Abstract
Repetitive transcranial magnetic stimulation (rTMS) of the inferior parietal cortex (IPC) increases resting-state functional connectivity (rsFC) of the hippocampus with the precuneus and other posterior cortical areas and causes proportional improvement of episodic memory. The anatomical pathway(s) responsible for the propagation of these effects from the IPC is unknown and may not be direct. In order to assess the relative contributions of candidate pathways from the IPC to the MTL via the parahippocampal cortex and precuneus, to the effects of rTMS on rsFC and memory improvement, we used diffusion tensor imaging to measure the extent to which individual differences in fractional anisotropy (FA) in these pathways accounted for individual differences in response. FA in the IPC-parahippocampal pathway and several MTL pathways predicted changes in rsFC. FA in both parahippocampal and hippocampal pathways was related to changes in episodic, but not procedural, memory. These results implicate pathways to the MTL in the enhancing effect of parietal rTMS on hippocampal rsFC and memory.
Keywords: rTMS, DWI, Hippocampus, resting-state functional connectivity, episodic memory
1. Introduction
Repetitive transcranial magnetic stimulation (rTMS) can modulate resting-state functional connectivity (rsFC) in brain networks (Freedberg et al., 2019; Halko et al., 2014; Hermiller et al., 2019; Nilakantan et al., 2017; Rastogi et al., 2017; Wang et al., 2014; Warren et al., 2019). By delivering rTMS to the inferior parietal cortex (IPC), Wang et al. (2014) increased rsFC between the hippocampus and a network of posterior cortical regions, most prominently the precuneus. The magnitude of this change was linearly related to improved episodic memory among individuals. These effects have been reproduced several times by the same (Hermiller et al., 2019; Nilakantan et al., 2017; Warren et al., 2019) and other (Freedberg et al., 2019; Tambini et al., 2018) laboratories, and they appear to be specifically associated with rsFC increases in a set of cortical regions with high baseline hippocampal rsFC. Using rTMS to increase memory network connectivity may help patients with pathologies that cause reduced efficiency in networks involved in episodic memory (e.g. Alzheimer disease; Greicius et al. 2004)
Previous studies have targeted the IPC sub-region with the strongest hippocampal rsFC in individual subjects because the hippocampus is the hub of the episodic memory network and because there is a related assumption that an IPC-hippocampus connection is central to the effect (Freedberg et al., 2019, 2020; Hermiller et al., 2019; Wang et al., 2014; Warren et al., 2019). One proposed route for propagation of hippocampal rsFC-reinforcing activity from the stimulated site is via retrosplenial and/or parahippocampal cortex to the hippocampus and thence, via divergent pathways, to regions connected to the hippocampus (Freedberg et al., 2019; Hebscher & Voss, 2020; Wang et al., 2014). To date, there is no experimental evidence for this hypothesis other than the finding that baseline rsFC between the IPC and the hippocampus predicts the changes in hippocampal rsFC and associative memory (Tambini et al., 2018).
High rsFC between regions can occur via intermediate nodes (Honey et al., 2009) where anatomical connectivity exists (Davis et al., 2017; Gong et al., 2009), and models based on anatomical connectivity account for the effects of TMS on network rsFC (Muldoon et al., 2016). Individual differences in pathway anatomy also have potential to explain the wide variability in the physiological (López-Alonso et al., 2014) and behavioral (Nicolo et al., 2015) responses to rTMS.
Diffusion tensor imaging (DTI) analysis of diffusion-weighted (DW) MRI can estimate the location of major WM pathways (via tractography) and quantify their properties. Fractional anisotropy (FA; Basser 1995; Jones et al. 2013) is a scalar value sensitive to axon diameter, fiber density, membrane permeability, myelination, and the intra-voxel orientational coherence of axons (Beaulieu, 2002) and can identify important differences in pathway organization between individuals (Cheng et al., 2012). FA has been used in conjunction with physiological methods (Betzel et al., 2014; Honey et al., 2009) to explain inter-individual rsFC variability.
In order to study the mechanism underlying the effect of IPC rTMS on hippocampal rsFC and episodic memory, we used DTI-based tractography to find the segments of the WM skeleton that most likely connect regions of interest (ROIs), including the stimulation point in the IPC, regions in the MTL, such as the hippocampus, and the precuneus. We then measured FA in pathways capable of transmitting the effect to the hippocampus and other nodes in the episodic memory network. Finally, we looked for associations between FA in these candidate pathways and the response to rTMS across individuals.
We measured FA in several independent candidate pathways linking the stimulation point to downstream regions. These pathways are hypothesized to be responsible for the effects of IPC rTMS on rsFC and episodic memory (Freedberg et al., 2019; Hebscher & Voss, 2020; Hermiller et al., 2020; Tambini et al., 2018; Wang et al., 2014; Warren et al., 2019) and include regions which support episodic memory (Ritchey & Cooper, 2020; Rudge & Warrington, 1991; Rugg & Vilberg, 2013; Scoville & Milner, 1957; Sestieri et al., 2011; Valenstein et al., 1987). The first connected the IPC to a region of the medial temporal lobe (MTL), including the parahippocampal and entorhinal areas. Anatomical tracing studies in primates have found robust connections between area 7 (IPC, or posterior parietal cortex) and the parahippocampal cortex (Cavada & Goldman-Rakic, 1989a; Mesulam et al., 1977; Pandya & Seltzer, 1982). The parahippocampal cortex projects to the caudal entorhinal cortex (Suzuki & Amaral, 1994) which then projects to the hippocampus via the perforant pathway (Amaral & Witter, 1989). Thus, we examined IPC-MTL connections in three segments (the “indirect route”): 1) IPC to parahippocampal cortex, 2) parahippocampal cortex to entorhinal cortex, and 3) entorhinal cortex to hippocampus. We also considered the contribution of the “direct route,” fibers connecting the IPC to the hippocampus directly, which have been found by anatomical tracing in primates (Ding et al., 2000; Rockland and Van Hoesen, 1999). The other pathway we examined, “the precuneus pathway,” connects the IPC to the precuneus, the region showing the most reproducible and robust hippocampal rsFC increase after IPC rTMS (Freedberg et al., 2019; Wang et al., 2014). Tracing studies in monkeys (Pandya and Seltzer 1982; Cavada and Goldman-Rakic 1989a; Morecraft et al. 2004) have shown projections from area 7 (the IPC) to area 7m (the precuneus) and from there to the MTL, and similar connections have been shown with DTI in humans (Takahashi et al., 2008), passing via the cingulum bundle (Wu et al., 2016). The precuneus has diverse cognitive functions (Cavanna & Trimble, 2006), but it is critical for episodic memory (Rudge & Warrington, 1991; Valenstein et al., 1987; von Cramon & Schuri, 1992). Enhancement of rsFC in a network functionally centered on the precuneus, rather than the hippocampus, is also an alternative explanation for the effect of IPC rTMS on memory. Finally, we also considered the contributions of the”retrosplenial pathway,” connecting the IPC to the retrosplenial area and from there to other MTL regions (Cavada and Goldman-Rakic, 1989a; Kobayashi and Amaral, 2003; Seltzer and Pandya, 1984; Suzuki and Amaral, 1994).
To investigate whether the effects of IPC rTMS on behavior were specific to episodic memory, we also tested procedural memory, which involves a largely separate system, based principally in a network of frontal and striatal regions (Knowlton et al., 1996; Poldrack et al., 1999). As an additional control to determine whether our results are specific to our tracts of interest, we substituted FA values in all our correlations with IPC-precentral FA. The precentral gyrus is not thought to be involved in any of our rTMS effects of interests. Thus, we expect FA correlations for our pathways of interest to be significantly greater than correlations using FA from our control tract.
2. Materials and Methods
2.1. Ethics statement
This experiment was approved by the NINDS CNS IRB. Written informed consent was obtained prior to study participation.
2.2. Participants
Forty-eight adults (20 female; mean age = 25.33 ± 4.80 yrs), free of neurological or psychiatric disorders or medications acting on the central nervous system, were included in the study. All participants passed screening for contraindications to TMS (Rossi et al., 2009) and MRI. In addition to the 18 participants who received three daily consecutive sessions of left IPC stimulation and 15 who received vertex stimulation, we included 12 participants from a previous study (Freedberg et al., 2020), who received either three (n= 6) or four (n = 6) days of stimulation to the left IPC, the other three participants in that study, which did not include behavioral testing, received 1 day of stimulation and were not included here. We used three days of stimulation in general due to logistical constraints. From the total of 48 participants, we excluded 13 for the following reasons: received only 1 day of stimulation (3); hippocampal seed used to locate the left IPC target was mistakenly placed in the parahippocampal cortex (3); failed to follow instructions during baseline behavior testing (1); excessive head motion during scanning (1); incomplete or technically poor DTI data acquisition (5). This left 35 participants (16 female; mean age = 25.31 ± 4.52 yrs). Of this group, 18 received three sessions of left IPC stimulation, six received four sessions of left IPC stimulation, and 11 received three sessions of vertex stimulation. Since participants in the previous study (Freedberg et al., 2020) did not receive behavioral testing, we report behavioral results from 12 participants from the left IPC stimulation group and 11 from the vertex group. See Table 1 for a summary of treatment duration and behavioral testing numbers. One participant from the left IPC group developed an explicit strategy during the baseline procedural memory test and we omitted their data for that test. All participants in the final data set reported being right-handed.
Table 1.
Participants included in the current study.
| Source | N | Number of stimulation days | Stimulation site | Number of Participants Excluded | Received Behavioral testing? |
|---|---|---|---|---|---|
| Freedberg et al. (2020) | 15 | 3 | |||
| 3 | 1 | IPC | 3 | No | |
| 6 | 3 | IPC | 0 | No | |
| 6 | 4 | IPC | 0 | No | |
| New participants | 33 | 10 | |||
| 18 | 3 | IPC | 6 | Yes | |
| 15 | 3 | Vertex | 4 | Yes | |
| Total after exclusion | 35 | ||||
| 18 | 3 | IPC | Yes (N = 12) | ||
| 6 | 4 | IPC | No (N = 6) | ||
| 11 | 3 dys | Vertex | Yes (N = 11) |
IPC = Inferior parietal cortex.
2.3. Procedures
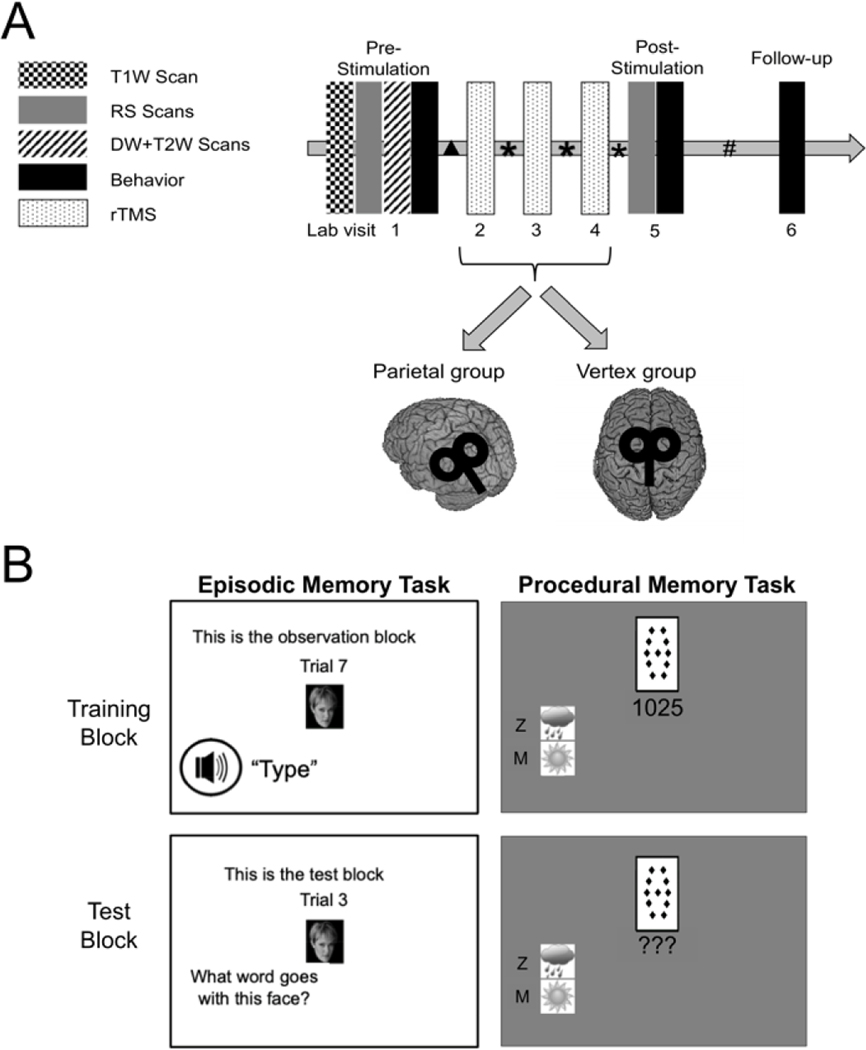
All participants underwent, in order: baseline MRI scanning, three or four consecutive daily rTMS sessions, and a post-stimulation MRI scan. Behavioral measures were taken immediately following each scan and 7–14 days after the last rTMS session (Figure 1A). Baseline MRI scanning included an anatomical localizer, structural scan (for functional scan co-localization with anatomy, and neuro-navigation), a resting state fMRI scan, DW, and T2 scans. Participants underwent their first rTMS session within 36 hours of baseline scanning. The second MRI session occurred on the day after the final rTMS session and within three hours of the time of day of the first scanning session. Participants were blinded to the specific intent of the study and to the stimulation condition.
Figure 1.
A. Timing of procedures and stimulation sessions. Six of the participants received four sessions of stimulation. DW = diffusion-weighted, RS = resting-state. Time between sessions: ▲ = 0 – 36 hours, * = 24 hours, # = 7–14 days. B. Memory tasks. Episodic memory task: Participants encoded face-word pairs during the training block and were asked to recall the paired word when shown the faces at testing. Procedural memory task: Participants were shown 14 arrangements of four cards and learned the probabilistic relationship between the cards and two possible outcomes through feedback. During testing, participants were tested on their ability to produce the optimal response for each arrangement.
2.4. fMRI and DWI acquisition
MRI was performed on a Siemen’s Magnetom 3T scanner, using a 16-channel head coil with foam padding to prevent head movement. Participants were fitted with earplugs and supplied with headphones to protect hearing. During resting fMRI scans, participants were instructed to lie still with their eyes open and to breathe and blink normally. Blood oxygen level-dependent (BOLD) data were recorded with a T2*-weighted gradient-echo echo-planar imaging sequence (EPI) and the following parameters: FOV = 220 × 220 mm, voxel size = 3.4 × 3.4 × 3.0 mm3, matrix size = 64 × 64, 36 interleaved axial slices per volume, TR = 2000 ms, TE = 27 ms, flip angle = 90º, GRAPPA = 2; number of volumes = 206 (scan length approx. 6.8 minutes).
DWI scans were acquired with a dual spin-echo EPI sequence and the following parameters: FOV = 256 × 256 mm, voxel size = 2.0 mm isotropic, matrix size = 128 × 128, 75 axial slices, TR = 17000 ms, TE = 98 ms, GRAPPA = 2. For each participant, two sets of DWIs were acquired with opposite phase encoding, one anterior-to-posterior (AP) and one posterior-to-anterior (PA). For each phase-encoded set, there were 80 total volumes: 60 directions with diffusion weighting factor b = 1100 s/mm2, 10 directions with b = 300 s/mm2, and 10 acquisitions with b = 0 s/mm2 (i.e., non-DW).
T2-weighted images with fat saturation were acquired with a turbo spin-echo sequence and the following parameters: FOV = 192 × 192 mm, voxel size = 1.0 × 1.0 × 1.7 mm3, matrix size of 192 × 192, 94 axial slices, TR = 8000 ms, TE = 89 ms, GRAPPA = 2. T1-weighted images were acquired with a magnetization-prepared rapid gradient echo sequence (MPRAGE) with AP phase encoding and the following parameters: FOV = 256 × 256 mm, voxel size = 1.0 mm isotropic, matrix size = 256 × 256, 176 sagittal slices, TR = 2530 ms, and TE = 3.03 ms, GRAPPA = 2.
2.5. Resting-state preprocessing
Resting-state data were preprocessed with the AFNI software package (Cox, 1996), version 20.1.05. Briefly, the first 5 volumes (out of 206 acquired) were removed to ensure that magnetization was stabilized (3dTcat). Preprocessing included, in order, de-spiking (3dDespike), slice-timing correction to the first slice (3dTshift), deobliquing (3dWarp), motion correction (3dvolreg), and functional/structural affine co-registration to Talairach space using the TT_N27 anatomical template and voxel resampling to 2 mm3 (align_epi_anat.py and 3dAllineate; Talairach and Tournoux, 1988). The estimates for motion correction, EPI to anatomical alignment, and transformation into standard space, were combined into a single transformation so that regridding of EPI data occurred only once. Next, EPI data were spatially smoothed using a 4 mm full width at half maximum (FWHM) Gaussian kernel (3dmerge). We then scaled each voxel time series to a mean of 100 with a range of 0–200 (3dTcat and 3dcalc; see Chen et al. 2017), regressed head motion from each voxel time series using the mean and derivatives of the 6 rigid-body parameter estimates, and detrended the data (3dDeconvolve, 3dTproject, 3dcalc; Power et al., 2012). Importantly, head motion correction and linear detrending were performed based on a single regression model in order to avoid reintroducing signal-related nuisance covariates. This problem can be caused by projecting data into a sequence of different subspaces (Lindquist et al., 2019). Prior to model regression, frames with movement displacement greater than 0.3 mm were censored. We used a threshold of 0.3 mm of average head displacement across all frames, including censored ones, during any scan to exclude participants (one participant). After preprocessing each participant’s baseline resting-state scan, we identified the hippocampal “seed” as the voxel maximally connected with a 15 mm sphere centered on the IPC for each participant (see Section 2.10, below).
2.6. ROI selection for rsFC calculations
We measured rsFC between the hippocampal seed, determined from the baseline resting-state scan, and a cluster of voxels in the precuneus. The boundaries of the precuneus were defined by reanalyzing the data from Wang et al. (2014). Their experimental design was similar to this, except their participants (n = 16) received both active and sham stimulation of the IPC, in separate sessions. Thus, participants underwent four scans (pre-active, post-active, pre-sham, and post-sham). First, we put the data from Wang et al. (2014) through the preprocessing pipeline described above. Then, we performed a whole brain analysis by contrasting the changes in hippocampal rsFC following active IPC and sham stimulation. We identified a cluster of voxels spanning the left precuneus and medial occipital lobe (p < 0.05, NN = 3) and excluded voxels outside the precuneus using the CA_N27_ML AFNI atlas to form a mask of the precuneus. We specifically isolated the precuneus cluster because the precuneus demonstrated the largest hippocampal-cortical rsFC changes in Wang et al. (2014). Additionally, we reproduced this hippocampal-precuneus rsFC change in our previous work (Freedberg et al., 2019). Thus, we felt it represented the best location for measuring hippocampal network rsFC changes. For the present study, average hippocampal rsFC was calculated in these voxels for each time point (pre- and post-stimulation) and for each participant, using the AFNI 3dBrickStat command and the correlation maps described above.
2.7. DWI preprocessing
The DW and anatomical datasets were preprocessed using a combination of FATCAT (Taylor & Saad, 2013) and TORTOISE (Pierpaoli et al. 2010; Irfanoglu et al. 2018; version 3.1.4). DW DICOMs were first converted into NIFTI format (with text gradient tables; fat_proc_convert_dcm_dwis), and both the T1W and T2W DICOM sets were also converted into NIFTI (fat_proc_convert_dcm_anat). Each participant’s T2W image was rigidly re-oriented (“axialized”) to the standard mni_icbm152_t2 reference data set included with the FATCAT software download (fat_proc_axialize_anat), and each participant’s T1W image was then also aligned to their re-oriented T2W image (fat_proc_align_anat_pair). DW images were visually inspected, and volumes corrupted by cardiac pulsation artifacts, signal dropout, motion artifact, ghosting, or spike/radiofrequency noise artifacts, were marked and removed (fat_proc_select_vols and fat_proc_filter_dwis) by authors CF or JM, and verified by MF. An average of 2.43 ± 2.35 volumes was discarded per participant (from 80 total). We then used TORTOISE’s DIFFPREP to correct for distortions caused by motion and eddy currents, in conjunction with DR-BUDDI (Irfanoglu et al., 2015) to correct for EPI distortions (B0 inhomogeneity effects). Finally, tensor fitting and tensor uncertainty estimation were performed in AFNI (fat_proc_dwi_to_dt), and DEC maps were generated for quality assurance (fat_proc_decmap). Data were inspected at multiple points of the processing pipeline for artifacts. Notably, the FATCAT and TORTOISE tools both use AFNI programs to generate images that are useful for quality control at each processing step (e.g., alignment, FA maps, etc.). Before and after undergoing preprocessing and tensor fitting, each participant’s DW image sets were inspected for artifacts.
2.8. Region of interest (ROI) selection for FA calculations
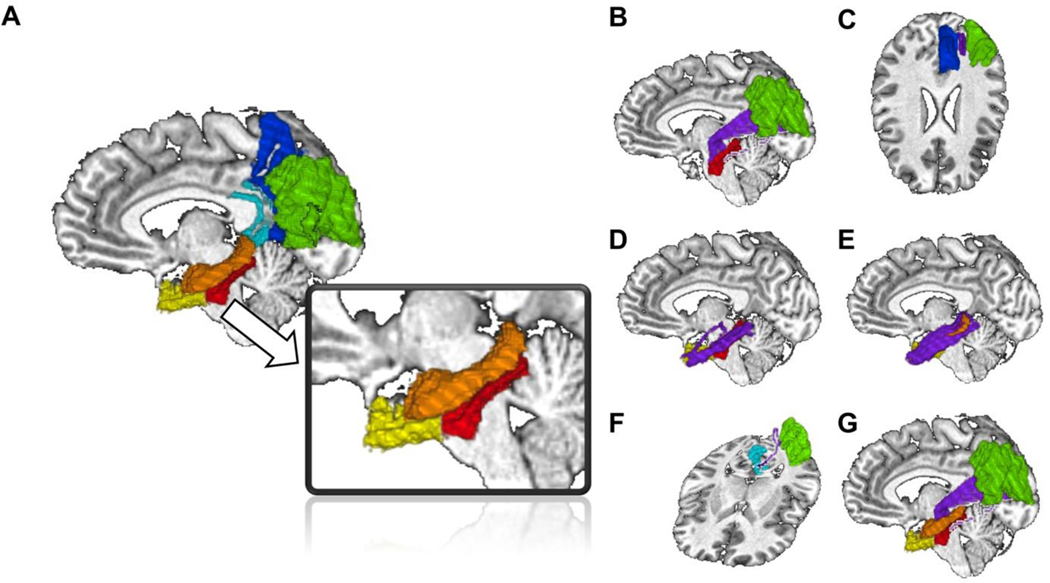
FA was calculated in WM pathways between specific pairs of gray matter (GM) ROIs and restricted to the stimulated (left) hemisphere. These GM ROIs were selected based on the theoretical mechanistic framework for rTMS-induced hippocampal network enhancement (Freedberg et al., 2019; Hebscher & Voss, 2020; Wang et al., 2014). For each participant, we identified GM ROIs for the stimulation point in the left IPC and the parahippocampal, entorhinal, and hippocampal areas by parcellating each participant’s T1W scan with the “recon-all” command in FreeSurfer (Dale et al., 1999) version 6.0.0; In particular, we used the Desikan (DK) atlas (Desikan et al. 2006) because it delineates gyral anatomy well. Note that the entorhinal cortex parcellation in this atlas corresponds to both the entorhinal and perirhinal cortices. For examples of these regions and our pathways of interest from a representative participant, see Figure 2.
Figure 2.
ROIs and tracts for FA calculations. A. ROIs, including the precuneus (blue), IPC (green), retrosplenial cortex (cyan), parahippocampal cortex (red), entorhinal cortex (yellow), and hippocampus (orange) overlaid on a single participant’s brain. Tracts used to calculate FA are shown in purple and include the IPC-parahippocampus (B), IPC-precuneus (C), parahippocampus-entorhinal (D), entorhinal-hippocampus (E), IPC-retrosplenial (F), and IPC-hippocampal pathways (G).
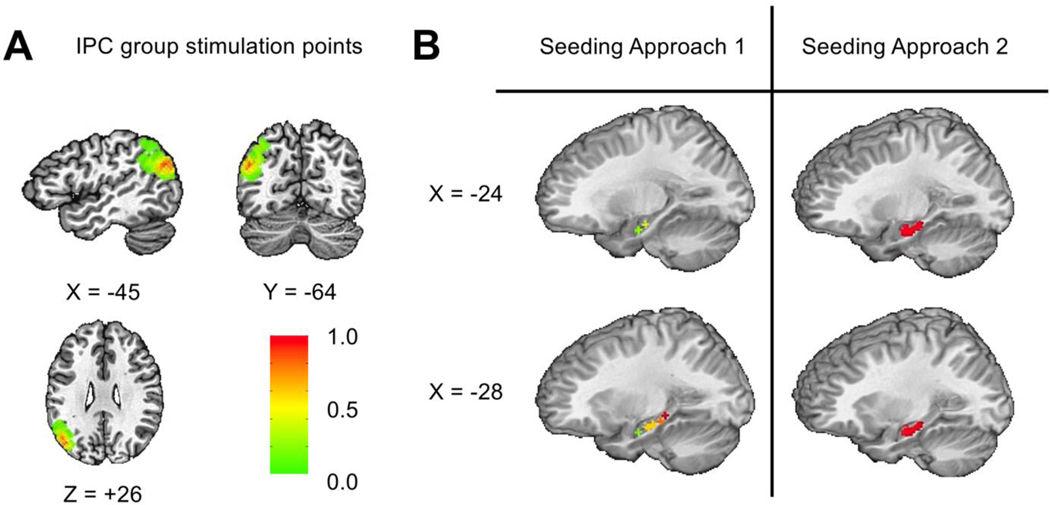
For the stimulation point GM ROI, we used the DK atlas region for the IPC, which had substantial overlap with each participant’s stimulation location. To quantify this overlap and justify our selection of the IPC to represent the stimulation location, we identified each participant’s stimulation location in Talairach space and created a 6 mm-radius sphere around it. We then transformed each participant’s FreeSurfer parcellation into Talairach space and calculated the number of intersecting voxels between each participant’s sphere and each region in the DK atlas. Finally, we averaged the percentage of overlapping voxels across participants and compared the percentage of overlap between regions in the DK atlas. On average, the atlas IPC region contained the greatest density of overlapping voxels across participants (39.30%), which was substantially more than neighboring regions, i.e., the supramarginal gyrus (7.06%) and superior parietal cortex (1.96%). All other regions had < 1% overlap. Stimulation locations are illustrated in Figure 3A, overlaid on a template brain. We chose the isthmus cingulate cortex from the DK atlas because it corresponds well to the retrosplenial cortex (see Figure 2).
Figure 3.
A. Distribution of stimulation points. Topographical distribution of stimulated voxels across the left IPC, shown on a template brain (TT_N27). Colors represent the proportion of voxels stimulated across participants. B. IPC group seeding approaches 1. Location of the six sampled seeds for 12 participants. 2. Region sampled to find the seed voxel maximally connected with the IPC for the remaining 12 IPC participants.
We had planned to examine the IPC-retrosplenial pathway, but were unable to produce viable tractography results in most of our participants for this pathway (see below) and thus could not analyze its FA. We also created a GM ROI of the precuneus and precentral gyrus in each participant, directly from the DK atlas. The precentral gyrus ROI was used to create a control tract between the IPC and the precentral gyrus.
2.9. Calculation of Fractional Anisotropy (FA)
We calculated FA in WM left hemisphere bundles estimated between GM ROIs using AFNI’s 3dTrackID command (Taylor et al., 2012). Prior to using 3dTrackID, GM ROIs were inflated by up to two voxels in all directions to provide a degree of intersection with WM pathways using 3dROIMaker, and to reduce the effects of regridding and alignment when mapping parcellations from the T1w dataset into the DTI grid space. Inflation was constrained by using the WM skeleton from DTI (where FA > 0.2) to limit over-inflation of ROIs into WM regions.
We performed probabilistic tractography between our GM ROIs using local uncertainties in diffusion tensor eigenvectors and the tensor-fitted data for each participant. 3dTrackID uses repeated iterations of whole brain tracking with the FACTID algorithm (Taylor et al., 2012) to estimate the location of WM pathways between ROIs, essentially parcellating the WM skeleton into most likely pathways between ROIs based on the participant’s own diffusion data. We used a standard map where FA > 0.2 to define the WM skeleton within which we estimated tracts. The maximum acceptable turn angle for a pathway was set to 60 degrees. We performed 1000 iterations of whole brain tracking and all voxels through which more than five pathways passed to connect a pair of targets were included to create WM pathways associated with each pair of targets. The mean and standard deviation of the FA for each pathway were automatically calculated by the FATCAT tracking function. This resulted in a single mean FA value for each viable pathway in each participant. Outlier FA values greater or less than 3 standard deviations from the mean were disregarded. This occurred in one participant (IPC group) for the IPC-parahippocampal pathway. Thus, the degrees of freedom for correlations using data in this pathway is reduced compared to other tracts.
2.10. rTMS and Stimulation Localization
As in previous studies (e.g. Wang et al. 2014), we individualized stimulation to the left IPC sub-region maximally connected to the left hippocampus. In each participant, the IPC target search volume was a sphere of 15 mm radius, cut to exclude non-brain voxels, around Talairach-Tournoux location x = −47, y = −68, z = +36 (LPI-SPM coordinate notation here and below). We used two approaches for determining the seed and stimulus location, both employing automated scripts to remove bias. For the first approach (12 participants), we chose the maximally connected hippocampal voxel from six pre-selected locations along the longitudinal aspect of the hippocampus in Talairach-Tournoux space (Seed 1: x = −26, y = −10, z = −17; Seed 2: x = −22, y = −16, z = −13; Seed 3: x = −30, y = −17, z = −14; Seed 4: x = −30, y = −22, z = −12; Seed 5: x = −30, y = −27, z = −9; Seed 6: x = −30, y = −32, z = −6; Figure 3B). In the second approach (12 participants), we selected the maximally connected one of 70 pre-selected voxels in the anterior hippocampus (Figure 3B). These included hippocampal voxels within 15 mm of the Talairach-Tournoux coordinates used by Wang et al. (2014; x = −26, y = −10, z = −17). This approach was intended to provide wider sampling within the hippocampus and to constrain the search to the part of the hippocampus related to the rTMS-induced episodic memory improvement (Wang et al., 2014).
In each approach, we created a 3 mm radius sphere around the coordinates of each voxel in the search and computed an average time series using the voxels in that sphere. For each seed, we searched the IPC sphere for the voxel with the maximum correlation with the hippocampal seed. We then chose the seed that had the highest connectivity with any voxel within the IPC sphere. The IPC voxel with maximal hippocampal connectivity was marked in standard space, and back-transformed to participant space using the inverse matrix of the original affine transformation. This location was transformed into a 3 mm radius sphere and overlaid on the participant’s structural MRI for rTMS targeting with the Brainsight frameless stereotaxic system. A stimulation trajectory was created in Brainsight so that the plane of the coil was tangential to the scalp and the induced current field was oriented perpendicular to the long axis of the gyrus containing the stimulation target. For control stimulation, we located the vertex using the 10–20 International system (Steinmetz et al., 1989) and held the coil tangential to the scalp with the junction of the coil lobes in the sagittal axis. Figure 1A shows an example of the coil orientation for the IPC group.
Because the hippocampal seeds were determined in standard space and then transformed to native space, some could have ended up outside of the hippocampus. To investigate whether this occurred, we measured the degree of overlap between each participant’s 3 mm hippocampal seed and the hippocampus derived from the participant’s individual parcellation with the DK atlas. Thus, we calculated the percentage of voxels used to target the hippocampus and define the time series of the hippocampal seed, that were co-localized with the hippocampus itself. Overall, 61.2 ± 0.14% (Range: 24–78%) of the volume of the seeds were co-localized with the hippocampus, an order of magnitude more than the closest structure, the parahippocampal cortex (6.6 ± 0.05%). This analysis shows that the time series used to calculate connectivity included at least 24% of hippocampal voxels for each participant.
2.11. rTMS
TMS was delivered with a MagStim Rapid2 stimulator (The MagStim Co., Ltd., Whitland, Dyfed, UK) through a Double AirFilm coil at 100% of the resting motor evoked potential threshold, determined immediately before the first rTMS session using the TMS Motor Threshold Assessment Tool (MTAT 2.0; http://www.clinicalresearcher.org/software.htm). TMS was delivered in 2-second trains at 20-Hz (40 pulses per train) with an inter-train interval of 28 seconds. Each session contained 40 trains, 1600 pulses, and lasted 20 minutes. These parameter values were similar to Wang et al. (2014), although those investigators used different equipment.
2.12. Memory Tasks
Each session of behavioral testing included an episodic memory task, a procedural memory task, a paired-associates task, and a battery of neuropsychological tests (Weintraub et al., 2013). Only the episodic and procedural memory tasks were analyzed for the current study. These two tasks were administered at the start of each behavioral testing session and their order was counterbalanced across participants to eliminate possible interfering effects (Poldrack et al., 2001; Poldrack & Packard, 2003).
2.12.1. Episodic memory task
The task was presented on a 22-inch monitor connected to a laptop running MATLAB (The MathWorks, Inc. Natick, MA, USA) and Psychtoolbox software (Brainard 1997; Pelli 1997; Figure 1B, left). The task had two sections: training and testing. During training, participants viewed 20 face-word pairs. The faces were presented for three seconds at the center of the screen as grayscale pictures (Althoff & Cohen, 1999) on a white background (size on screen = 2.5 × 2.5 inches). The paired words were presented aurally one second after each face. Participants were instructed to remember the 20 face-word pairs and told that they would be asked to recall the word paired with each face verbally. After a one-minute delay. Participants were shown each face and requested to say the paired word. There was no time limit and the order of face-word pairs was different than in the training phase of the task. The proportion of successfully remembered pairs was noted after each session.
Participants saw a different set of stimuli in each behavioral session and the order of the three possible sets was counterbalanced across participants. Each set included ten male and ten female faces. Words were nouns three to eight letters long with written Kucera-Francis frequencies of 200 to 2000 and concreteness ratings of 300 to 700 (MRC Psycholinguistic Database; www.psych.rl.ac.uk).
2.12.2. Procedural Memory Task
This task, based on the Weather Prediction Task (Knowlton et al., 1994), tests the ability to learn and use implicit, probabilistic, associations between events. It was performed on the same laptop computer used to administer the episodic memory task with MATLAB and Psychtoolbox software (Figure 1B). Participants were told they would learn to predict one of two fictional weather outcomes (rain or sunny, hot or cold) or whether symptoms (rash, headache, sneezing, or fatigue) were associated with two fictional diseases (“nermitis” or “caldosis”), based on the presentation of four “cards” containing arbitrary stimuli (size on screen = 0.9 × 1.6 inches). Each card was probabilistically associated with the outcomes, based on how often it was shown and its reinforcement rate (Table 2). For each task session, each card was associated with one of the outcomes at a rate of 76, 57, 43, and 20%, as in Knowlton et al. (1994). These probabilities can be calculated for each card using the following equation and the values in Table 2:
where P (Arrangement) is the probability that that card will appear on any trial, and P (Outcome) is the reinforcement rate for one of the two outcomes. Thus for Card 1 (arrangements 8–14) the numerator was 0.343 and the denominator was 0.454, so 0.343/0.454 = 0.76, or 76%. Participants were not told these probabilities in order to discourage use of an explicit strategy.
Table 2.
Procedural task training contingencies. Columns 2–5 from the left indicate the presence (1) or absence (0) of each card for each arrangement (Column 1). The probability that that an arrangement would appear is shown in Column 5. The reinforcement rate for each arrangement is indicated in Column 6.
| Arrangement | Card | P ( Arrangement) | P (Outcome) | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| 1 | 0 | 0 | 0 | 1 | 0.140 | 0.15 |
| 2 | 0 | 0 | 1 | 0 | 0.084 | 0.38 |
| 3 | 0 | 0 | 1 | 1 | 0.087 | 0.10 |
| 4 | 0 | 1 | 0 | 0 | 0.084 | 0.62 |
| 5 | 0 | 1 | 0 | 1 | 0.064 | 0.18 |
| 6 | 0 | 1 | 1 | 0 | 0.047 | 0.50 |
| 7 | 0 | 1 | 1 | 1 | 0.041 | 0.21 |
| 8 | 1 | 0 | 0 | 0 | 0.140 | 0.85 |
| 9 | 1 | 0 | 0 | 1 | 0.058 | 0.50 |
| 10 | 1 | 0 | 1 | 0 | 0.064 | 0.82 |
| 11 | 1 | 1 | 0 | 1 | 0.032 | 0.43 |
| 12 | 1 | 1 | 0 | 0 | 0.087 | 0.90 |
| 13 | 1 | 1 | 0 | 1 | 0.032 | 0.57 |
| 14 | 1 | 1 | 1 | 0 | 0.041 | 0.79 |
On each trial, participants saw an arrangement of 1–3 cards and were instructed to press one of two keys on a standard keyboard to make their predictions. Correct predictions were rewarded with points. Participants started with 1,000 points and were awarded 25 points after a successful prediction or penalized by 25 points for incorrect predictions or failure to respond within 5 seconds. A prompting tone sounded two seconds after card presentation. Participants were instructed to use the strategy they felt would optimize their point winning. There were three training blocks of 50 trials, followed by a single block of 42 trials where learning was assessed in the absence of feedback.
During training, each trial began with the presentation of cards at the top of the screen. There were 14 possible unique card arrangements which occurred with a set frequency (Table 2, P (Arrangements)). Each card was presented at a different screen position in each array to encourage participants to associate outcomes with card identities, not locations. A running point count was presented near the center of the screen in black and changed to green for successful, and red for unsuccessful, predictions. A key indicating the key-outcome mapping was shown during all trials.
Learning was calculated from performance on the test block, during which all aspects of the task were the same as training but there was no time limit, the audio prompt was disabled, and the point count was replaced with question marks. Responses were scored correct when the participant chose the outcome most often associated with a successful prediction for that card arrangement during training. Participants responded to each of the 14 arrangements three times for a total of 42 trials. Since two of the arrangements predicted an outcome 50% of the time, trials with this arrangement were discarded from analysis. The task was performed in each of the three behavioral testing sessions with a different set of associations and the order was counterbalanced across participants.
2.13. Statistical Analyses
We reasoned that pathways responsible for rTMS-induced changes in hippocampal rsFC would have FA correlations with rsFC that were greater for the IPC than the vertex group (stimulation-specificity) and greater for those pathways compared to the control tract (IPC-precentral) in the IPC group (tract-specificity). We also posited that pathways responsible for rTMS-induced changes in episodic memory should also meet those criteria and also show correlations greater for episodic than procedural memory in the IPC group (memory-specificity).
Each analysis included a contrast of two correlations to form a Z-statistic for observational data (“ZObs”) and the creation of a Z-statistic distribution for those data. The ZObs value formed the test statistic for a permutation test. A distribution of Z-statistics was derived by reshuffling the data labels and re-contrasting the correlations 50,000 times. For all correlations, we used non-parametric Spearman’s correlations employing the corr function in Matlab (Takeuchi, 2020), which executes the Pearson’s correlation after ranking.
Stimulation-specificity:
ZObs values were derived by contrasting FA-rsFC correlations between groups (IPC vs. vertex). The distribution of Z-statistics was created by shuffling the group labels across participants and re-contrasting the FA-memory correlations.
Memory-specificity:
ZObs values were derived by contrasting FA-memory correlations between memory types (episodic vs. procedural). The distribution of Z-statistics was created by shuffling the memory labels within participants and re-contrasting the FA-rsFC correlations.
Tract-specificity:
ZObs values were derived by contrasting FA-rsFC and FA-memory correlations between our tracts of interest (e.g. IPC-parahippocampus) and our control tract. The distribution of Z-statistics was created by shuffling the tract labels within participants and re-contrasting the tract correlations.
2.14. Data and code availability statement
Data values including rsFC, episodic and procedural memory change values, FA in the five pathways and control tract, and preprocessing and analysis code are available on github (https://github.com/mfreedberg84/Hippocampal_Stimulation_and_FA).
3. Results
These results are part of a larger data set on changes in brain connectivity and behavior following hippocampal network-targeted stimulation. Thus, we did not apply significance testing to the subset of data presented in the current work, but list effect sizes (Cohen’s d) and standard deviations for our behavioral measures in Table 3. Baseline episodic (IPC group =0.22±0.16; vertex group = 0.33±0.16; p = 0.10) and procedural memory (IPC group = 0.65±0.09; vertex group = 0.63±0.09; p = 0.61) did not differ significantly between groups.
Table 3.
Effect sizes (Cohen’s d) and standard deviations (parentheses) for behavioral data. Episodic memory score changes are represented as the average change in proportion of remembered pairs. Procedural memory score changes are represented as the average change in proportion of optimal decisions.
| Contrast | Episodic Memory Change | Procedural Memory Change | ||
|---|---|---|---|---|
| One day change | One week change | One day change | One week change | |
| Parietal | 0.49 (0.14) | 0.38 (0.16) | −0.14 (0.14) | −0.18 (0.12) |
| Vertex | −0.31 (0.15) | −0.06 (0.14) | 0.07 (0.12) | 0.08 (0.11) |
| Group Difference | 0.79 (0.15) | 0.46 (0.15) | −0.22 (0.13) | −0.26 (0.12) |
Results below are presented individually for the five tracts and are summarized in Table 4. Although we report individual correlations, our hypotheses rely on the contrast of correlations between groups (IPC vs. vertex), memory tasks (episodic vs. procedural), and tracts (Episodic memory network tracts vs. IPC-precentral tract) using permutation tests.
Table 4.
Connectivity (rsFC) and memory (EM) outcomes for all tracts.
| Tract | Effect | Group-Specific? | Memory-Specific? | Tract-Specific | interpretation |
|---|---|---|---|---|---|
| IPC-Parahippocampus | rsFC | Yes p < 0.05 | WA | Yes p < 0.005 | Pathway a strongly related to rsFC and EM changes |
| EM (one day) | Trend p = 0.05 | Yes p < 0.01 | Yes p < 0.05 | ||
| EM (one week) | Yes p < 0.01 | Yes p < 0.001 | Yes p < 0.005 | ||
| Parahippocampus-Entorhinal | rsFC | Yes p < 0 05 | N/A | Yes p < 0.05 | Pathway is strongly related to rsFC changes |
| EM (one day) | No ns | No ns | No ns | ||
| EM (one wee) | No ns | No ns | No ns | ||
| Entorhlnal-Hippocampus | rsFC | Trend p = 0.06 | N/A | Trend P = 0.06 | Pathway may be related to rsFC changes |
| EM (one day) | No ns | No ns | No ns | ||
| EM (one week) | No ns | Trend p = 0 07 | No ns | ||
| IPC-Hippocampus | rsFC | No ns | N/A | Yes p< 0.001 | Pathway is related to episodic memory changes |
| EM (one day) | No ns | No ns | No ns | ||
| EM (one week) | Yes p < 0 05 | Yes p < 0.05 | Yes p < 0.005 | ||
| IPC-Precuneus | rsFC | Trend p = 0.09 | N/A | Yes p = 0.05 | Pathway may be related to rsFC changes |
| EM (one day) | Trend p = 0 08 | Trend P = 0.05 | No ns | ||
| EM (one week) | No ns | Yes P < 0.05 | Yes p < 0.05 |
EM = Episodic memory. ns = Not significant.
3.1. Indirect Route: IPC-Parahippocampus Tract
3.1.1. Stimulation-specificity
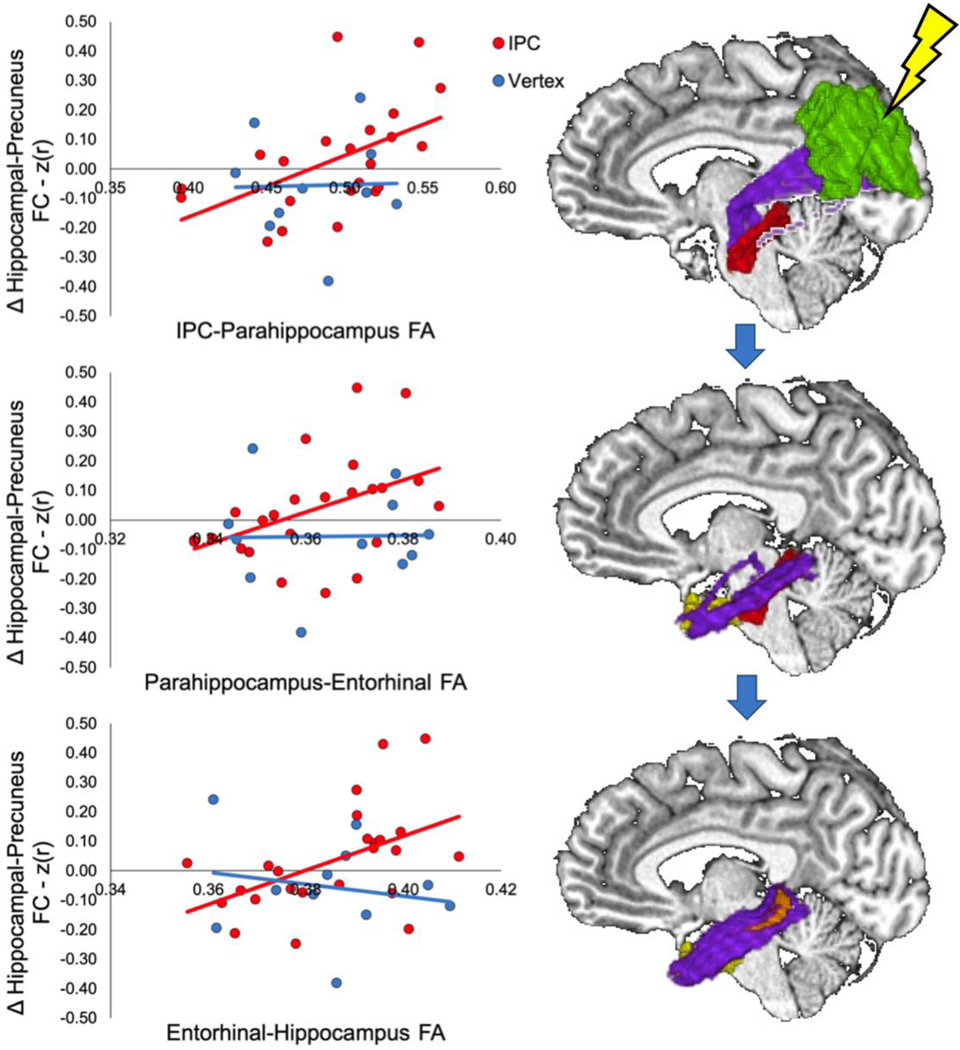
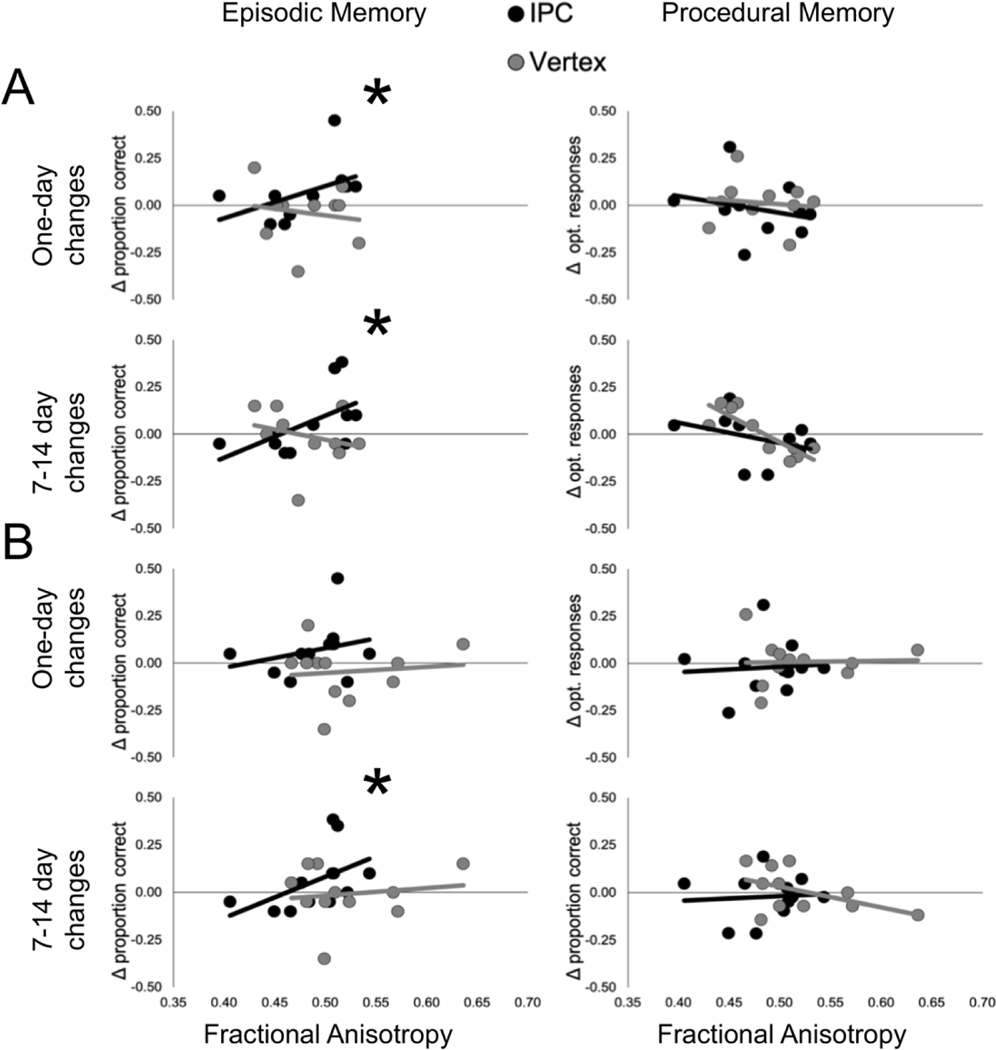
We found a significant correlation between IPC-parahippocampus FA and rTMS-induced changes in hippocampal-precuneus rsFC for the IPC (r(22) = 0.58, p = 0.005; Figure 4), but not vertex, group (r(8) = −0.02, p = 0.973; Figure 4). The correlation for the IPC group was significantly greater than for the vertex group (ZObs = 0.674, Upper 5% of distribution = 0.659, p = 0.047). One day after stimulation, IPC-parahippocampus FA predicted rTMS-induced episodic memory changes for the IPC (r(9) = 0.70, p = 0.017; Figure 5A), but not the vertex, group (r(8) = −0.16, 656; Figure 5A). There was a trend difference between correlations (ZObs = 1.023, Upper 5% of distribution = 1.042, p = 0.054). A trend correlation between FA and episodic memory change was found for the IPC group one week after stimulation (r(9) = 0.55, p = 0.078; Figure 5A), but not the vertex group (r(8) = −0.40, p = 0.247; Figure 5A). The correlation for the IPC group was significantly greater compared to that for the vertex group (ZObs = 1.051, Upper 5% of distribution = 0.694, p = 0.01).
Figure 4.
Left: Scatter plots of FA-rsFC associations in tracts where the correlation values differed significantly by group. ROIs (right) include the IPC (green), parahippocampal cortex (red), entorhinal cortex (yellow), and hippocampus (orange) with corresponding tracts in purple. The lightning bolt represents stimulation location in the IPC. Arrows between images represent hypothetical path of signal from the IPC to the hippocampus.
Figure 5.
Correlations between FA and rTMS-induced changes in episodic and procedural memory for (A) the IPC-parahippocampus and (B) IPC-hippocampus pathways. Asterisks show significant correlation differences between groups.
3.1.2. Memory-specificity
One day after stimulation, FA was not significantly related to procedural memory (r(8) = −0.49, p = 0.154, Figure 5A). The correlation for episodic memory was significantly stronger than for procedural memory (ZObs = 1.397, Upper 5% of distribution = 0.976, p = 0.007, Figure 5A). One week after stimulation, IPC-parahippocampus FA was weakly related to procedural memory (r(8) = −0.579, p = 0.079, Figure 5A) and the correlations for episodic and procedural memory were significantly different (ZObs = 1.284, Upper 5% of distribution = 0.684, p = 0.0001, Figure 5A).
3.1.3. Tract-specificity
No significant correlation was found between IPC-precentral FA and rTMS-induced rsFC changes (r(20) = −0.16, p = 0.453) and the correlation for IPC-parahippocampus FA with rsFC change was significantly greater (ZObs = 0.822, Upper 5% of distribution = 0.481, p = 0.002). IPC-precentral FA and episodic memory change were not correlated one day after stimulation (r(9) = −0.02, p = 0.956) and the correlation for the IPC-parahippocampus FA with episodic memory change was significantly greater (ZObs = 0.879, Upper 5% of distribution = 0.754, p = 0.015). One week after stimulation, IPC-precentral FA was not correlated with episodic memory change (r(9) = −0.35, p = 0.294) and the correlation for IPC-parahippocampus FA was significantly greater (ZObs = 0.986, Upper 5% of distribution = 0.709, p = 0.003).
3.2. Indirect Route: Parahippocampus-Entorhinal Tract
3.2.1. Stimulation-specificity
We found a significant correlation between parahippocampus-entorhinal FA and rTMS-induced change in hippocampal-precuneus rsFC for the IPC (r(22) = 0.50, p = 0.014), but not the vertex, group (r(9) = −0.04, p = 0.924; Figure 4). The correlation for the IPC group was significantly greater than that for the vertex group (ZObs = 0.579, Upper 5% of distribution = 0.549, p = 0.03). One day after stimulation, parahippocampus-entorhinal FA did not predict rTMS-induced episodic memory change for the IPC (r(10) = 0.14, p = 0.664) or vertex groups (r(9) = −0.34, p = 0.301), and the correlations were not significantly different (ZObs = 0.499, Upper 5% of distribution = 0.844, p = 0.183). One week after stimulation, no significant correlation between FA and episodic memory changes was found for the IPC (r(10) = 0.31, p = 0.334) or the vertex group (r(9) = −0.004, p = 0.989) and the correlations were not significantly different (ZObs = 0.320, Upper 5% of distribution = 0.782, p = 0.278).
3.2.2. Memory-specificity
One day after stimulation, parahippocampus-entorhinal FA was not significantly correlated with episodic (r(10) = 0.14, p = 0.664) or procedural memory (r(9) = 0.03, p = 0.926). No significant difference between correlations was found (ZObs = 0.109, Upper 5% of distribution = 0.758, p = 0.435). One week after stimulation, no significant correlation was found for episodic (r(10) = 0.31, p = 0.334) or for procedural memory (r(9) = −0.05, p = 0.883) and the correlations were not significantly different (ZObs = 0.366, Upper 5% of distribution = 0.633, p = 0.167).
3.2.3. Tract-specificity
The correlation between parahippocampal-entorhinal FA and rsFC changes was significantly greater than for the control tract (ZObs = 0.708, Upper 5% of distribution = 0.676, p = 0.041) and the association between episodic memory and FA was not significantly different between tracts one day (ZObs = 0.160, Upper 5% of distribution = 1.033, p = 0.399) or one week after stimulation (ZObs = 0.679, Upper 5% of distribution = 1.094, p = 0.148).
3.3. Indirect Route: Entorhinal-Hippocampus Tract
3.3.1. Stimulation-specificity
We found a significant correlation between entorhinal-hippocampus FA and rTMS-induced change in hippocampal-precuneus rsFC for the IPC (r(22) = 0.49, p = 0.015; Figure 4), but not vertex group (r(9) = −0.12, p = 0.734; Figure 4). There was a trend difference in correlations between groups (ZObs = 0.659, Upper 5% of distribution = 0.701, p = 0.063). One day after stimulation, entorhinal-hippocampus FA did not predict rTMS-induced episodic memory changes for the IPC (r(10) = 0.359, p = 0.252) or vertex groups (r(9) = −0.27, p = 0.427) and the correlations were not significantly different (ZObs = 0.650, Upper 5% of distribution = 0.839, p = 0.10). One week after stimulation, there was a trend correlation between FA and episodic memory changes for the IPC (r(10) = 0.50, p = 0.097), but not the vertex, group (r(9) = 0.15, p = 0.663). No difference in correlation was found between groups at one week (ZObs = 0.401, Upper 5% of distribution = 0.774, p = 0.197).
3.3.2. Memory-specificity
One day after stimulation, entorhinal-hippocampus FA was not correlated with episodic (r(10) = 0.36, p = 0.252) or procedural memory change (r(9) = 0.005, p = 0.989). No difference between the correlations for the two memory-types was found (ZObs = 0.371, Upper 5% of distribution = 0.609, p = 0.182). One week after stimulation, there was a weak association between FA and episodic memory change (r(10) = 0.50, p = 0.097), but not between FA and procedural memory (r(9) = −0.05, 894). A trend difference was found between correlations (ZObs = 0.596, Upper 5% of distribution = 0.704, p = 0.069).
3.3.3. Tract-specificity
One day after stimulation, the correlation between rsFC and entorhinal-hippocampal FA was slightly greater than for the control tract (ZObs = 0.706, Upper 5% of distribution = 0.723, p = 0.055). However, no difference in correlation between FA and episodic memory was found between tracts one day (ZObs = 0.395, Upper 5% of distribution = 1.158, p = 0.289) or one week after stimulation (ZObs = 0.914, Upper 5% of distribution = 1.229, p = 0.108).
3.4. Direct Route: IPC-Hippocampal Tract
3.4.1. Stimulation-specificity
We found a significant correlation between IPC-hippocampus FA and rTMS-induced change in hippocampal-precuneus rsFC for the IPC (r(22) = 0.49, p = 0.017), but not the vertex, group (r(9) = 0.10, p = 0.776). Nevertheless, the correlation for the IPC group was not significantly greater than that for the vertex group (ZObs = 0.429, Upper 5% of distribution = 0.608, p = 0.125). One day after stimulation, IPC-hippocampus FA was not significantly associated with rTMS-induced episodic memory changes for the IPC (r(10) = 0.34, p = 0.283) or vertex groups (r(9) = −0.13, p = 0.696) and the correlations were not significantly different (ZObs = 0.486, Upper 5% of distribution = 0.705, p = 0.131). A significant correlation between FA and episodic memory changes was found one week after stimulation for the IPC group (r(10) = 0.71, p = 0.009), but not the vertex group (r(9) = −0.13, p = 0.683). The correlation for the IPC group was significantly greater compared to the vertex group (ZObs = 1.036, Upper 5% of distribution = 0.765, p = 0.014).
3.4.2. Memory-specificity
One day after stimulation, FA was not related to procedural memory (r(9) = 0.08, p = 0.821, Figure 5B) and the correlations did not differ by memory type (ZObs = 0.274, Upper 5% of distribution = 0.456, p = 0.17, Figure 5B). One week after stimulation, FA was not related to procedural memory (r(9) = 0.10, p = 0.768, Figure 5B). The correlations for episodic and procedural memory were significantly different (ZObs = 0.795, Upper 5% of distribution = 0.772, p = 0.049, Figure 5B).
3.4.3. Tract-specificity
One day after stimulation, the correlation between FA and hippocampal rsFC changes was significantly greater for the IPC-hippocampus tract compared to the control tract (ZObs = 0.695, Upper 5% of distribution = 0.361, p = 0.001). No difference in the correlations of FA and episodic memory was found tracts one day after stimulation (ZObs = 0.370, Upper 5% of distribution = 0.969, p = 0.250). The correlation for the IPC-hippocampus pathway was significantly greater than for the control pathway one week after stimulation (ZObs = 1.259, Upper 5% of distribution = 0.813, p = 0.002).
3.5. Precuneus Pathway: IPC-Precuneus Tract
3.5.1. Stimulation-specificity
We found a significant correlation between IPC-precuneus FA and rTMS-induced changes in hippocampal-precuneus rsFC for the IPC, (r(22) = 0.54, p = 0.006) but not the vertex, group (r(9) = −0.004, p = 0.989) and a trend difference was found between correlations (ZObs = 0.614, Upper 5% of distribution = 0.774, p = 0.094). One day after stimulation, IPC-precuneus FA was weakly associated with rTMS-induced episodic memory change for the IPC group (r(10) = 0.57, p = 0.051), but not the vertex, group (r(8) = −0.09, p = 0.791). A trend difference between correlations as found (ZObs = 0.746, Upper 5% of distribution = 0.887, p = 0.079). One week after stimulation, a significant correlation between FA and episodic memory change was found for the IPC (r(10) = 0.622, p = 0.031), but not the vertex, group (r(9) = −0.51, p = 0.106), but the difference between correlations was not significant (ZObs = 0.160, Upper 5% of distribution = 0.694, p = 0.353).
3.5.2. Memory-specificity
One day after stimulation, IPC-precuneus FA correlated marginally with episodic (r(10) = 0.57, p = 0.051), but not procedural, memory (r(9) = 0.01, p = 0.979) and there was a trend difference between these correlations (ZObs = 0.645, Upper 5% of distribution = 0.641, p = 0.051). One week after stimulation, IPC-precuneus FA was significantly correlated with episodic (r(10) = 0.62, 0.031), but not procedural, memory (r(9) = −0.15, p = 0.667) and the correlations were significantly different (ZObs = 0.875, Upper 5% of distribution = 0.826, p = 0.036).
3.5.3. Tract-specificity
For the IPC group, the correlations of FA and rsFC change were significantly different between the IPC-precuneus and control tracts (ZObs = 0.775, Upper 5% of distribution = 0.631, p = 0.017). The correlations of FA and episodic memory change were not significantly different between tracts one day after stimulation (ZObs = 0.673, Upper 5% of distribution = 0.873, p = 0.117), but a significant difference emerged one week after stimulation (ZObs = 1.091, Upper 5% of distribution = 1.018, p = 0.038).
4. Discussion
Our study links the effects of IPC rTMS on hippocampal-cortical rsFC with FA in WM pathways from the IPC to the MTL, passing through the parahippocampal and entorhinal cortex. FA in all three pathways from the IPC to the hippocampus, via the parahippocampus and entorhinal cortex, were related to rTMS-induced rsFC changes. Furthermore, FA in both the IPC-parahippocampus and IPC-hippocampal pathways was significantly related to episodic memory change. These effects were specific to IPC stimulation and episodic memory and were not present in a control tract from the stimulation target to an uninvolved cortical area. Finally, FA in a direct pathway from the IPC to the precuneus showed only a weak association with the rTMS-induced changes.
The hypothesized mechanism for IPC rTMS-induced enhancement of hippocampal rsFC and episodic memory is propagation of activity and subsequent synaptic changes via either the IPC-parahippocampal or IPC-retrosplenial pathways (Freedberg et al., 2019; Hebscher & Voss, 2020; Wang et al., 2014). Although we were unable to measure IPC-retrosplenial FA, IPC-parahippocampal FA was related to hippocampal-cortical rsFC and episodic memory changes one day and one week after stimulation. These results confirm the hypothesis and indicate that this pathway is important for the effect with one caveat: Although the parahippocampus-entorhinal cortex, and entorhinal cortex-hippocampus pathways were implicated in the rsFC changes, they were not associated with the corresponding improvement in episodic memory. Thus, we cannot say whether pathways indirectly connecting the IPC stimulation site to the hippocampus are responsible for the behavioral effect.
We found that FA in the direct route was related to rTMS-induced episodic memory improvement one week after stimulation. However, because we did not find any correlation with rsFC or episodic memory changes the day after stimulation, this pathway is likely not critical for improvement at the one-day time point. Rather, our results provide stronger evidence that indirect MTL pathways are involved in the acute effects of stimulation on connectivity and memory.
The low correlations for the IPC-precuneus pathway imply a secondary role in the effect of IPC rTMS on hippocampal rsFC and episodic memory and we were not surprised by this finding. Although lateral parietal regions, such as the IPC and angular gyrus, and medial regions, such as the precuneus and posterior cingulate cortex, are commonly co-activated on fMRI by episodic memory tasks (Addis et al., 2007; Benoit & Schacter, 2015; Ritchey & Cooper, 2020; Rugg & Vilberg, 2013; Sestieri et al., 2011), the improvements in memory observed after IPC rTMS could occur via an alternative route involving these areas. We note that it is possible this pathway is involved, but we were not able to exclude it using this analysis.
Differences in pathway FA explained only 22–30% of the variability in rTMS-induced rsFC change between individuals in the IPC group, so there must be other important sources of variability (Ridding & Ziemann, 2010). These include other individual factors, such as the cortical thickness of the stimulated region (Conde et al., 2012). State-specific factors that influence the efficacy of TMS include diurnal endocrine fluctuations (Sale et al., 2008) and menstrual cycle phase (Smith et al., 1999). We kept rTMS sessions to the same approximate time of day. However, differences in timing between participants may have also contributed to variability in response.
A design limitation of this study is that we examined FA only in the pathways most likely to conduct the effects of IPC stimulation on hippocampal-cortical rsFC and memory, based on the anatomical and fMRI literature. Although there are several parietal (Cavada & Goldman-Rakic, 1989a), prefrontal (Cavada & Goldman-Rakic, 1989b), and MTL (Amaral & Witter, 1989; Suzuki & Amaral, 1994) regions that project to the hippocampus, we prioritized our search to avoid an excessive number of comparisons and false positive findings. A second intrinsic limitation is the limited extent to which DTI and FA can be used to characterize WM organization in voxels with crossing fibers (Alexander et al., 2001, 2002), which may exist in 63–90% of WM voxels (Jeurissen et al., 2013). High angular resolution diffusion imaging (HARDI) models can be used to improve tractography through regions of crossing fibers but requires many diffusion directions and introduces WM tracking difficulties itself (because many voxels will then contain several possible propagation pathways). We chose to acquire fewer diffusion directions in order to use the robust EPI distortion correction method in DR-BUDDI, which requires collecting DTI data in two different phase-encoding directions. This was a necessary step, as uncorrected EPI-related distortions significantly affect fiber trajectories (Irfanoglu et al., 2012). Third, although there are properties of microstructure that determine anisotropy and conduction in WM, such as fiber density and myelination, FA is related to multiple properties of microstructure, not all of which may be related to rTMS efficacy in ways accounted for by our hypothesis.
In this study, we used non-parametric permutation tests to account for the differences in sample sizes between groups and the small sample size overall. We also treated all pathways independently rather than as a network. However, it is possible that we may have found a different pattern of results using other analysis techniques, such as pathway (Gitelman et al., 2003; McIntosh & Gonzalez-Lima, 1994; Roebroeck et al., 2005) or network (Rubinov & Sporns, 2010) analyses. Undersampling can increase the false positive rate and inflate effect sizes in some cases (Button et al., 2013; Yarkoni, 2009). This would seriously weaken our conclusion on the relative contributions of the MTL pathways, had it come from an exploratory search for significant correlations with FA. However, the goal of this study was not discovery, but to use the variation in FA to investigate the relative contributions of known pathways with high prior likelihood of involvement in the behavioral and FC effects of IPC stimulation. The IPC and parahippocampus are robustly connected (Cavada & Goldman-Rakic, 1989a; Mesulam et al., 1977; Pandya & Seltzer, 1982) and parahippocampus rsFC is related to the effectiveness of IPC stimulation (Tambini et al., 2018). We also note that, currently, there is no consensus on the sample required to show a correlation between FA and rsFC. For example, Betzel et al. (2014) studied changes in structural and functional connectivity across the lifespan in more than 100 individuals from a publicly available data set, while Honey et al. (2009) used data from five participants to study similar associations. The main constraint on sample size in our study was the multiday stimulation regimen. While our number of participants is low, it is not unusual for studies combining rTMS and fMRI.
5. Conclusion
This study shows that several MTL pathways play a significant and specific role in the behavioral and imaging changes produced by IPC rTMS. To date, enhancement of episodic memory network connectivity and memory performance by IPC rTMS is the only noninvasive neuromodulation finding with robust and reproducible results, clinical applicability, and a mechanistic biomarker/surrogate endpoint whose intensity correlates with clinical response. Understanding more about its mechanism will enable further clinical and scientific advances in this area.
7. Acknowledgments
We would like to thank Dr. Joel Voss at Northwestern University for sharing his data for this study.
6. Funding
This research was supported (in part) by the Intramural Research Program of the NIH, NINDS and the Department of Defense in the Center for Neuroscience and Regenerative Medicine (CNRM-70–3904).
References
- Addis DR, Wong AT, & Schacter DL (2007). Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 45(7), 1363–1377. 10.1016/j.neuropsychologia.2006.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, & Parker DL (2001). Analysis of partial volume effects in diffusion-tensor MRI. Magnetic Resonance in Medicine, 45(5), 770–780. 10.1002/mrm.1105 [DOI] [PubMed] [Google Scholar]
- Alexander DC, Barker GJ, & Arridge SR (2002). Detection and modeling of non-Gaussian apparent diffusion coefficient profiles in human brain data. Magnetic Resonance in Medicine, 48(2), 331–340. 10.1002/mrm.10209 [DOI] [PubMed] [Google Scholar]
- Althoff RR, & Cohen NJ (1999). Eye-movement-based memory effect: A reprocessing effect in face perception. Journal of Experimental Psychology: Learning Memory and Cognition, 25(4), 997–1010. 10.1037/0278-7393.25.4.997 [DOI] [PubMed] [Google Scholar]
- Amaral DG, & Witter MP (1989). The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience, 31(3), 571–591. 10.1016/0306-4522(89)90424-7 [DOI] [PubMed] [Google Scholar]
- Basser PJ (1995). Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine, 8(7), 333–344. 10.1002/nbm.1940080707 [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system - A technical review. NMR in Biomedicine, 15(7–8), 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Benoit RG, & Schacter DL (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. 10.1016/j.neuropsychologia.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo XN, & Sporns O (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage, 102(P2), 345–357. 10.1016/j.neuroimage.2014.07.067 [DOI] [PubMed] [Google Scholar]
- Brainard D (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, & Munafò MR (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Cavada C, & Goldman-Rakic PS (1989a). Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. Journal of Comparative Neurology, 287(4), 393–421. 10.1002/cne.902870402 [DOI] [PubMed] [Google Scholar]
- Cavada C, & Goldman-Rakic PS (1989b). Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. Journal of Comparative Neurology, 287(4), 422–445. 10.1002/cne.902870403 [DOI] [PubMed] [Google Scholar]
- Cavanna AE, & Trimble MR (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(3), 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Chen G, Taylor PA, & Cox RW (2017). Is the statistic value all we should care about in neuroimaging? NeuroImage, 147(October 2016), 952–959. 10.1016/j.neuroimage.2016.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Wang Y, Sheng J, Kronenberger WG, Mathews VP, Hummer TA, & Saykin AJ (2012). Characteristics and variability of structural networks derived from diffusion tensor imaging. NeuroImage, 61(4), 1153–1164. 10.1016/j.neuroimage.2012.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde V, Vollmann H, Sehm B, Taubert M, Villringer A, & Ragert P (2012). Cortical thickness in primary sensorimotor cortex influences the effectiveness of paired associative stimulation. NeuroImage, 60(2), 864–870. 10.1016/j.neuroimage.2012.01.052 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI : Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Davis SW, Luber B, Murphy DLK, Lisanby SH, & Cabeza R (2017). Frequency-specific neuromodulation of local and distant connectivity in aging and episodic memory function. Human Brain Mapping, 38(12), 5987–6004. 10.1002/hbm.23803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, & Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen G, & Rockland KS (2000). Inferior parietal lobule projections to the presubiculum and neighboring ventromedial temporal cortical areas. Journal of Comparative Neurology, 425(4), 510–530. [DOI] [PubMed] [Google Scholar]
- Freedberg M, Reeves JA, Toader AC, Hermiller MS, Voss JL, & Wassermann EM (2019). Persistent enhancement of hippocampal network connectivity by parietal rTMS is reproducible. ENeuro, 6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg M, Reeves J, Toader A, Hermiller M, Kim E, Haubenberger D, Cheung Y, Voss J, & Wassermann E (2020). Optimizing hippocampal-cortical network modulation via repetitive transcranial magnetic stimulation: A dose-finding study using the continual reassessment method. Neuromodulation, 23(3), 366–372. 10.1111/ner.13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, & Friston KJ (2003). Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. NeuroImage, 19(1), 200–207. 10.1016/S1053-8119(03)00058-2 [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, & Beaulieu C (2009). Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cerebral Cortex, 19(3), 524–536. 10.1093/cercor/bhn102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, & Menon V (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4637–4642. 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, & Pascual-Leone A (2014). Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. Journal of Neuroscience, 34(36), 12049–12056. 10.1523/JNEUROSCI.1776-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebscher M, & Voss JL (2020). Testing network properties of episodic memory using non-invasive brain stimulation. Current Opinion in Behavioral Sciences, 32, 35–42. 10.1016/j.cobeha.2020.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiller MS, Chen YF, Parrish TB, & Voss JL (2020). Evidence for Immediate Enhancement of Hippocampal Memory Encoding by Network-Targeted Theta-Burst Stimulation during Concurrent fMRI. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 40(37), 7155–7168. 10.1523/JNEUROSCI.0486-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiller MS, VanHaerents S, Raij T, & Voss JL (2019). Frequency-specific noninvasive modulation of memory retrieval and its relationship with hippocampal network connectivity. Hippocampus, 29(7), 595–609. 10.1002/hipo.23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, & Hagmann P (2009). Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 2035–2040. 10.1073/pnas.0811168106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfanoglu MO, Modi P, Nayak A, Hutchinson EB, Sarlls J, & Pierpaoli C (2015). DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. NeuroImage, 106, 284–299. 10.1016/j.neuroimage.2014.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfanoglu MO, Nayak A, Jenkins J, & Pierpaoli C (2017). TORTOISE v3: Improvements and new features of the NIH diffusion MRI processing pipeline. In ISMRM 25th annual meeting. Honolulu, HI. 10.1016/j.neuroimage.2016.08.016.5. [DOI] [Google Scholar]
- Irfanoglu MO, Walker L, Sarlls J, Marenco S, & Pierpaoli C (2012). Effects of image distortions originating from susceptibility variations and concomitant fields on diffusion MRI tractography results. NeuroImage, 61(1), 275–288. 10.1016/j.neuroimage.2012.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier JD, Jones DK, & Sijbers J (2013). Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping, 34(11), 2747–2766. 10.1002/hbm.22099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, & Turner R (2013). White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage, 73, 239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, & Squire LR (1996). A neostriatal habit learning system in humans. Science, 273(5280), 1399–1402. 10.1126/science.273.5280.1399 [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, & Gluck MA (1994). Probabilistic classification learning in amnesia. Learning and Memory, 1(2), 106–120. 10.1101/lm.1.2.106 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, & Amaral DG (2003). Macaque monkey retrosplenial cortex: II. Cortical afferents. Journal of Comparative Neurology, 466(1), 48–79. 10.1002/cne.10883 [DOI] [PubMed] [Google Scholar]
- Lindquist MA, Geuter S, Wager TD, & Caffo BS (2019). Modular preprocessing pipelines can reintroduce artifacts into fMRI data. Human Brain Mapping, 40(8), 2358–2376. 10.1002/hbm.24528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Alonso V, Cheeran B, Río-Rodríguez D, & Fernández-Del-Olmo M (2014). Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimulation, 7(3), 372–380. 10.1016/j.brs.2014.02.004 [DOI] [PubMed] [Google Scholar]
- McIntosh AR, & Gonzalez-Lima F (1994). Structural equation modeling and its application to network analysis in functional brain imaging. Human Brain Mapping, 2(1–2), 2–22. 10.1002/hbm.460020104 [DOI] [Google Scholar]
- Mesulam MM, Van Hoesen GW, Pandya DN, & Geschwind N (1977). Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: A study with a new method for horseradish peroxidase histochemistry. Brain Research, 136(3), 393–414. 10.1016/0006-8993(77)90066-X [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, & Pandya DN (2004). Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. Journal of Comparative Neurology, 469(1), 37–69. 10.1002/cne.10980 [DOI] [PubMed] [Google Scholar]
- Muldoon SF, Pasqualetti F, Gu S, Cieslak M, Grafton ST, Vettel JM, & Bassett DS (2016). Stimulation-based control of dynamic brain networks. PLoS Computational Biology, 12(9). 10.1371/journal.pcbi.1005076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolo P, Ptak R, & Guggisberg AG (2015). Variability of behavioural responses to transcranial magnetic stimulation: Origins and predictors. Neuropsychologia, 74(January), 137–144. 10.1016/j.neuropsychologia.2015.01.033 [DOI] [PubMed] [Google Scholar]
- Nilakantan AS, Bridge DJ, Gagnon EP, VanHaerents SA, & Voss JL (2017). Stimulation of the posterior cortical-hippocampal network enhances precision of memory recollection. Current Biology, 27(3), 465–470. 10.1016/j.cub.2016.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, & Seltzer B (1982). Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. Journal of Comparative Neurology, 204(2), 196–210. 10.1002/cne.902040208 [DOI] [PubMed] [Google Scholar]
- Pelli DG (1997). The video toolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 10.1163/156856897X00366 [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu M, Barnett A, Basser P, Chang L-C, Koay C, Pajevic S, Rohde G, Sarlls J, & Wu M (2010). TORTOISE: An integrated software package for processing of diffusion MRI data. In ISMRM 18th annual meeting (Vol. 5). Stockholm, Sweden. [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Moyano JC, Myers C, & Gluck MA (2001). Interactive memory systems in the human brain. Nature, 414(6863), 546–550. 10.1038/35107080 [DOI] [PubMed] [Google Scholar]
- Poldrack R, & Packard M (2003). Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia, 41(3), 245–251. 10.1016/S0028-3932(02)00157-4 [DOI] [PubMed] [Google Scholar]
- Poldrack R, Prabhakaran V, Seger C. a, & Gabrieli JD (1999). Striatal activation during acquisition of a cognitive skill. Neuropsychology, 13(4), 564–574. 10.1037/0894-4105.13.4.564 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi A, Cash R, Dunlop K, Vesia M, Kucyi A, Ghahremani A, Downar J, Chen J, & Chen R (2017). Modulation of cognitive cerebello-cerebral functional connectivity by lateral cerebellar continuous theta burst stimulation. NeuroImage, 158(March), 48–57. 10.1016/j.neuroimage.2017.06.048 [DOI] [PubMed] [Google Scholar]
- Ridding MC, & Ziemann U (2010). Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. Journal of Physiology, 588(13), 2291–2304. 10.1113/jphysiol.2010.190314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, & Cooper RA (2020). Deconstructing the posterior medial episodic network. Trends in Cognitive Sciences, 1–15. 10.1016/j.tics.2020.03.006 [DOI] [PubMed] [Google Scholar]
- Rockland KS, & Van Hoesen GW (1999). Some temporal and parietal cortical connections converge in CA1 of the primate hippocampus. Cerebral Cortex, 9(3), 232–237. 10.1093/cercor/9.3.232 [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, & Goebel R (2005). Mapping directed influence over the brain using Granger causality and fMRI. NeuroImage, 25(1), 230–242. 10.1016/j.neuroimage.2004.11.017 [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Avanzini G, Bestmann S, Berardelli A, Brewer C, Canli T, Cantello R, Chen R, Classen J, Demitrack M, Di Lazzaro V, Epstein CM, George MS, … Ziemann U (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Rudge P, & Warrington E (1991). Selective impairment of memory and visual impairment in splenial tumours. Brain, 114(1), 349–360. 10.1093/brain/114.1.349 [DOI] [PubMed] [Google Scholar]
- Rugg MD, & Vilberg KL (2013). Brain networks underlying episodic memory retrieval. Current Opinion in Neurobiology, 23(2), 255–260. 10.1016/j.conb.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, & Nordstrom MA (2008). Cortisol inhibits neuroplasticity induction in human motor cortex. Journal of Neuroscience, 28(33), 8285–8293. 10.1523/JNEUROSCI.1963-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville W, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery & Psychiatry, 20(11), 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, & Pandya DN (1984). Further observations on parieto-temporal connections in the rhesus monkey. Experimental Brain Research, 55(2), 301–312. 10.1007/BF00237280 [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, & Shulman GL (2011). Episodic memory retrieval, parietal cortex, and the default mode network: Functional and topographic analyses. Journal of Neuroscience, 31(12), 4407–4420. 10.1523/JNEUROSCI.3335-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Keel J, Greenberg B, Adams L, Schmidt P, Rubinow D, & Wassermann E (1999). Menstrual cycle effects on cortical excitability. Neurology, 53(9), 2069–2072. 10.1212/WNL.53.9.2069 [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Fürst G, & Meyer BU (1989). Craniocerebral topography within the international 10–20 system. Electroencephalography and Clinical Neurophysiology, 72(6), 499–506. 10.1016/0013-4694(89)90227-7 [DOI] [PubMed] [Google Scholar]
- Suzuki WA, & Amaral DG (1994). Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. Journal of Neuroscience, 14(3 II), 1856–1877. 10.1523/jneurosci.14-03-01856.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Ohki K, & Kim DS (2008). Dissociated pathways for successful memory retrieval from the human parietal cortex: Anatomical and functional connectivity analyses. Cerebral Cortex, 18(8), 1771–1778. 10.1093/cercor/bhm204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach P, & Tournoux J (1988). A stereotactic coplanar atlas of the human brain. New York: Thieme. [Google Scholar]
- Tambini A, Nee D, & D’Esposito M (2018). Hippocampal-targeted theta burst stimulation enhances associative memory formation. Journal of Cognitive Neuroscience, 30(10), 1452–1472. 10.1162/jocn_a_01300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Cho KH, Lin CP, & Biswal BB (2012). Improving DTI tractography by including diagonal tract propagation. PLoS ONE, 7(9). 10.1371/journal.pone.0043415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, & Saad ZS (2013). FATCAT: (An Efficient) functional and tractographic connectivity analysis toolbox. Brain Connectivity, 3(5), 523–535. 10.1089/brain.2013.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, & Watson RT (1987). Retrosplenial amnesia. Brain, 110(6), 1631–1646. 10.1093/brain/110.6.1631 [DOI] [PubMed] [Google Scholar]
- von Cramon DY, & Schuri U (1992). The septo-hippocampal pathways and their relevance to human memory: A case report. Cortex, 28(3), 411–422. 10.1016/S0010-9452(13)80151-7 [DOI] [PubMed] [Google Scholar]
- Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, Hermiller MS, & Voss JL (2014). Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science, 345(6200), 1054–1057. 10.1126/science.1252900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KN, Hermiller MS, Nilakantan AS, & Voss JL (2019). Stimulating the hippocampal posteriormedial network enhances task-dependent connectivity and memory. ELife, 8, 1–21. 10.7554/eLife.49458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen S, Heaton R, Tulsky D, Zelazo P, Bauer P, Carlozzi N, Slotkin J, Blitz D, Wallner-Allen K, Fox N, Beaumont J, Munas D, Nowinski C, Richler J, Deocampo J, … Gershon R (2013). Cognition assessment using the NIH toolbox. Neurology, 80(11), S54–S64. 10.1212/WNL.0b013e3182872e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sun D, Wang Y, Wang Y, & Ou S (2016). Segmentation of the cingulum bundle in the human brain: A new perspective based on DSI tractography and fiber dissection study. Frontiers in Neuroanatomy, 10(SEP), 1–16. 10.3389/fnana.2016.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T (2009). Big correlations in little studies. Perspect. Psychol. Stud, 4(3), 294–298. 10.1111/j.1745-6924.2009.01127.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data values including rsFC, episodic and procedural memory change values, FA in the five pathways and control tract, and preprocessing and analysis code are available on github (https://github.com/mfreedberg84/Hippocampal_Stimulation_and_FA).