Abstract
Leiomyomas are the most common uterine benign tumor, and their malignant counterpart leiomyosarcomas are extremely rare. Despite this, a preoperative diagnosis could be useful for safe surgical minimally invasive management. At present, some clinical and ultrasound findings help recognizing lesions at risk of malignancy. We tried to implement a technique for the preoperative diagnosis for lesions at risk performing ultrasound-guided biopsies of suspected lesions in ten patients. Among them, one case was diagnosed as malignant by the needle biopsy. All patients underwent surgery for myomectomy or hysterectomy, and the histology was confirmed in all cases. No complications occurred. The review of the literature shows other similar experiences of preoperative biopsy of uterine lesions, showing good results for the differential diagnosis between uterine sarcoma and leiomyoma. In our experience, despite the small number of patients enrolled, this technique is safe and effective to plan minimally invasive surgery of uterine fibroids.
Keywords: Biopsies, differential diagnosis, leiomyoma, leiomyosarcoma, ultrasound
INTRODUCTION
Leiomyomas represent the most common uterine benign tumor, and their malignant counterpart leiomyosarcomas are extremely rare, with a prevalence from 0.01% to 0.06% of all fibroids. Considering the high rate of intervention in women with fibroids and despite the low incidence of sarcomas, a preoperative diagnosis should be necessary for safe surgical management. Since the Food and Drug Administration warning against power morcellation of 2014, the attention toward the risk of dissemination of malignancy during myomectomies or hysterectomies has widely increased. The use of power morcellation has been reduced, and at the same time, the rate of laparotomies has risen, along with all known risks of an invasive surgery compared to laparoscopy.[1] A histologic preoperative diagnosis could be the solution to decide the proper surgical technique, to adequately perform minimally invasive uterine surgery.
TECHNIQUE AND CASE PRESENTATION
We report our case series of a preoperative minimally invasive diagnostic procedure for fibroids with uncertain clinical aspects. We retrospectively reviewed the cases of ten patients, who underwent clinical and ultrasound evaluation at our Gynecology Unit from December 2014 to December 2018. All patients had ultrasound findings of uterine fibroid with one or more features of suspicion for a malignant origin, such as postmenopausal age, a growing single uterine lesion, inhomogeneous with mixed echogenic, and hypoechoic areas, no acoustic shadowing, or irregular vessel distribution in the tumor.[2] The patients with multiple lesions were not included in the study. After the clinical evaluation, we performed biopsies of each suspected mass. Local anesthesia was given on the site of the needle injection, either abdominal or vaginal/paracervical. Biopsies were ultrasound-guided either with transvaginal or transabdominal approach, depending on the position of the mass. An 18G Tru-Cut needle has been used [Figure 1]. We performed four to five biopsies for each fibroid to reduce the sampling error. On the basis of the formalin-fixed specimen histology, we, therefore, could tailor the surgery on the patient with the safest and less invasive approach. For each patient, the time elapsed between preoperative biopsies and surgery varied from 30 to 60 days. Seven patients underwent either biopsy and definitive surgery to remove the uterine mass; they all had clinical risk factors or ultrasound findings suspicious for leiomyosarcoma [patients characteristics are summarized in Table 1].
Figure 1.
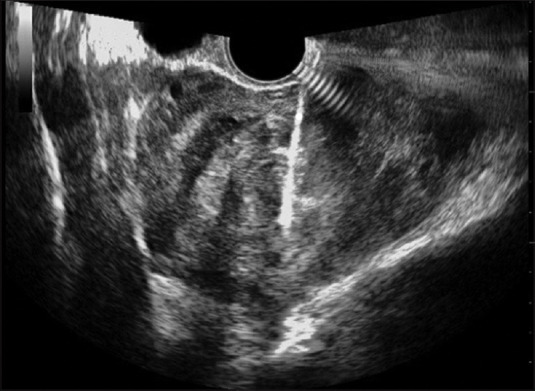
Ultrasound frame of the needle taking a fibroid sample
Table 1.
Patients’ characteristics and histological findings
| Patient | Age | BMI | Menopause | Clinical features | Ultrasound features | Biopsy | Treatment | Preoperative histology | Postoperative histology |
|---|---|---|---|---|---|---|---|---|---|
| A | 33 | 37 | No | Pelvic pain | 17 cm single fibroid rapidly growing | Yes | Abdominal myomectomy | Typical leiomyoma | Typical leiomyoma |
| B | 40 | 33 | No | Pelvic pain | Single mass, growing lesion, inhomogeneous content, irregular vessel distribution | Yes | Abdominal myomectomy | Typical leiomyoma | Typical leiomyoma |
| C | 41 | 19 | No | Menometrorrhagia | Single mass, growing lesion | Yes | TLH - BS with in-bag morcellation | Typical leiomyoma | Typical leiomyoma |
| D | 42 | 30 | No | No pregnancy desire | 8 cm single fibroid, inhomogeneous content, irregular vessel distribution | Yes | TLH - BS with in-bag morcellation | Cellular leiomyoma | Cellular leiomyoma |
| E | 42 | 36 | No | No complaints | Single mass, growing lesion, irregular vessel distribution | Yes | TVH - BS | Typical leiomyoma | Typical leiomyoma |
| F | 46 | 21 | No | Dyspareunia, pelvic pain, no response to UPA | Single 8 cm fibroid, not reducing after UPA | Yes | TLH - BS with in-bag morcellation | Cellular leiomyoma | Cellular leiomyoma |
| G | 50 | 30 | Yes | No complaints | Single mass, growing lesion, inhomogeneous content, no acoustic shadowing | No | TAH - BS | / | Leiomyoma with scleral-hyaline degeneration |
| H | 51 | 22 | Yes | No complaints | Single mass, colliquation, no acoustic shadowing | Yes | TLH - BS with in-bag morcellation | Typical leiomyoma | Typical leiomyoma |
| I | 55 | 23 | Yes | Previous hysterectomy for leiomyomas | Solid single mass with acoustic shadowing and inhomogeneous content | Yes | Abdominal bilateral oophorectomy | Leiomyoma | Ovarian leiomyoma |
| L | 58 | 22 | Yes | Total hysterectomy + oophorectomy for leiomyosarcoma 13 years before | Solid inhomogeneous pelvic mass | Yes | Chemotherapy | Leiomyosarcoma | / |
TLH: Total laparoscopic hysterectomy, TVH: Total vaginal hysterectomy, TAH: Total abdominal hysterectomy, BS: Bilateral salpingectomy, BMI: Body mass index, UPA: Ulipristal acetate
In all the cases, the definitive histological examination confirmed the preoperative bioptical diagnosis: In five cases (patients A, B, C, E, and H), the mass was diagnosed either preoperatively or postoperatively as typical leiomyoma [Figure 2a]; in two cases (patients D and F), the preoperative diagnosis of cellular leiomyoma was confirmed after surgery [Figure 2b]. One patient (patient I) already had a hysterectomy for uterine fibroids in the past, and on our examination, she was found to have a solid pelvic mass with acoustic shadowing but inhomogeneous aspect which seemed to origin from the cervical stump. She underwent abdominal computed tomography (CT) which showed a 10 cm solid mass originating from the pelvis, with the aspects of disproliferation. We then performed ultrasound-guided biopsies and the pathological analysis defined the specimens as leiomyoma. Afterward, the patient underwent a positron emission tomography total body which confirmed a low glucose hypercaptation of the mass. The patient underwent a longitudinal laparotomy for the removal of the mass, which eventually turned out to origin from the left ovary. The definitive histology was ovarian leiomyoma. In one case (patient G), a 50-year-old patient was found to have a uterine 6 cm subserosal fibroid with uneven appearance, no acoustic echoes, which had grown 2 cm for the past control 6 months before. We could not be able to perform the biopsy: the needle could not pierce the mass because of its hardness. Therefore, the patient underwent total laparotomic hysterectomy and salpingectomy. The histologic result demonstrated a leiomyoma with scleral-hyaline degeneration [Figure 2c]. The last patient (patient L), a 58-year-old woman, had a history of total hysterectomy and bilateral oophorectomy for uterine leiomyosarcoma 13 years before. On current ultrasound evaluation, we appreciated a solid dishomogeneous pelvic mass, suggestive for a relapse. The bioptic diagnosis turned out to be leiomyosarcoma, as expected. Subsequently, we performed diagnostic laparoscopy for the staging of the neoplasm, but the patient decided not to undergo resection of the neoplasm. Afterwards, the patient underwent adjuvant chemotherapy, and currently, she is still on remission and under CT follow-up.
Figure 2.
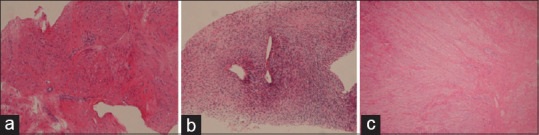
Formalin-fixed tissue sections of patient B, typical leiomyoma (a), patient D, cellular leiomyoma (b), and patient G, leiomyoma with scleral-hyaline degeneration (c)
DISCUSSION
In all seven cases, which underwent either a successful biopsy or surgery to remove the mass of the histological examination and confirmed the preoperative diagnosis as leiomyoma or its benign variants. The histologic result of the mass which we have not been able to pierce (leiomyoma with scleral-hyaline degeneration) could explain the reason why it had been impossible to perform the biopsy. However, this eventuality is quite uncommon and, in our opinion, should not limit the implementation of this procedure, which in turn demonstrated to be a useful tool for differential diagnosis between benign and malignant smooth muscle uterine tumors in our experience. For the patient with a history of leiomyosarcoma, the procedure resulted to be effective to give a histologic diagnosis without performing surgery; in such a manner, we could be able to plan our management strategy outside the operating room. Among all patients enrolled no complications, such as intraperitoneal hemorrhage, infection, or injury to adjacent structures have been observed. Moreover, there has not been any macroscopic sign of seeding of bioptical fragments and intraperitoneal spread of the disease in any of the patients who underwent surgery after preoperative biopsy.
The review of the literature showed other similar experiences implementing procedures which could be able to give a differential diagnosis between benign and malignant smooth muscle cell uterine tumors. Tulandi and Ferenczy[3] reported two cases of intraoperative percutaneous needle biopsy of uterine fibroids during laparoscopy. As we did, they used a Tru-Cut needle to perform the biopsy, but in their experience, the samplings were studied on frozen sections as they needed an immediate analysis before undertaking morcellation or converting into laparotomy. Since the role of the frozen section in the diagnosis of myometrial disease is controversial,[4] in our opinion, there could arise some doubts on the reliability of this procedure. To date, the only reliable demonstrated technique for the diagnosis of leiomyosarcoma is formalin-fixed tissue analysis. Therefore, we chose to distinguish a preoperative diagnostic time from the actual surgical one. Moreover, in Tulandi's experience, the procedure was implemented in two patients with no particular risk factors or features of suspicion for malignancy, except for the general risk of a uterine fibroid to undercover a leiomyosarcoma. Kawamura et al.[5] suggest the use of preoperative ultrasound-guided transcervical biopsies to differentiate uterine leiomyomas from leiomyosarcomas. Their experience on 453 cases demonstrated a sensitivity of 100% and a specificity of 98.6%, with positive and negative predictive values of 58% and 100%, respectively, of the procedure. Their experience could be useful because of its reproducibility and the possibility of this technique to reach submucous fibroids, which with our approach would have been difficult to realize. On the other hand, a transcervical approach hardly permits to reach subserosal fibroids. Therefore, the two approaches could be considered complementary in their technical effectiveness. Tamura et al.[6] report their experience on 63 cases of preoperative ultrasound-guided needle biopsy of uterine tumors having characteristics suspected for malignancy on magnetic resonance imaging (MRI). They used an 18G needle and performed three biopsies for each tumor. They were able to detect 12 cases of malignancy, with the procedure having a sensitivity of 91.7% and a specificity of 100% on patients that afterward underwent surgery. We propose a preoperative procedure, which allows the analysis of a formalin-fixed tissue section, without the limits of the frozen section; we used a transabdominal or transvaginal approach to be able to reach also deep-seated subserosal or intramural leiomyomas. We performed multiple biopsies for each fibroid, to reduce the sampling errors; we chose to make 4–5 biopsies for each tumor but an evidence-based number of samplings needs to be demonstrated. To elect patients worthy of undertaking the procedure, we used the clinical and ultrasound parameters, whereas Tamura et al. used MRI findings. Yet, there is no accordance on which characteristics are more suspicious for uterine malignancy,[7] there forward it will be necessary to better standardize the clinical and instrumental characteristics of fibroids suspected to be malignant and for this reason eligible to preoperative histologic assessment. In our experience, this procedure has been demonstrated to be safe and relatively easy to implement, but larger studies are needed to state its reproducibility, safety, and effectiveness as a tool in the diagnostic course of uterine fibroids.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Harris JA, Sammarco AG, Swenson CW, Uppal S, Kamdar N, Campbell D, et al. Are perioperative bundles associated with reduced postoperative morbidity in women undergoing benign hysterectomy? Retrospective cohort analysis of 16,286 cases in Michigan. Am J Obstet Gynecol. 2017;216:502. doi: 10.1016/j.ajog.2016.12.173. e1-e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu TI, Yen TC, Lai CH. Clinical presentation and diagnosis of uterine sarcoma, including imaging. Best Pract Res Clin Obstet Gynaecol. 2011;25:681–9. doi: 10.1016/j.bpobgyn.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Tulandi T, Ferenczy A. Biopsy of uterine leiomyomata and frozen sections before laparoscopic morcellation. J Minim Invasive Gynecol. 2014;21:963–6. doi: 10.1016/j.jmig.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Leibsohn S, d’Ablaing G, Mishell DR, Schlaerth JB. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968–74. doi: 10.1016/0002-9378(90)91298-q. [DOI] [PubMed] [Google Scholar]
- 5.Kawamura N, Ichimura T, Ito F, Shibata S, Takahashi K, Tsujimura A, et al. Transcervical needle biopsy for the differential diagnosis between uterine sarcoma and leiomyoma. Cancer. 2002;94:1713–20. doi: 10.1002/cncr.10382. [DOI] [PubMed] [Google Scholar]
- 6.Tamura R, Kashima K, Asatani M, Nishino K, Nishikawa N, Sekine M, et al. Preoperative ultrasound-guided needle biopsy of 63 uterine tumors having high signal intensity upon T2-weighted magnetic resonance imaging. Int J Gynecol Cancer. 2014;24:1042–7. doi: 10.1097/IGC.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 7.Van den Bosch T, Coosemans A, Morina M, Timmerman D, Amant F. Screening for uterine tumours. Best Pract Res Clin Obstet Gynaecol. 2012;26:257–66. doi: 10.1016/j.bpobgyn.2011.08.002. [DOI] [PubMed] [Google Scholar]


