Abstract
The capacity of metronidazole to inhibit the growth of Mycobacterium tuberculosis was tested in in vitro and in vivo mouse models. In vitro addition of metronidazole to cultures of infected bone marrow-derived macrophages had no effect, nor did it increase the reduction in bacterial load due to isoniazid. In vivo, metronidazole did not reduce bacterial numbers in the lungs of aerosol-infected mice during the active stage of the disease, during a phase of containment, or after prolonged isoniazid therapy (Cornell model). A small but significant reduction was seen if metronidazole therapy was given during an established chronic disease state 100 days after aerosol administration. These data indicate that under most conditions M. tuberculosis organisms are not in a metabolic state in which they are susceptible to the action of metronidazole and, hence, that this drug would be of limited clinical value.
In many individuals, Mycobacterium tuberculosis infection takes the form of a latent disease state. At some time thereafter, often for poorly understood reasons, the disease may reactivate and give rise to an active and often fatal infection. In some patients, this may occur even after the individual has been given prolonged chemotherapy. Animal models have been used to address the latter issue (6), but the basis for why this minor population of bacilli still survive remains unclear.
What has been equally unclear for some time is the physiological status of these surviving bacilli in the infected host. A growing body of data indicates that the phagosomal environment may not be as hostile as was once believed (16), and there is a growing appreciation of the capacity of major bacterial cell wall lipoglycans and complex carbohydrates to scavenge oxygen and nitrogen radicals (1), in turn explaining the resistance of many clinical isolates to these materials (14).
Furthermore, the bacterium may actively adapt in order to survive. The existence of latent or stationary-phase genes has been established (4), although whether these are operative or even needed in mycobacteria has yet to be shown. Another possibility is that, faced with the low oxygen tension that is probably operative in the host granuloma, the bacterium is forced to use anaerobic metabolic pathways in order to generate energy (via NADH). This hypothesis, recently proposed by Wayne (20), is based upon the activation of glyoxylic acid pathway enzymatic activity in bacilli exposed to a hypoxic environment in vitro. This in turn has led to the suggestion that drugs such as metronidazole that preferentially target anaerobic organisms may be effective against M. tuberculosis under these conditions. The action of this class of drugs is still not precisely understood, but the susceptible organism must have the ability to reduce metronidazole under low redox potential conditions to produce a metabolite which is believed to cause DNA strand breakage, thus preventing bacterial replication (7–9).
In this study, metronidazole was tested for its capacity to kill M. tuberculosis under a variety of in vitro and in vivo conditions. In general, the results were negative, although in one specific situation an apparent antimicrobial effect was seen.
Female C57BL/6 mice were obtained from the Jackson Laboratories, Bar Harbor, Maine, and were used when they were 8 weeks of age. All animals were housed in a level III biosafety facility. M. tuberculosis Erdman was grown and was stored as described previously (11). Mice were exposed to the infection with an aerosol generation device (Glas-Col, Terre Haute, Ind.) that deposited approximately 100 viable bacilli into the lungs (2). The course of the infection was monitored against time by plating serial dilutions of individual whole-lung homogenates on nutrient 7H11 nutrient agar and counting bacterial colony formation after 3 weeks incubation at 37°C in humidified air. Drugs were dissolved in distilled water and given by gavage. Because the length of time for gavage in the Cornell model exceeded the length of time that we were permitted to perform gavage, each of the drugs was included in the water supply at 100 mg/liter (mean water consumption approximates this to be 25 mg/kg of body weight/day) and mice were allowed to drink water ad libitum (12). To induce the regrowth of the surviving bacilli, mice were given hydrocortisone sulfate (1 mg/day) subcutaneously for 7 days. Differences in bacterial loads were compared by the Student t test or the Wilcoxon rank test (for the Cornell model).
Bone marrow-derived macrophages were cultured in vitro as described previously (17). Briefly, bone marrow cells were cultured in fibroblast-conditioned media for 10 days and were then washed and infected with M. tuberculosis. Four hours later the monolayer was washed again to remove noningested bacilli. The drugs were then added at the indicated concentrations, and the cells were cultured for another 8 days, after which the monolayers were lysed and plated as described above.
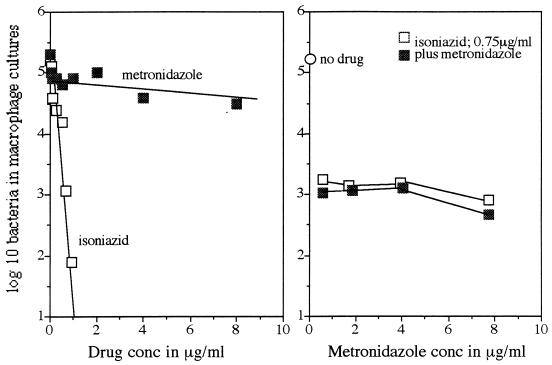
The capacity of metronidazole to inhibit the growth of M. tuberculosis in murine macrophages is indicated in Fig. 1. Isoniazid inhibited the growth of the Erdman strain in a dose-dependent manner, whereas increasing concentrations of metronidazole had no effect. The addition of metronidazole to cultures already inhibited by a single concentration of isoniazid also did not decrease bacterial numbers any further.
FIG. 1.
Effect of increasing doses of drug on growth of M. tuberculosis Erdman within bone marrow-derived macrophage monolayers. The right panel shows the results of an experiment in which increasing doses of metronidazole were added to a fixed dose of isoniazid (0.75 μg) previously determined to inhibit 99% of the bacterial inoculum. Data are expressed as the mean numbers of bacteria recovered from three separate culture wells; standard errors of the means did not exceed 0.5.
The effects of metronidazole treatment begun at the time (day 30) at which acquired immunity causes the active disease to slow and give rise to a state of chronic disease in vivo are indicated in Fig. 2. Metronidazole (15 mg/kg) had no effect on the growth of the Erdman strain. The possibility that the dose of drug was too low was discounted by pharmacokinetic analysis of mice, which showed an average peak serum metronidazole level of 5.9 μg/ml, thus falling within the range of 4.6 to 11.3 μg/ml seen in humans given therapeutic doses of this drug.
FIG. 2.
Failure of metronidazole (15 mg/kg/day) given daily from day 30 through day 80 after aerosol administration to inhibit growth of M. tuberculosis Erdman in the lungs. ■, controls; □, metronidazole therapy. Data indicate mean numbers of bacteria recovered (n = 4); standard errors of the means did not exceed 0.3.
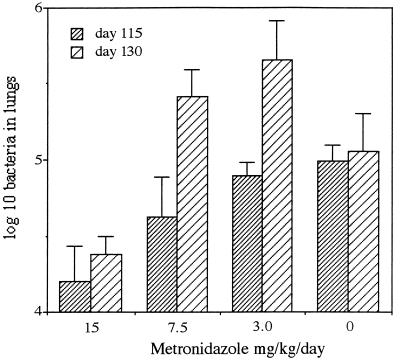
At approximately 80 to 100 days after aerosol administration, mice establish a chronic disease state in which no overt changes in bacterial load can usually be detected (13). Administration of metronidazole (15 mg/kg) during this period of time caused a relatively small but statistically significant reduction in bacterial counts (Fig. 3).
FIG. 3.
Capacity of metronidazole (15 mg/kg/day) to inhibit growth of M. tuberculosis Erdman during the chronic stage of disease. Treatment was given from day 100 to day 130, with bacterial numbers assessed on days 115 and 130. Data are mean standard error of the mean numbers of bacteria recovered (n = 4).
Finally, we examined the effect of the addition of metronidazole to the therapy stage of the Cornell model (10) in vivo. This model was originally developed to try to explain why a small number of bacteria survive after prolonged chemotherapy, and many consider it a model (albeit, a still not well explained model) of persisting bacteria that may be in a latent or dormant state. In this model mice are infected with M. tuberculosis and then after several weeks are begun on a protracted course of chemotherapy until bacteria can no longer be detected (by colony counts) in target organs. Because very small numbers of bacteria persist, cortisol is then given in order to immunosuppress the animal and induce the regrowth of bacterial persistors. Hence, if the addition of metronidazole to the isoniazid therapy further reduced the numbers of persisting bacilli, then fewer mice would show evidence of regrowth and/or fewer bacterial colonies would be recovered from the lungs. As indicated in Table 1, however, metronidazole therapy did not significantly influence bacterial persistence in two separate experiments.
TABLE 1.
Addition of metronidazole to Cornell model
| Expt no. and treatment | No. of mice with regrowth/no. of mice tested | No. of colonies recovered (mean ± SEM) |
|---|---|---|
| Expt 1 | ||
| Isoniazid only | 6/8 | 35 ± 12 |
| Isoniazid plus metronidazole | 4/6 | 17 ± 18 |
| Expt 2 | ||
| Isoniazid only | 19/25 | 42 ± 7 |
| Isoniazid plus metronidazole | 16/24 | 37 ± 5 |
Mice were infected by aerosol with approximately 100 viable M. tuberculosis Erdman organisms. After 60 days the mice were given isoniazid alone or both drugs for 100 days. No bacteria could be detected in the lungs at 100 days. After 20 days of rest the mice were given cortisol once daily for 1 week, and then after another 7 days the mice were killed and their lungs were harvested and plated (whole-organ homogenate on 15-cm petri dishes). No differences in terms of the numbers of mice with reactivated infections or in the actual colony counts that were recovered were seen (P > 0.4; Wilcoxon rank test).
The results of this study indicate that in most situations the addition of metronidazole to antimycobacterial therapy does not significantly increase the level of bacterial clearance. The results support the hypothesis that, in general, bacteria in the infected host are not predominantly in a state of anaerobic metabolism, at least as it may pertain to susceptibility to metronidazole, even though the bacterial curves may indicate a state of chronic or latent disease. In this regard, we have previously shown that the lung granuloma in infected mice is in an apparent state of flux, despite minimal changes in bacterial load (15). Bacterial metabolism may be considerably slowed, and cell wall elongation may have shut down, but there is no evidence as yet that the oxygen tension has become so low that anaerobic pathways are required. In addition, the idea that these bacteria survive over very long periods of time by becoming truly latent is not supported by our observation that bacteria harvested from chronically infected mice and bacteria taken directly from frozen cultures in vitro grow identically when inoculated into gamma interferon gene-disrupted mice (3).
Having said that, metronidazole had a small but significant effect during a phase of the infection in which we speculate (there is as yet no real hard evidence) that the surviving bacteria are in a general state of nonreplication (resulting in a chronic disease state), probably as a result of the sustained production of gamma interferon that occurs in the lungs at that time (2). As a result, a proportion of these bacilli may be experiencing adverse intraphagosomal conditions (low partial O2 pressure, low pH) which force them into a metabolic state in which they are susceptible to the action of metronidazole.
Perhaps the conditions most suited to such mechanisms would be a combined onslaught by host immunity and administered chemotherapy, as in the Cornell model (taking into account the assumption, which is as yet unproven, that this model does indeed model the events in infected humans). Yet here, in two separate studies, metronidazole had no effect on the eventual outcome of the model.
These data therefore provide useful information which supports the hypothesis that the “persistor” bacilli in the Cornell model do not survive by entering a state of anaerobic metabolism and that the local oxygen tension is not low enough to switch on anaerobic pathways and make the bacilli susceptible to metronidazole, as data from the laboratory of Wayne and colleagues indicate (19–23).
In this regard, it is still not understood how a few bacilli survive the prolonged exposure to isoniazid therapy. We are hesistant to invoke the “much granules” hypothesis (18) here, but certainly, a reasonable working hypothesis is that a few mycobacteria survive by building only a partially complete cell wall, perhaps as an adaptation to lower levels of metabolic energy sources. Thus, if some mycobacteria construct the peptidoglycan core and perhaps the arabinogalactan layer but do not synthesize the full mycolic acid layer beyond that, then these bacilli may then be resistant to isoniazid, given the target for this drug within mycolic acid biosynthesis and elongation. Once the local conditions (oxygen tension?) are then more conducive to regrowth, then the residual infection reactivates. Under these conditions, the bacilli synthesize the full cell wall, explaining why they are subsequently found to be isoniazid susceptible.
In summary, therefore, metronidazole therapy of mice infected by aerosol exposure to M. tuberculosis was only minimally effective and was then effective only under very specific conditions. Unless surrogate clinical markers can be developed to identify individuals who are in this disease state, then widespread use of this drug seems unwarranted. An exception to this may be advanced disease, in which clinical improvement with metronidazole therapy has been reported (5).
Acknowledgments
This study was supported by NIH programs AI-45244 and AI-45239.
We thank Brian Kelly for contributions to this work and Phillip Chapman, Department of Statistics, Colorado State University, for help with analysis of the Cornell model data.
ADDENDUM IN PROOF
After the manuscript was submitted, similar conclusions were reported in another article (J. Dhillon, B. W. Allen, Y. M. Hu, A. R. Coates, and D. A. Mitchison, Int. J. Tuberc. Lung Dis. 2:736–742, 1998).
REFERENCES
- 1.Chan J, Fan X D, Hunter S W, Brennan P J, Bloom B R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper A M, Callahan J E, Keen M, Belisle J T, Orme I M. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tubercle Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 3.Cooper, A. M., and I. M. Orme. Unpublished observations.
- 4.DeMaio J, Zhang Y, Ko C, Bishai W R. Mycobacterium tuberculosis sigF is part of a gene cluster with similarities to the Bacillus subtilis sigF and sigB operons. Tubercle Lung Dis. 1997;78:3–12. doi: 10.1016/s0962-8479(97)90010-1. [DOI] [PubMed] [Google Scholar]
- 5.Desai C R, Heera S, Patel A, Babrekar A B, Mahashur A A, Kamat S R. Role of metronidazole in improving response and specific drug sensitivity in advanced pulmonary tuberculosis. J Assoc Physicians India. 1989;37:694–697. [PubMed] [Google Scholar]
- 6.Dhillon J, Dickinson J M, Sole K, Mitchison D A. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob Agents Chemother. 1996;40:552–555. doi: 10.1128/aac.40.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards D I. The action of metronidazole on DNA. J Antimicrob Chemother. 1977;3:43–48. doi: 10.1093/jac/3.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Knight R C, Skolimoowski I M, Edwards D I. Effect of reduced metronidazole on DNA. Biochem Pharmacol. 1978;27:2089–2093. doi: 10.1016/0006-2952(78)90277-0. [DOI] [PubMed] [Google Scholar]
- 9.Lau A H, Lam N P, Piscitelli S C, Wilkes L, Danzinger L H. Clinical pharmacokinetics of metronidazole and other nitroimidazole anti-infectives. Clin Pharmacokinet. 1992;23:328–364. doi: 10.2165/00003088-199223050-00002. [DOI] [PubMed] [Google Scholar]
- 10.McCune R M, Tompsett R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med. 1956;104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 12.Orme I M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988;140:3589–3593. [PubMed] [Google Scholar]
- 13.Orme I M, McMurray D N. The immune response to tuberculosis in animals. In: Rom W N, Garay S, editors. Tuberculosis infections. New York, N.Y: Little, Brown, & Company; 1996. pp. 269–280. [Google Scholar]
- 14.Rhoades E R, Orme I M. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhoades E R, Frank A A, Orme I M. Progression of pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tubercle Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 16.Russell D G, Sturgill-Koszycki S, Vanheyningen T, Collins H, Schaible U E. Why intracellular parasitism need not be a degrading experience for Mycobacterium. Phil Trans R Soc London. 1997;352:1303–1310. doi: 10.1098/rstb.1997.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner P S, Furney S K, Jacobs M K, Klopman G, Ellner J J, Orme I M. A bone marrow derived murine macrophage model for evaluating efficacy of anti-myocobacterial drugs under relevant physiological conditions. Antimicrob Agents Chemother. 1994;38:2557–2563. doi: 10.1128/aac.38.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanford J L. Much’s granules revisited. Tubercle. 1987;68:241–242. doi: 10.1016/0041-3879(87)90063-8. [DOI] [PubMed] [Google Scholar]
- 19.Wayne L G. Dynamics of submerged growth of Mycobacterium tuberculosis under aerobic and microaerophilic conditions. Am Rev Respir Dis. 1976;114:807–811. doi: 10.1164/arrd.1976.114.4.807. [DOI] [PubMed] [Google Scholar]
- 20.Wayne L G. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 21.Wayne L G, Diaz G A. Autolysis and secondary growth of Mycobacterium tuberculosis in submerged culture. J Bacteriol. 1967;93:1374–1381. doi: 10.1128/jb.93.4.1374-1381.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wayne L G, Hayes L G. An in vivo model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wayne L G, Sramek H A. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1994;38:2054–2058. doi: 10.1128/aac.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wayne L G, Lin K-Y. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]