Abstract
While technological innovations are the invariable crux of speculation about the future of critical care, they cannot replace the clinician at the bedside. This article summarizes the work of the Society of Critical Care Medicine–appointed multiprofessional task for the Future of Critical Care. The Task Force notes that critical care practice will be transformed by novel technologies, integration of artificial intelligence decision support algorithms, and advances in seamless data operationalization across diverse healthcare systems and geographic regions and within federated datasets. Yet, new technologies will be relevant and meaningful only if they improve the very human endeavor of caring for someone who is critically ill.
Keywords: critical care, future, innovations, patient-centered care, technology
HISTORICAL CONSIDERATIONS
Hypothesizing about the future of critical care is a difficult task. Predictions should be informed by the past while also advancing aspirations that are both pragmatic and inspirational. In 1854, during the Crimean War, Florence Nightingale enacted one of the first principles of critical care by placing the most seriously injured patients closest to the nursing station to allow for more attentive monitoring (1–3). However, it was not until the polio epidemic during the early 1950s and the advent of respiratory care units for polio patients confined to iron lungs that critical care medicine emerged. Max Harry Weil and Herbert Shubin in Los Angeles and Peter Safar in Baltimore created the first ICUs in the late 1950s. Since those early days, ICU organization and critical care practice have evolved extensively. Critical care clinicians have adapted to emerging science by implementing new techniques and have changed clinical practice when accumulating evidence failed to support previously accepted theories. Indeed, some aspects of care, such as the use of corticosteroids for sepsis, have had constantly changing cycles of acceptance and rejection driven by new and accumulating data or novel reconceptualization (4, 5). Regardless of ever-changing critical care practices and delivery, the critically ill patient remains the focal point in both the past and the future of critical care.
INTRODUCTION
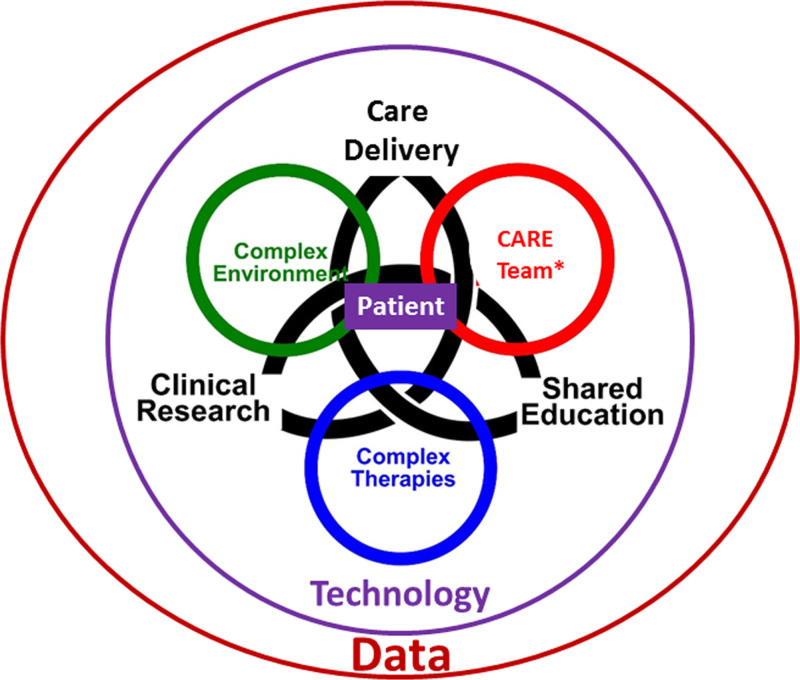
Innovations in technology are invariably the crux of any speculation about the future of critical care. Although new technologies—along with better exchange and use of data—will undoubtedly shape the future of critical care, they cannot replace the astute knowledge and humanistic clinician-patient interaction at the bedside. In the future, the multiprofessional critical care team will expand even further to harnessing emerging technology and data science tools across the continuum of critical illness from development to recovery. Future healthcare will seek to mitigate the current challenges of ineffective resource utilization, professional burnout, uneven distribution of services, and newly emergent complex syndromes/diseases by embracing smart technology, devices, and medications. Concurrent to this, clinicians will continue to ensure humanistic, patient-centered care with the right treatment for the right patient at the right time. Innovations will be meaningful only if clinical outcomes are optimized while patients and their families are treated humanely, compassionately, and equitably (Fig. 1).
Figure 1.
Ideal state of the future ICU care team within a learning healthcare environment. *Care team is defined as all healthcare professionals and family or relevant support system. The family or relevant support system comprises individuals who can provide comfort to the patient outside of healthcare staff and may include direct family, friends, significant others, and/or religious leaders. Healthcare professionals include physicians, advanced practice providers, nurses, pharmacists, nutritionists, social services professionals, spiritual leaders, and physical, respiratory, and occupational health therapists.
A GLIMPSE OF THE FUTURE
A 59-year-old woman with fever and cough was diagnosed with influenza A by her primary care provider (PCP) 5 days ago. The PCP prescribed a wearable noninvasive continuous monitoring device. Today, the telehealth monitoring center is alerted by a surveillance artificial intelligence (AI) algorithm that demonstrates subtle signs of progressively worsening respiratory distress. A drone delivers initial therapies in response to this alert, but over the next few hours, the patient continues to worsen. The telehealth monitoring center contacts her, and the speech signal software detects irregularities that indicate respiratory decline. A mobile emergency care team is deployed to her home for further evaluation. The telehealth monitoring center notifies the patient’s emergency contact and updates the contact on the next steps for care. The mobile emergency care team diagnoses bacterial pneumonia. Antibiotics are started but continuous monitoring and genetic and molecular biomarkers assessed through AI algorithms alert her clinical team that the patient is still at increased risk for respiratory failure and shock. The mobile emergency care unit continues treatment while transporting the patient to a hospital with appropriate capabilities and capacity. On arrival, her hospital care team begins an anti-inflammatory regimen that is personalized to her host response, the pneumonia, and the unique characteristics of the pathogen. To optimize efficacy and minimize toxicity, her medications are selected and dosed based on her genotype, patient-specific variables, and dynamic measures of drug response. She progresses to acute respiratory failure requiring intubation and is supported with mechanical ventilation and infusion pumps equipped with closed-loop monitoring to enhance weaning and optimization of doses to effect. Her family is notified and, in compliance with institutional review board and ethics protocols, they consent to enrolling her in an embedded, multicenter, adaptive, clinical platform trial that is simultaneously assessing six interventions. All of her health data are securely aggregated with those from other patients with pneumonia for pandemic surveillance, continuous validation of machine learning algorithms, tracking of quality metrics, development of patient registries for certain diseases and conditions, clinical trial screening and participation, and public health monitoring.
HARNESSING DATA AND TECHNOLOGY TO IMPROVE DELIVERY OF CARE
Acquisition of data is integral to both modern life and modern healthcare. In the future, healthcare data will be harmonized and shared in real time to help healthcare professionals deliver precision care to improve both patient and population outcomes. Currently each patient in an ICU generates over 1,000 data points per day, in addition to conventional vital signs (6), and this will only increase with expanded use of advanced and remote monitoring systems from outside the ICU and hospital. Genomic and biological biomarkers as well as psychologic and social data will contribute to each patient’s multifaceted data environment. As is happening in asthma (7) and cancer (8), precision medicine will enable identification and differential management of unique critical care endotypes (9) and responses to acute injury. Pharmacogenomic data will be combined with pharmacokinetic and pharmacodynamic predictive models to select a personalized medication regimen that incorporates prognostic factors and individualized patient phenotypes to mitigate potential side effects and optimize the likelihood of response to therapy. Technologies will be leveraged to better track and disseminate patients’ social history and treatment preferences. Discussions undertaken in the prehospital setting regarding important patient preferences—such as advance directives, surrogate decision-maker(s), religion/spirituality, cultural beliefs, and preferred coping strategies—will be communicated across geographically and professionally diverse care teams and readily available to inform treatment choices.
Future paradigm shifts will be in data use rather than data acquisition. Data will not simply be stored in a database but will be actively processed for better real-time clinical decision-making. Vast amounts of data can lead to cognitive overload, which in turn can contribute to medical errors and burnout (10). Thus, it will be technology-assisted improvements in data management, integration, and presentation that will ultimately transform critical care practice. Data will be readily accessible in multifaceted permutations, such as individual patients, various patient groups, diagnoses, and/or time periods. Data trends and projections will be contextualized both within and across hospitalizations, patient life spans, and/or varying patient populations. Context-based clinical decision support systems can alert for early recognition of conditions and greater implementation of evidence-based therapies.
Data science and AI algorithms integrated into the electronic health record and assisted by real-time point-of-care testing will aid in rapid medical diagnostics, identify patients at high risk for acute deterioration or development of complications, and guide personalized treatment. The algorithms that accomplish these feats will use secure technology to identify and track a patient’s risk for decompensation, organ-specific injury (e.g., renal failure), intervention-specific complications or side effects (e.g., pneumothorax), guideline adherence, and clinical trial eligibility. It may also explain emergent events in real time. Data models will provide possible avenues of action, including potential effects on trajectories and recommendations to mitigate risks with subtle nudges to optimize delivery of patient-, clinician-, and unit-level quality measures. This will occur while integrating patient/caregiver preferences.
AI algorithms already exist to better recognize early signs of organ dysfunction, speech and vocal biomarkers indicative of dyspnea, asthma exacerbation, cognitive impairment, and high risk for clinical deterioration (11, 12). Still, in the future, data acquisition technology will be automated to provide more personalized, real-time prevention and treatment recommendations. AI algorithms using closed-loop iterative models will enable minute-to-minute titration of organ support treatments, such as continuous renal replacement, infusion pumps, mechanical ventilators, perfusion circuits, nutritional support, and pain or sedation support (13). Such technologies are currently in their infancy but, with enhanced algorithms, they will become more reliable, accurate, and able to promote better patient outcomes.
On a population level, better data acquisition and large-scale data analysis for patients with similar conditions located across disparate geographical sites will enable hospitals to evolve into true learning healthcare systems (14, 15). These systems will combine patient data, clinician-specific data and competencies, and healthcare system data including staffing, patient census, and supply availability to optimize healthcare delivery and improve equity. This can occur only when large-scale data across different institutions and healthcare systems can be securely combined into a single identity-protected mega-dataset for widespread use (16, 17). Such large-scale federation of patient, clinician, and healthcare system data will refine practices in different environments and help detect and mitigate disparities in healthcare delivery. Practice variations both within and across healthcare settings will be captured and analyzed to inform potential new areas of therapy and intervention (18). Indeed, one consequence of the COVID-19 pandemic has been the rapid adoption of international, multiplatform, adaptive, pragmatic clinical trials (19, 20). These large, federated international datasets will constitute an influential platform for clinical research design and integration. These datasets will allow for global pathogen surveillance and better detection of emerging pandemics. They will improve emergency responses to mass casualty events, natural disasters, and military conflicts and enable minute-by-minute awareness of situation severity as well as more efficient and equitable resource allocation across local, regional, and national healthcare agencies (21).
Challenges related to innovations in data acquisition include the need for rigorous and transparent safeguarding of patient privacy and ensuring equitable use of data, devices, and technology. During the next years to decades, patients, family members, clinicians, medical researchers, administrators, health policy advocates, and government and regulatory entities will need to partner together to demand and develop better transparency and assurance around data security, privacy, and sharing to protect patient health and well-being (22, 23). Current financial and banking practices already share such data with medical data requiring similar, if not even higher, security. Emerging strategies using blockchain technology have demonstrated that it is possible to federate data in ways that address security concerns (24). Yet as the use of such data increases, we must also demand that novel technologies be used equitably. Such potentially transcendent technology could alleviate disparities but could just as easily exacerbate them, particularly if technology and expertise are concentrated in institutions that are already well resourced rather than that in resource-limited environments that could benefit most (25).
THE CASE CONTINUES
Throughout the patient’s hospitalization, her current and past records are seamlessly linked and available in an accessible format to all clinicians in real time and across the spectrum of hospital care settings and teams. Past discussions with other clinicians concerning her values and goals of care are available, allowing her current clinical team to meet her stated wishes and continue these discussions as needed. Although her multiprofessional care team may change as she is transferred in and out of the ICU, team members effectively communicate with each other throughout. The patient and her family and friends are considered part of her care team, and her family and friends can learn about and assist in her clinical care, if they wish. Devices in the patient’s room allow her to see and interact with geographically distant family members and her spiritual leader. She and her family also use these devices to speak with other patients and support groups, receive information about her condition, communicate with her care team, view her current therapies, play music, and display images of her choosing. Bed motion and position sensors report a dashboard of patient activity, including the percentage of time the head of bed is less than 30%, the last time she was turned, agitation episodes, sleep quality, and ambient light and noise levels that conflict with her natural circadian rhythms. Sedative pharmacotherapy is less frequently needed because it has been increasingly replaced with technology that mitigates delirium development by engaging with the patient through conversation, games, massage, reading, ICU diary entry, orientation, and oral intake, as appropriate.
The patient recovers from her pneumonia and is extubated. Before discharge, a post-ICU clinic visit is arranged to help her continue her recovery beyond the ICU. Hospitalization records, with pertinent information related to potential ICU-related morbidities, are accessed electronically by her PCP and her other outpatient clinicians. She is discharged home with wearable monitors for prediction and early detection of myocardial ischemia, sepsis, and other conditions that may occur after acute, severe pneumonia.
TOWARD A MORE PATIENT-CENTERED CRITICAL CARE TEAM AND ENVIRONMENT
The critical care team of the future will continue to be multiprofessional, with diverse generalists, therapists, specialists, and subspecialists seamlessly collaborating toward the common goal of optimal and humanistic patient care in a learning healthcare system (Table 1). The team’s wearable technology will optimize staffing patterns by tracking and mitigating excessive workloads while monitoring for mental and physical fatigue and distraction that could worsen patient care and/or exacerbate clinician burnout (26). In addition to multiprofessional diversity, critical care teams of the future will also readily reflect the cultural, ethnic, gender identity, and geographic diversity of the communities they serve (27) and be trained and skilled in communication approaches that are both patient- and family-centered as well as culturally competent and sensitive (28). Patients’ families and friends will be further integrated into the ICU team and actively encouraged to participate in patient care (29). Geographically distant family members will be able to virtually follow the patient’s course and communicate with the clinical team in real time and via messaging. Family and friends can provide specific information of what a restorative environment might consist of for the patient (e.g., family photographs, a personal diary, and nature imagery) (30) and help to emotionally support the patient and care team throughout the hospitalization.
TABLE 1.
ICU Team of the Future Functioning Within a Learning Healthcare System
| Workplace Challenges for the ICU Team | ||
|---|---|---|
| Complex Patients | Complex Environment | Complex Therapies |
| Complex chronic comorbidities | Increasing technology | Closed-loop titration systems |
| Chronic invasive devices | Expanding documentation | Inflammatory response manipulation |
| Out-of-hospital monitoring | Risk of information overload | Stress response modulation |
| Compromised immune status | Challenges of continuity | Maintaining patient participation |
| Bimodal extended life span | Danger of alarm fatigue | Monitoring expected trajectory |
| Increased illness severity | Ensuring adequate handoff | New therapies to minimize postintensive care syndrome |
| Telehealth from the ICU | Addressing patient, family, and clinicians | |
| Diurnal rhythm | ||
| Workforce shortages | ||
| Rising cost of healthcare | ||
| Intercalated Elements of a Learning Healthcare System | ||
| Patient Care | Shared Education | Clinical Research |
| Multidisciplinary | Just-in-time simulation | Links to national databases |
| Patient- and family-centered | Frequent debriefing sessions | Continuous QI |
| Evidence-based | Joint case reviews | All patients potential research subjects |
| Ultrasonography proficiency | Burnout risk awareness | Dashboards for QI activity |
| Holistic and humanistic | Monitoring of provider competency | |
| Characteristics of the ICU Team of the Future | ||
| Individual | Team | Administration |
| Enjoys out-of-box challenges | Promotes situational awareness | Provides adequate staffing |
| Nurtures resilience | Encourages team members | Supports multifaceted QI |
| Engages in nonwork renewal | Encourages family as part of ICU team | Seeks suggestions for improvement |
| Values humanism in care | Avoids wasteful practices | Maintains awareness of front line |
| Practices personal wellness | Practices cross-training | Monitors staff turnover |
| Has ICU personality | Has multiprofessional and collaborative members | Facilitates/funds national networking |
| Provides patient and family support and comfort | Offers lifestyle improvement perks | |
QI = quality improvement.
Telehealth technology will be leveraged to facilitate ready consultation and communication with geographically distant specialists and subspecialists (31–33). Therapists from diverse disciplines (e.g., respiratory, occupational, physical, cognitive, and rehabilitation) will collaborate with critical care clinicians to develop and enact therapeutic plans that may start in the ICU and continue to outpatient and home-based settings (34, 35). Pharmacists will provide medication consultation throughout hospitalization. Dietitians will use patient-specific nutritional data from technologies such as indirect calorimetry, ultrasound, or MRI-evaluated muscles mass to inform individualized nutrition care plans (36). Critically ill patients who are cognizant but unable to verbalize will use technology-assisted devices to communicate (37).
The ICU environment of the future will be designed to support the physical, emotional, and spiritual needs of patients and family members and mitigate their risks for postintensive care syndrome (PICS), PICS-family, and PICS-pediatric (38–40). Wearable technologies and home-based rehabilitation programs will also better identify and ameliorate these syndromes (41). Future ICU design will distinguish between clinical and nonclinical areas to better integrate humanistic objects; the optimal setting will optimize physical, emotional, and mental well-being for the patient, family, and critical care team (42). Equipment and medical devices will be smaller and less obtrusive. The ICU bed will likely be able to draw specimens for laboratory tests, perform diagnostic imaging, and receive and administer medications. The bed may look like a full-body device that is worn, providing constant passive movement to ensure muscles tone. (43) Critical care in the future may not be limited to a physical hospital location; ancillary critical care environments will potentially include a patient’s home, field hospitals, or low-resource environments with virtual input from experts around the world (44, 45).
EMERGING TECHNOLOGY ADOPTION, EQUITY, AND THE ROLE OF PROFESSIONAL SOCIETIES IN THE FUTURE
Through advocacy, policy, and education, patients, families, clinicians, public health officials, government entities, and professional societies will need to drive innovation and change and to identify and mitigate barriers to critical care progress as well as critical care disparities (Table 2). Indeed, thoughtful and strategic alliances among diverse stakeholders will be essential for effective and equitable dissemination of evidence-informed critical care innovations.
TABLE 2.
Overcoming Barriers
| Domain | Approaches to barrier mitigation |
|---|---|
| Enhance collaboration and communication | Professional societies, government entities, public health officials, critical care clinicians, and patients and their families will need to come together to drive change and innovation to promote the optimal health outcomes of all. Communication and collaboration will be essential in creating the ideal future |
| Evolving technologies | All technologies and algorithms will need to be constantly, iteratively, and systematically evaluated for accuracy, validity, false alerts, and potential ingrained gender, racial, and/or socioeconomic biases |
| Critical care practitioners, patients, and their families will also need to embrace a more integral role in the development, testing, validation, and implementation of technology and emerging artificial intelligence models to ensure that the models are useful, useable, enhance the value of care, and improve patient and family outcomes and experiences | |
| Enhanced security of health data | Security features will need to evolve to protect health-related data; this is imperative to protecting against potential collapse of healthcare systems or, more importantly, unnecessary patient demise (22, 23) |
| Evolving research techniques | Well-designed randomized clinical trials will need to unequivocally demonstrate that novel algorithms and technologies effectively and equitably improve clinically relevant and patient-centered outcomes |
| Provider responsibilities to improve patient care | Critical care professionals will need to be open to adopting and implementing novel and emerging technologies, particularly when evidence supports that such technologies likely improve patient care and/or outcomes |
| Equally important, professionals will need to reject or deescalate emerging technologies and processes when evidence indicates lack of benefit or potentially worse outcomes | |
| Innovative educational models | Training of critical care professionals will need to include education on application of new technology and data science and interpretation of data and outputs of smart algorithms |
As rapid evolution of technology will change the practice of critical care, it will also transform the ways professional societies support critical care practitioners and communities (Table 3). Critical care has always been a technology- and data-dependent field. As big data and technology potentially revolutionize critical care practice, professional societies are well positioned to partner with patients, families, practitioners, researchers, industry leaders, policymakers, and administrators to ensure that humanistic, high-value, ever-improving patient care remains the central goal for the future of critical care medicine.
TABLE 3.
Role of Professional Societies in the Future
| Domain | Approaches |
|---|---|
| Improve outcomes for a diverse population of critically ill and injured patients | Potentiate critical care research both through research funding portfolios and by enhancing knowledge dissemination |
| Develop real-time updates to clinical practice guidelines | |
| Advocate for equitable and inclusive critical care, particularly in underresourced care settings | |
| Expand and support a global network of critical care professionals | Develop collaborative relationships between and across local and global clinician organizations and critical care professionals |
| Facilitate better development of practical and innovative tele-critical care networks | |
| Advance multimodal educational opportunities for diverse healthcare professionals | |
| Advocate for patients, families, and critical care professionals | Partner with governmental entities, private foundations, nonprofit organizations, associations, industry, and other groups to better advocate for critically ill patients, their families, and their critical care practitioners |
| Build and support innovative programs that potentiate thriving of critical illness survivors and their families | |
| Lead in public communications promoting critical care awareness |
CONCLUSIONS
It is not a futuristic concept to build a multiprofessional patient-centered critical care team that provides the right care to the right patient at the right time through the entire continuum of acute illness and injury. This is a timeless core principle of critical care medicine and comprises the “Right Care, Right Now” motto for the Society of Critical Care Medicine. What will change in the future is how we use data, devices, and new technologies to continue to strive toward that goal. These anticipated changes will occur throughout healthcare, though critical care may be at the forefront because of its data-heavy, complex environment and high-acuity, time-sensitive interventions. It is also our belief that critical care medicine practitioners are uniquely positioned to advocate for and lead the development and implementation of these future efforts to ensure that patient-centered care and patient, family, and clinician experience remain the central focus. For more information on the Society of Critical Care Medicine Future of Critical Care Task Force timeline, process, and discussion topics, please see Appendix A, http://links.lww.com/CCX/A947, and Appendix B, http://links.lww.com/CCX/A948.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Gong has received compensation from Regeneron for service as a member of a data safety and monitoring board. Dr. Wong holds equity and management roles in Ataia Medical. Dr. Brown chairs a data safety monitoring board for Hamilton Ventilators, and his employer, Intermountain Healthcare, has been paid for his research services by Faron, Sedana, and Janssen. Dr. DePriest received payment in the last year from Baxter for two recorded educational lectures addressing critical care nutrition and enteral nutrition. Dr. Narayan serves on Advisory Board and possesses stock for Medcura [topical hemostat company]. Dr. Provencio serves as a paid consultant and has grant funding from Minnetronix and serves as a paid consultant for Cryothermics. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Shankar-Hari M, Wunsch H, Rowan K, et al. : Reflections on critical care’s past, present, and future. Crit Care Med 2021; 49:1855–1865 [DOI] [PubMed] [Google Scholar]
- 2.Weil MH, Tang W: From intensive care to critical care medicine: A historical perspective. Am J Respir Crit Care Med 2011; 183:1451–1453 [DOI] [PubMed] [Google Scholar]
- 3.Weil MH, Shoemaker WC: Pioneering contributions of Peter Safar to intensive care and the founding of the Society of Critical Care Medicine. Crit Care Med 2004; 32:S8–S10 [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Bellissant E, Bollaert PE, et al. : Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev 2019; 12:CD002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeschke R, Angus DC: Living with uncertainty in the intensive care unit: Should patients with sepsis be treated with steroids? JAMA 2009; 301:2388–2390 [DOI] [PubMed] [Google Scholar]
- 6.Herasevich V, Litell J, Pickering B: Electronic medical records and mHealth anytime, anywhere. Biomed Instrum Technol 2012; Suppl:45–48 [DOI] [PubMed] [Google Scholar]
- 7.Schoettler N, Strek ME: Recent advances in severe asthma: From phenotypes to personalized medicine. Chest 2020; 157:516–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsimberidou AM, Fountzilas E, Nikanjam M, et al. : Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat Rev 2020; 86:102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong HR, Atkinson SJ, Cvijanovich NZ, et al. : Combining prognostic and shock responsive to corticosteroids. Crit Care Med 2016; 44:e1000–e1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weir CR, Nebeker JR. Critical issues in an electronic documentation system. AMIA Annu Symp Proc. 2007; 2007:786–790 [PMC free article] [PubMed] [Google Scholar]
- 11.Fagherazzi G, Fischer A, Ismael M, et al. : Voice for health: The use of vocal biomarkers from research to clinical practice. Digit Biomark 2021; 5:78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churpek MM, Yuen TC, Winslow C, et al. : Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med 2014; 190:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branson RD: Automation of mechanical ventilation. Crit Care Clin 2018; 34:383–394 [DOI] [PubMed] [Google Scholar]
- 14.Morris AH, Stagg B, Lanspa M, et al. : Enabling a learning healthcare system with automated computer protocols that produce replicable and personalized clinician actions. J Am Med Inform Assoc 2021; 28:1330–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey JD, Courtright KR, Rice TW, et al. : What can a learning healthcare system teach us about improving outcomes? Curr Opin Crit Care 2021; 27:527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estiri H, Klann JG, Weiler SR, et al. : A federated EHR network data completeness tracking system. J Am Med Inform Assoc 2019; 26:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens KA, Anderson N, Lin CP, et al. : Implementing partnership-driven clinical federated electronic health record data sharing networks. Int J Med Inform 2016; 93:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoto M, Oakes M, Stuart E, et al. : Analytical methods for a learning health system: 2. Design of observational studies. EGEMS (Wash DC) 2017; 5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, et al. : Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021; 385:777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators; Lawler PR, Goligher EC, Berger JS, et al. : Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021; 385:790–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh DP, Ma TF, Ip HS, et al. : Artificial intelligence and avian influenza: Using machine learning to enhance active surveillance for avian influenza viruses. Transbound Emerg Dis 2019; 66:2537–2545 [DOI] [PubMed] [Google Scholar]
- 22.Sabin S: Hospital ransomware attacks now have deadly consequences. Politico. October 4, 2021. Available at: https://www.politico.com/newsletters/weekly-cybersecurity/2021/10/04/hospital-ransomware-attacks-now-have-deadly-consequences-798002. Accessed November 4, 2021
- 23.Storm D: MEDJACK: Hackers hijacking medical devices to create backdoors in hospital networks. Computerworld. June 8, 2015. Available at: https://www.computerworld.com/article/2932371/medjack-hackers-hijacking-medical-devices-to-create-backdoors-in-hospital-networks.html. Accessed November 4, 2021
- 24.Warnat-Herresthal S, Schultze H, Shastry KL, et al. ; COVID-19 Aachen Study (COVAS); Deutsche COVID-19 Omics Initiative (DeCOI): Swarm learning for decentralized and confidential clinical machine learning. Nature 2021; 594:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tossas-Milligan KY, Winn RA: Breaking the cycle of health inequities: The bioethics of data. J Health Care Poor Underserved 2019; 30:86–90 [DOI] [PubMed] [Google Scholar]
- 26.Rosen MA, Dietz AS, Lee N, et al. : Sensor-based measurement of critical care nursing workload: Unobtrusive measures of nursing activity complement traditional task and patient level indicators of workload to predict perceived exertion. PLoS One 2018; 13:e0204819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sprung CL, Jennerich AL, Joynt GM, et al. : The influence of geography, religion, religiosity and institutional factors on worldwide end-of-life care for the critically ill: The WELPICUS study. J Palliat Care 2021 Apr 5. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Markin A, Cabrera-Fernandez DF, Bajoka RM, et al. : Impact of a simulation-based communication workshop on resident preparedness for end-of-life communication in the intensive care unit. Crit Care Res Pract 2015; 2015:534879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyskiel RM, Chang BH, Alday AA, et al. : Towards expanding the acute care team: Learning how to involve families in care processes. Fam Syst Health 2015; 33:242–249 [DOI] [PubMed] [Google Scholar]
- 30.Nadkarni NM: After the fall: The tapestry of disturbance and recovery. Crit Care Med 2017; 45:348–355 [DOI] [PubMed] [Google Scholar]
- 31.Subramanian S, Pamplin JC, Hravnak M, et al. : Tele-critical care: An update from the Society of Critical Care Medicine Tele-ICU Committee. Crit Care Med 2020; 48:553–561 [DOI] [PubMed] [Google Scholar]
- 32.Fathi JT, Modin HE, Scott JD. Nurses advancing telehealth services in the era of healthcare reform. Online J Issues Nurs. 2017; 22:228488818 [Google Scholar]
- 33.Davidson R, Barrett DI, Rixon L, et al. ; ACT Program: How the integration of telehealth and coordinated care approaches impact health care service organization structure and ethos: Mixed methods study. JMIR Nurs 2020; 3:e20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholls J, MacKenzie C, Braund R: Preventing drug-related adverse events following hospital discharge: The role of the pharmacist. Integr Pharm Res Pract 2017; 6:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirkham HS, Clark BL, Paynter J, et al. : The effect of a collaborative pharmacist-hospital care transition program on the likelihood of 30-day readmission. Am J Health Syst Pharm 2014; 71:739–745 [DOI] [PubMed] [Google Scholar]
- 36.Looijaard WGPM, Molinger J, Weijs PJM: Measuring and monitoring lean body mass in critical illness. Curr Opin Crit Care 2018; 24:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson KM, O’Neill Zimmerman T, Light J: Visual attention to cued targets in simulated aided augmentative and alternative communication displays for individuals with intellectual and developmental disabilities. J Speech Lang Hear Res 2021; 64:1726–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikkelsen ME, Still M, Anderson BJ, et al. : Society of Critical Care Medicine’s international consensus conference on prediction and identification of long-term impairments after critical illness. Crit Care Med 2020; 48:1670–1679 [DOI] [PubMed] [Google Scholar]
- 39.Needham DM, Davidson J, Cohen H, et al. : Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med 2012; 40:502–509 [DOI] [PubMed] [Google Scholar]
- 40.Manning JC, Pinto NP, Rennick JE, et al. : Conceptualizing post intensive care syndrome in children-the PICS-p framework. Pediatr Crit Care Med 2018; 19:298–300 [DOI] [PubMed] [Google Scholar]
- 41.Major ME, Dettling-Ihnenfeldt D, Ramaekers SPJ, et al. : Feasibility of a home-based interdisciplinary rehabilitation program for patients with post-intensive care syndrome: The REACH study. Crit Care 2021; 25:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesecioglu J. Improving the patient’s environment: The ideal intensive care unit. Réanimation 2015; 24:341–343 [Google Scholar]
- 43.Halpern NA, Anderson DC, Kesecioglu J: ICU design in 2050: Looking into the crystal ball! Intensive Care Med 2017; 43:690–692 [DOI] [PubMed] [Google Scholar]
- 44.Sessler CN: Evolution of ICU design: Smarter is better. Chest 2014; 145:205–206 [DOI] [PubMed] [Google Scholar]
- 45.Amaral AC, Rubenfeld GD: The future of critical care. Curr Opin Crit Care 2009; 15:308–313 [DOI] [PubMed] [Google Scholar]