI] [PubMed] [Google Scholar]
- [38].Roe CM, Mintun MA, Ghoshal N, Williams MM, Grant EA, Marcus DS, Morris JC (2010) Alzheimer disease identification using amyloid imaging and reserve variables: Proof of concept. Neurology 75, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11, 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meng X, D’Arcy C (2012) Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 7, e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, Nowinski CJ, Cantu RC, McKee AC, Stern RA (2012) Chronic traumatic encephalopathy: Neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav 6, 244–254. [DOI] [PubMed] [Google Scholar]
- [42].Alosco ML, Mez J, Kowall NW, Stein TD, Goldstein LE, Cantu RC, Katz DI, Solomon TM, Kiernan PT, Murphy L, Abdolmohammadi B, Daneshvar D, Montenigro PH, Nowinski CJ, Stern RA, McKee AC (2017) Cognitive reserve as a modifier of clinical expression in chronic traumatic encephalopathy: a preliminary examination. J Neuropsychiatry Clin Neurosci 29, 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van Loenhoud AC, Wink AM, Groot C, Verfaillie SCJ, Twisk J, Barkhof F, van Berckel B, Scheltens P, van der Flier WM, Ossenkoppele R (2017) A neuroimaging approach to capture cognitive reserve: application to Alzheimer’s disease. Hum Brain Mapp 38, 4703–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jones R, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y (2011) Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc 17, 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, Hinton L, DeCarli C (2010) Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 133, 2196–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zahodne LB, Manly JJ, Brickman AM, Siedlecki KL, DeCarli C, Stern Y (2013) Quantifying cognitive reserve in older adults by decomposing episodic memory variance: replication and extension. J Int Neuropsychol Soc 19, 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Serra L, Bruschini M, Di Domenico C, Gabrielli GB, Marra C, Caltagirone C, Cercignani M, Bozzali M (2017) Memory is not enough: the neurobiological substrates of dynamic cognitive reserve. J Alzheimers Dis 58, 171–184. [DOI] [PubMed] [Google Scholar]
- [48].Reed BR, Dowling M, Tomaszewski Farias S, Sonnen J, Strauss M, Schneider JA, Bennett DA, Mungas D (2011) Cognitive activities during adulthood are more important than education in building reserve. J Int Neuropsychol Soc 17, 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Beyer L, Meyer-Wilmes J, Schönecker S, Schnabel J, Sauerbeck J, Scheifele M, Prix C, Unterrainer M, Catak C, Pogarell O, Palleis C, Perneczky R, Danek A, Buerger K, Bartenstein P, Levin J, Rominger A, Ewers M, Brendel M (2021) Cognitive reserve hypothesis in frontotemporal dementia: A FDG-PET study. Neuroimage Clin 29, 102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zahodne LB, Manly JJ, Brickman AM, Narkhede A, Griffith EY, Guzman VA, Schupf N, Stern Y, Erica Y, Guzman VA, Schupf N, Stern Y (2016) Is residual memory variance a valid method for quantifying cognitive reserve? A longitudinal application. Neuropsychologia 77, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bocancea DI, van Loenhoud AC, Groot C, Barkhof F, van der Flier WM, Ossenkoppele R (2021) Measuring resilience and resistance in aging and Alzheimer disease using residual methods: a systematic review and meta-analysis. Res Methods Neurol 97, 474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Seichepine DR, Stamm JM, Daneshvar DH, Riley DO, Baugh CM, Gavett BE, Tripodis Y, Martin B, Chaisson C, McKee AC, Cantu RC, Nowinski CJ, Stern RA (2013) Profile of self-reported problems with executive functioning in college and professional football players. J Neurotrauma 30, 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R, Katz DI, Cantu RC, Stern RA (2014) Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Katz DI, Bernick C, Dodick DW, Mez J, Mariani ML, Adler CH, Alosco ML, Balcer LJ, Banks SJ, Barr WB, Brody DL, Cantu RC, Dams-O’Connor K, Geda YE, Jordan BD, McAllister TW, Peskind ER, Petersen RC, Wethe JV, Zafonte RD, Foley ÉM, Babcock DJ, Koroshetz WJ, Tripodis Y, McKee AC, Shenton ME, Cummings JL, Reiman EM, Stern RA (2021) National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology 96, 848–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].United States Department of Labor (1991) Dictionary of Occupational Titles Fourth, Government Printing Office, Washington. [Google Scholar]
- [56].Wilkinson GS, Robertson GJ, Psychological Assessment Resources Inc (2006) Wide range achievement test (WRAT4), Psychological Assessment Resources Inc, Lutz, FL. [Google Scholar]
- [57].Reitan RM (1992) Trail making test: Manual for Administration and Scoring, Reitan Neuropsychology Laboratory, Tucson, AZ. [Google Scholar]
- [58].Stern RA, White T (2003) Neuropsychological Assessment Battery, Psychological Assessment Resources, Inc., Lutz, FL. [Google Scholar]
- [59].Lezak MD, Howieson DB, Bigler ED, Tranel D (2012) Neuropsychological assessment, Oxford University Press, New York. [Google Scholar]
- [60].Beck AT, Steer RA, Brown GK (1996) Manual for the BDI-II, San Antonio, TX. [Google Scholar]
- [61].Roth RM, Isquith PK, Gioia GA (2005) BRIEF-A : behavior rating inventory of executive function-adult version: professional manual, Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- [62].Lepage C, Muehlmann M, Tripodis Y, Hufschmidt J, Stamm J, Green K, Wrobel P, Schultz V, Weir I, Alosco ML, Baugh CM, Fritts NG, Martin BM, Chaisson C, Coleman MJ, Lin AP, Pasternak O, Makris N, Stern RA, Shenton ME, Koerte IK (2019) Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav 13, 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hart JJ, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E, Conover H, Mansinghani S, Lu H, Cullum CM (2013) Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol 70, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bernick C, Shan G, Zetterberg H, Banks S, Mishra VR, Bekris L, Leverenz JB, Blennow K (2020) Longitudinal change in regional brain volumes with exposure to repetitive head impacts. Neurology 94, e232–e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Alosco ML, Koerte IK, Tripodis Y, Mariani M, Chua AS, Jarnagin J, Rahimpour Y, Puzo C, Healy RC, Martin B, Chaisson CE, Cantu RC, Au R, Mcclean M, Mckee AC, Lin AP, Shenton ME, Killiany RJ, Stern RA (2018) White matter signal abnormalities in former National Football League players. Alzheimers Dement (Amst) 10, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- [67].Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad SciUSA 97, 11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30, 1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Guenette JP, Stern RA, Tripodis Y, Chua AS, Schultz V, Sydnor VJ, Somes N, Karmacharya S, Lepage C, Wrobel P, Alosco ML, Martin BM, Chaisson CE, Coleman MJ, Lin AP, Pasternak O, Makris N, Shenton ME, Koerte IK (2018) Automated versus manual segmentation of brain region volumes in former football players. Neuroimage Clin 18, 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].R Core Team (2020) R: A Language Environment for Statistical Computing
- [71].Murman DL (2015) The impact of age on cognition. Semin Hear 36, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Glisky EL (2007) Changes in cognitive function in human aging. In Brain Aging: Models, Methods, and Mechanisms, Riddle DR, ed. Boca Raton (FL). [Google Scholar]
- [73].Harada CN, Love MCN, Triebel K (2013) Normal cognitive aging. Clin Geriatr Med 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Potter GG, Plassman BL, Burke JR, Kabeto MU, Langa KM, Llewellyn DJ, Rogers MAM, Steffens DC (2009) Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement 5, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zahodne LB, Manly JJ, Azar M, Brickman AM, Glymour MM (2016) Racial disparities in cognitive performance across mid and late adulthood: analyses in two cohort studies. J Am Geriatr Soc 64, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Barnes LL, Bennett DA (2014) Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health Aff (Millwood) 33, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM, McClean MD, Tripodis Y, Stern RA (2015) Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 84, 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pettigrew C, Soldan A, Li S, Lu Y, Wang M-C, Selnes OA, Moghekar A, O’Brien R, Albert M, BIOCARD Research Team (2013) Relationship of cognitive reserve and APOE status to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Cogn Neurosci 4, 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].O’Donoghue MC, Murphy SE, Zamboni G, Nobre AC, Mackay CE (2018) APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex 104, 103–123. [DOI] [PubMed] [Google Scholar]
- [80].Reas ET, Laughlin GA, Bergstrom J, Kritz-Silverstein D, Barrett-Connor E, McEvoy LK (2019) Effects of APOE on cognitive aging in community-dwelling older adults. Neuropsychology 33, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bracco L, Piccini C, Baccini M, Bessi V, Biancucci F, Nacmias B, Bagnoli S, Sorbi S (2007) Pattern and progression of cognitive decline in Alzheimer’s disease: Role of premorbid intelligence and ApoE genotype. Dement Geriatr Cogn Disord 24, 483–491. [DOI] [PubMed] [Google Scholar]
- [82].Andreotti C, Hawkins KA (2015) RBANS norms based on the relationship of age, gender, education, and WRAT-3 reading to performance within an older African American sample. Clin Neuropsychol 29, 442–445. [DOI] [PubMed] [Google Scholar]
- [83].Franzen MD, Burgess EJ, Smith-Seemiller L (1997) Methods of estimating premorbid functioning. Arch Clin Neuropsychol 12, 711–738. [PubMed] [Google Scholar]
- [84].Pintzka CWS, Hansen TI, Evensmoen HR, Håberg AK (2015) Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Front Neurosci 9, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Voevodskaya O, Simmons A, Nordenskold R, Kullberg J, Ahlstrom H, Lind L, Wahlund L-O, Larsson E-M, Westman E, Alzheimer’s Disease Neuroimaging Initiative (2014) The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front Aging Neurosci 6, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].MacPherson SE, Healy C, Allerhand M, Spanò B, Tudor-Sfetea C, White M, Smirni D, Shallice T, Chan E, Bozzali M, Cipolotti L (2017) Cognitive reserve and cognitive performance of patients with focal frontal lesions. Neuropsychologia 96, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MS V, Wright CB (2009) Construct validity of cognitive reserve in a multi-ethnic cohort: the Northern Manhattan Study. J Int Neuropsychol Soc 15, 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Opdebeeck C, Martyr A, Clare L (2016) Cognitive reserve and cognitive function in healthy older people: a metaanalysis. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 23, 40–60. [DOI] [PubMed] [Google Scholar]
- [89].Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W (2019) Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol 138, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zivadinov R, Polak P, Schweser F, Bergsland N, Hagemeier J, Dwyer MG, Ramasamy DP, Baker JG, Leddy JJ, Willer BS (2018) Multimodal imaging of retired professional contact sport athletes does not provide evidence of structural and functional brain damage. J Head Trauma Rehabil 33, E24–E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Iverson GL, Caccese JB, Merz ZC, Büttner F, Terry DP (2021) Age of first exposure to football is not associated with later-in-life cognitive or mental health problems. Front Neurol 12, 647314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Scarmeas N, Levy G, Tang M-X, Manly JJ, Stern Y (2001) Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 57, 2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Valenzuela MJ, Sachdev P (2006) Brain reserve and dementia: a systematic review. Psychol Med 36, 441–454. [DOI] [PubMed] [Google Scholar]
- [94].Shapiro ME, Mahoney JR, Peyser D, Zingman BS, Verghese J (2014) Cognitive reserve protects against apathy in individuals with human immunodeficiency virus. Arch Clin Neuropsychol 29, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Altieri M, Trojano L, Gallo A, Santangelo G (2020) The relationships between cognitive reserve and psychological symptoms: a cross-sectional study in healthy individuals. Am J Geriatr Psychiatry 28, 404–409. [DOI] [PubMed] [Google Scholar]
- [96].Colangeli S, Boccia M, Verde P, Guariglia P, Bianchini F, Piccardi L (2016) Cognitive reserve in healthy aging and Alzheimer’s disease: a meta-analysis of fMRI studies. Am J Alzheimers Dis Other Demen 31, 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, Bennett DA (2013) Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 80, 1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL (2003) Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60, 1909–1915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


